Abstract
Depending on genetic sensitivity to it, stress may affect depressive symptomatology differentially. Applying the stress-diathesis hypothesis to older adults, we postulate: (1) recent stress will associate with increased depressive symptoms levels and (2) this effect will be greater for individuals with at least one short allele of the serotonin transporter gene promoter region (5-HTTLPR). Further, we employ a design that addresses specific limitations of many prior studies that have examined the 5-HTTLPR x SLE relation, by: (a) using a within-person repeated-measures design to address fluctuations that occur within individuals over time, increase power for detecting GxE, and address GE correlation; (b) studying reports of exogenous stressful events (those unlikely to be caused by depression) to help rule out reverse causation and negativity bias, and in order to assess stressors that are more etiologically relevant to depressive symptomatology in older adults. The sample is drawn from the Health and Retirement Study, a U.S. population-based study of older adults (N=28,248; mean age = 67.5; 57.3% female; 80.7% Non-Hispanic White, 14.9% Hispanic/Latino, 4.5% African American; genetic subsample = 12,332), from whom measures of depressive symptoms and exogenous stressors were collected biannually (1994–2010). Variation in the 5-HTTLPR was characterized via haplotype, using two single nucleotide polymorphisms (SNPs). Ordered logit models were constructed to predict levels of depressive symptoms from 5-HTTLPR and stressors, comparing results of the most commonly applied statistical approaches (i.e., comparing allelic and genotypic models, and continuous and categorical predictors) used in the literature. All models were stratified by race/ethnicity. Overall, results show a main effect of recent stress for all ethnic groups, and mixed results for the variation in 5-HTTLPRxStress interaction, contingent upon statistical model used. Findings suggest there may be a differential effect of stressors and 5-HTTLPR on depressive symptoms by ethnicity, but further research is needed particularly when using a haplotype to characterize variation in 5-HTTLPR in population-based sample with a diverse ethnic composition.
Keywords: 5-HTTLPR, depressive symptoms, stressful life events, race differences, older adults, GxE
Introduction
Many individuals experience depressive symptomatology following major life events. However, this relationship may be stronger among those who have genetic sensitivity to environmental stress (Karg et al. 2011). This transactional relationship follows the stress-diathesis hypothesis, which postulates that some individuals possess inherent vulnerability, possibly due to genetic sources, such that they are disproportionately and negatively affected by environmental stressors (Beck 1995; Mazure et al. 2002). Prior research has found that one source of genetic vulnerability is having short alleles of a microsatellite marker, the serotonin transporter gene-linked polymorphic promoter region (5-HTTLPR, for 5-hydroxytryptamine transporter-linked polymorphic region) (Caspi et al. 2003; Karg et al. 2011; Kendler et al. 2005; Munafò et al. 2009; Risch et al. 2009; Sharpley et al. 2014; Uher and McGuffin 2010). Yet, evidence has been conflicting, with some studies concluding the short alleles of 5-HTTLPR have no main effect, do not moderate the effect of stressful life events (SLEs), or confer a protective effect on depression-related outcomes (Chorbov et al. 2007; Gillespie et al. 2005; Laucht et al. 2009; Middeldorp et al. 2007; Peyrot et al. 2013; Power et al. 2010; Surtees et al. 2006). A handful of studies to date have been conducted to examine the moderator effect of 5-HTTLPR on a range of SLEs and CES-D in older aged cohorts, yielding mixed results (Goldman et al. 2010; Kim et al. 2007; Power et al. 2010; Surtees et al. 2006). Older adults constitute a group that is likely to experience multiple major life events with respect to medical, financial and interpersonal circumstances (Fiske et al. 2009; Kraaij et al. 2002) and experience greater consequences from them (Fiske et al. 2009). Additionally, studies conducted in twins strongly support that the magnitude of impact that stressors and genetics confer on depressive symptoms in later life is through an interaction between the two (Kendler et al. 1995; Tennant 2002). Efforts to better understand this effect in older individuals can potentially contribute to developing more targeted and age-specific interventions for the prevention of depression as well as the treatment, whether through behavioral or pharmacologic means.
The neurotransmitter serotonin is crucial for regulating multiple physiological processes including mood, sleep, eating, and sexual activity. The serotonin transporter (5-HTT) protein is responsible for facilitating the reuptake of serotonin at the synapses, thereby terminating its activity. It is believed that variants of the functional polymorphism of 5-HTTLPR influence affective disorders and modulate the serotonergic response to stress through the differing levels of reuptake, which is dictated by the level of transcriptional activity of the transporter protein from these variants. The polymorphisms of the SLC6A4 gene are comprised of differing lengths of repeated sequences, initially characterized with bi-allelic nomenclature as either a long (419 bp) or short (375 bp) variant (Heils et al. 1996). The short variant exhibits less transcriptional activity (Lesch et al. 1996) and therefore elicits less serotonin reuptake compared to the long variant. There is evidence that other variants exist (Delbruck et al. 1997; Frisch et al. 2000; Gelernter et al. 1997; Nakamura et al. 2000), although they are seldom examined in genetic association studies because of their rare frequency (Goldman et al. 2010). The most common approach for studying the effect of the polymorphism on psychiatric phenotypes, including depression, has been with the bi-allelic structures which yield three genotypes: long/long (L/L), short/long (L/S), or short/short (S/S).
Over the past decade, a plethora of studies have aimed to replicate findings from a 2003 study by Caspi et al (Caspi et al. 2003), now cited over 6,100 times in the literature. Study investigators were one of the first to report on a significant gene-by-environment interaction (GxE) for a psychiatric phenotype. One of the GxE interactions tested was between the 5-HTTLPR genotypes and a range of SLE types on depression. Study participants for the investigation were drawn from a representative birth cohort in New Zealand, and asked to report the number and types of SLEs (e.g., related to employment, housing, health, finances, relationships) experienced in the last five years, and on past-year depressive symptoms. Authors report that participants who had SS or SL genotypes experienced disproportionately more depression symptoms than those with the LL genotype. Findings from the majority of studies since the Caspi et al report are mixed and summarized in four of the recent meta-analyses (Karg et al. 2011; Munafò et al. 2009; Risch et al. 2009; Sharpley et al. 2014; Uher and McGuffin 2010), which also had mixed findings.
From the first two meta-analyses (based on 5 and 14 studies, respectively, with 4 overlapping studies), authors concluded that the positive results between 5-HTTLPR and SLEs on major depressive disorder were consistent with chance findings (Munafò et al. 2009; Risch et al. 2009). Conversely, authors of the third (Karg et al. 2011) and fourth (Sharpley et al. 2014) meta-analyses (based on 54 and 81 studies, respectively, including all those from the prior two meta-analyses with Sharpley et al including 27 studies published subsequent to the Karg et al. analysis) concluded there was robust evidence that 5-HTTLPR moderates the relationship between SLEs and depression, with the S allele conferring greater risk for developing depression given the presence of SLEs. Despite the divergent findings, authors of all four meta-analyses and prior reviews (Monroe and Reid 2008; Uher and McGuffin 2010; Zammit and Owen 2006) highlight that two primary sources of conflicting findings in studies on this topic are the method for measuring environmental stress and the statistical approaches to modeling the GxE effect.
In one extensive review, authors note that stressors assessed should be those that are most etiologically relevant to depression, and meet several criteria for depressogenic SLEs, such as being acute with a distinct timing of onset, considered a major event such that it causes some threat to the individual, an event that primarily affects the individual respondent, and is recent (Monroe and Reid 2008). Additionally, the temporal sequence of events should be clear such that the SLE precedes the depressive symptoms rather than the symptoms leading to stressful events (reverse causation) as addressed in several prior studies (Kim et al. 2007; Peyrot et al. 2013). Few studies have addressed the issue of GE correlation, to distinguish whether people with genetic susceptibility differ from those with heightened emotionality in general (Caspi et al. 2003; Kendler et al. 2005; Kilpatrick et al. 2007; Peyrot et al. 2013). Finally, the issue of negativity bias, or whether depressive symptoms result in greater reporting of recent events, is discussed in very few of the prior studies. Addressing these in a study on SLEs and depressive symptoms would add more confidence to the findings.
A second, yet critical, issue characterizing the prior research on 5-HTTLPR and SLE concerns the statistical approaches used to test the interaction (Munafò et al. 2009). The majority of studies assessed the allelic effect (number of S alleles), but a few reported a genotypic effect (S/S, S/L vs. L/L or, alternatively, S/S vs. S/L, L/L) (Cervilla et al. 2007; Grabe et al. 2005; Kendler et al. 2005). Moreover, several studies modeled the interactions with SLEs on a cumulative count of stressors (Gillespie et al. 2005; Kim et al. 2007; Surtees et al. 2006), whereas others modeled SLEs by level of implied or measured stressfulness (Kendler et al. 2005; Ritchie et al. 2009). Lastly, the majority of the prior studies used measures of depressive symptomatology at a single time-point as the outcome while few included more than one assessment of depression per individual to account for within-person fluctuations over time (Araya et al. 2009; Caspi et al. 2003; Chorbov et al. 2007; Jacobs et al. 2006; Kendler et al. 2005; Kilpatrick et al. 2007; Kim et al. 2007; Middeldorp et al. 2007; Ming et al. 2013; Wilhelm et al. 2006).
Issues highlighted in the meta-analyses and reviews revolve around heterogeneity in methodology applied to the design of each attempted replication. Thus, a central goal of the present study is to compare results of statistical approaches that test the main and interaction effects through both allelic and genotypic analyses, as well as continuous and categorical analyses for SLEs. Notably, we do this while employing a design that addresses specific limitations of many prior studies that have examined the 5-HTTLPR x SLE relationship: (a) using a within-person repeated-measures design to address fluctuations that occur within individuals over time, increase power for detecting GxE (Wong et al., 2003), and address GE correlation; and (b) examining reports of exogenous stressful events (those unlikely to be caused by depression) to help rule out reverse causation and negativity bias. Furthermore, in the current study, we examine the 5-HTTLPR main effect sand GxE effect by assessing recent SLEs that are etiologically relevant to depressive symptomatology among a U.S. population-based sample of ethnically diverse older adults. Examining effects in an older sample is important because it has been speculated that life developmental stages may contribute to the heterogeneity in findings among younger samples (Uher and McGuffin 2010). Furthermore, there is evidence that mental health outcomes exhibit greater divergence between ethnic groups in older adulthood (Jackson et al. 2010; Keyes 2009). Thus, in accordance with the stress-diathesis hypothesis, we postulate that in older adults (1) recent exogenous stressors will be associated with increased depressive symptoms overall and (2) this effect will be greater for individuals with one or more short 5-HTTLPR alleles.
