Abstract
The development of genetic and molecular biology tools permitting the connection of specific genes to their functions has accelerated our understanding of molecular pathways underlying health and disease. The resulting gains in knowledge have propelled gene targeting to the forefront of promising therapeutic strategies. Here we discuss the uniquely powerful and adaptable approach of morpholino-driven modification of normal and mutant gene expression as a pathway to health.
Keywords: vivo-morpholino, oligonucleotide, gene silencing, ATP-sensitive potassium channel, skeletal muscle
Modern medicine is rapidly evolving based on the development of multiple technologies that have permitted pinpointing specific molecular determinants of disease pathophysiology. Identification of such targets offers unprecedented potential for development of novel therapeutics, yet traditional pharmacologic approaches often lack the temporal, spacial and molecular specificity needed to achieve ideal outcomes. In this context, genetic silencing and editing techniques have emerged as powerful, specific, and highly adaptable strategies with the capacity for treating a wide variety of diseases. This approach may be used to interfere with disease processes promoted by the actions of normal “wild-type” molecular components, as well as to diminish the consequences of harmful mutations and disease modifying polymorphisms, or to battle gene expression by infectious organisms. Although gene knockdown or editing techniques have been successful in research applications for decades, having served as an important method for advancing our understanding of normal development and disease pathophysiology, central challenges to widespread clinical translation due to issues of targeting, systemic delivery in adults, stability, and cell-penetration, are just recently being overcome.
Here we discuss the use of morpholinos and their adaptations, particularly Vivo-morpoholinos - an approach with particular advantages that shows promise for bringing custom gene knockdown and editing into mainstream medicine.
Structure, mechanism of action and toxicity of morpholono oligonucleotides
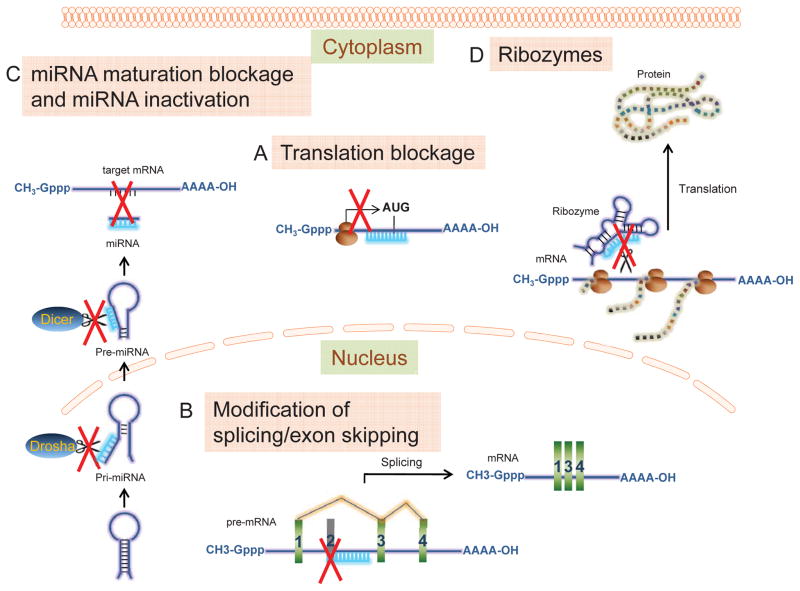
Invented in 1989, morpholino oligonucleotides are “uncharged stereoregular nucleic acid analogs” (1). These are molecules similar to RNA or DNA in that they comprise the same nitrogenous bases, although bound to morpholine rings instead of ribose or deoxyribose rings, and linked through uncharged phosphorodiamidate groups instead of anionic phosphates. They are typically 18–30 bases in length and bind to targeted RNA sequences by base-pairing. However, unlike siRNA, these phosphorodiamidate morpholino oligomers (PMOs or Morpholinos) do not result in degradation of their target RNA since their backbone structure is foreign and not recognized by cellular nucleases. Rather Morpholinos act by “steric blocking”: binding to a target sub-sequence within the RNA thereby getting in the way of molecules that might otherwise interact with the RNA. As a result, various biological processes may be modulated or prevented from occurring: pre-mRNA splicing, mRNA translation, miRNA maturation and activity, ribosomal frameshifting, and/or ribozyme activity [(2–4), Fig. 1]. The uncharged nature of the phosphorodiamidate groups avoids adverse interactions with cellular proteins and the molecules do not appear to activate the innate immune response (4). Morpholinos, injected or electroporated into embryos at various stages, have been invaluable in defining the role of specific mRNA transcripts in development (4).
