To the Editor
We report a 9 year-old Chinese girl, born full term with no family history of recurrent infections or immunodeficiency. She had received hepatitis B and BCG vaccinations at birth without complications. She had multiple infections in infancy including Klebsiella pneumoniae urinary tract infection at 1 month and another two episodes of Klebsiella pneumoniae bacteremia at 2 and 10 months of age, all treated with intravenous ceftriaxone with good recovery and documented clearance. However at 10 months, after presenting with fever and a macular rash hepatosplenomegaly, she was diagnosed with extensive abdominal lymphadenopathy on CT scan. The abdominal lymph node biopsy showed granulomatous formation and sensitive Mycobacterium tuberculosis complex was cultured [1]. The intestinal tuberculosis was treated with rifampicin, isoniazid and pyrazinamide for 12 months with resolution of abdominal lymphadenopathy. At this time, the skin biopsy was clear of mycobacterial species. Immunophenotyping and immunoglobulins levels were normal at the time (Supplementary table 1). The patient’s blood was tested for IFN-γ and IL-12 production after stimulation with BCG, BCG+IL-12 and BCG+IFN-γ, respectively and showed a diminished production of both IFN-γ and IL-12 after BCG+IL-12 and BCG+IFN-γ, respectively as compared to the travel control (Supplementary table 2).
Six months after stopping anti-tuberculosis therapy, the patient had tuberculosis infection of the skin manifesting as a painful erythematous plaque with scabbed nodules over the face (lupus vulgaris) (Figure 1), elbows, shins and calves, and the skin biopsy demonstrated acid-fast bacilli, though no specific organism was cultured. Lupus vulgaris in children is very rare, and is almost exclusively due to infections by Mycobacterium tuberculosis or Mycobacterium bovis. She was commenced on isoniazid, rifampicin, pyrazinamide and clarithromycin for 15 months to cover for atypical mycobacterial organisms as well. Subcutaneous recombinant IFN-γ (rIFN-γ) was trialled at 20 mcg/m2 twice per week with good response. When anti-tuberculosis medications were changed to dual therapy after 12 months, there was recurrence of intestinal tuberculosis based on CT findings and granulomatous formation on lymph node biopsy (no organism cultured). Ethambutol and moxifloxacin