Methods
Participants
The sample was drawn from the Health and Retirement Study (HRS), a nationally representative sample of households of older Americans in the 48 contiguous United States. Data collection for the HRS began in 1992, targeting individuals born 1931–1941 and their spouses, regardless of spousal birth year. In 1998, the sample was merged with the Assets and Health Dynamics Among the Oldest Old (AHEAD) study that had begun in 1993, targeting the 1890–1923 birth cohorts. That year, two new birth cohorts were added to the HRS (cohorts 1924–1930 and 1942–1947). The spouses of participants were also included irrespective of their birth year. The newly combined sample of 21,384 respondents represents all Americans born between 1890 and 1947 and their spouses. New cohorts continue to be added to the HRS every 6 years. The response rate at baseline was 70–81% and the retention rate for the combined cohorts is 88% (HRS 2011). Participants are interviewed biennially on a broad range of economic, psychological, and physical health measures. For the present study, we included all participants who were interviewed in wave 2 (1994) through 10 (2010) and were at least 50 years old at any wave of data collection. Spouses that were younger than age 50 were excluded because they were not part of the planned sampling frame that would contribute to the representativeness of the sample. Wave 1 (1992) was excluded because the measure used to assess depressive symptoms differed from that used in all remaining waves. The phenotypic sample is comprised of 28,248 adults who, at their first assessment wave for HRS, were between ages 50 and 104 (mean = 63.0; SD = 10.6), 57.3% female; 80.7% Non-Hispanic White (NHW), 14.9% Hispanic/Latino (HL), 4.5% African American (AA).
Measures
Depressive Symptoms
Participants were asked to rate their depressive symptoms using a modified version of the well-known Center for Epidemiologic Studies-Depression Scale (CES-D) inventory. The original inventory (Radloff 1977; Radloff 1991; Zimmerman and Coryell 1994) used a scoring system, in which the 20 CES-D items are scored on a 0–3 scale, and these scores are summed over the 20 items to form a CES-D total score ranging from 0 to 60 (Santor and Coyne 1997). However, for administration with the HRS, the CES-D was abbreviated to an 8-item inventory. The 8 items were selected for their psychometric properties in assessing the continuum of depressive symptoms compared to the full scale, (Kohout et al. 1993) and demonstrate very similar construct and external validity to the original inventory (Steffick 2002; Turvey et al. 1999). Each item on the scale asked about how the respondent felt in the last week. The form of the questions reads “Much of the time in the last week I felt…” with a response set of yes or no. The number of depressive symptoms was calculated from a sum total of depressive symptoms. Scores ranged from 0 to 8, with more symptoms suggesting worse affective functioning.
The CES-D scale was not meant to be used to clinically diagnose depression, rather it serves to assess an elevated level of depressive symptoms that occur in different diagnostic categories of depression, and can serve as an indicator of depression risk (Radloff 1977). In order to examine the determinants that contribute to reaching higher risk levels for depression, we used ordinal categories with which to compare differing levels of symptomatology. This also minimizes the problem of zero-inflation because a large proportion of respondents (~44%) report 0 symptoms at multiple time-points. Similar to prior studies (Cuijpers et al. 2004; Poulin et al. 2005), we created cut-points for the sum score for the symptom categories: no risk (score of 0), low (scores of 1 or 2), medium (scores of 3 or 4), and high (scores of 5 or greater) risk. For all analyses, all available measurement occasions were categorized into levels of depressive symptoms, including a range of 1 to 10 occasions per person (mean = 5.2), and marginal frequencies of 44.1%, 33.5%, 12.1%, and 10.3%, respectively.
Exogenous Stressful Life Events (SLEs)
HRS participants were asked at every wave if they had experienced specific events since the last wave. Thus, recent stressors were defined as those that occurred within the last two years, prior to each assessment of CES-D items. In order to minimize the effect of reverse causation, recent stressors that were unlikely to be caused by prior depressive symptoms were included in the panel of exogenous SLEs. These were coded as a set of eleven possible binary variables for three interpersonal stressors (spouse died, parent died, divorce), five medical stressors (had a stroke or heart attack, became disabled, diagnosed with cancer, had a nursing home stay), and three financial stressors (unemployment, income shock, asset shock) described in detail below.
Variables for interpersonal SLEs indicated the respondent’s divorce, loss of spouse, and parent. For example, one item asked, “Have you been divorced or widowed since [last wave]? If yes, in what month and year were you widowed?” Variables for medical SLEs assessed whether the respondent had experienced a nursing home stay, acute medical event (i.e., heart attack, stroke, cancer), or had become disabled. For example, participants were asked, “Since the last interview, has a doctor told you had a [new cancer or malignant tumor, excluding minor skin cancer/ heart attack or myocardial infarction/ stroke]” and “In what year was your most recent [cancer diagnosed/ heart attack/ stroke]? In what month was that?” The assessment of disability was provided by activities of daily living (ADLs). Respondents who reported no difficulty performing each of six ADLs (i.e., walking across a room, dressing, bathing or showering, eating, getting in or out of bed, and using the toilet) met the criterion for no disability.
Variables for financial SLEs included three constructs. The first was in regard to having been unemployed, and was assessed every other wave since 2004 with the question, “Have you been unemployed and looking for work for longer than 3 months at some point in the past five years? If Yes, what year?” The remaining financial constructs were calculated from a series of questions that asked about income and assets, then coded as binary variables for income shock and asset shock. These constructs indicate whether respondents experienced a reduction in total household income of 30% or more since the last wave, or the value of their household wealth (excluding secondary residence) had been reduced by 30% or more since the last wave. Detailed descriptions on the panel of financial questions, verification of income and assets, and calculations are provided in RAND documentation (RAND 2014).
The eleven possible SLE items were summed to create a count variable of stressors that had occurred prior to each assessment period. Because few respondents had experienced 3 or more SLEs in a given period (see Table 1), the items were coded into successive categories of 0 SLEs, 1 SLE, 2 SLEs, or 3 or more SLEs.
Table 1.
Sample characteristics at first assessment, by race/ethnic group
| Phenotypic Sample (N=28,248) | Genotypic Sample (N=12,332) | |||||
|---|---|---|---|---|---|---|
| AA (N=1,259) | HL (N=4,204) | NHW (N=22,785) | AA (N=570) | HL (N=1,616) | NHW (N=10,146) | |
| Age: M (SD) | 58.95 (9.09) | 62.03 (10.32) | 63.42 (10.61) | 56.43 (6.77) | 57.92 (7.43) | 59.33 (8.10) |
| Female (%) | 56.55 | 61.32 | 56.62 | 60.00 | 63.86 | 57.93 |
| CES-D Depressive symptoms: M (SD) | 2.07 (2.31) | 1.93 (2.16) | 1.37 (1.92) | 1.97 (2.23) | 1.82 (2.15) | 1.16 (1.79) |
| Levels of depressive symptoms (%) | ||||||
| 0 symptoms | 33.52 | 33.37 | 47.72 | 35.44 | 36.26 | 52.81 |
| 1–2 symptoms | 34.71 | 37.13 | 32.11 | 33.51 | 36.32 | 30.89 |
| 3–4 symptoms | 13.42 | 14.44 | 11.05 | 14.39 | 13.86 | 8.96 |
| 5–8 symptoms | 18.35 | 15.06 | 9.13 | 16.67 | 13.55 | 7.34 |
| Stressful life events (SLEs): M (SD) | 0.34 (0.70) | 0.47 (0.76) | 0.32 (0.62) | 0.36 (0.71) | 0.46 (0.73) | 0.33 (0.62) |
| 0 | 75.54 | 66.48 | 74.43 | 75.61 | 65.97 | 74.03 |
| 1 | 16.68 | 22.79 | 19.49 | 15.44 | 23.70 | 19.95 |
| 2 | 6.20 | 8.40 | 4.63 | 7.19 | 8.48 | 5.00 |
| 3 or more | 1.59 | 2.33 | 1.16 | 1.75 | 1.86 | 1.03 |
| 5-HTTLPR genotype (%) | ||||||
| Long/Long (L/L) | -- | -- | -- | 20.85 | 78.63 | 20.65 |
| Long/Short (L/S) | -- | -- | -- | 50.71 | 20.06 | 60.39 |
| Short/Short (S/S) | -- | -- | -- | 28.45 | 1.31 | 18.97 |
| 5-HTTLPR alleles (%) | ||||||
| L allele | -- | -- | -- | 46.20 | 88.66 | 50.84 |
| S allele | -- | -- | -- | 53.80 | 11.34 | 49.16 |
Notes.
SD = standard deviation; AA = African-American; HL = Hispanic/Latino; NHW = Non-Hispanic White; Age range for the phenotypic sample is 50 to 104; Age range for the genotypic sample is 50 to 93; Depressive symptoms ranged from 0 to 8; Stressful life events ranged from 0 to 8 and include events that occurred in the two years prior to assessment.
5-HTTLPR Genotype
Genetic sensitivity was characterized by the number of short alleles of 5-HTTLPR. Genotype data were available for N=12,507 participants who provided DNA samples and were accessed from the National Center for Biotechnology Information Genotypes and Phenotypes Database (dbGaP). Participants in the genotypic subsample, who were also assessed for depressive symptoms and SLE (N=12,332) were 58.8% female; 82.3% Non-Hispanic White, 13.1% Hispanic/Latino, 4.6% African American, and at their first assessment, age M=59.0, SD=8.0, range 50–93. DNA samples from HRS participants were collected in 2006 from buccal swabs using the Qiagen Autopure method. Samples were genotyped by the NIH Center for Inherited Disease Research (CIDR) using the Illumina HumanOmni2.5–4v1 array (San Diego, CA), with standard quality control procedures applied (HRS 2012), including CIDR technical filters (Laurie et al. 2010). Next, genotyped data were phased and imputed in two steps, using SHAPEIT2 (Delaneau et al. 2013) and IMPUTE2 (Howie et al. 2009; Li et al. 2009), respectively. Further details regarding the phasing and imputation process of HRS data are available from CIDR (CIDR 2012). From the ~2.4 million single nucleotide polymorphisms (SNPs) sequenced, nearly 21 million SNPs were imputed to the 1000 Genomes reference panel. Although the two SNPs used to characterize variation in 5-HTTLPR, rs2129785 and rs11867581, were directly genotyped for HRS on the Illumina chip array, we constructed haplotypes with these SNPs for each individual using the phased dosage data. Dosage data were extracted using plink 1.9 (Chang et al. 2014), using several probability thresholds (from 0.10 to 0.49) for hard coding, with each threshold yielding identical results in allele frequencies. Of note, the extracted allele frequencies from phased dosage data were identical to overall frequencies from the directly genotyped data.
The two SNPs were selected as tagging SNPs due to their resulting haplotype being in high linkage disequilibrium (LD; r2=0.78) with the SLC6A4 polymorphic promoter region. The two tag SNPs were assessed for consistency with Hardy-Weinberg Equilibrium (HWE). Stratified by ethnicity, there was no evidence for deviation from HWE as observed allele frequencies for neither rs2129785 (AA χ2=1.06, HL χ2=0.33, NHW χ2=0.02; p’s>0.05) nor rs11867581 (AA χ2=3.29; HL χ2=1.73, NHW χ2=0.25; p’s>0.05) differed from the expected allele frequencies.
Haplotype combinations of these SNPs were initially identified by Vinkhuyzen et al (Vinkhuyzen et al. 2011) and used subsequently by others (Seneviratne et al. 2013) to characterize the 5-HTTLPR alleles based on relative transcriptional efficiency, as either long (L) or short (S): 5′-HTTLPR-L: (rs2129785:A-rs11867581:G) and (rs2129785:G-rs11867581:A) and 5′-HTTLPR-S: (rs2129785:A-rs11867581:A). The present study was consistent with the Vinkhuyzen and Seneviratne reports in that there were no individuals with the (rs2129785:G-rs11867581:G) haplotype. Resulting frequencies for the estimated 5-HTTLPR alleles and genotypes are provided at the bottom of Table 1. After identifying individual genotypes, genetic sensitivity was characterized as levels, from lowest to highest sensitivity, by having no short alleles (homozygous L/L), one short allele (heterozygous S/L), or two short alleles (homozygous S/S) of 5-HTTLPR.