Figure 1. Mechanisms of action of anti-sense morpholino oligomers.
A) Translational blockage. PMOs block the translation initiation complex binding sites on mRNA and prevent translation from occurring. B) Modification of splicing/exon skipping. PMOs block splice sites on pre-mRNA, prevent recognition of these sites by the spliceosome that in turn causes exon skipping. C) miRNA maturation blockage and miRNA inactivation. PMOs may block maturation enzyme cleavage sites (i.e. Drosha, Dicer) on pri- or pre-miRNA to prevent its maturation. PMOs may complementarily bind to mature miRNA and prevent it from binding to target mRNA. D) Ribozymes. PMOs may bind to enzymatically active RNAs (ribozymes) blocking their active sites and preventing them from cleaving their target mRNAs.
In vivo delivery of Morpholinos
In the majority of cases, Morpholinos require assistance (microinjections, electroporation, or association with delivery molecules) for their efficient intracellular entry. In order to improve intracellular delivery of Morpholinos, covalent conjugation to arginine-rich cell penetrating peptides to form peptide-linked phosphorodiamidate Morpholino oligomers (PPMOs, Sarepta Therapeutics, Inc., Cambridge, MA), or to octaguanidinium dendrimer scaffolds to form Vivo-morpholinos (Gene Tools, LLC, Philomath, OR), was developed (2–4). These compounds may be delivered intravenously, intraperitoneally or locally to various tissues, and show rapid and efficient entry to cells at relatively low doses making them powerful tools for gene editing “therapy” in vivo.
In vivo gene silencing/editing with Morpholinos
Unmodified Morpholinos (PMOs) demonstrate promising therapeutic potential against Duchenne muscular dystrophy. Duchenne muscular dystrophy is a severe X-chromosome-linked genetic disease caused by mutations in the gene DMD that encodes dystrophin, a protein involved in maintaining the integrity of skeletal and cardiac myocyte membranes. Mutations in DMD alter the structure or function of dystrophin or prevent any functional dystrophin from being produced. Dystrophin deficient muscles are subject to membrane destabilization, muscle deterioration and loss. PMOs have been designed to cause translational skipping of exons. Fortuitously, these unmodified Morpholinos can cross the pathologically leaky cellular membrane of dystrophin deficient myocytes, resulting in restoration of a functional, though abbreviated, dystrophin protein that improves muscle and cardiac function [(2–4), Table 1]. This technology is currently under evaluation in clinical and pre-clinical trials.
Table 1.
