Abstract
Purpose
Perioperative stroke is associated with significant morbidity and mortality, with an incidence that may be underappreciated. In this review, we examine the significance, pathophysiology, risk factors, and evidence-based recommendations for prevention and management of perioperative stroke.
Source
This is a narrative review based on literature from the PubMed Database regarding perioperative stroke across a broad surgical population. The Society for Neuroscience in Anesthesiology and Critical Care recently published evidence-based recommendations for perioperative management of patients at high risk for stroke; these recommendations were analyzed and incorporated into this review.
Principal Findings
The incidence of overt perioperative stroke is highest in patients presenting for cardiac and major vascular surgery, although preliminary data suggest that the incidence of covert stroke may be as high as 10% in non-cardiac surgery patients. The pathophysiology of perioperative stroke involves different pathways. Thrombotic stroke can result from increased inflammation and hypercoagulability, cardioembolic stroke can result from disease states such as atrial fibrillation, and tissue hypoxia from anemia can result from the combination of anemia and beta-blockade. Across large-scale database studies, common risk factors for perioperative stroke include advanced age, history of cerebrovascular disease, ischemic heart disease, congestive heart failure, atrial fibrillation, and renal disease. Recommendations for prevention and management of perioperative stroke are evolving, though further work is needed to clarify the role of proposed modifiable risk factors such as perioperative anticoagulation, anti-platelet therapy, appropriate transfusion thresholds, and perioperative beta-blockade.
Conclusions
Perioperative stroke carries a significant clinical burden. The incidence of perioperative stroke may be higher than previously recognized, and there are diverse pathophysiologic mechanisms. There are many opportunities for further investigation of perioperative stroke pathophysiology, prevention, and management.
INTRODUCTION
Stroke is responsible for approximately 6.2 million deaths annually making cerebrovascular disease a leading global cause of premature death and disability.1 Additionally, cerebrovascular disease is projected to be the second leading cause of death worldwide by the year 2030.2 Given the global burden, many efforts have focused on the prevention and treatment of stroke and other sequelae of cerebrovascular disease. One area of particular concern is the perioperative setting, where patients may be at particular risk of stroke.3 In the United States alone, significant increases (14–47%) in demand for surgical services are expected over the coming years,4 and it follows that the number of perioperative strokes may increase accordingly. Perioperative stroke in high-risk cardiovascular surgery has been well-documented, with an incidence ranging from approximately 1.9–9.7%.5 Currently, the incidence of perioperative ischemic stroke (IS) in non-cardiac, non-neurologic, and non-major vascular surgery ranges from approximately 0.1% to 1.9% depending on associated risk factors.6, 7 Pilot data from the Neurovision study, however, suggest that the incidence of covert stroke in high-risk non-cardiac surgery patients may be as high as 10%.8 This is relevant because clinically silent cerebral ischemia has been proportionally correlated with postoperative cognitive impairment in cardiac surgery patients.9 In addition to the potentially under-appreciated incidence and significance of perioperative stroke, recent data have demonstrated that mortality from perioperative stroke may be particularly high, with an approximate incidence ranging from 20% to 60% depending on type of stroke, operation, and patient.10–12 As such, interest has grown in the identification of those at risk for perioperative stroke as well as potentially modifiable risk factors. This focus has culminated in a consensus statement by the Society of Neuroscience in Anesthesiology an Critical Care (SNACC) for perioperative care of non-cardiac, non-neurological surgery patients at high risk of stroke.13
The SNACC Consensus Statement defines perioperative stroke as a brain infarction of ischemic or hemorrhagic etiology that occurs during surgery or within 30 days after surgery.13 The remainder of this review will thus focus on stroke based on this definition. Specifically, the pathophysiology and risk factors of perioperative stroke will be reviewed, and the recently released SNACC perioperative stroke recommendations will also be reviewed. Lastly, directions for future investigation will be suggested.
PATHOPHYSIOLOGY
Because stroke is caused by a diverse array of etiologies, different stroke subtypes may be a function of varying pathophysiologic pathways. Thus, discussion of the pathophysiology of perioperative stroke begins first with a framework of stroke etiology classification. Though stroke is classified in many different ways (e.g., arterial vs. venous and ischemic vs. hemorrhagic), for the purpose of this review, perioperative stroke will be classified as either ischemic or hemorrhagic.
Ischemic Stroke
Categorization of IS based on etiology has been outlined in the Org 10172 Acute Stroke Treatment (TOAST) study.14 The TOAST Classification system divides IS subtype into large-artery atherosclerosis, cardioembolism, small-artery occlusion (lacunar), stroke of other determined etiology, and stroke of undetermined etiology. Much of the recent literature on perioperative stroke focuses on IS, as the incidence of ischemic perioperative stroke seems to be higher than that of hemorrhagic stroke (HS).15, 16 As such, pathophysiologic cascades that may facilitate perioperative IS have been considered in this context. Though Ng et al.17 reviewed major studies that reported the etiologies of perioperative stroke in the non-cardiac surgery setting, estimated subtype incidences are difficult to ascertain, as many population-based studies do not analyze stroke subtypes with that level of granularity.6, 7, 18 Based on available data, thrombosis, embolism, anemic tissue hypoxia, and cerebral hypoperfusion have all been described as etiologic pathways contributing to perioperative IS.10, 19–21 Below, we will review three of the major pathophysiologic mechanisms of IS – thrombosis, cardioembolism, and anemic tissue hypoxia.
Large- and Small-Vessel Occlusion: Thrombosis
Surgery precipitates systemic inflammation and hypercoagulability,22–24 and this state may contribute to thrombogenesis and vessel plaque rupture in the perioperative setting. Further, patients receiving anticoagulation or antiplatelet therapy preoperatively may be at risk for rebound hypercoagulation25 and subsequent IS upon withdrawal.26 Taken together, this indicates that some patients – especially those on preoperative anticoagulation or antiplatelet therapy – may be at increased risk for perioperative thromboembolic events via a hypercoaguable state, which may be driven by both surgical intervention and rebound hypercoagulation. This hypercoaguable state may be exacerbated by systemic inflammation, which is also increased perioperatively.22, 23, 27 An elevation of inflammatory biomarkers has been shown to predict future stroke,28–30 and it follows that anti-inflammatory measures may conceivably reduce stroke risk. One such possible anti-inflammatory intervention is statin administration, which has been associated with decreased perioperative stroke across multiple surgical populations.31, 32 Though statins act through various pathways, there is evidence to suggest that the anti-inflammatory effects in particular confer stroke protection.33–37 Given the clinical evidence, inflammatory and hypercoaguable factors may indeed combine to increase thrombogenic stroke risk perioperatively.