Preliminary Analyses and Attrition
A total of 28,248 individuals and 148,253 observations were included in preliminary analysis (full phenotypic sample). However, as some individuals leave the study at every wave, assessing whether the sample remains representative of the population of older individuals in the U.S. is important. Preliminary analyses showed that the percentage of missing data varied by year and individual measure. Across waves 2 through 10, there were 10,649 (7.2%) of the 148,253 level-1 observations for CESD score missing, which translates into 5,117 of the 28,248 (18.1%) individuals with missing data at least one of the 9 waves. Percentages of individuals with missing data varied by year, ranging between 5.9% in 1998 to 15.6% in 2002. In some cases, the variation was due to individuals who skipped an assessment year, then returned, while in other cases, individuals permanently left the study because they chose to or died (cumulative mortality rate for HRS is 11.3%). None of the main variables of interest for the present study were identified as significantly associated with attrition or missing data. In order to avoid losing participants with sporadically missing data, or in only some years after leaving the study, for all aims, we employ a longitudinal modeling method that uses all available data, including the repeat measurements on all participants (Kapteyn et al. 2006).
Statistical Analyses
Depressive symptomatology was tested as an ordinal variable in order to assess symptom level experienced. Because of the four ordered levels of the dependent variable, CES-D, the level-1 within-person variation in stress and depressive symptoms over time, and use of continuous (age) and categorical (gender, genotype) level-2 predictors, we constructed multinomial cumulative logit random effects models (using PROC GLIMMIX in SAS) to test the study hypotheses. The cumulative logit model assumes proportional odds, or that a common effect is associated with each covariate across all CES-D levels, though allowing for different intercepts. Thus, when the effect of a covariate (age, sex) on level of CES-D symptomatology is interpreted, inference should be made with respect to all levels. For instance, a significant overall effect of age would imply the same age effect at each level of CES-D. Although the proportional odds assumption generally provides a way to reduce the number of parameters and calculate odds ratios (ORs) and 95% confidence intervals (CIs) for each unit increase in covariates, the assumption was not met for all main predictors. For genotype, the proportional odds assumption was met for two subgroups, HL (χ2=5.06, df=2, p=0.08) and NHW (χ2=0.76, df=2, p=0.68), but not for AA (χ2=49.10, df=2, p<0.0001). Thus, as described within each set of results, we implemented a second set of analyses using dummy coded variables (thermometer-type coding) to examine the effects of 5-HTTLPR at each level of S allele (i.e., two short alleles to one, and one short allele to no short alleles). Further, because both the frequencies of CES-D levels shown in the charts and estimated variation in 5-HTTLPR genotypes (provided in Table 1) differed across race/ethnic groups, we stratified all analyses by these subgroups.
Association of recent SLEs and CES-D levels
To test the first hypothesis, that depressive symptoms increase as a function of SLEs, separate models were constructed, for each of the three race/ethnic groups, adjusted for age (mean-centered) and sex. Each set of models was tested for the full phenotypic samples and then replicated in the genotypic subsample. To achieve the goal of comparing statistical approaches, we constructed the first set of models using the degree of freedom trend test, by entering SLEs as a continuous variable (1 degree of freedom test). For the next set of models, we dummy coded the SLE variable such that the individual effect of each additional SLE could be compared to the referent category of having reported no SLEs (3 degree of freedom test). Each reported OR and 95% confidence interval is interpreted as the effect of a one level change in the covariate on the odds of being in a higher CES-D level (more symptoms) rather than a lower level, adjusting for other covariates. To summarize the variation in CES-D accounted for by predictors, pseudo-R squared values were calculated by comparing the covariance parameter estimate of a null (intercept only) model to that from the full model (containing predictors).
Main effect of 5-HTTLPR and interaction between 5-HTTLPR and SLEs on CES-D levels
To test the second hypothesis, we tested both the main effect and interactive effect of 5-HTTLPR x SLE effect on CES-D level, separately for each race/ethnic group using a forward stepwise approach. In the context of our large sample size and repeated measured design, the prevalence of exposure and depressive symptoms within our sample, we had high power (>95%) to detect similar findings reported in the Caspi et al study within each race/ethnic subsample. Described in further detail below, first, we tested the main effects of 5-HTTLPR, adjusted for age and sex; second we added recent SLEs; third we added an interaction term of 5-HTTLPRxSLE.
First, we assessed the main effect of the variation in 5-HTTLPR genotypes. The main effect was assessed in two ways, both as an allelic and genotypic effect. For the allelic effect, we examined a dosage effect of 0, 1, or 2 short alleles using a 1 degree of freedom test, which provided a test of the multiplicative effect of the S allele since these were logit models. For the genotypic effect, we used a 2 degree of freedom test, which provided a test of the multiplicative effect of the L/S and S/S genotypes compared to the referent L/L genotype. In the second step, we tested whether there was a dominance effect of 5-HTTLPR (described below). Third, we added SLEs retaining the dominance effect of the genotype found. In these models testing the main effect of SLEs, we tested SLEs as both a continuous predictor (1 degree of freedom test) and categorical predictor (3 degree of freedom test). Fourth we added the interaction term, comparing the commonly used statistical approaches of testing each of the allelic and genotypic effects of 5-HTTLPR as moderators of both the continuously-coded and categorically-coded SLEs on the depressive symptom outcome.
With regard to step two, if there was evidence of a main effect in either the allelic and genotypic analysis, we assessed 5-HTTLPR for a dominance effect, or whether the L or S allele was dominant within the heterozygous genotype. This was achieved by creating dummy variables using a thermometer-coding approach (Gyurak et al. 2013; Haase et al. 2013; Kendler et al. 2004; Kendler et al. 2005). This way of coding did not alter results from the models, yet provided a method of retaining the ordinal structure of the variable while identifying whether one allele was contributing proportionally more to the effect on CES-D. Thermometer coding yielded two variables, G1 and G2, with individuals coded as follows: two long alleles coded as 0, 0; one long and one short allele coded as 0, 1; and two short alleles coded as 1, 1, respectively. This enabled us to compare the S/S group to the S/L group, and the S/L group to the L/L group. For interpretation, a significant association with the G1 variable indicates dominance of the S allele and that individuals with the L/L genotype differ from individuals with the S/L or S/S genotypes. Conversely, a significant association with the G2 variable indicates dominance of the L allele and that individuals with S/S genotype differ from those with the S/L and L/L genotypes. If a dominance effect was found, we collapsed the genotypes into two classifications. For example, if there was a significant effect of the G2 variable only, we collapse the groups into two (L/L and L/S together, and S/S alone), reflecting the implication that the genotype is acting through dominance from the L alleles. Statistical analyses were conducted using SAS software, version 9.4 (SAS Institute, Cary, NC).
GE Correlation
To address the issue of GE correlation, we constructed logit models to evaluate the association between the proportion of S alleles (as the dependent variable) and number of SLEs (independent variable). This considers the possibility that those who tend to accumulate a greater number of stressors do so because of a genetic predisposition, and this may be independent of levels of CES-D symptomatology. Thus, if a significant effect of SLEs result in predicting genotype, we interpret this as there being a correlational effect of genotype and SLEs on probability of CES-D levels.
Results
Sample and genotype
Table 1 provides means, standard deviation, and frequencies on key variables from the first assessment point for individuals in the phenotypic and genotypic samples. Generally, the genotypic samples were slightly younger, lower in mean for depressive symptoms, and similar with respect to mean of SLEs to the phenotypic sample. Table 1 also provides the differential frequency distribution of short and long 5-HTTLPR variants by race/ethnic subgroup (Short alleles: AA=54%, HL=11%, NHW=49%).
Inclusion in the genetic sample was examined for its relationship to the main study variables. Inclusion status was weakly related to being younger (r=−0.15), but after adjusting for age, inclusion status was not predicted by higher CES-D level (r=−0.08), number of SLEs reported (r=−0.02), being female (r=0.01), or belonging to ethnic subgroups of AAs (r=−0.02), HLs (r=−0.05), or NHWs (r=0.06).
Association of recent SLEs and CES-D levels
Results testing the first hypothesis using the 1 degree of freedom trend test (continuous SLE variable) are presented in Table 2a. Findings were consistent across the NHW and HL samples and supported our first hypothesis. In the genotypic sample, when using a continuous measure of SLEs in the first set of models (Table 2a), each additional SLE conferred increased odds of being at a higher CES-D level among the AA (OR=1.17, 95% CI 1.07, 1.29), HL (OR=1.19, 95% CI 1.13, 1.26), and NHW (OR=1.26, 95% CI 1.23, 1.29) samples. Females had greater odds of being in a higher CES-D level across all models. Older age contributed very little to CES-D level, increasing the odds by 1% among NHWs and was not significant among the HL and AA subgroups. Results in the genotypic sample replicated the results in the phenotypic sample fairly closely, and thus only results of the genotypic sample are shown.
Table 2.
The effect of recent stressors on levels of depressive symptoms in the genotypic samples
| A. Effect of SLEs as a continuous variable on CES-D level, adjusting for age and sex | ||||||
|---|---|---|---|---|---|---|
| African-Americans | Hispanic/Latino | Non-Hispanic White | ||||
| OR (95% CI) | OR (95% CI) | OR (95% CI) | ||||
| Age (per year) | 0.99 | (0.98, 1.01) | 0.99 | (0.99, 1.00) | 1.01 | (1.01, 1.02) |
| Female (vs. Male) | 2.02 | (1.42, 2.88) | 1.55 | (1.25, 1.92) | 1.87 | (1.73, 2.04) |
| SLEs | 1.17 | (1.07, 1.29) | 1.19 | (1.13, 1.26) | 1.26 | (1.23, 1.29) |
| B. Effect of SLEs as a categorical variable on CES-D level, controlled for age and sex | ||||||
|---|---|---|---|---|---|---|
| African-Americans | Hispanic/Latino | Non-Hispanic White | ||||
| OR (95% CI) | OR (95% CI) | OR (95% CI) | ||||
| Age (per year) | 0.99 | (0.98, 1.01) | 0.99 | (0.99, 1.00) | 1.01 | (1.01, 1.02) |
| Female (vs. Male) | 2.02 | (1.42, 2.88) | 1.55 | (1.25, 1.92) | 1.87 | (1.73, 2.04) |
| 1 SLE (vs. 0) | 1.18 | (0.99, 1.40) | 1.17 | (1.07, 1.28) | 1.17 | (1.13, 1.22) |
| 2 SLEs (vs. 0) | 1.22 | (0.96, 1.55) | 1.35 | (1.19, 1.54) | 1.51 | (1.43, 1.60) |
| 3 SLEs (vs. 0) | 2.17 | (1.39, 3.38) | 1.96 | (1.55, 2.49) | 2.56 | (2.30, 2.85) |
Notes.
OR = odds ratios; 95% CI = 95% confidence intervals.
The genotypic samples include N=10,146 with 70,826 observations for NHWs; N=1,616 with 10,699 observations for HLs; N=570 with 3,191 observations for AAs.
Results testing the first hypothesis using the 3 degree of freedom test (dummy coded SLE variable) are shown in Table 2b. In this second set of models, each level of SLEs was predictive of higher CES-D in HLs and NHWs (Table 2b). However, in the genotypic AA sample, experiencing only three SLEs or more was significant, contributing to a 117% increased odds for CES-D level (OR=2.17, 95% CI 1.39, 3.39). The combined contribution of age, sex, and exogenous stressors contributed to 4.9%, 2.8%, and 3.0% of the variation in probability of CES-D level for the AA, HL, and NHW genotypic samples, respectively.