were added to her treatment regimen with good response. These four anti-tuberculosis medications were continued for 3 years together with subcutaneous rIFN-γ injection. Despite weaning to a very low, likely suboptimal dose of rIFN-γ, she was free from both mycobacterial and interestingly, pyogenic infections for more than 3 years. It was at this point when a mutation in IκBα (S36Y) was diagnosed by whole exome sequencing (WES), and stem cell transplantation was offered, but declined by parents in view of stability.
Figure 1.
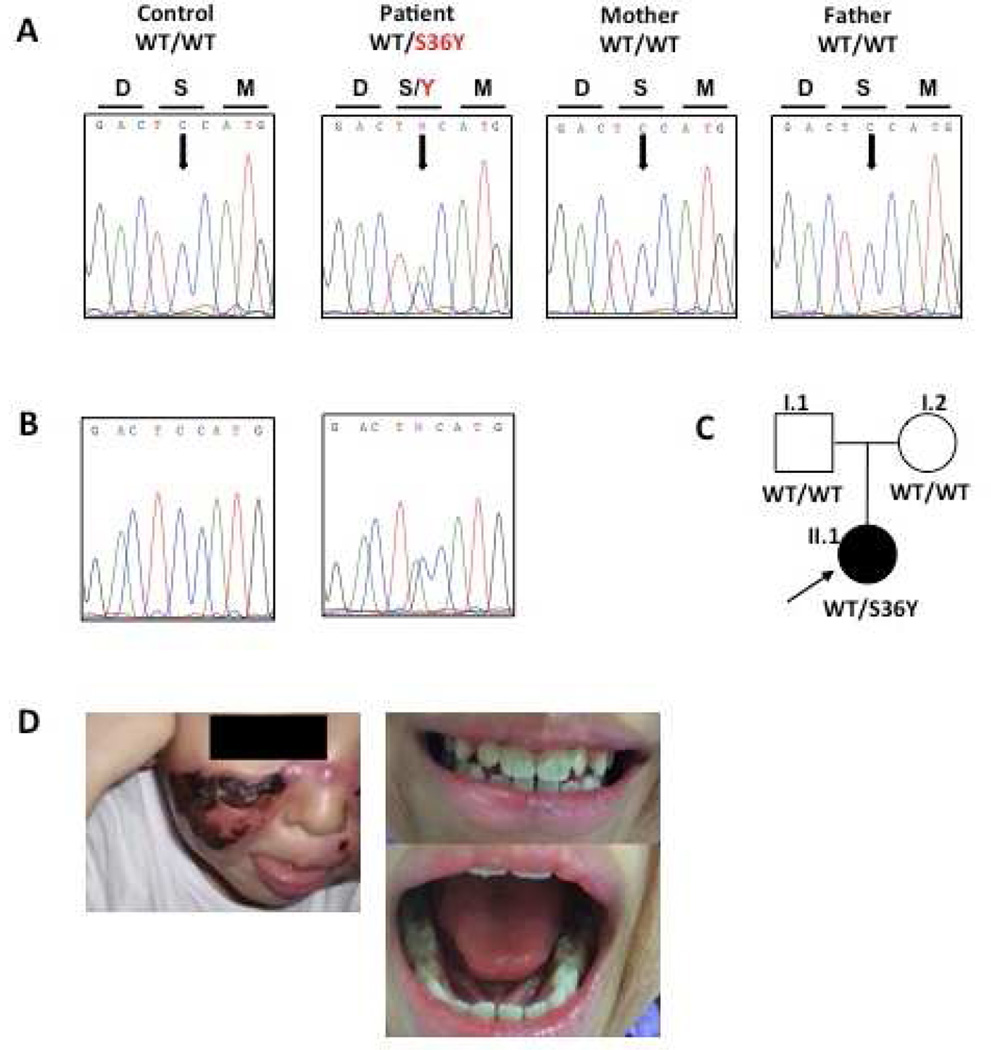
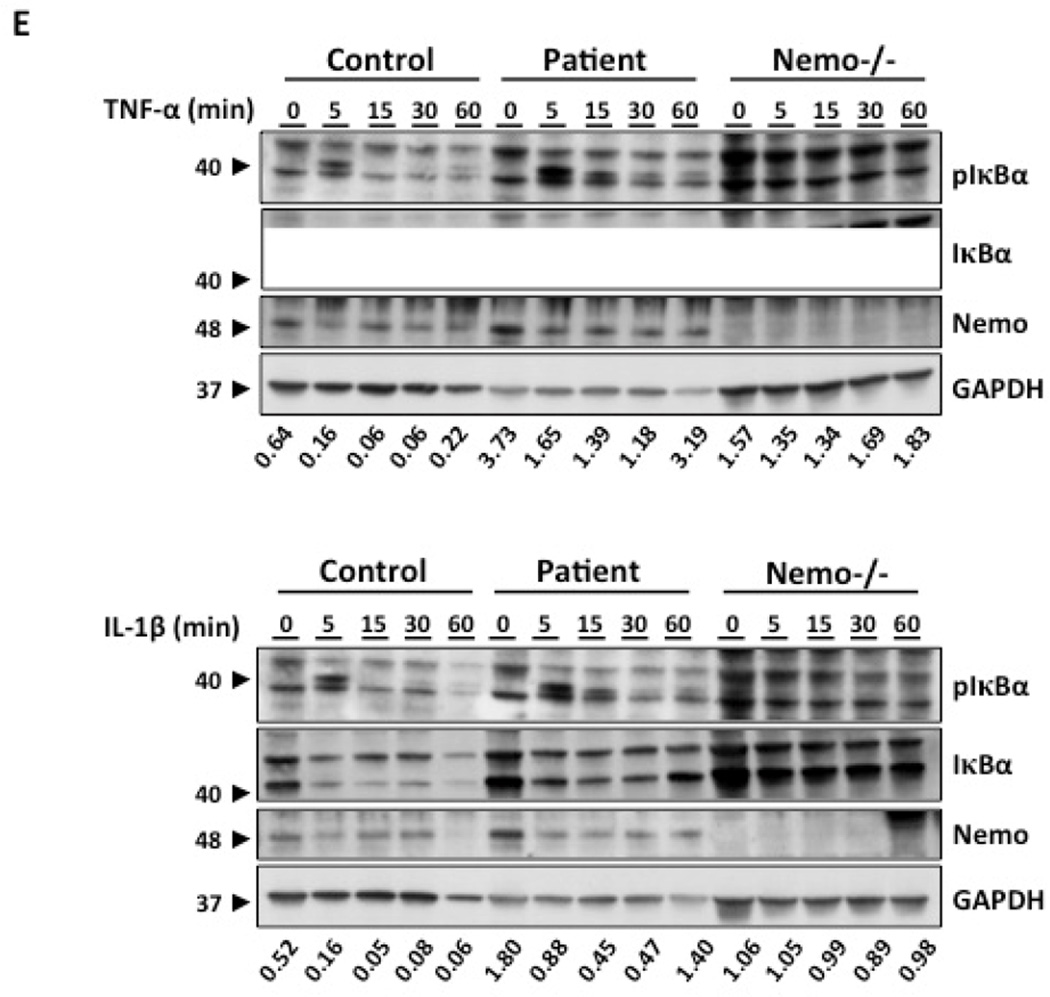
Identification and functional characterization of a heterozygous de novo NFKBIA mutation. (A–B) Electropherogram and (C) familial segregation showing de novo heterozygous S36Y (c.107C>A) mutation (indicated in red) in the patient’s gDNA from granulocytes (A) and dermal fibroblasts (B), and wild-type (WT) alleles in both parents. (D) Picture of the patient’s face and teeth (E) Immunoblot analysis of phospho-Ser32 IκBα, IκBα degradation and NEMO from a healthy control and the patient´s fibroblasts after stimulation with TNF-α (20 ng/ml) and IL-1β (10 ng/ml). Numbers below the western blot indicate the ratio between GAPDH and IKBA in the patient compared with the control after stimulation, and analyzed by densitometry.
At 8 years old, when anti-tuberculosis medications had already been stopped, and 4 months after stopping subcutaneous rIFN-γ there was recurrence of infections including Pseudomonas aeruginosa pansinusitis and notable complications of bronchiectasis diagnosed on high resolution CT. Subcutaneous rIFN-γ was recommenced at 50 mcg/m2 three times per week, however one month after she developed Mycobacterium abscessus left knee septic arthritis and tibial osteomyelitis for which she was treated with intravenous amikacin, cefoxitin and oral clarithromycin based on sensitivities. She has had no further infections on anti-tuberculosis therapy, rIFN-γ, anti-microbial prophylaxis with Bactrim (after desensitization for challenge-proven allergy) and intravenous immunoglobulin replacement (due to poor pneumococcal antibody responses) and will continue until stem cell transplantation. Physical examination revealed an intellectually normal child with failure to thrive (height and weight less than 3rd percentile) and