Cardioembolism
Ischemic stroke of embolic origin is often due to cardioembolism. In this review, cardioembolism refers to any embolic phenomenon originating from the heart, including both valvular and non-valvular sources. In the perioperative setting, atrial fibrillation combined with a hypercoaguable state (as discussed above) may be a source of cardioembolic phenomena. Atrial fibrillation has indeed been a consistent risk factor for perioperative stroke across various surgical populations.5, 6, 38, 39 An additional source of cardioembolic stroke is cardiovascular manipulation of the heart and aortic arch, both of which occur during major cardiac and some vascular surgery (e.g., endovascular stent grafting). These surgeries have, in fact, been linked with a relatively high incidence of perioperative stroke, with embolic stroke representing a relatively high proportion of these events.5, 38
Other Determined Stroke Etiologies – Anemia-Associated Tissue Hypoxia
One potential mechanism by which IS may occur involves cerebral hypoxia in the setting of hemodilution and anemia.40 With anemic states, increased cardiac output (CO) and cerebral blood flow (CBF) act as compensatory mechanisms to preserve oxygenation.41–46 In the setting of non-specific beta-blocker (i.e. metoprolol) use and anemia, both CO and cerebral vasodilation become impaired, which may result in cerebral tissue hypoxia.46–48 Mechanistically, animal models have demonstrated that the β2-mediated reduction in cerebral vasodilatory function may be integral to the reduction in cerebral oxygenation.47, 48 In animal studies, minimizing β2-mediated cerebrovascular antagonism improved cerebral oxygenation in the setting of anemia.49 Ultimately, this aberrant physiology may render vulnerable brain regions at risk for ischemia and stroke. Indeed, clinical data have demonstrated an increased stroke risk in surgical patients on beta-blockade with hemoglobin levels below 9 g/dL; those taking metoprolol were at highest risk.20 Stroke risk was also increased in the POISE trial in patients who experienced significant bleeding.21
Hemorrhagic Stroke
According to the American Heart Association and American Stroke Association (AHA/ASA), stroke of hemorrhagic etiology is attributable to a focal collection of blood within the brain parenchyma, subarachnoid space, or ventricular system that is not caused by trauma.50 Perioperatively, this may plausibly occur via factors such as uncontrolled hypertension, cerebral vascular malformations, and administration of anticoagulant or antiplatelet therapy. Fortunately, however, this seems to be an infrequent occurrence, as HS represents approximately only 1–4% of all perioperative strokes as demonstrated by incidence data.15, 16 As such, much of the research into the mechanisms of perioperative stroke to date centers on IS, which will remain the focus for the rest of this review.
RISK FACTORS
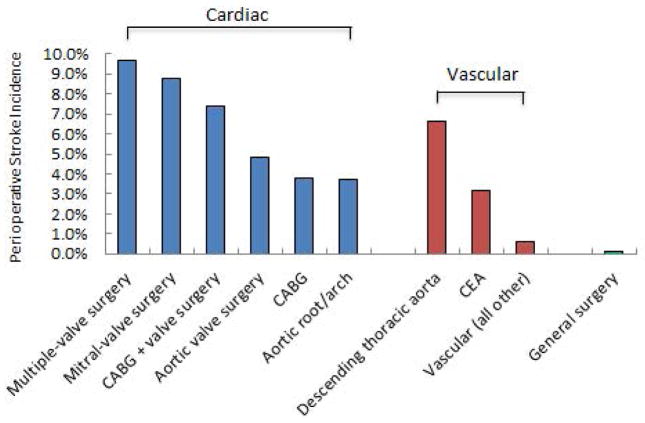
Many risk factors for perioperative stroke have been elucidated over the years, and several of those factors seem to fit well within the pathophysiologic framework as outlined above. These risk factors have largely been derived from case series and large database studies, 5–7, 51 as the rarity of perioperative stroke makes prospective data collection and analysis difficult. In particular, factors that reflect pre-existing vascular disease or propagation of vascular disease have been consistently demonstrated across studies. Examples include advanced age, previous stroke or transient ischemic attacks, coronary artery disease, and renal disease (Table 1).6, 7, 51 These factors may reflect less cerebrovascular reserve and thus higher susceptibility to deleterious cerebral thromboembolic phenomena. Major cardiovascular surgery has a relatively high incidence of perioperative stroke (Figure 3),5, 52 as these surgeries carry the additional risk of cardioembolism from cardiac and vascular manipulation. Along similar lines, atrial fibrillation serves as a risk factor for perioperative embolic stroke.5, 6, 38, 39 Recently, in fact, patients with new-onset atrial fibrillation after non-cardiac surgery were shown to be at increased risk of IS beyond the perioperative setting alone.53.
Table 1.
Stroke risk factors across various surgical populations
| Study | Year | Population | Patients (n) | OR (CI, 95%) |
|---|---|---|---|---|
| Sharifpour et al. | 2013 | Vascular surgery (noncarotid) | 47,750 | |
| Acute renal failure | 2.03 (1.39–2.97) | |||
| History of TIA, stroke, existing hemiplegia | 1.72 (1.29–2.30) | |||
| Female sex | 1.47 (1.12–1.93) | |||
| History of cardiac diseasea | 1.42 (1.07–1.87) | |||
| Age | 1.02 (1.01–1.04) | |||
| Mashour et al. | 2011 | Non-cardiac, non-vascular, non-neurologic surgery | 523,059 | |
| Age > 62 years | 3.9 (3.0–5.0) | |||
| MI within 6 months | 3.8 (2.4–6.0) | |||
| Acute renal failure | 3.6 (2.3–5.8) | |||
| History of stroke | 2.9 (2.3–3.8) | |||
| Dialysis | 2.3 (1.6–3.4) | |||
| Bateman et al. | 2009 | Hemicolectomy, THA, lobectomy/segmental lung resection patients | 371,641 | |
| Renal disease | 2.98 (2.52–3.54) | |||
| Atrial fibrillation | 1.95 (1.69–2.26) | |||
| History of stroke | 1.64 (1.25–2.14) | |||
| Valvular disease | 1.54 (1.25–1.90) | |||
| CHF | 1.44 (1.21–1.70) | |||
| Bucerius et al. | 2003 | Cardiac surgery | 16,184 | |
| High transfusion requirement | 6.04 (5.05–7.23) | |||
| History of cerebrovascular disease | 3.55 (2.71–4.66) | |||
| Preoperative infection | 2.39 (1.69–3.40) | |||
| Urgent operation | 1.47 (1.23–1.76) | |||
| CPB >2 hours | 1.42 (1.17–1.72) | |||
Defined as myocardial infarction within 6 months, history of cardiac revascularization, or congestive heart failure within 30 days.
CI = confidence interval; TIA = transient ischemic attack; MI = myocardial infarction; THA = total hip arthroplasty; CHF = congestive heart failure; CPB = cardiopulmonary bypass
Perioperative beta-blockade has emerged as a risk factor for stroke across the general surgical population.16, 20, 21, 54 Both prospective randomized controlled trials and retrospective observational studies have demonstrated an increased risk of stroke in patients taking beta-blockers in the perioperative setting.16, 20, 21 Further, there may be a differentially increased risk with relatively non-selective beta-blockers.16, 20 As previously outlined, this may be due to impaired cerebral vasodilation and cardiac output in the setting of malperfusion and non-selective beta-blockade.46–48
SNACC CONSENSUS STATEMENT
In 2014, the Society for Neuroscience in Anesthesiology and Critical Care (SNACC) released the first set of recommendations regarding care for patients at high-risk for perioperative stroke in the setting of non-cardiac, non-neurologic surgery.13 Based on a review of the literature as well as expert opinion, recommendations to minimize the risk of perioperative stroke in the preoperative, intraoperative, and postoperative periods were presented. Considerations for prevention and management of stroke across the perioperative spectrum are reviewed below. These factors are derived from both the SNACC Consensus Statement as well as from other subsequent influential studies.55–57
PREOPERATIVE MANAGEMENT
Phenotype of the High-Risk Patient
Prevention of perioperative stroke begins with the identification of high-risk patients in the preoperative setting. By initially stratifying risk based on the type of surgery, non-cardiac, non-neurologic, and non-major vascular surgery are all associated with the lowest incidence of perioperative stroke – approximately 1 per 1000 cases (0.1%).7 This is the population on which the SNACC Consensus Statement largely focuses. The incidence then climbs with major vascular and cardiac surgery, with incidences reported as high as 5.6% and 9.7%, respectively.5, 58 In addition to the type of surgery for which the patient is presenting, patient-specific risk factors play a role as well. Across major epidemiologic studies examining stroke risk factors in the general surgical population, advanced age, history of renal failure, history of stroke, and cardiac disease all confer an increased risk of perioperative stroke.6, 7 The existence of such conditions in a patient’s past medical history should alert the perioperative physician to an increased risk of cerebrovascular compromise, which should raise the index of suspicion for stroke in the setting of postoperative neurologic changes. Thus, the phenotype of the high risk patient may appear as someone presenting for major surgery (especially cardiovascular) with comorbidities such as advanced age, renal dysfunction, history of cerebrovascular compromise, and history of cardiac disease.