To further examine why the impact of different levels of SLEs differed by ethnic group, we conducted ad hoc analysis in the genotypic sample such that SLEs were categorized into types of stressors: interpersonal, medical, and financial. Results showed that only interpersonal (OR=1.56, 95% CI 1.21, 2.01) and medical (OR=1.38, 95% CI 1.02, 1.87) SLEs were significant for CES-D in AAs, while financial SLEs were not (OR=1.08, 95% CI 0.97, 1.21). When financial SLEs were removed from the total aggregate sum score, we found that 1 SLE compared to 0 was significant (OR=1.37, 95% CI 1.09, 1.72) as was 2 SLEs compared to 0 (OR=3.51, 95% CI 1.82, 6.77). When stratified by type of SLEs for HLs, medical stressors (OR=1.71, 95% CI 1.56, 1.87) increased risk of depression to a greater degree than did interpersonal (OR=1.44, 95% CI 1.29, 1.59) or financial SLEs (OR=1.09, 95% CI 1.04, 1.13). For NHWs, interpersonal stressors (OR=1.73, 95% CI 1.65, 1.81) increased risk of depression to a greater degree than did medical (OR=1.61, 95% CI 1.55, 1.67) or financial SLEs (OR=1.09, 95% CI 1.07, 1.11).
Main effect of 5-HTTLPR and interaction between 5-HTTLPR and SLEs on CES-D levels
Figures 1a through 1c depict the differences in distribution of SLEs and CES-D levels by calculated 5-HTTLPR genotype, separately for each ethnic subgroup. Data in these figures are not adjusted for the effects of age, sex, or within person variation over time, but suggest that for AAs, the S/S genotype associates with less depressive symptomatology irrespective of level of stressor exposure, whereas for HLs the S/S genotype associates with greater symptomatology, also irrespective of level of stressor exposure. For NHWs, the S/S genotype does not differ in association to CES-D level compared to the L/S or L/L genotypes, at any level of stressor exposure.
Figure 1.
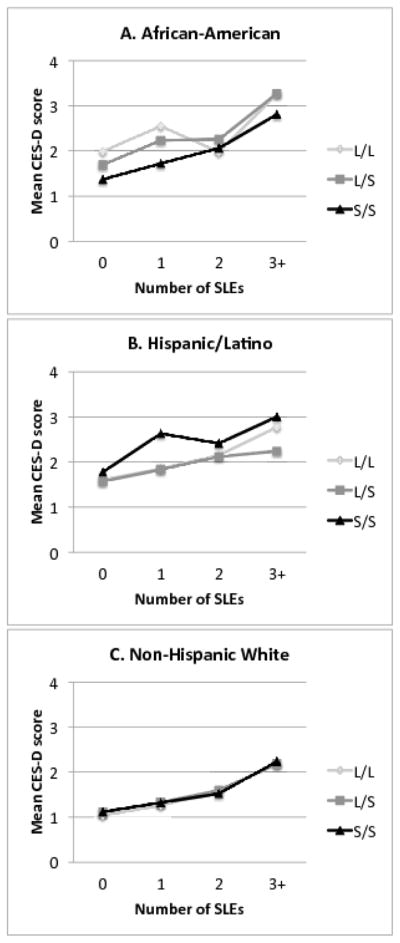
Figures 1a–1c. These figures depict the mean CES-D score, in relation to the number of stressful life events (SLEs) experienced, for each genotype. The mean scores include all available observations for each individual, and are not adjusted for age, sex, or within-person variation.
Results evaluating the second hypothesis for this study were mixed with little evidence overall to support the hypothesis of a GxE interaction. Of note, because empirical evidence exists for the interaction effects of sex and age on 5-HTTLPR in predicting depressive symptoms (e.g., Brummet et al., 2008; Sjober et al 2006), we examined these relationships and did not find evidence of either. Findings from testing the second hypothesis will be discussed below, separately by ethnic subgroup, using the stepwise approach previously described.
African-American sample
In the allelic analysis (Table 3a) we found a significant main effect of 5-HTTLPR, but with an unexpected direction of effect. Each additional S allele decreased the odds of being at a higher CES-D level by 36% (OR=0.64, 95% CI 0.50, 0.82), holding age and sex constant. In the genotypic analysis (Table 3b), we found that the protective effect held for the S/S group only (OR=0.42, 95% CI 0.26, 0.69) and not the L/S group (OR=0.73, 95% CI 0.46, 1.14) among AAs.
Table 3.
Results of models testing the main effects of 5-HTTLPR and SLEs on CES-D levels in all ethnicities, adjusted for age and sex
| A. Main effect of the 5-HTTLPR S allele | ||||||
|---|---|---|---|---|---|---|
| African-American | Hispanic/Latino | Non-Hispanic White | ||||
| OR (95% CI) | OR (95% CI) | OR (95% CI) | ||||
| Age | 1.00 | (0.98, 1.01) | 1.00 | (0.99, 1.00) | 1.02 | (1.01, 1.02) |
| Female (vs. Male) | 2.04 | (1.43, 2.92) | 1.53 | (1.23, 1.91) | 1.88 | (1.73, 2.04) |
| 5-HTTLPR (per S allele) | 0.64 | (0.50, 0.82) | 0.98 | (0.78, 1.23) | 1.03 | (0.96, 1.09) |
| B. Main genotypic effects | ||||||
|---|---|---|---|---|---|---|
| African-American | Hispanic/Latino | Non-Hispanic White | ||||
| OR (95% CI) | OR (95% CI) | OR (95% CI) | ||||
| L/S (vs. L/L) | 0.73 | (0.46, 1.14) | 0.91 | (0.70, 1.18) | 1.05 | (0.95, 1.17) |
| S/S (vs. L/L) | 0.42 | (0.26, 0.69) | 1.50 | (0.61, 3.69) | 1.05 | (0.92, 1.20) |
| C. Main genotypic effects testing dominance of the L allele | ||||||
|---|---|---|---|---|---|---|
| African-American | Hispanic/Latino | Non-Hispanic White | ||||
| OR (95% CI) | OR (95% CI) | OR (95% CI) | ||||
| S/S (vs. L/L or L/S) | 0.52 | (0.36, 0.77) | 1.53 | (0.62, 3.76) | 1.01 | (0.91, 1.12) |
| D. Main genotypic (reflecting dominance structure) and continuous SLE effects | ||||||
|---|---|---|---|---|---|---|
| African-American | Hispanic/Latino | Non-Hispanic White | ||||
| OR (95% CI) | OR (95% CI) | OR (95% CI) | ||||
| S/S (vs. L/L or L/S) | 0.53 | (0.36, 0.78) | 1.49 | (0.61, 3.64) | 1.02 | (0.92, 1.13) |
| SLEs | 1.18 | (1.07, 1.30) | 1.19 | (1.13, 1.26) | 1.26 | (1.23, 1.29) |
| E. Main genotypic (reflecting dominance structure) and ordinal SLE effects | ||||||
|---|---|---|---|---|---|---|
| African-American | Hispanic/Latino | Non-Hispanic White | ||||
| OR (95% CI) | OR (95% CI) | OR (95% CI) | ||||
| S/S (vs. L/L or L/S) | 0.53 | (0.36, 0.77) | 1.48 | (0.61, 3.62) | 1.02 | (0.92, 1.13) |
| 1 SLE (vs. 0) | 1.19 | (1.00, 1.41) | 1.17 | (1.06, 1.28) | 1.18 | (1.13, 1.22) |
| 2 SLEs (vs. 0) | 1.22 | (0.96, 1.56) | 1.36 | (1.19, 1.54) | 1.51 | (1.43, 1.60) |
| 3 SLEs (vs. 0) | 2.15 | (1.37, 3.38) | 1.94 | (1.53, 2.46) | 2.59 | (2.33, 2.88) |
Notes.
OR = odds ratios; 95% CI = 95% confidence intervals. Significant allelic and genotypic effects are bolded. The effects of age and sex did not change across models, thus they are shown in 3a only.
Testing a dominance effect among AAs, we found a significant effect of the G2 variable (OR=0.58, 95% CI 0.38, 0.87), but not the G1 variable (OR=0.73, 95% CI 0.46, 1.14). After collapsing the genotypes (reflecting the implication that the genotype is acting through dominance from the L alleles), the 5-HTTLPR dominance effect can be interpreted as, those who possessed the S/S genotype (versus the L/L or L/S genotype) were at a 48% (OR=0.52, 95% CI 0.36, 0.77) decreased odds for being at a higher level of CES-D (shown in Table 3c).
Testing the effects of genotype with the continuous SLEs, we found that both the effect of the S/S genotype (OR=0.53, 95% CI 0.36, 0.78) versus alternate genotypes as well as SLEs (OR=1.18, 95% CI 1.07, 1.30) were significant among AAs (Table 3d). Testing the genotype with SLEs tested as ordinal predictor, we found that the S/S (OR=0.53, 95% CI 0.36, 0.77) versus alternative genotypes was significant and when only three or more SLEs were reported (OR=2.15, 95% CI 1.37, 3.38), the effect of SLEs were significant for AAs (Table 3d). In this model, age, sex, genotype, and SLEs explained 7.3% of the variation in probability of CES-D level for AAs. Testing the interaction term between variation in 5-HTTLPR and SLEs, there was no evidence of an interaction with SLEs when entered as either the continuous (F(1)=1.22, p=0.27) or ordinal scale (F(3)=0.37, p=0.78).
Hispanic-Latino sample
In the stepwise process of testing for main and interaction effects in HLs, we first tested the main effect of the 5-HTTLPR, then added the main effects of SLEs, and lastly tested the interaction between the two. From analyses of the genetic effects, there was no evidence for a main effect of variation in 5-HTTLPR on CES-D in either the allelic (OR=0.98, 95% CI 0.78, 1.23; Table 3a) or genotypic (L/S vs L/L OR=0.91, 95% CI 0.70, 1.18; S/S vs L/L OR=1.50, 95% CI 0.61, 3.69; Table 3b) analyses. There was an effect of sex, such that females had a 53% (OR=1.53, 95% CI 1.23, 1.91) increased odds of being at a higher CES-D level. This effect remained consistent across models. No dominance effect of 5-HTTLPR was evident (Table 3c). Effects of SLEs were significant when entered either as a continuous (Table 3d) or ordinal variable (Table 3e). Similarly, no effect was evident for the GxE effect when SLEs were included as continuous (F(1)=0.13, p=0.71) or ordinal (F(3)=0.48, p=0.69).
Non-Hispanic White sample
We conducted the same stepwise process as completed in AAs and HLs. In step one, there was no evidence of a main effect of 5-HTTLPR variation in either the allelic (OR=1.03, 95% CI 0.96, 1.09; Table 3a) or the genotypic (L/S vs L/L OR=1.05, 95% CI 0.91, 1.12; S/S vs L/L OR=1.05, 95% CI 0.92, 1.12; see Table 3b) analyses. Older age contributed very little to the odds of being at a higher CES-D level (OR=1.02, 95% CI 1.01, 1.02), whereas sex (from male to female) increased the odds by 88% (OR=1.88, 95% CI 1.73, 2.04). These effects remained consistent across models. In step two, no dominance effect of 5-HTTLPR (comparing the effect of the S/S genotype to the other two) was evident (Table 3c). In step three, effects for both the continuous and ordinal SLEs scales were significant (Tables 3d and 3e). For example, each additional SLE increased the odds of being in a higher CES-D level by 26% (OR=1.26, 95% CI 1.23, 1.29).