| Disease/process | Organism | Type of PMO modification | Target gene/tissue | Mechanism | PMID (Year) |
|---|---|---|---|---|---|
| Disease models | |||||
| Myotonic dystrophy type 1 | Mouse | Vivo | PKM2/skeletal muscle | Modification of splicing | 23901116 (2013) |
| Muscle hypertrophy | Mouse | Vivo | Myostatin/skeletal muscle | Exon skipping | 20924365 (2011) |
| Hyperbilirubinemia in hypertension | Mouse | Vivo | UGT1A1/liver | Translation blockage | 20668235 (2010) |
| Hypothalamic-pituitary-adrenal axis suppression | Rat | Vivo | CRH/hypothalamus | Translation blockage | 21979757 (2012) |
| Systemic hyperphenylalaninemia | Mouse | Vivo | Pah/liver, kidneys | Exon skipping | 25226162 (2014) |
| Intermediate spinal muscular atrophy | Mouse | Unmodified, Vivo | SMN2/skeletal muscle, spinal cord | Modification of splicing | 23339722 (2013) 26264577 (2015) |
| Muscular dystrophies | |||||
| Duchene muscular dystrophy | Dog | Unmodified | Dystrophin/skeletal muscle | Exon skipping | 19288467 (2009) |
| Duchene muscular dystrophy | Mouse | CPP | Dystrophin/skeletal muscle, heart | Exon skipping | 18806224 (2008) 18545222 (2008) 18784278 (2008) 19692354 (2009) 20700113 (2010) 19815563 (2010) 21505427 (2011) 22683468 (2012) |
| Duchene muscular dystrophy | Mouse | Vivo | Human dystrophin transgene/skeletal muscle | Exon skipping | 21611204 (2011) |
| Duchene muscular dystrophy | Mouse | Unmodified | Dystrophin/skeletal muscle | Exon skipping | 16444267 (2006) 16285002 (2006) 19469709 (2009) 21102560 (2010) |
| Duchene muscular dystrophy | Mouse | Vivo | Dystrophin/skeletal muscle | Multiple exon skipping | 22869723 (2012) 25647512 (2014) |
| Duchene muscular dystrophy | Dog | Vivo | Dystrophin/skeletal muscle | Multiple exon skipping | 22888777 (2012) |
| Duchene muscular dystrophy | Human | Unmodified | Dystrophin/skeletal muscle | Exon skipping | 19713152 (2009) 21784508 (2011) 23907995 (2013) 26086759 (2015) |
| Facioscapulohumeral muscular dystrophy | Mouse | Vivo | PITX1/skeletal muscle | Translation blockage | 24232919 (2014) |
| Duchene muscular dystrophy | Mouse | CPP | Dystrophin, myostatin/skeletal muscle | Exon skipping | 24942628 (2014) 25959011 (2015) |
| Spinal muscular atrophy | Mouse | Unmodified, Vivo | SMN2/skeletal muscle | Modification of splicing | 24781211 (2014) 24636820 (2014) |
| Cancer | |||||
| Cancer/breast cancer | Mouse | Vivo | STAT3/tumor | Modification of splicing | 22006329 (2011) |
| Acute lymphoblastic leukemia | Mouse | Vivo | Gfi1/tumor | Translation blockage | 23410974 (2013) |
| Squamous cell carcinoma | Mouse | Vivo | IL-10/immune cells | Translation blockage | 25990221 (2015) |
| Nijmegen Breakage Syndrome | Mouse | Vivo | Nibrin/tumor | Modification of splicing | 26265251 (2015) |
| Colon cancer | Mouse | Vivo | Flt-1/tumor | Modification of splicing | 25534570 (2015) |
| Eye pathology | |||||
| Corneal angiogenesis | Mouse | Unmodified, Vivo | Flt-1/corneal | Modification of splicing | 22199251 (2012) 22438952 (2012) |
| Corneal angiogenesis | Mouse | Vivo | KDR/corneal | Modification of splicing | 22997228 (2013) |
| Heart diseases | |||||
| Dilated cardiomyopathy (DCM) | Mouse | Vivo | TTN/heart | Exon skipping | 25759365 (2015) |
| Modulation of physiological processes | |||||
| Energy efficiency | Mouse | Vivo | KATP channel/skeletal muscle | Translation blockage | 25648265 (2015) |
| Physical activity | Mouse | Vivo | DRD1, Vmat2, Glut4/skeletal muscle, brain | Translation blockage | 23630592 (2013) |
| Physical activity | Mouse | Vivo | Anxa6, Casq1/skeletal muscle | Translation blockage | 24505100 (2014) |
| Alcohol seeking | Mouse | Vivo | Orexin/hypothalamus | Translation blockage | 24806225 (2014) 25329297 (2014) |