Identifying Modifiable Risk Factors
Although many risk factors for perioperative stroke are largely non-modifiable, the discussion below encompasses elements the perioperative physician may be able to modulate for risk reduction. As may become evident, there are noticeably few large-scale, randomized, prospective trials available to help guide management for the factors below. Possible modifiable risk factors, along with proposed strategies for minimizing perioperative stroke risk, are outlined in the sections below and presented in Table 2.
Table 2.
Proposed Perioperative Modifiable Risk Factors
| Candidate Modifiable Risk Factor | Possible Risk Reduction Strategy |
|---|---|
| Recent stroke55 | Delay elective surgery ≥ 9 months after stroke |
| Intraoperative cerebral hypoxia79, 80 | Cerebral oxygenation protocol |
| Hypotension83 | Avoid >30% decrease in MAP from baseline |
| High thromboembolism riska, 93 | Evidence-based bridging protocol |
| GA, orthopedic joint replacement81, 82 | Consider neuraxial technique if possible |
| Beta-blockade20 | Transfuse to Hgb ≥ 9 if patient on β-blockade |
Per the American College of Chest Physicians Guidelines96; MAP = mean arterial pressure; GA = general anesthesia; Hgb = hemoglobin
Recent Stroke
In 2014, Jorgensen et al. presented observational cohort data regarding postoperative risk of major adverse cardiovascular events (MACE) after recent IS.55 The data come from the Danish National Patient Register – a national registry of prospectively collected data from patients in the Danish healthcare system. In this study, patients who had a recent history of IS also demonstrated increased risk of MACE and mortality within 30 days of the operation. Further, patients were also at risk for postoperative IS, and this risk progressively decreased the longer the duration of time between stroke and subsequent surgery; stroke < 3 months prior, odds ratio (OR) 67.6 (95% confidence interval [CI], 52.27 to 87.42), stroke 3 to <6 months prior, OR 24.02 (95% CI, 15.03 to 38.39), and stroke 6 to <12 months prior, OR 10.39 (95% CI, 6.18 to 17.44). The increased risk of perioperative stroke appeared to return to that of patients with a remote history of prior stroke between 9 and 12 months. One criticism was the lack of a nonsurgery control group.59 Indeed, increased risk of stroke recurrence has been demonstrated within 12 months of incident stroke without surgery.60, 61 Nonetheless, probing for history of recent stroke seems reasonable. As time between stroke symptom onset and intervention is critical, any conditions that obscure the ability to evaluate for stroke symptoms (e.g., recovery from general anesthesia), may place patients at higher risk. As such, elective cases should be delayed and a risk/benefit analysis should be conducted in these scenarios given (1) the challenges present with stroke diagnosis and management after surgery and anesthesia and (2) the 8-fold increase in mortality with perioperative stroke.7 Further investigation into the optimal timing of surgery after stroke is certainly warranted.
Perioperative Beta-Blockade
Evidence demonstrates a reduced risk of MACE with perioperative beta-blockade, though this may come at the expense of increased stroke risk in non-cardiac surgery patients.54, 62 In the 2014 American College of Cardiology/American Heart Association (ACC/AHA) Guidelines, the authors recommend a risk-benefit analysis for perioperative beta-blockade on a case-by-case basis.62 Specifically, the suggestion is made to weigh the risk of MACE against the risk of perioperative stroke to guide the decision-making with regards to perioperative betablockade management. In cases where the risk of MACE may be higher than that of perioperative stroke, perioperative beta-blockade may be beneficial, whereas in cases where stroke risk is higher than that of MACE, a strategy involving aggressive beta-blockade may be harmful. This distinction is certainly not always easy to delineate clinically. An initial step may be identifying patients on beta-blockade who are at high-risk for cerebrovascular ischemia based on patient-and surgery-specific risk factors. For example, as mentioned above, the combination of anemia and beta-blockade may place patients at risk for stroke. As such, patients on preoperative beta-blockade presenting for surgery with a high risk of major hemorrhage may be at increased risk. Further investigation may be informative.
Anticoagulant and Antiplatelet Therapy
In general, the risk of excessive perioperative bleeding is weighed against the risk of thromboembolism, though a clear, diametric clinical distinction is not always present. For patients on anticoagulation for conditions such as atrial fibrillation, the ACC/AHA Guidelines recommend discontinuation of anticoagulation for ≥ 48 hours for major surgery.62 The American College of Chest Physicians (ACCP) recommends continued perioperative anticoagulation for patients at high risk for venous thromboembolism.63 At this point, it is unclear if aggressive perioperative anticoagulation would reduce the risk of postoperative stroke,39 and further investigation would be of benefit for clinical decision-making. With regards to antiplatelet therapy, both observational and interventional data have demonstrated a cerebroprotective effect of aspirin in cardiac surgery patients.64, 65 In non-cardiac surgery patients, the PeriOperative Ischemic Evaluation (POISE)-2 Trial demonstrated a reduced incidence of stroke in patients who started aspirin therapy during the course of the study,66 though this benefit was not seen in patients who had already been on aspirin therapy. Further, the authors noted that this was likely a spurious subgroup effect in the initiation stratum due to the combination of small sample size, unexpectedly large benefit of aspirin, benefits not previously demonstrated in previous studies, and hypothesized direction opposite to the observed finding.66, 67
INTRAOPERATIVE MANAGEMENT
From the perspective of the anesthesiologist, an evidence-based intraoperative strategy to minimize stroke risk may indeed sound appealing. To that end, different intraoperative anesthetic techniques, pharmacologic strategies, and physiologic strategies have been studied with the intent of minimizing postoperative stroke risk. Below, we review some of the major studies examining intraoperative considerations that may impact the risk of perioperative stroke.