In step four, there were mixed findings on the moderator effect of the genotype (Tables 4a–4f). Incorporating a dominance effect of genotype, there was no evidence to support the interaction when SLEs were entered either as continuous (F(1)=1.89, p=0.17; see Table 4a) or ordinal variables (F(3)=0.84, p=0.47; see Table 4b). Support for the interaction was found in the allelic (F(1)=6.05, p=.014; Table 4c) and genotypic analysis (F(2)=3.41, p=.033; Table 4e), but only when the interaction was tested using a continuous indicator for SLEs. The interaction was not detected when SLEs were coded categorically, either with the allelic analysis (F(3)=2.00, p=0.11; Table 4d) or the genotypic analysis (F(6)=1.57, p=0.15; Table 4f).
Table 4.
Results of models testing the GxE effects on CES-D levels in the Non-Hispanic White sample, adjusted for age and sex
| A. GxE dominance effect of 5-HTTLPR and the interaction with continuous SLEs | |||||
|---|---|---|---|---|---|
| OR (95% CI) | F-Value | df | p-value | ||
| S/S (vs. L/L or L/S) | 1.02 | (0.92, 1.13) | 0.52 | 1 | 0.47 |
| SLEs (per SLE) | 1.26 | (1.23, 1.29) | 366.14 | 1 | <0.0001 |
| 5-HTTLPR x SLEs | -- | -- | 1.89 | 1 | 0.17 |
| B. GxE dominance effect of 5-HTTLPR and the interaction with ordinal SLEs | |||||
|---|---|---|---|---|---|
| OR (95% CI) | F-Value | df | p-value | ||
| S/S (vs. L/L or L/S) | 0.58 | (0.37, 0.90) | 0.00 | 1 | 0.98 |
| 1 SLE (vs. 0) | 1.18 | (1.13, 1.22) | 133.13 | 3 | <0.0001 |
| 2 SLEs (vs. 0) | 1.51 | (1.43, 1.60) | |||
| 3 SLEs (vs. 0) | 2.59 | (2.33, 2.88) | |||
| 5-HTTLPR for SLEs: 0 | 1.04 | (0.93, 1.17) | 0.84 | 3 | 0.47 |
| 5-HTTLPR for SLEs: 1 | 1.00 | (0.89, 1.13) | |||
| 5-HTTLPR for SLEs: 2 | 0.93 | (0.80, 1.09) | |||
| 5-HTTLPR for SLEs: 3 | 1.03 | (0.77, 1.35) | |||
| 5-HTTLPR x SLEs | -- | -- | |||
| C. GxE with allelic effect of 5-HTTLPR and the interaction with continuous SLEs | |||||
|---|---|---|---|---|---|
| OR (95% CI) | F-Value | df | p-value | ||
| 5-HTTLPR (per S allele) | 1.03 | (0.96, 1.10) | 2.52 | 1 | 0.11 |
| SLEs (per SLE) | 1.26 | (1.23, 1.29) | 177.25 | 1 | <0.0001 |
| 5-HTTLPR x SLEs | -- | -- | 6.05 | 1 | 0.0139 |
| D. GxE with allelic effect of 5-HTTLPR and the interaction with ordinal SLEs | |||||
|---|---|---|---|---|---|
| OR (95% CI) | F-Value | df | p-value | ||
| 5-HTTLPR (per S allele) | -- | -- | 0.11 | 1 | 0.74 |
| 1 SLE (vs. 0) | 1.18 | (1.13, 1.22) | 64.49 | 3 | <0.0001 |
| 2 SLEs (vs. 0) | 1.51 | (1.43, 1.60) | |||
| 3 SLEs (vs. 0) | 2.58 | (1.32, 2.87) | |||
| S allele for 0 SLE | 1.05 | (0.98, 1.13) | 2.00 | 3 | 0.11 |
| S allele for 1 SLE | 1.03 | (0.49, 1.11) | |||
| S allele for 2 SLEs | 0.96 | (0.87, 1.06) | |||
| S allele for 3 SLEs | 0.92 | (0.77, 1.09) | |||
| 5-HTTLPR x SLEs | -- | -- | |||
| E. GxE with genotypic effect of 5-HTTLPR and the interaction with continuous SLEs | |||||
|---|---|---|---|---|---|
| OR (95% CI) | F-Value | df | p-value | ||
| L/S (vs. L/L) | 1.05 | (0.95, 1.17) | 1.61 | 2 | 0.20 |
| S/S (vs. L/L) | 1.06 | (0.93, 1.20) | |||
| SLE at L/L | 1.33 | (1.27, 1.39) | 338.26 | 1 | <0.0001 |
| SLE at L/S | 1.25 | (1.21, 1.29) | |||
| SLE at S/S | 1.22 | (1.16, 1.28) | |||
| 5-HTTLPR x SLEs | -- | -- | 3.41 | 2 | 0.033 |
| F. GxE with genotypic effect of 5-HTTLPR and the interaction with ordinal SLEs | |||||
|---|---|---|---|---|---|
| OR (95% CI) | F-Value | df | p-value | ||
| L/S (vs. L/L) | 0.98 | (0.86, 1.10) | 0.12 | 2 | 0.89 |
| S/S (vs. L/L) | 0.98 | (0.84, 1.14) | |||
| 1 SLE (vs. 0) | 1.17 | (1.12, 1.22) | 127.17 | 3 | <0.0001 |
| 2 SLEs (vs. 0) | 1.51 | (1.42, 1.61) | |||
| 3 SLEs (vs. 0) | 2.70 | (2.40, 3.05) | |||
| 5-HTTLPR x SLEs | -- | -- | 1.57 | 6 | 0.15 |
Notes.
OR = odds ratios; 95% CI = 95% confidence intervals; df = degrees of freedom. Significant effects are in bold. The effects of age and sex did not change across models, therefore they are shown in the prior table, 3a, only.
Lastly, addressing the issue of GE correlation, we found no evidence of a correlation between SLEs reported and genotype in the AA group (OR=0.97, 95% CI 0.77, 1.23), HL group (OR=1.02, 95% CI 0.87, 1.18), or NHW group (OR=1.00, 95% CI 0.94, 1.06).
Discussion
This study is the largest to have been conducted on the effect of 5-HTTLPR and stress interaction in predicting depression symptoms. Findings from prior studies on this topic have been remarkably conflicting. In the present study, we were able to implement and compare results for the most common statistical approaches used to examine this GxE effect in the literature. At the same time, we were able to address issues of CES-D fluctuations over time within the same individual, GE correlation, reverse causation, and negativity bias, many of which are not consistently addressed by other study designs. From our analyses, we found that neither the effect of the genotype nor stress was consistent across race/ethnic groups. There was a significant effect of more SLEs predicting higher depressive symptom levels in all race/ethnic groups when SLEs were assessed as a continuous variable. However, when using a categorical SLE variable, only experiencing three or more SLEs had an effect. Among African-Americans, there was no evidence of GxE interaction, but there was a main effect of genotype, in the opposite direction expected. Among Hispanic/Latinos, recent stress consistently predicted CES-D level, but variation in 5-HTTLPR showed no evidence for a main effect or GxE effect. Among Non-Hispanic Whites, recent stress predicted CES-D level, but no main effect of genotype. Also in Non-Hispanic Whites, there was some evidence for a GxE effect when SLEs were continuously coded, but not when categorically coded. Comparisons across the statistical methods employed here suggest possible reasons for conflicting findings in the literature. Further testing of these models is worthwhile, comparing results from ethnic subsamples, with the 5-HTTLPR length characterized through a direct genotyping processes.
Our findings that recent stressors contribute to higher depressive symptom levels is consistent with prior research on older adults (Alexopoulos 2005; Fiske et al. 2009; Kraaij et al. 2002; Surtees et al. 2006). However, our results suggest that the type of stressor matters (interpersonal and medical vs. financial), plus more stressors (two or more) have markedly greater depressogenic effects among African-Americans than does a single stressor. This coincides somewhat with findings in the literature on the “ethnic paradox”, in which older African-Americans show relatively fewer depressive symptoms than members of other groups, despite experiencing more stressful environments and greater physical morbidity (Jackson et al. 2010; Keyes 2009). Moreover, evidence suggests that unhealthy behaviors moderate (buffer) the effects of stress, thereby compensating for what would otherwise be increased depressive symptoms (Jackson et al. 2010). Examining such three-way gene-by-environment-by-behavioral interactions is beyond the scope of this study, though such evidence suggests plausible mechanisms to explain our finding. Also important with this finding is that it was detected only by the statistical approach of modeling categories of SLEs, as levels of exposure (versus a continuous count of stressors).
On the characterization of the 5-HTTLPR genotypes, some discussion is necessary with regard to the method used to estimate the bi-allelic motif in each of our three subsamples. Although we apply the best method currently known for characterizing the 5-HTTLPR on SNP data from commercially available chip arrays, it is possible that using the two-SNP haplotype that is in imperfect LD with 5-HTTLPR harbors some degree of error in characterizing the bi-allelic genotype. Initially, the tag SNPs were identified for their high LD with 5-HTTLPR based on an Australian sample (Vinkhuyzen et al, 2011; Wray et al., 2010; Medland et al., 2009). Comparing the allele frequencies of HRS Non-Hispanic Whites (S=49%, L=51%) to those reported in other studies focused on participants from European descent (range of values for S allele=40–47%; (Brummett et al. 2008; Caspi et al. 2003; Chipman et al. 2007; Chorbov et al. 2007; Gelernter et al. 1997; Li et al. 2013; Nakamura et al. 2000; Otte et al. 2007; Rees et al. 1997; Ritchie et al. 2009; Scheid et al. 2007; Schild et al. 2014; Seneviratne et al. 2013; Seneviratne et al. 2009; Williams et al. 2003; Yang et al. 2013); for table of allele frequencies across studies see Supplemental material), the HRS sample included very similar frequencies. However, sample ascertainment for HRS was designed to represent the US population of adults age 50 and over. Thus, although is unclear as to whether one study sample better represents the genotype distribution for 5-HTTLPR among Non-Hispanic Whites versus another, the haplotype appears to provide a good approximation of the alleles.
In contrast, when comparing the HRS Hispanic/Latino allelic variation (S=11%, L=89%) with other studies focused on participants from predominantly Spanish-speaking countries (range of values for S allele=43–60%; (Schild et al. 2014; Seneviratne et al. 2009); for table of allele frequencies across studies see Supplemental material), the HRS sample clearly contained fewer S alleles. However, when comparing the HRS Hispanic/Latino sample to the Mexican-American sample from 1000 Genomes (S=3%; Consortium 2012), the frequencies are more similarly and predominantly comprised of Long alleles. It is unclear whether these discrepancies in frequencies is an effect of measurement, due to lower reliability of the two-SNP haplotype for characterizing 5-HTTLPR in HLs (i.e., low LD in ethnic Latinos), or an effect of sampling, due to the ascertainment of the sample itself (e.g., survival or attrition issues in the HRS HL subsample, recruitment methods applied when oversampling HL participants, greater composition of individuals with Mexican ancestry). In either case, given this large discrepancy in allelic variation for HLs in the HRS versus other studies, no clear conclusions can be drawn from results of analyses run in the HL subsample for this study; and thus, few interpretations will be discussed with regard to 5-HTTLPR findings among HLs.