| Other genetic disorders | |||||
| Pompe disease | Mouse | CPP | Gys1/skeletal muscle | Exon skipping | 25350581 (2014) |
| X-linked agammaglobulinemia | Mouse | CPP | BTK/B lymphocytes | Exon skipping | 25105368 (2014) |
| Huntington’s disease | Mouse | Unmodified | HTT/brain | Translation blockage | 25035419 (2014) |
Abbreviations: PKM2 - pyruvate kinase; UGT1A1 - UDP glucuronosyltransferase 1 family polypeptide A1; CRH - corticotropin-releasing hormone; Pah - phenylalanine hydroxylase; PITX1 - paired-like homeodomain transcription factor 1; STAT3 - signal transducer and activator of transcription 3; Gfi1 - growth factor independence 1; IL-10 – interleukin 10; Flt-1 - vascular endothelial growth factor receptor 1; KDR - vascular endothelial growth factor receptor 2; TTN – titin; KATP channel - ATP-sensitive potassium channel; DRD1 - dopamine receptor D1; Vmat2 - vesicular monoamine transporter 2; Glut4 - glucose transporter type 4; Anxa6 – annexin 6; Casq1 – calsequestrin 1; Gys1 - muscle specific glycogen synthase; BTK - Bruton’s tyrosine kinase; HTT – huntingtin;
However, delivery through “competent” cell membranes in other disease states requires the modified morpholinos: PPMOs or/and Vivo-morpholinos. PPMOs have been used in multiple studies in animal models against genetic, viral and bacterial diseases [(3, 4), Table 1]. They are being developed as pharmaceuticals (Sarepta Therapeutics, Inc) but have not yet reached clinical trial stage. A limitation of PPMOs may be dose-dependent renal toxicity above a threshold concentration (4).
Vivo-morpholinos appear to be the most effective approach for in vivo delivery, tissue distribution and antisense effect of current gene knock-down/editing agents as therapeutics or to create disease models as demonstrated by numerous animal studies [(3, 4), Table 1]. Vivo-morpholinos consistently result in at least 50% gene knockdown (3, 4), although the efficiency of gene modification in brain and vasculature following systemic delivery is limited. However, like PPMOs, no Vivo-morpholino has yet reached the clinical trial stage. Because of their lipophilic properties and chemical and biological stability, Vivo-morpholinos irreversibly penetrate cell membranes and provide a prolonged effect on the target gene of 17 weeks or more (3, 4). As such, Vivo-morpholino targeting of Dmd in mdx mice (an animal model of Duchenne muscular dystrophy) demonstrated substantial amelioration of pathology during long term administration (2, 4). Since multiple mutations can cause Duchenne muscular dystrophy, a cocktail of Vivo-morpholino oligomers has been developed that leads to multiple exon-skipping and could thus serve as a more universal and effective approach to treatment than unmodified Morpholinos (2, 3).
The only significant reported toxicity from systemic Vivo-morpholino administration occurred following intravenous delivery of oligomers containing sequences capable of 3′-5′ hybridization. These particular Vivo-morpholinos exhibited dendrimer clustering causing increased blood viscosity and clot formation leading to death (Table 1 – PMID 24505100 and 24806225). This toxicity may be overcome by careful design and testing of oligomer sequences with low self-hybridization potential or alternatively by vehicle (saline)-based masking of charges on the dendrimers. In addition, guanidinium has significant toxic effects at higher doses, however these appear to be minimal at the Vivo-morpholino concentrations sufficient for substantial gene knock-down (3, 4). Further studies directed at short- and long-term toxicities are needed for Vivo-morpholino studies to be translated to human use.