Anesthetic and Monitoring Techniques
Various anesthetic and neuromonitoring techniques have been studied in patients undergoing surgical procedures associated with high risk of stroke, such as carotid endarterectomy (CEA). In over 3,000 patients presenting for CEA, the GALA Trial randomized patients to general (GA) vs. local anesthesia (LA) in a multicenter, randomized controlled format.68 The trial could not show any definitive difference in stroke outcomes between those two groups. A secondary analysis of this trial did not demonstrate an increased risk of stroke in patients exposed to nitrous oxide,69 despite the known increase in plasma homocysteine levels associated with its use.70 This finding was reaffirmed in patients undergoing major non-cardiac surgery, where the use of nitrous oxide did not confer an increased risk of stroke.71 In patients who are indeed undergoing high-risk procedures like CEA under GA, neuromonitoring techniques such electroencephalography (EEG) and somatosensory evoked potential monitoring (SSEP) allow detection of intraoperative ischemia.72, 73 EEG, however, may allow for faster and more sensitive detection of ischemia as compared to SSEP in these cases.72, 74 A review of these techniques, as well as a discussion of their respective benefits and drawbacks, can be found elsewhere.74–76 Assessment of regional cerebral oxygenation (rSO2) is an additional intraoperative neuromonitoring technique, where near-infrared spectroscopy (NIRS) is used to indirectly measure cerebral tissue oxygenation.77, 78 In cardiac surgery patients, preliminary data indicate that intraoperative rSO2 monitoring may reduce perioperative stroke risk,79, 80 though further studies are needed to confirm or refute these findings.
Lastly, investigators have recently assessed perioperative stroke rates in orthopedic surgery patients receiving either general or neuraxial anesthesia for various joint arthroplasty procedures.81, 82 In both studies, general anesthesia was associated with higher perioperative stroke risk. Based on study design – a large, retrospective database study82 and a prospective, observational cohort study81 – no inferences regarding causality can be drawn. Nonetheless, the notion that anesthetic technique may modulate perioperative stroke risk in certain patient populations certainly deserves further investigative consideration.
Physiologic Management
Optimal intraoperative physiologic management may play a role in stroke prevention. Maintaining blood pressure near preoperative baseline values may help lower stroke risk, though supporting evidence is limited. Two large, retrospective, database studies have examined the relationship between intraoperative blood pressure and postoperative stroke.16, 83 Bijker et al. demonstrated an association between intraoperative hypotension and postoperative stroke when mean arterial pressure (MAP) was reduced by 30% (per minute) compared to baseline (OR 1.01; 99.9% CI, 1.00 to 1.03).83 This OR expresses the increase in stroke risk per minute of defined intraoperative hypotension (30% MAP reduction). In a subsequent retrospective, database study, Mashour et al. found associations between postoperative stroke and intraoperative hypotension 20% below baseline for both systolic blood pressure (SBP) and MAP (median values measured over 10-minute epochs).16 Reasons for the discrepancy between these studies are unclear, but might relate to differences in the definition of baseline blood pressure as well as the duration of hypotension. Indeed, definitions of intraoperative hypotension and baseline blood pressure vary widely in the literature, as does the studied duration of perioperative hypotension.84 As an additional consideration, Bijker et al. also noted significant variance in postoperative blood pressure recording intervals and lack of detailed characterization of postoperative hypotensive severity.83 For these reasons, postoperative blood pressure data were excluded from their analysis. Studying the effects of hypotension and perioperative stroke outcomes thus becomes difficult when considering the rarity of stroke, the lack of standardized definitions for baseline blood pressure and intraoperative hypotension, and time period during which hypotension may be most deleterious (i.e. intraoperative vs. postoperative).
Avoiding extremes in plasma glucose concentration may also help reduce stroke risk. Ghandi et al. found an increased risk of postoperative stroke with tight intraoperative glucose control in cardiac surgery patients.85 Conversely, Doenst et al. demonstrated an increased risk of composite adverse events – which included stroke – with hyperglycemia during cardiac surgery.86 At present, few data are otherwise available to guide intraoperative glucose management, especially in the non-cardiac surgery literature.
Intraoperative Beta-Blockade
As discussed previously, the findings of the POISE Trial led many to re-think the approach to perioperative beta-blockade. At the time, few data existed relating risk of perioperative stroke to specific beta-blockers, dosing regimens, and notably, effects of intraoperative beta-blockade. In a retrospective investigation of 57,218 patients, Mashour et al.16 found a 3.3-fold (95% CI, 1.4 to 7.8; P = 0.003) unadjusted increased risk of perioperative stroke in patients who received intraoperative metoprolol. This finding was not demonstrated with other beta-blockers studied. Postulated mechanisms for this association include impaired cerebral tissue oxygen delivery via decreased cerebral vasodilation and impaired CO in the setting of hemodilution.46–48 At present, few other data exist regarding intraoperative beta-blockade and cerebrovascular outcomes. Decisions regarding intraoperative beta-blockade may be made on a case-by-case basis depending on the patient, type of surgical intervention, and ongoing physiologic considerations as previously described.
POSTOPERATIVE MANAGEMENT
In general, only approximately 5–15% of perioperative strokes occur intraoperatively or in the immediate (i.e. apparent in the PACU) postoperative setting.16, 51 Indeed, most postoperative strokes present at least 24 hours after surgery.16, 51 Given this timeline, continued clinical vigilance for stroke symptoms along with timely neurology consultation is paramount for successful postoperative stroke diagnosis and management. Emergent neuroimaging should be obtained in parallel with stroke rapid response team activation, if available. A multidisciplinary discussion involving neurology, the primary surgical service, and possibly both interventional neuroradiology and anesthesiology input should ensue. Providers should seek expeditious evaluation and resource mobilization, as in-hospital stroke may be associated with worse outcomes compared to community-onset stroke.57 Indeed, Saltman et al. noted delayed symptom recognition, delayed neuroimaging, lower rates of thrombolysis, and worse stroke severity with in-hospital stroke patients compared to those with community-onset stroke.57 Surgical patients represented nearly 50% of all in-hospital strokes in this study. Thus, timely and optimal perioperative stroke management may be crucial in these high-risk patients. Glucose should be checked, as both hypo- and hyperglycemia have been associated with worse stroke outcomes.85, 87 Aspirin should be considered prior to discharge if surgically feasible, given improved outcomes when used for secondary prevention.88 A proposed perioperative stroke management checklist is outlined in Table 3.
Table 3.
Perioperative Ischemic Stroke Management NIHSS = National Institutes of Health Stroke Scale; rTPA = recombinant tissue plasminogen activator; ASA = acetylsalicylic acid (aspirin )
| Perioperative Ischemic Stroke Checklist |
|---|
|
Treatment for postoperative stroke remains a source of ongoing active discussion. According to the 2013 AHA/ASA Guidelines, patients who have undergone recent major surgery may be candidates for intravenous fibrinolysis, though a careful risk-benefit analysis should first be conducted.89 These guidelines also speculate that selective intra-arterial thrombolysis may be warranted in a select group of surgical patients who may be at high risk for systemic hemorrhage. Several case series have demonstrated a favorable safety profile of intra-arterial intervention across diverse surgical populations.90–92 Catheter-based mechanical interventions may also be appropriate in these select populations, especially given recent data demonstrating improved functional outcomes and reduction in mortality among select patients.93 Ultimately, further investigation is warranted.