When comparing the allelic variation in HRS African-Americans (S=54%, L=46%) with African-American participants in other studies (range of values for S allele=23–48%; (Brummett et al. 2008; Cicchetti and Rogosch 2014; Gelernter et al. 1997; Hu et al. 2006; Walsh et al. 2014; Williams et al. 2003; Yang et al. 2013); for table of allele frequencies across studies see Supplemental material), the HRS AA sample was more similar in 5-HTTLPR variation reported in studies in which participants were recruited from Michigan or Georgia (range of values for S allele=53–54%; Walsh et al. 2014), compared to studies in which participants were recruited from Connecticut, Tennessee, Mississippi, or Arkansas (range of values for S allele=70–77%; Gelernter et al. 1997; Walsh et al. 2014; Yang et al. 2013). Interestingly, all studies varied greatly from the African subsample reported in 1000 Genomes (S=99%; Consortium 2012). From these results, it is unclear as to whether the HRS subsample better represents the genotype distribution for 5-HTTLPR compared to other studies, or if the haplotype is truly a good approximation of 5-HTTLPR given the unknown LD. Although, we apply the best method currently known for characterizing the variation in 5-HTTLPR from a SNP array, findings from the analyses on AAs require replication in a sample using directly genotyped 5-HTTLPR alleles.
With regard to the main effects of the variation in 5-HTTLPR on depressive symptoms, our null findings in Non-Hispanic Whites are consistent with prior reports in older adults (Goldman et al. 2010; Kim et al. 2007; Power et al. 2010; Surtees et al. 2006), conducted in East Asian and French samples. In contrast, our finding that the S/S genotype is protective in African-Americans is a novel one among AAs specifically, as it has been reported previously only in other ethnic samples (e.g., Carli et al. 2011; Chorbov et al. 2007; Laucht et al. 2009; Phillips-Bute et al. 2008; Ritchie et al. 2009); among males but not females in (Sjöberg et al. 2006). Nonetheless, the result warrants further consideration, for which we offer two possible explanations. As observed by Taylor et al (Taylor et al. 2006), protective effects of the S/S genotype are typically detected in samples that also report positive early or recent life experiences. One hypothesis that has gained traction in the last several years is the notion that the S/S genotype not only confers sensitivity to negative environments, but also to positive environments (Bakermans-Kranenburg and Van IJzendoorn 2007; Belsky et al. 2009; Belsky and Pluess 2009; Ellis et al. 2011). Support for the differential susceptibility hypothesis has been demonstrated by Taylor and colleagues, who found that young adults with the S/S genotype who faced recent adversity tended to exhibit fewer depressive symptoms when also reporting positive experiences when compared to their L/S and L/L counterparts (Taylor et al. 2006). Therefore, without also assessing recent positive life events or supportive environmental factors, we would not be able to detect this effect.
A second explanation for the unexpected finding concerns the classification of genotypes. It is possible that the bi-allelic characterization of 5-HTTLPR for this study classifies the effects of rarer long alleles that have been shown to have lower transcriptional efficacy, similar to the S alleles. One of these variants has been identified as the G allele of rs25531 (Kraft et al. 2005; Wendland et al. 2006), and exemplifies one limitation to using sequenced chip data as it has been shown that accuracy with which to tag this rarer allele is low (Vinkhuyzen et al. 2011). Relevant to our unexpected finding however, is that although more rare in individuals of European ancestry (frequency between 0.09 to 0.14), the G allele has frequencies as high as 0.24 in African-Americans, and exhibits some evidence of codominance with the L and S alleles (Hu et al. 2005). Although this explanation is credible, a case can be made against this explanation. If it were likely that we had classified these rare alleles into the L/L genotype and there were a high frequency of them in our sample, one would expect our sample to have a disproportionately higher frequency of L/L or L/S genotypes overall, compared to other African-American samples. This does not seem to be the case as the genotypes in our African-American sample were 21% L/L, 51% L/S, and 28% S/S compared to the frequencies in other studies for African-Americans, which have been estimated be 53% L/L, 37% L/S, and 9% S/S (Goldman et al. 2010). Another study examining the frequency of alleles in the African-Americans in the U.S. calculated frequencies of the S/S genotype to be as low as 4 to 10% (Lotrich, 2003). Nonetheless, it would be worthwhile for future population-based studies to include a tri-allelic characterization of 5-HTTLPR that has been directly genotyped in AAs to assess this possible explanation. Also, future studies in which the 5-HTTLPR variant is directly genotyped in AAs are needed to confirm our result.
With regard to the interaction effect of 5-HTTLPR variation and SLEs, there was no effect among African-Americans and not a consistent effect among Non-Hispanic Whites. The findings among African-Americans corroborates multiple studies that have found no GxE effect in older adults (Power et al. 2010; Surtees et al. 2006), yet the inconsistent results among Non-Hispanic Whites corroborate findings of the conflicting meta-analyses (Karg et al. 2011; Munafò et al. 2009; Risch et al. 2009). The findings among Non-Hispanic Whites could arguably be interpreted with their being a significant interaction if we had implemented only one type of statistical modeling, the 1 degree of freedom multiplicative model. Also, it is possible that we would have detected a significant interaction given a different set of conditions, such as assessing a broader range of acute stressors likely to occur, including hospitalization from accidents or falls, other medical diagnoses, changes in living arrangements, interpersonal stressors, or chronic stressors (Monroe and Reid 2008). It is possible that the restricted range of our predictors impedes possible positive findings; yet with the current design, we have been able to minimize spurious effects that would otherwise be due to GE correlation, in which the genotype is invariably related to an individual’s chosen environment that may be precipitously stressful. Thus, measuring only exogenous stressors provides an objective method of assessing GxE while addressing the temporal issue whereby SLEs precede CES-D symptoms and thus symptom levels do not beget the reporting of SLEs.
A potential limitation of this study concerns the use of a proxy haplotype to estimate variation in 5-HTTLPR. One concern is that a statistical algorithm based on imperfect LD was used to estimate the polymorphism rather than direct genotyping of the variant. A second concern is the applicability of the algorithm to the U.S. ethnic subsamples. It must be noted that the approach in this study is currently the best available approximation for 5-HTTLPR using genotyped data from a chip array (see Vinkhuyzen et al., 2011) and incorporating available LD data from sources such as HapMap and 1000 Genomes. Particularly among the NHW and AA samples, the variation of alleles was similar to multiple studies in which the variant was directly genotyped. However, within the HL sample, questions arise from the resulting allelic frequencies that require more in depth investigation, such as comparing the haplotype approach to directly genotyped individuals within the same HL sample. Such in depth investigation would be useful for all subsamples. Another potential limitation concerns the dependent variable of depressive symptomatology. While the modified CES-D scale confers some restricted variation in capturing all domains of depressive symptomatology compared to the longer versions, the scale has been validated to the longer inventory. That we found evidence of a relationship with exogenous SLEs across subsamples as well as with genotype in African-Americans leads us to believe that the measure adequately represents the intended assessment of depressive symptomatology. Furthermore, the majority of studies on this topic have used a dichotomously coded depression outcome. In our study, assessing levels of CES-D in lieu of a binary outcome enables us to assess how exogenous stressors are impacting the experiences of older individuals who are more prone to having these types of events occur. Third, as previously mentioned, measuring only exogenous stressors, such that excluding the effect of chronic or daily stressors may be less robust on overall depressive symptomatology and the feelings incurred by subjective stressors. However, our decision to use only recent and exogenous SLEs exhibits a strength in this design, as it also minimizes the issue that higher depressive symptoms over time could lead to either the reporting of more SLEs, or causing more SLEs to occur. Fourth, despite the power gained from using a repeated-measures design (Wong et al., 2003), when calculating post hoc power, using a continuous outcome of depressive symptoms, allelic effect of 5-HTTLPR, and continuous measure of SLE exposure, we were limited in detecting a GxE effect. In the largest subgroup of NHW, we had 99%, 81%, and 32% power to detect the E, G, and GxE effect, respectively (in AAs and HLs, there was 31% and 7% power, respectively to detect the GxE effect). Finally, genotypic frequencies may be unique to the subsamples retained within the U.S. Health and Retirement Study for DNA sampling; therefore findings may not be externally applicable to other race/ethnic subpopulations. Nonetheless, the findings address the potential reason for conflicting results in prior research that has included different ethnic subpopulations.
Despite the lack of consistent findings for a GxE effect, the finding that genotype associates with CES-D among African-Americans offers implications for further research, including the need for replication among an older African-American sample in which the 5-HTTLPR variant was directly genotyped. Also, because one plausible explanation of the protective effect involves behaviors (ethnic paradox) and other environmental support factors (potential differential susceptibility interactions), further investigation of these alternate explanations would be needed for appropriate tailoring of targeted interventions (e.g., culturally and age-appropriate support interventions for older African-Americans). Irrespective of genotype, depression symptoms have greater consequences in older adults than in younger adults, and our findings on the effect of “uncontrollable” life stress on depressive symptoms among older adults, suggests there may be differential need for supportive resources given the types of stressors most depressogenic in each ethnic group (interpersonal for African-Americans and Non-Hispanic Whites, medical for Hispanic/Latinos). Further examination on the levels and types of SLEs that confer depressogenic effects in the different ethnic groups will be important for informing intervention efforts.
Supplementary Material
Acknowledgments
This research was supported by the National Institute on Aging, of the National Institutes of Health, under award numbers F32AG048681 and P30AG17265. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Note: The Health and Retirement Study genetic data is sponsored by the National Institute on Aging (grant numbers U01AG009740, RC2AG036495, and RC4AG039029) and was conducted by the University of Michigan.