scarring over the face (a consequence of facial lupus vulgaris which she had at age 3). Respiratory examination reveals bilateral crepitations and wheezes in keeping with bilateral bronchiectasis. There were no features of anhidrotic ectodermal dysplasia (EDA) such as hypohidrosis, sparse hair, dental abnormalities, coarse skin or osteopetrosis.
The immunological results (Supplementary tables 1 and 2) show that the patient does not display an overt functional T cell defect and a specific defect in the IL-12/IFN-γ loop was not identified. WES was thus performed on gDNA from the patient. We identified a heterozygous nucleotide substitution (c.107C>A) in exon 1 of NFKBIA (encoding IκBα), leading at the protein level to a missense mutation, replacing a Serine at position 36 by a Tyrosine (S36Y). The mutation was confirmed by Sanger sequencing, on PCR fragment amplified from gDNA extracted from granulocytes and dermal fibroblasts (Figure 1). The mutant and WT allele in the patient were of same amplitude, suggesting equal amount of both alleles. The mutation was not found in any public database (1,000G, ExAc, dbSNP). Familial segregation analysis (Figure 1) showed that both parents are wild-type for the substitution; the mutation thus appeared de novo in the patient (Figure 1). This mutation leads to a gain-of-function of IκBα and was reported in a patient with mild AD-EDA-ID and non-infectious systemic inflammation [2, 3]. We analyzed the IκBα phosphorylation and subsequent degradation in primary fibroblasts from the patient, a healthy control and a NEMO-deficient cell line after TNF-α (20 ng/ml) and IL-1β (10 ng/ml) stimulation. We found that IκBα was phosphorylated on Serine 32 after TNF-α stimulation in control and in patient’s cells, as compared with a NEMO-deficient patient. However, we observe an impaired, but not abolished IκBα degradation in the patient’s cells compared to the healthy control (positive control) and the NEMO-deficient cells (negative control). The same results were obtained after stimulation with IL-1β. Altogether, we have identified a de novo heterozygous gain-of-function mutation in NFKBIA, affecting the serine at position 36. The mutation does not impair the phosphorylation of IκBα on Serine 32, but affect partially its degradation, leading to impaired NF-κB signaling (Figure 1).