In terms of blood pressure management in IS patients, the 2013 AHA/ASA Guidelines recommend permissive hypertension up to 220/120 mm Hg if not being considered for thrombolytic reperfusion therapy and if not contraindicated by co-existing medical conditions (i.e. acute aortic dissection, acute myocardial infarction, etc.).89 If intravenous thrombolytic therapy is being considered, however, recommendations are for controlled blood pressure reduction to <185/110 mm Hg.89 Since the release of these guidelines, the results of the China Antihypertensive Trial in Acute Ischemic Stroke (CATIS) have been released. From a starting mean [standard deviation (SD)] SBP of 166.7 (17.3) mm Hg, there was no clinical benefit to blood pressure reduction to 144.7 (15.0) mm Hg at 24 hours or 137.3 (11.8) mm Hg at 7 days. 56 Thus, at present, a SBP goal approximately between 140–185 mmHg may be reasonable. Certainly, avoiding blood pressure extremes may help avoid further neurologic insult, as has been observed in patients after receiving thrombolysis.94
CONCLUSIONS
Perioperative stroke has attracted renewed attention over the past few years, as associated morbidity and mortality remain high, and potentially modifiable risk factors have been identified. Significant investigative work remains to be done in the areas of perioperative beta-blockade, anticoagulation, antiplatelet management, and intraoperative management. The SNACC Consensus Statement represents a succinct yet thorough review of the current literature supporting preventative and management approaches to perioperative stroke.
Figure 1.
Ischemic stroke risk as a function of surgical procedure. Stroke incidences are calculated composite averages derived from representative literature from cardiac,5, 97, 98 vascular,51, 98–100 and general surgery.6, 7 CABG = coronary artery bypass graft; CEA = carotid endarterectomy
IMPLICATION STATEMENT.
Perioperative stroke is associated with significant morbidity and mortality. In this narrative review, we will discuss the incidence, pathophysiology, risk factors, and evidence-based recommendations for prevention and management of perioperative stroke.
Acknowledgments
Funding source: Department of Anesthesiology, University of Michigan Health System
Footnotes
Conflicts of interest: none
References
- 1.Mendis S, Puska P, Norvving B. Global Atlas on Cardiovascular Disease Prevention and Control. Geneva: World Health Organization Publications; 2011. [Google Scholar]
- 2.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong GY, Warner DO, Schroeder DR, et al. Risk of surgery and anesthesia for ischemic stroke. Anesthesiology. 2000;92:425–32. doi: 10.1097/00000542-200002000-00024. [DOI] [PubMed] [Google Scholar]
- 4.Etzioni DA, Liu JH, Maggard MA, Ko CY. The aging population and its impact on the surgery workforce. Ann Surg. 2003;238:170–7. doi: 10.1097/01.SLA.0000081085.98792.3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bucerius J, Gummert JF, Borger MA, et al. Stroke after cardiac surgery: a risk factor analysis of 16,184 consecutive adult patients. Ann Thorac Surg. 2003;75:472–8. doi: 10.1016/s0003-4975(02)04370-9. [DOI] [PubMed] [Google Scholar]
- 6.Bateman BT, Schumacher HC, Wang S, Shaefi S, Berman MF. Perioperative acute ischemic stroke in noncardiac and nonvascular surgery: incidence, risk factors, and outcomes. Anesthesiology. 2009;110:231–8. doi: 10.1097/ALN.0b013e318194b5ff. [DOI] [PubMed] [Google Scholar]
- 7.Mashour GA, Shanks AM, Kheterpal S. Perioperative stroke and associated mortality after noncardiac, nonneurologic surgery. Anesthesiology. 2011;114:1289–96. doi: 10.1097/ALN.0b013e318216e7f4. [DOI] [PubMed] [Google Scholar]
- 8.Mrkobrada M, Hill MD, Chan MT, et al. Abstract tmp9: The neurovision pilot study: non-cardiac surgery carries a significant risk of acute covert stroke. Stroke. 2013:44. [Google Scholar]
- 9.Barber PA, Hach S, Tippett LJ, et al. Cerebral ischemic lesions on diffusion-weighted imaging are associated with neurocognitive decline after cardiac surgery. Stroke. 2008;39:1427–33. doi: 10.1161/STROKEAHA.107.502989. [DOI] [PubMed] [Google Scholar]
- 10.Parikh S, Cohen JR. Perioperative stroke after general surgical procedures. N Y State J Med. 1993;93:162–5. [PubMed] [Google Scholar]
- 11.Landercasper J, Merz BJ, Cogbill TH, et al. Perioperative stroke risk in 173 consecutive patients with a past history of stroke. Arch Surg. 1990;125:986–9. doi: 10.1001/archsurg.1990.01410200044006. [DOI] [PubMed] [Google Scholar]
- 12.Biteker M, Kayatas K, Turkmen FM, Misirli CH. Impact of perioperative acute ischemic stroke on the outcomes of noncardiac and nonvascular surgery: a single centre prospective study. Can J Surg. 2014;57:E55–61. doi: 10.1503/cjs.003913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mashour GA, Moore LE, Lele AV, Robicsek SA, Gelb AW. Perioperative care of patients at high risk for stroke during or after non-cardiac, non-neurologic surgery: consensus statement from the Society for Neuroscience in Anesthesiology and Critical Care*. J Neurosurg Anesthesiol. 2014;26:273–85. doi: 10.1097/ANA.0000000000000087. [DOI] [PubMed] [Google Scholar]
- 14.Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 15.Likosky DS, Marrin CA, Caplan LR, et al. Determination of etiologic mechanisms of strokes secondary to coronary artery bypass graft surgery. Stroke. 2003;34:2830–4. doi: 10.1161/01.STR.0000098650.12386.B3. [DOI] [PubMed] [Google Scholar]
- 16.Mashour GA, Sharifpour M, Freundlich RE, et al. Perioperative metoprolol and risk of stroke after noncardiac surgery. Anesthesiology. 2013;119:1340–6. doi: 10.1097/ALN.0b013e318295a25f. [DOI] [PubMed] [Google Scholar]
- 17.Ng JL, Chan MT, Gelb AW. Perioperative stroke in noncardiac, nonneurosurgical surgery. Anesthesiology. 2011;115:879–90. doi: 10.1097/ALN.0b013e31822e9499. [DOI] [PubMed] [Google Scholar]
- 18.Popa AS, Rabinstein AA, Huddleston PM, et al. Predictors of ischemic stroke after hip operation: a population-based study. J Hosp Med. 2009;4:298–303. doi: 10.1002/jhm.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Limburg M, Wijdicks EF, Li H. Ischemic stroke after surgical procedures: clinical features, neuroimaging, and risk factors. Neurology. 1998;50:895–901. doi: 10.1212/wnl.50.4.895. [DOI] [PubMed] [Google Scholar]