References
- Alexopoulos GS. Depression in the elderly. Lancet. 2005;365(9475):1961–1970. doi: 10.1016/S0140-6736(05)66665-2. [DOI] [PubMed] [Google Scholar]
- Araya R, Hu X, Heron J, Enoch MA, Evans J, Lewis G, Nutt D, Goldman D. Effects of stressful life events, maternal depression and 5-HTTLPR genotype on emotional symptoms in pre-adolescent children. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2009;150(5):670–682. doi: 10.1002/ajmg.b.30888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, Van IJzendoorn MH. Research review: Genetic vulnerability or differential susceptibility in child development: The case of attachment. Journal of Child Psychology and Psychiatry. 2007;48(12):1160–1173. doi: 10.1111/j.1469-7610.2007.01801.x. [DOI] [PubMed] [Google Scholar]
- Beck JS. Cognitive therapy. Wiley Online Library; 1995. [Google Scholar]
- Belsky J, Jonassaint C, Pluess M, Stanton M, Brummett B, Williams R. Vulnerability genes or plasticity genes quest. Molecular psychiatry. 2009;14(8):746–754. doi: 10.1038/mp.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychol Bull. 2009;135(6):885. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Brummett BH, Boyle SH, Siegler IC, Kuhn CM, Ashley-Koch A, Jonassaint CR, Züchner S, Collins A, Williams RB. Effects of environmental stress and gender on associations among symptoms of depression and the serotonin transporter gene linked polymorphic region (5-HTTLPR) Behavior genetics. 2008;38(1):34–43. doi: 10.1007/s10519-007-9172-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carli V, Mandelli L, Zaninotto L, Roy A, Recchia L, Stoppia L, Gatta V, Sarchiapone M, Serretti A. A protective genetic variant for adverse environments? The role of childhood traumas and serotonin transporter gene on resilience and depressive severity in a high-risk population. European Psychiatry. 2011;26(8):471–478. doi: 10.1016/j.eurpsy.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Cervilla J, Molina E, Rivera M, Torres-Gonzalez F, Bellon J, Moreno B, Luna J, Lorente J, Mayoral F, King M. The risk for depression conferred by stressful life events is modified by variation at the serotonin transporter 5HTTLPR genotype: evidence from the Spanish PREDICT-Gene cohort. Molecular psychiatry. 2007;12(8):748–755. doi: 10.1038/sj.mp.4001981. [DOI] [PubMed] [Google Scholar]
- Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ. Second-generation PLINK: rising to the challenge of larger and richer datasets. 2014 doi: 10.1186/s13742-015-0047-8. arXiv preprint arXiv:14104803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipman P, Jorm A, Prior M, Sanson A, Smart D, Tan X, Easteal S. No interaction between the serotonin transporter polymorphism (5-HTTLPR) and childhood adversity or recent stressful life events on symptoms of depression: Results from two community surveys. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2007;144(4):561–565. doi: 10.1002/ajmg.b.30480. [DOI] [PubMed] [Google Scholar]
- Chorbov VM, Lobos EA, Todorov AA, Heath AC, Botteron KN, Todd RD. Relationship of 5-HTTLPR genotypes and depression risk in the presence of trauma in a female twin sample. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2007;144(6):830–833. doi: 10.1002/ajmg.b.30534. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. Genetic moderation of child maltreatment effects on depression and internalizing symptoms by serotonin transporter linked polymorphic region (5-HTTLPR), brain-derived neurotrophic factor (BDNF), norepinephrine transporter (NET), and corticotropin releasing hormone receptor 1 (CRHR1) genes in African American children. Dev Psychopathol. 2014;26(4 Pt 2):1219–1239. doi: 10.1017/S0954579414000984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CIDR. CIDR Health and Retirement Study: Imputation Report - 1000 Genomes Project reference panel. University of Washington; Seattle, WA: 2012. pp. 1–25. [Google Scholar]
- Consortium GP. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491(7422):56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuijpers P, de Graaf R, van Dorsselaer S. Minor depression: risk profiles, functional disability, health care use and risk of developing major depression. Journal of affective disorders. 2004;79(1):71–79. doi: 10.1016/S0165-0327(02)00348-8. [DOI] [PubMed] [Google Scholar]
- Delaneau O, Zagury J-F, Marchini J. Improved whole-chromosome phasing for disease and population genetic studies. Nature methods. 2013;10(1):5–6. doi: 10.1038/nmeth.2307. [DOI] [PubMed] [Google Scholar]
- Delbruck SJ, Wendel B, Grunewald I, Sander T, Morris-Rosendahl D, Crocq MA, Berrettini WH, Hoehe MR. A novel allelic variant of the human serotonin transporter gene regulatory polymorphism. Cytogenetics and cell genetics. 1997;79(3–4):214–220. doi: 10.1159/000134726. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, Van IJzendoorn MH. Differential susceptibility to the environment: An evolutionary??? neurodevelopmental theory. Development and psychopathology. 2011;23(1):7. doi: 10.1017/S0954579410000611. [DOI] [PubMed] [Google Scholar]
- Fiske A, Wetherell JL, Gatz M. Depression in older adults. Annual review of clinical psychology. 2009;5:363–389. doi: 10.1146/annurev.clinpsy.032408.153621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch A, Finkel B, Michaelovsky E, Sigal M, Laor N, Weizman R. A rare short allele of the serotonin transporter promoter region (5-HTTLPR) found in an aggressive schizophrenic patient of Jewish Libyan origin. Psychiatric genetics. 2000;10(4):179–183. doi: 10.1097/00041444-200010040-00005. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Kranzler H, Cubells JF. Serotonin transporter protein (SLC6A4) allele and haplotype frequencies and linkage disequilibria in African-and European-American and Japanese populations and in alcohol-dependent subjects. Hum Genet. 1997;101(2):243–246. doi: 10.1007/s004390050624. [DOI] [PubMed] [Google Scholar]
- Gillespie NA, Whitfield JB, Williams B, Heath AC, Martin NG. The relationship between stressful life events, the serotonin transporter (5-HTTLPR) genotype and major depression. Psychol Med. 2005;35(01):101–111. doi: 10.1017/s0033291704002727. [DOI] [PubMed] [Google Scholar]
- Goldman N, Glei DA, Lin YH, Weinstein M. The serotonin transporter polymorphism (5-HTTLPR): allelic variation and links with depressive symptoms. Depression and anxiety. 2010;27(3):260–269. doi: 10.1002/da.20660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabe HJ, Lange M, Wolff B, Völzke H, Lucht M, Freyberger H, John U, Cascorbi I. Mental and physical distress is modulated by a polymorphism in the 5-HT transporter gene interacting with social stressors and chronic disease burden. Molecular psychiatry. 2005;10(2):220–224. doi: 10.1038/sj.mp.4001555. [DOI] [PubMed] [Google Scholar]
- Gyurak A, Haase CM, Sze J, Goodkind MS, Coppola G, Lane J, Miller BL, Levenson RW. The effect of the serotonin transporter polymorphism (5-HTTLPR) on empathic and self-conscious emotional reactivity. Emotion. 2013;13(1):25. doi: 10.1037/a0029616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase CM, Saslow LR, Bloch L, Saturn SR, Casey JJ, Seider BH, Lane J, Coppola G, Levenson RW. The 5-HTTLPR polymorphism in the serotonin transporter gene moderates the association between emotional behavior and changes in marital satisfaction over time. Emotion. 2013;13(6):1068. doi: 10.1037/a0033761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heils A, Teufel A, Petri S, Stöber G, Riederer P, Bengel D, Lesch KP. Allelic variation of human serotonin transporter gene expression. Journal of neurochemistry. 1996;66(6):2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009;5(6):e1000529. doi: 10.1371/journal.pgen.1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HRS. Sample Sizes and Response Rates. University of Michigan; Ann Arbor, MI: 2011. [Accessed March 15, 2015]. pp. 1–13. http://hrsonline.isr.umich.edu/sitedocs/sampleresponse.pdf. [Google Scholar]
- HRS. Quality control report for genotypic data. University of Washington; St. Louis, MO: 2012. [Accessed March 2015]. pp. 1–44. http://hrsonline.isr.umich.edu/sitedocs/genetics/HRS_QC_REPORT_MAR2012.pdf. [Google Scholar]
- Hu X, Oroszi G, Chun J, Smith TL, Goldman D, Schuckit MA. An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcohol Clin Exp Res. 2005;29(1):8–16. doi: 10.1097/01.alc.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, Xu K, Arnold PD, Richter MA, Kennedy JL, Murphy DL, Goldman D. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet. 2006;78(5):815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JS, Knight KM, Rafferty JA. Race and unhealthy behaviors: chronic stress, the HPA axis, and physical and mental health disparities over the life course. Am J Public Health. 2010;100(5):933–939. doi: 10.2105/AJPH.2008.143446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs N, Kenis G, Peeters F, Derom C, Vlietinck R, Van Os J. Stress-related negative affectivity and genetically altered serotonin transporter function: evidence of synergism in shaping risk of depression. Arch Gen Psychiatry. 2006;63(9):989–996. doi: 10.1001/archpsyc.63.9.989. [DOI] [PubMed] [Google Scholar]
- Kapteyn A, Michaud P-C, Smith JP, Van Soest A. Effects of attrition and non-response in the Health and Retirement Study. Institute for the Study of Labor; Germany: 2006. pp. 1–43. IZA Discussion Papers No 2246. [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Arch Gen Psychiatry. 2011;68(5):444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Kessler RC, Walters EE, MacLean C, Neale MC, Heath AC, Eaves LJ. Stressful life events, genetic liability, and onset of an episode of major depression in women. Am J Psychiatry. 1995;152(6):833–842. doi: 10.1176/ajp.152.6.833. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kuhn JW, Prescott CA. Childhood sexual abuse, stressful life events and risk for major depression in women. Psychol Med. 2004;34(08):1475–1482. doi: 10.1017/s003329170400265x. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kuhn JW, Vittum J, Prescott CA, Riley B. The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression: a replication. Arch Gen Psychiatry. 2005;62(5):529–535. doi: 10.1001/archpsyc.62.5.529. [DOI] [PubMed] [Google Scholar]
- Keyes CL. The Black-White paradox in health: flourishing in the face of social inequality and discrimination. Journal of Personality. 2009;77(6):1677–1706. doi: 10.1111/j.1467-6494.2009.00597.x. [DOI] [PubMed] [Google Scholar]
- Kilpatrick DG, Koenen KC, Ruggiero KJ, Acierno R, Galea S, Resnick HS, Roitzsch J, Boyle J, Gelernter J. The serotonin transporter genotype and social support and moderation of posttraumatic stress disorder and depression in hurricane-exposed adults. Am J Psychiatry. 2007;164(11):1693–1699. doi: 10.1176/appi.ajp.2007.06122007. [DOI] [PubMed] [Google Scholar]
- Kim Y, Schulz R, Carver CS. Benefit finding in the cancer caregiving experience. Psychosomatic Medicine. 2007;69(3):283–291. doi: 10.1097/PSY.0b013e3180417cf4. [DOI] [PubMed] [Google Scholar]
- Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D depression symptoms index. Journal of Aging and Health. 1993;5(2):179–193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- Kraaij V, Arensman E, Spinhoven P. Negative life events and depression in elderly persons a meta-analysis. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2002;57(1):P87–P94. doi: 10.1093/geronb/57.1.p87. [DOI] [PubMed] [Google Scholar]
- Kraft JB, Slager SL, McGrath PJ, Hamilton SP. Sequence analysis of the serotonin transporter and associations with antidepressant response. Biological psychiatry. 2005;58(5):374–381. doi: 10.1016/j.biopsych.2005.04.048. [DOI] [PubMed] [Google Scholar]