Morpholinos can also be used to alter expression of normal genes to generate a desired therapeutic effect. A recent study from our laboratory illustrates the potential clinical range and power of Vivo-morpholino technology in this regard (5). The goal of this project was to modify the energy efficiency of skeletal muscles to promote thermogenesis and increased caloric consumption as a strategy to combat obesity. Recently, we discovered that muscle energy efficiency is regulated by sarcolemmal ATP-sensitive potassium (KATP) channels, formed through association of Kir6.2 pore-forming and SUR2A regulatory sulfonylurea receptor subunits. Specifically, we found that potassium efflux via sarcolemmal KATP channels was activated by low-intensity muscle activity and opposed depolarizing currents thus shortening action potential duration and limiting calcium influx. The resulting decrease in calcium-dependent functions translates into less energy used for contractions, calcium re-sequestration, and heat production. Conversely, loss of normal Kir6.2 function augments ATP turnover and elicits an extra energetic cost of muscle performance. Pharmacologic blockade of skeletal muscle KATP channels to increase cellular energy expenditure as a treatment for obesity would be a logical clinical translation of these findings. Unfortunately, no KATP channel antagonist is selective for skeletal muscle KATP channels which share the same isoform composition as cardiac KATP channels and local administration of a KATP channel blocker would need to be repeated frequently and risk systemic leak with potentially detrimental consequences on cardiac energetic efficiency and stress resistance. To overcome these limitations, we employed intramuscular injections of anti-Kir6.2 Vivo-morpholino (5). We found that three once weekly local intramuscular injections of anti-Kir6.2 Vivo-morpholino into bilateral limb muscles of mice caused the effective knock-down of KATP channels accompanied by altered membrane electrical response and augmented force generation only in targeted muscles while KATP channel expression and function in adjacent muscles and heart remained unaffected, presumably due to the rapid intracellular transit of the Vivo-morpholino. Even in response to low-intensity activities, treated mice increased their muscle and body temperature as well as oxygen consumption significantly more that controls, although with only a mild decrement in maximal exercise tolerance. This study serves as a proof-of-principle that Vivo-morpholino technology can be used successfully to target normal components of molecular pathways in a local manner to achieve a desired clinical result, in this case modification of energy efficiency in select skeletal muscles.
Conclusion
Morpholino-based oligomers are effective, stable and highly adaptable anti-sense DNA analogs for gene silencing and editing. The development of cell-penetrating modifications has greatly facilitated their applicability for in vivo use by permitting systemic and/or precise local delivery in adult subjects. Their exceptional duration of biologic effect further enhances their utility. These unique properties make them very attractive foundations for the development of therapeutic agents and animal disease models through targeting the expression of normal and mutant genes. Further definition of potential toxicities, as well as safe and effective methods of application, will be essential for clinical translation.
Acknowledgments
The authors are supported by the National Institutes of Health [HL113089 to Denice Hodgson-Zingman (University of Iowa), DK092412 to Leonid Zingman (University of Iowa)], and the VA Merit Review Program [1I0BX000718 to Leonid Zingman (University of Iowa)].
Footnotes
Conflicts of Interest
There are no conflicts of interest.
Author Contributions
All authors contributed to the concept, background research, and writing of this article.
References
- 1.Stirchak EP, Summerton JE, Weller DD. Uncharged stereoregular nucleic acid analogs: 2. Morpholino nucleoside oligomers with carbamate internucleoside linkages. Nucleic Acids Res. 1989;17(15):6129–41. doi: 10.1093/nar/17.15.6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kole R, Krieg AM. Exon skipping therapy for Duchenne muscular dystrophy. Advanced drug delivery reviews. 2015;87:104–7. doi: 10.1016/j.addr.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Moulton JD, Jiang S. Gene knockdowns in adult animals: PPMOs and vivo-morpholinos. Molecules. 2009;14(3):1304–23. doi: 10.3390/molecules14031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moulton HM. In vivo delivery of morpholino oligos by cell-penetrating peptides. Current pharmaceutical design. 2013;19(16):2963–9. doi: 10.2174/1381612811319160010. [DOI] [PubMed] [Google Scholar]
- 5.Koganti SR, Zhu Z, Subbotina E, Gao Z, Sierra A, Proenza M, et al. Disruption of KATP channel expression in skeletal muscle by targeted oligonucleotide delivery promotes activity-linked thermogenesis. Molecular therapy : the journal of the American Society of Gene Therapy. 2015;23(4):707–16. doi: 10.1038/mt.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]