- 20.Ashes C, Judelman S, Wijeysundera DN, et al. Selective beta1-antagonism with bisoprolol is associated with fewer postoperative strokes than atenolol or metoprolol: a single-center cohort study of 44,092 consecutive patients. Anesthesiology. 2013;119:777–87. doi: 10.1097/ALN.0b013e3182a17f12. [DOI] [PubMed] [Google Scholar]
- 21.Group PS, Devereaux PJ, Yang H, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371:1839–47. doi: 10.1016/S0140-6736(08)60601-7. [DOI] [PubMed] [Google Scholar]
- 22.Schietroma M, Carlei F, Mownah A, et al. Changes in the blood coagulation, fibrinolysis, and cytokine profile during laparoscopic and open cholecystectomy. Surg Endosc. 2004;18:1090–6. doi: 10.1007/s00464-003-8819-0. [DOI] [PubMed] [Google Scholar]
- 23.Khafagy HF, Hussein NA, Madkour ME, et al. Perioperative effects of anesthesia and surgery on inflammation-coagulation interaction. Life Science Journal. 2014;11:7. [Google Scholar]
- 24.Collins GJ, Jr, Barber JA, Zajtchuk R, Vanek D, Malogne LA. The effects of operative stress on the coagulation profile. Am J Surg. 1977;133:612–6. doi: 10.1016/0002-9610(77)90022-8. [DOI] [PubMed] [Google Scholar]
- 25.Cundiff DK. Clinical evidence for rebound hypercoagulability after discontinuing oral anticoagulants for venous thromboembolism. Medscape J Med. 2008;10:258. [PMC free article] [PubMed] [Google Scholar]
- 26.Broderick JP, Bonomo JB, Kissela BM, et al. Withdrawal of antithrombotic agents and its impact on ischemic stroke occurrence. Stroke. 2011;42:2509–14. doi: 10.1161/STROKEAHA.110.611905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stouthard JM, Levi M, Hack CE, et al. Interleukin-6 stimulates coagulation, not fibrinolysis, in humans. Thromb Haemost. 1996;76:738–42. [PubMed] [Google Scholar]
- 28.Rost NS, Wolf PA, Kase CS, et al. Plasma concentration of C-reactive protein and risk of ischemic stroke and transient ischemic attack: the Framingham study. Stroke. 2001;32:2575–9. doi: 10.1161/hs1101.098151. [DOI] [PubMed] [Google Scholar]
- 29.Everett BM, Kurth T, Buring JE, Ridker PM. The relative strength of C-reactive protein and lipid levels as determinants of ischemic stroke compared with coronary heart disease in women. J Am Coll Cardiol. 2006;48:2235–42. doi: 10.1016/j.jacc.2006.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–9. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 31.Kuhn EW, Liakopoulos OJ, Stange S, et al. Preoperative statin therapy in cardiac surgery: a meta-analysis of 90,000 patients. Eur J Cardiothorac Surg. 2014;45:17–26. doi: 10.1093/ejcts/ezt181. discussion. [DOI] [PubMed] [Google Scholar]
- 32.Antoniou GA, Hajibandeh S, Hajibandeh S, et al. Meta-analysis of the effects of statins on perioperative outcomes in vascular and endovascular surgery. J Vasc Surg. 2015;61:519–32 e1. doi: 10.1016/j.jvs.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 33.Everett BM, Glynn RJ, MacFadyen JG, Ridker PM. Rosuvastatin in the prevention of stroke among men and women with elevated levels of C-reactive protein: justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) Circulation. 2010;121:143–50. doi: 10.1161/CIRCULATIONAHA.109.874834. [DOI] [PubMed] [Google Scholar]
- 34.Engstrom G, Lind P, Hedblad B, et al. Effects of cholesterol and inflammation-sensitive plasma proteins on incidence of myocardial infarction and stroke in men. Circulation. 2002;105:2632–7. doi: 10.1161/01.cir.0000017327.69909.ff. [DOI] [PubMed] [Google Scholar]
- 35.Ridker PM, Rifai N, Clearfield M, et al. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med. 2001;344:1959–65. doi: 10.1056/NEJM200106283442601. [DOI] [PubMed] [Google Scholar]
- 36.Liakopoulos OJ, Choi YH, Haldenwang PL, et al. Impact of preoperative statin therapy on adverse postoperative outcomes in patients undergoing cardiac surgery: a meta-analysis of over 30,000 patients. Eur Heart J. 2008;29:1548–59. doi: 10.1093/eurheartj/ehn198. [DOI] [PubMed] [Google Scholar]
- 37.McGirt MJ, Perler BA, Brooke BS, et al. 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors reduce the risk of perioperative stroke and mortality after carotid endarterectomy. J Vasc Surg. 2005;42:829–36. doi: 10.1016/j.jvs.2005.08.039. discussion 36–7. [DOI] [PubMed] [Google Scholar]
- 38.Likosky DS, Caplan LR, Weintraub RM, et al. Intraoperative and postoperative variables associated with strokes following cardiac surgery. Heart Surg Forum. 2004;7:E271–6. doi: 10.1532/HSF98.20041035. [DOI] [PubMed] [Google Scholar]
- 39.Kaatz S, Douketis JD, Zhou H, Gage BF, White RH. Risk of stroke after surgery in patients with and without chronic atrial fibrillation. J Thromb Haemost. 2010;8:884–90. doi: 10.1111/j.1538-7836.2010.03781.x. [DOI] [PubMed] [Google Scholar]
- 40.McLaren AT, Marsden PA, Mazer CD, et al. Increased expression of HIF-1alpha, nNOS, and VEGF in the cerebral cortex of anemic rats. Am J Physiol Regul Integr Comp Physiol. 2007;292:R403–14. doi: 10.1152/ajpregu.00403.2006. [DOI] [PubMed] [Google Scholar]
- 41.Brannon ES, Merrill AJ, Warren JV, Stead EA. The Cardiac Output in Patients with Chronic Anemia as Measured by the Technique of Right Atrial Catheterization. J Clin Invest. 1945;24:332–6. doi: 10.1172/JCI101610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duke M, Abelmann WH. The hemodynamic response to chronic anemia. Circulation. 1969;39:503–15. doi: 10.1161/01.cir.39.4.503. [DOI] [PubMed] [Google Scholar]
- 43.Tu YK, Liu HM. Effects of isovolemic hemodilution on hemodynamics, cerebral perfusion, and cerebral vascular reactivity. Stroke. 1996;27:441–5. doi: 10.1161/01.str.27.3.441. [DOI] [PubMed] [Google Scholar]
- 44.Korosue K, Ishida K, Matsuoka H, et al. Clinical, hemodynamic, and hemorheological effects of isovolemic hemodilution in acute cerebral infarction. Neurosurgery. 1988;23:148–53. doi: 10.1227/00006123-198808000-00003. [DOI] [PubMed] [Google Scholar]
- 45.Korosue K, Heros RC. Mechanism of cerebral blood flow augmentation by hemodilution in rabbits. Stroke. 1992;23:1487–92. doi: 10.1161/01.str.23.10.1487. discussion 92–3. [DOI] [PubMed] [Google Scholar]
- 46.Ragoonanan TE, Beattie WS, Mazer CD, et al. Metoprolol reduces cerebral tissue oxygen tension after acute hemodilution in rats. Anesthesiology. 2009;111:988–1000. doi: 10.1097/ALN.0b013e3181b87f0e. [DOI] [PubMed] [Google Scholar]
- 47.Hare GM, Worrall JM, Baker AJ, et al. Beta2 adrenergic antagonist inhibits cerebral cortical oxygen delivery after severe haemodilution in rats. Br J Anaesth. 2006;97:617–23. doi: 10.1093/bja/ael238. [DOI] [PubMed] [Google Scholar]