- Laucht M, Treutlein J, Blomeyer D, Buchmann AF, Schmid B, Becker K, Zimmermann US, Schmidt MH, Esser G, Rietschel M. Interaction between the 5-HTTLPR serotonin transporter polymorphism and environmental adversity for mood and anxiety psychopathology: evidence from a high-risk community sample of young adults. International Journal of Neuropsychopharmacology. 2009;12(6):737–747. doi: 10.1017/S1461145708009875. [DOI] [PubMed] [Google Scholar]
- Laurie CC, Doheny KF, Mirel DB, Pugh EW, Bierut LJ, Bhangale T, Boehm F, Caporaso NE, Cornelis MC, Edenberg HJ. Quality control and quality assurance in genotypic data for genome-wide association studies. Genet Epidemiol. 2010;34(6):591–602. doi: 10.1002/gepi.20516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274(5292):1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Li JJ, Berk MS, Lee SS. Differential susceptibility in longitudinal models of gene–environment interaction for adolescent depression. Development and psychopathology. 2013;25(4pt1):991–1003. doi: 10.1017/S0954579413000321. [DOI] [PubMed] [Google Scholar]
- Li Y, Willer C, Sanna S, Abecasis G. Genotype imputation. Annu Rev Genomics Hum Genet. 2009;10:387–406. doi: 10.1146/annurev.genom.9.081307.164242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazure CM, Maciejewski PK, Jacobs SC, Bruce ML. Stressful life events interacting with cognitive/personality styles to predict late-onset major depression. The American journal of geriatric psychiatry. 2002;10(3):297–304. [PubMed] [Google Scholar]
- Middeldorp CM, de Geus EJ, Beem AL, Lakenberg N, Hottenga J-J, Slagboom PE, Boomsma DI. Family based association analyses between the serotonin transporter gene polymorphism (5-HTTLPR) and neuroticism, anxiety and depression. Behavior genetics. 2007;37(2):294–301. doi: 10.1007/s10519-006-9139-7. [DOI] [PubMed] [Google Scholar]
- Ming Q-s, Zhang Y, Chai Q-l, Chen H-y, Hou C-j, Wang M-c, Wang Y-p, Cai L, Zhu X-z, Yi J-y. Interaction between a serotonin transporter gene promoter region polymorphism and stress predicts depressive symptoms in Chinese adolescents: a multi-wave longitudinal study. BMC psychiatry. 2013;13(1):142. doi: 10.1186/1471-244X-13-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe SM, Reid MW. Gene-environment interactions in depression research: genetic polymorphisms and life-stress polyprocedures. Psychol Sci. 2008;19(10):947–956. doi: 10.1111/j.1467-9280.2008.02181.x. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Durrant C, Lewis G, Flint J. Gene× environment interactions at the serotonin transporter locus. Biological psychiatry. 2009;65(3):211–219. doi: 10.1016/j.biopsych.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Ueno S, Sano A, Tanabe H. The human serotonin transporter gene linked polymorphism (5-HTTLPR) shows ten novel allelic variants. Molecular psychiatry. 2000;5(1):32–38. doi: 10.1038/sj.mp.4000698. [DOI] [PubMed] [Google Scholar]
- Otte C, McCaffery J, Ali S, Whooley MA. Association of a serotonin transporter polymorphism (5-HTTLPR) with depression, perceived stress, and norepinephrine in patients with coronary disease: the Heart and Soul Study. 2007 doi: 10.1176/appi.ajp.2007.06101617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrot WJ, Middeldorp CM, Jansen R, Smit JH, de Geus EJ, Hottenga JJ, Willemsen G, Vink JM, Virding S, Barragan I, Ingelman-Sundberg M, Sim SC, Boomsma DI, Penninx BW. Strong effects of environmental factors on prevalence and course of major depressive disorder are not moderated by 5-HTTLPR polymorphisms in a large Dutch sample. J Affect Disord. 2013;146(1):91–99. doi: 10.1016/j.jad.2012.08.044. [DOI] [PubMed] [Google Scholar]
- Phillips-Bute B, Mathew JP, Blumenthal JA, Morris RW, Podgoreanu MV, Smith M, Stafford-Smith M, Grocott HP, Schwinn DA, Newman MF. Relationship of genetic variability and depressive symptoms to adverse events after coronary artery bypass graft surgery. Psychosomatic medicine. 2008;70(9):953. doi: 10.1097/PSY.0b013e318187aee6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin C, Hand D, Boudreau B, Santor D. Gender differences in the association between substance use and elevated depressive symptoms in a general adolescent population. Addiction. 2005;100(4):525–535. doi: 10.1111/j.1360-0443.2005.01033.x. [DOI] [PubMed] [Google Scholar]
- Power T, Stewart R, Ancelin M-L, Jaussent I, Malafosse A, Ritchie K. 5-HTTLPR genotype, stressful life events and late-life depression: no evidence of interaction in a French population. Neurobiology of aging. 2010;31(5):886–887. doi: 10.1016/j.neurobiolaging.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Radloff LS. the Ces-D scale. Applied psychological measurement. 1977;1(3):385–401. [Google Scholar]
- Radloff LS. The Use of the Center for Epidemiologic Studies Depression Scale in Adolescents and Young-Adults. J Youth Adolesc. 1991;20(2):149–166. doi: 10.1007/BF01537606. [DOI] [PubMed] [Google Scholar]
- RAND. RAND Enhanced Fat Files. Center for the Study of Aging, RAND Corporation; Santa Monica, CA: 2014. [Google Scholar]
- Rees M, Norton N, Jones I, McCandless F, Scourfield J, Holmans P, Moorhead S, Feldman E, Sadler S, Cole T. Association studies of bipolar disorder at the human serotonin transporter gene (hSERT; 5HTT) Molecular psychiatry. 1997;2(5):398–402. doi: 10.1038/sj.mp.4000256. [DOI] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang K-Y, Eaves L, Hoh J, Griem A, Kovacs M, Ott J, Merikangas KR. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. Jama. 2009;301(23):2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie K, Jaussent I, Stewart R, Dupuy A-M, Courtet P, Ancelin M-L, Malafosse A. Association of adverse childhood environment and 5-HTTLPR genotype with late-life depression. J Clin Psychiatry. 2009;70(9):1281. doi: 10.4088/JCP.08m04510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santor DA, Coyne JC. Shortening the CES–D to improve its ability to detect cases of depression. Psychological Assessment. 1997;9(3):233. [Google Scholar]
- Scheid J, Holzman C, Jones N, Friderici K, Nummy K, Symonds L, Sikorskii A, Regier M, Fisher R. Depressive symptoms in mid-pregnancy, lifetime stressors and the 5-HTTLPR genotype. Genes, Brain and Behavior. 2007;6(5):453–464. doi: 10.1111/j.1601-183X.2006.00272.x. [DOI] [PubMed] [Google Scholar]
- Schild AH, Nader IW, Pietschnig J, Voracek M. Ethnicity moderates the association between 5-HTTLPR and national suicide rates. Archives of suicide research. 2014;18(1):1–13. doi: 10.1080/13811118.2013.803447. [DOI] [PubMed] [Google Scholar]
- Seneviratne C, Franklin J, Beckett K, Ma JZ, Ait-Daoud N, Payne TJ, Johnson BA, Li MD. Association, interaction, and replication analysis of genes encoding serotonin transporter and 5-HT3 receptor subunits A and B in alcohol dependence. Hum Genet. 2013;132(10):1165–1176. doi: 10.1007/s00439-013-1319-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seneviratne C, Huang W, Ait-Daoud N, Li MD, Johnson BA. Characterization of a functional polymorphism in the 3′ UTR of SLC6A4 and its association with drinking intensity. Alcoholism: Clinical and Experimental Research. 2009;33(2):332–339. doi: 10.1111/j.1530-0277.2008.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpley CF, Palanisamy SK, Glyde NS, Dillingham PW, Agnew LL. An update on the interaction between the serotonin transporter promoter variant (5-HTTLPR), stress and depression, plus an exploration of non-confirming findings. Behavioural brain research. 2014;27:389–105. doi: 10.1016/j.bbr.2014.07.030. [DOI] [PubMed] [Google Scholar]
- Sjöberg RL, Nilsson KW, Nordquist N, Öhrvik J, Leppert J, Lindström L, Oreland L. Development of depression: sex and the interaction between environment and a promoter polymorphism of the serotonin transporter gene. International Journal of Neuropsychopharmacology. 2006;9(4):443–449. doi: 10.1017/S1461145705005936. [DOI] [PubMed] [Google Scholar]
- Steffick DE. Mental health and labor market outcomes. University of Michigan; 2002. [Google Scholar]
- Surtees PG, Wainwright NW, Willis-Owen SA, Luben R, Day NE, Flint J. Social adversity, the serotonin transporter (5-HTTLPR) polymorphism and major depressive disorder. Biological psychiatry. 2006;59(3):224–229. doi: 10.1016/j.biopsych.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Way BM, Welch WT, Hilmert CJ, Lehman BJ, Eisenberger NI. Early family environment, current adversity, the serotonin transporter promoter polymorphism, and depressive symptomatology. Biological psychiatry. 2006;60(7):671–676. doi: 10.1016/j.biopsych.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Tennant C. Life events, stress and depression: a review of recent findings. Australian and New Zealand Journal of Psychiatry. 2002;36(2):173–182. doi: 10.1046/j.1440-1614.2002.01007.x. [DOI] [PubMed] [Google Scholar]
- Turvey CL, Wallace RB, Herzog R. A revised CES-D measure of depressive symptoms and a DSM-based measure of major depressive episodes in the elderly. International Psychogeriatrics. 1999;11(02):139–148. doi: 10.1017/s1041610299005694. [DOI] [PubMed] [Google Scholar]
- Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the etiology of depression: 2009 update. Molecular psychiatry. 2010;15(1):18–22. doi: 10.1038/mp.2009.123. [DOI] [PubMed] [Google Scholar]
- Vinkhuyzen AA, Dumenil T, Ryan L, Gordon SD, Henders AK, Madden PA, Heath AC, Montgomery GW, Martin NG, Wray NR. Identification of tag haplotypes for 5HTTLPR for different genome-wide SNP platforms. Molecular psychiatry. 2011;16(11):1073. doi: 10.1038/mp.2011.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh K, Uddin M, Soliven R, Wildman DE, Bradley B. Associations between the SS variant of 5-HTTLPR and PTSD among adults with histories of childhood emotional abuse: Results from two African American independent samples. Journal of affective disorders. 2014;16:191–96. doi: 10.1016/j.jad.2014.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland JR, Martin BJ, Kruse MR, Lesch KP, Murphy DL. Simultaneous genotyping of four functional loci of human SLC6A4, with a reappraisal of 5-HTTLPR and rs25531. Mol Psychiatry. 2006;11(3):224–226. doi: 10.1038/sj.mp.4001789. [DOI] [PubMed] [Google Scholar]
- Wilhelm K, Mitchell PB, Niven H, Finch A, Wedgwood L, Scimone A, Blair IP, Parker G, Schofield PR. Life events, first depression onset and the serotonin transporter gene. The British Journal of Psychiatry. 2006;188(3):210–215. doi: 10.1192/bjp.bp.105.009522. [DOI] [PubMed] [Google Scholar]
- Williams RB, Marchuk DA, Gadde K, Barefoot JC, Grichnik K, Helms MJ, Kuhn CM, Lewis JG, Schanberg SM, Stafford-Smith M. Serotonin-related gene polymorphisms and central nervous system serotonin function. Neuropsychopharmacology. 2003 doi: 10.1038/sj.npp.1300054. [DOI] [PubMed] [Google Scholar]
- Wong M, Day N, Luan J, Chan K, Wareham N. The detection of gene–environment interaction for continuous traits: should we deal with measurement error by bigger studies or better measurement? Int J Epidemiol. 2003;32(1):51–57. doi: 10.1093/ije/dyg002. [DOI] [PubMed] [Google Scholar]
- Yang Z, Seneviratne C, Wang S, Ma JZ, Payne TJ, Wang J, Li MD. Serotonin transporter and receptor genes significantly impact nicotine dependence through genetic interactions in both European American and African American smokers. Drug Alcohol Depen. 2013;129(3):217–225. doi: 10.1016/j.drugalcdep.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit S, Owen MJ. Stressful life events, 5-HTT genotype and risk of depression. Br J Psychiatry. 2006;188:199–201. doi: 10.1192/bjp.bp.105.020644. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Coryell W. Screening for major depressive disorder in the community: A comparison of measures. Psychological Assessment. 1994;6(1):71. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