- 48.El Beheiry MH, Heximer SP, Voigtlaender-Bolz J, et al. Metoprolol impairs resistance artery function in mice. J Appl Physiol (1985) 2011;111:1125–33. doi: 10.1152/japplphysiol.01340.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu T, Beattie WS, Mazer CD, et al. Treatment with a highly selective beta(1) antagonist causes dose-dependent impairment of cerebral perfusion after hemodilution in rats. Anesth Analg. 2013;116:649–62. doi: 10.1213/ANE.0b013e318280e26d. [DOI] [PubMed] [Google Scholar]
- 50.Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064–89. doi: 10.1161/STR.0b013e318296aeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharifpour M, Moore LE, Shanks AM, et al. Incidence, predictors, and outcomes of perioperative stroke in noncarotid major vascular surgery. Anesth Analg. 2013;116:424–34. doi: 10.1213/ANE.0b013e31826a1a32. [DOI] [PubMed] [Google Scholar]
- 52.Goodney PP, Likosky DS, Cronenwett JL Vascular Study Group of Northern New E. Factors associated with stroke or death after carotid endarterectomy in Northern New England. J Vasc Surg. 2008;48:1139–45. doi: 10.1016/j.jvs.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 53.Gialdini G, Nearing K, Bhave PD, et al. Perioperative atrial fibrillation and the long-term risk of ischemic stroke. JAMA. 2014;312:616–22. doi: 10.1001/jama.2014.9143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blessberger H, Kammler J, Domanovits H, et al. Perioperative beta-blockers for preventing surgery-related mortality and morbidity. Cochrane Database Syst Rev. 2014;9:CD004476. doi: 10.1002/14651858.CD004476.pub2. [DOI] [PubMed] [Google Scholar]
- 55.Jorgensen ME, Torp-Pedersen C, Gislason GH, et al. Time elapsed after ischemic stroke and risk of adverse cardiovascular events and mortality following elective noncardiac surgery. JAMA. 2014;312:269–77. doi: 10.1001/jama.2014.8165. [DOI] [PubMed] [Google Scholar]
- 56.He J, Zhang Y, Xu T, et al. Effects of immediate blood pressure reduction on death and major disability in patients with acute ischemic stroke: the CATIS randomized clinical trial. JAMA. 2014;311:479–89. doi: 10.1001/jama.2013.282543. [DOI] [PubMed] [Google Scholar]
- 57.Saltman AP, Silver FL, Fang J, Stamplecoski M, Kapral MK. Care and Outcomes of Patients With In-Hospital Stroke. JAMA Neurol. 2015 doi: 10.1001/jamaneurol.2015.0284. [DOI] [PubMed] [Google Scholar]
- 58.Maatz W, Kohler J, Botsios S, John V, Walterbusch G. Risk of stroke for carotid endarterectomy patients with contralateral carotid occlusion. Ann Vasc Surg. 2008;22:45–51. doi: 10.1016/j.avsg.2007.07.034. [DOI] [PubMed] [Google Scholar]
- 59.Powers WJ. Time since stroke and risk of adverse outcomes after surgery. JAMA. 2014;312:1930. doi: 10.1001/jama.2014.13422. [DOI] [PubMed] [Google Scholar]
- 60.Dhamoon MS, Sciacca RR, Rundek T, Sacco RL, Elkind MS. Recurrent stroke and cardiac risks after first ischemic stroke: the Northern Manhattan Study. Neurology. 2006;66:641–6. doi: 10.1212/01.wnl.0000201253.93811.f6. [DOI] [PubMed] [Google Scholar]
- 61.Brown DL, Lisabeth LD, Roychoudhury C, Ye Y, Morgenstern LB. Recurrent stroke risk is higher than cardiac event risk after initial stroke/transient ischemic attack. Stroke. 2005;36:1285–7. doi: 10.1161/01.STR.0000165926.74213.e3. [DOI] [PubMed] [Google Scholar]
- 62.Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;64:e77–137. doi: 10.1016/j.jacc.2014.07.944. [DOI] [PubMed] [Google Scholar]
- 63.Guyatt GH, Akl EA, Crowther M, et al. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:7S–47S. doi: 10.1378/chest.1412S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cao L, Silvestry S, Zhao N, Diehl J, Sun J. Effects of preoperative aspirin on cardiocerebral and renal complications in non-emergent cardiac surgery patients: a sub-group and cohort study. PLoS One. 2012;7:e30094. doi: 10.1371/journal.pone.0030094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mangano DT Multicenter Study of Perioperative Ischemia Research G. Aspirin and mortality from coronary bypass surgery. N Engl J Med. 2002;347:1309–17. doi: 10.1056/NEJMoa020798. [DOI] [PubMed] [Google Scholar]
- 66.Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014;370:1494–503. doi: 10.1056/NEJMoa1401105. [DOI] [PubMed] [Google Scholar]
- 67.Sun X, Briel M, Walter SD, Guyatt GH. Is a subgroup effect believable? Updating criteria to evaluate the credibility of subgroup analyses. BMJ. 2010;340:c117. doi: 10.1136/bmj.c117. [DOI] [PubMed] [Google Scholar]
- 68.Group GTC, Lewis SC, Warlow CP, et al. General anaesthesia versus local anaesthesia for carotid surgery (GALA): a multicentre, randomised controlled trial. Lancet. 2008;372:2132–42. doi: 10.1016/S0140-6736(08)61699-2. [DOI] [PubMed] [Google Scholar]
- 69.Sanders RD, Graham C, Lewis SC, et al. Nitrous oxide exposure does not seem to be associated with increased mortality, stroke, and myocardial infarction: a non-randomized subgroup analysis of the General Anaesthesia compared with Local Anaesthesia for carotid surgery (GALA) trial. Br J Anaesth. 2012;109:361–7. doi: 10.1093/bja/aes164. [DOI] [PubMed] [Google Scholar]
- 70.Myles PS, Chan MT, Leslie K, et al. Effect of nitrous oxide on plasma homocysteine and folate in patients undergoing major surgery. Br J Anaesth. 2008;100:780–6. doi: 10.1093/bja/aen085. [DOI] [PubMed] [Google Scholar]
- 71.Myles PS, Leslie K, Chan MT, et al. The safety of addition of nitrous oxide to general anaesthesia in at-risk patients having major non-cardiac surgery (ENIGMA-II): a randomised, single-blind trial. Lancet. 2014;384:1446–54. doi: 10.1016/S0140-6736(14)60893-X. [DOI] [PubMed] [Google Scholar]
- 72.Pinkerton JA., Jr EEG as a criterion for shunt need in carotid endarterectomy. Ann Vasc Surg. 2002;16:756–61. doi: 10.1007/s10016-001-0208-3. [DOI] [PubMed] [Google Scholar]
- 73.Manninen P, Sarjeant R, Joshi M. Posterior tibial nerve and median nerve somatosensory evoked potential monitoring during carotid endarterectomy. Can J Anaesth. 2004;51:937–41. doi: 10.1007/BF03018896. [DOI] [PubMed] [Google Scholar]
- 74.Fielmuth S, Uhlig T. The role of somatosensory evoked potentials in detecting cerebral ischaemia during carotid endarterectomy. Eur J Anaesthesiol. 2008;25:648–56. doi: 10.1017/S0265021508003967. [DOI] [PubMed] [Google Scholar]
- 75.Pennekamp CW, Moll FL, de Borst GJ. The potential benefits and the role of cerebral monitoring in carotid endarterectomy. Curr Opin Anaesthesiol. 2011;24:693–7. doi: 10.1097/ACO.0b013e32834c7aa1. [DOI] [PubMed] [Google Scholar]
- 76.Moritz S, Kasprzak P, Arlt M, Taeger K, Metz C. Accuracy of cerebral monitoring in detecting cerebral ischemia during carotid endarterectomy: a comparison of transcranial Doppler sonography, near-infrared spectroscopy, stump pressure, and somatosensory evoked potentials. Anesthesiology. 2007;107:563–9. doi: 10.1097/01.anes.0000281894.69422.ff. [DOI] [PubMed] [Google Scholar]
- 77.Wahr JA, Tremper KK, Samra S, Delpy DT. Near-infrared spectroscopy: theory and applications. J Cardiothorac Vasc Anesth. 1996;10:406–18. doi: 10.1016/s1053-0770(96)80107-8. [DOI] [PubMed] [Google Scholar]
- 78.Jobsis FF. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science. 1977;198:1264–7. doi: 10.1126/science.929199. [DOI] [PubMed] [Google Scholar]
- 79.Murkin JM, Adams SJ, Novick RJ, et al. Monitoring brain oxygen saturation during coronary bypass surgery: a randomized, prospective study. Anesth Analg. 2007;104:51–8. doi: 10.1213/01.ane.0000246814.29362.f4. [DOI] [PubMed] [Google Scholar]
- 80.Goldman S, Sutter F, Ferdinand F, Trace C. Optimizing intraoperative cerebral oxygen delivery using noninvasive cerebral oximetry decreases the incidence of stroke for cardiac surgical patients. Heart Surg Forum. 2004;7:E376–81. doi: 10.1532/HSF98.20041062. [DOI] [PubMed] [Google Scholar]
- 81.Mortazavi SM, Kakli H, Bican O, et al. Perioperative stroke after total joint arthroplasty: prevalence, predictors, and outcome. J Bone Joint Surg Am. 2010;92:2095–101. doi: 10.2106/JBJS.I.00940. [DOI] [PubMed] [Google Scholar]
- 82.Memtsoudis SG, Sun X, Chiu YL, et al. Perioperative comparative effectiveness of anesthetic technique in orthopedic patients. Anesthesiology. 2013;118:1046–58. doi: 10.1097/ALN.0b013e318286061d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bijker JB, Persoon S, Peelen LM, et al. Intraoperative hypotension and perioperative ischemic stroke after general surgery: a nested case-control study. Anesthesiology. 2012;116:658–64. doi: 10.1097/ALN.0b013e3182472320. [DOI] [PubMed] [Google Scholar]
- 84.Bijker JB, Gelb AW. Review article: the role of hypotension in perioperative stroke. Can J Anaesth. 2013;60:159–67. doi: 10.1007/s12630-012-9857-7. [DOI] [PubMed] [Google Scholar]
- 85.Gandhi GY, Nuttall GA, Abel MD, et al. Intensive intraoperative insulin therapy versus conventional glucose management during cardiac surgery: a randomized trial. Ann Intern Med. 2007;146:233–43. doi: 10.7326/0003-4819-146-4-200702200-00002. [DOI] [PubMed] [Google Scholar]
- 86.Doenst T, Wijeysundera D, Karkouti K, et al. Hyperglycemia during cardiopulmonary bypass is an independent risk factor for mortality in patients undergoing cardiac surgery. J Thorac Cardiovasc Surg. 2005;130:1144. doi: 10.1016/j.jtcvs.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 87.McGirt MJ, Woodworth GF, Brooke BS, et al. Hyperglycemia independently increases the risk of perioperative stroke, myocardial infarction, and death after carotid endarterectomy. Neurosurgery. 2006;58:1066–73. doi: 10.1227/01.NEU.0000215887.59922.36. discussion -73. [DOI] [PubMed] [Google Scholar]
- 88.CAST: randomised placebo-controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke. CAST (Chinese Acute Stroke Trial) Collaborative Group. Lancet. 1997;349:1641–9. [PubMed] [Google Scholar]
- 89.Jauch EC, Saver JL, Adams HP, Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 90.Chalela JA, Katzan I, Liebeskind DS, et al. Safety of intra-arterial thrombolysis in the postoperative period. Stroke. 2001;32:1365–9. doi: 10.1161/01.str.32.6.1365. [DOI] [PubMed] [Google Scholar]
- 91.Moazami N, Smedira NG, McCarthy PM, et al. Safety and efficacy of intraarterial thrombolysis for perioperative stroke after cardiac operation. Ann Thorac Surg. 2001;72:1933–7. doi: 10.1016/s0003-4975(01)03030-2. discussion 7–9. [DOI] [PubMed] [Google Scholar]
- 92.Katzan IL, Masaryk TJ, Furlan AJ, et al. Intra-arterial thrombolysis for perioperative stroke after open heart surgery. Neurology. 1999;52:1081–4. doi: 10.1212/wnl.52.5.1081. [DOI] [PubMed] [Google Scholar]
- 93.Goyal M, Demchuk AM, Menon BK, et al. Randomized Assessment of Rapid Endovascular Treatment of Ischemic Stroke. N Engl J Med. 2015 doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 94.Ahmed N, Wahlgren N, Brainin M, et al. Relationship of blood pressure, antihypertensive therapy, and outcome in ischemic stroke treated with intravenous thrombolysis: retrospective analysis from Safe Implementation of Thrombolysis in Stroke-International Stroke Thrombolysis Register (SITS-ISTR) Stroke. 2009;40:2442–9. doi: 10.1161/STROKEAHA.109.548602. [DOI] [PubMed] [Google Scholar]
- 95.Darvish-Kazem S, Douketis JD. Perioperative management of patients having noncardiac surgery who are receiving anticoagulant or antiplatelet therapy: an evidence-based but practical approach. Semin Thromb Hemost. 2012;38:652–60. doi: 10.1055/s-0032-1326781. [DOI] [PubMed] [Google Scholar]
- 96.Epstein AE, Alexander JC, Gutterman DD, et al. Anticoagulation: American College of Chest Physicians guidelines for the prevention and management of postoperative atrial fibrillation after cardiac surgery. Chest. 2005;128:24S–7S. doi: 10.1378/chest.128.2_suppl.24s. [DOI] [PubMed] [Google Scholar]
- 97.Anyanwu AC, Filsoufi F, Salzberg SP, Bronster DJ, Adams DH. Epidemiology of stroke after cardiac surgery in the current era. J Thorac Cardiovasc Surg. 2007;134:1121–7. doi: 10.1016/j.jtcvs.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 98.Higgins J, Lee MK, Co C, Janusz MT. Long-term outcomes after thoracic aortic surgery: a population-based study. J Thorac Cardiovasc Surg. 2014;148:47–52. doi: 10.1016/j.jtcvs.2013.07.028. [DOI] [PubMed] [Google Scholar]
- 99.Axelrod DA, Stanley JC, Upchurch GR, Jr, et al. Risk for stroke after elective noncarotid vascular surgery. J Vasc Surg. 2004;39:67–72. doi: 10.1016/j.jvs.2003.08.028. [DOI] [PubMed] [Google Scholar]
- 100.Bonati LH, Dobson J, Featherstone RL, et al. Long-term outcomes after stenting versus endarterectomy for treatment of symptomatic carotid stenosis: the International Carotid Stenting Study (ICSS) randomised trial. Lancet. 2015;385:529–38. doi: 10.1016/S0140-6736(14)61184-3. [DOI] [PMC free article] [PubMed] [Google Scholar]