This is the first reported case of a female child with absolutely no features of EDA who was found to have a heterozygous missense variation mutation in IκBα (S36Y) resulting in significant immunodeficiency only. Immune cells as well as dermal fibroblasts from the patient carry the heterozygous missense mutation, apparently at the same level. Out of the eight patients previously reported (Supplementary Table 3), all, except a patient with mosaicism [4] and our own, had at least mild features of EDA. The patient we report does not display any clinical signs of EDA. For reasons still unknown, there is a considerable variability in the extent of the severity of the EDA clinical signs in IκBα-mutated patient (Supplementary Table 3). In addition, variability in terms of EDA clinical signs is also observed in patients with mutation in another component of the NF-κB signaling pathway, IKBKB, as well as in NEMO [5]. Recurrent mycobacterial diseases (tuberculous and non-tuberculous mycobacteria) is observed in our patient. Surprisingly, the other patient described with the exact same mutation (S36Y) by Yoshioka et al [2], display only mild EDA and had an episode of mycobacterial disease. All the other GOF-IκBα patients displayed EDA and were free of mycobacterial disease (Supplementary table 3). It is also interesting to note that our patient was free of all infections during three years (not only mycobacterial) when she was under IFN-γ treatment. Altogether, IκBα mutations should be suspected in children with bacterial and mycobacterial infections even without EDA clinical presentation. In addition, treatment with rIFN-γ injection may be proposed to prevent all infections.
Supplementary Material
Acknowledgments
We would like to thank all the member of the Laboratory of Human Genetics of Infectious Diseases from both Paris and New York. A special thank you is extended to Tatiana Kochetkov for the cell culture help and to Yelena Nemirovskaya for the outstanding administrative support. The laboratory of Human Genetics of Infectious Diseases is supported by grants from the National Institute of Allergy and Infectious Diseases (NIAID) (grant n° R37AI095983 and P01AI061093), the National Center for Research Resources and the National Center for Advancing Sciences (NCATS) (grant n° 8UL1TR000043), the Rockefeller University, the St. Giles Foundation, Institut National de la Santé et de la Recherche Médicale (INSERM), University Paris Descartes, the European Research Council (ERC-2010-AdG-268777) and the French National Research Agency (ANR) (TBPATHGEN grant n°ANR-14-CE14-0007-01) and ANR under the “Investments for the future” program (grant n° ANR-10-IAHU-01).
References
- 1.Boisson-Dupuis S, et al. Inherited and acquired immunodeficiencies underlying tuberculosis in childhood. Immunological reviews. 2015;264(1):103–120. doi: 10.1111/imr.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoshioka T, et al. Autosomal dominant anhidrotic ectodermal dysplasia with immunodeficiency caused by a novel NFKBIA mutation, p.Ser36Tyr, presents with mild ectodermal dysplasia and non-infectious systemic inflammation. Journal of clinical immunology. 2013;33(7):1165–1174. doi: 10.1007/s10875-013-9924-z. [DOI] [PubMed] [Google Scholar]
- 3.Boisson B, Quartier P, Casanova JL. Immunological loss-of-function due to genetic gain-of-function in humans: autosomal dominance of the third kind. Current opinion in immunology. 2015;32:90–105. doi: 10.1016/j.coi.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janssen R, et al. The same IkappaBalpha mutation in two related individuals leads to completely different clinical syndromes. The Journal of experimental medicine. 2004;200(5):559–568. doi: 10.1084/jem.20040773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Picard C, Casanova JL, Puel A. Infectious diseases in patients with IRAK-4, MyD88, NEMO, or IkappaBalpha deficiency. Clinical microbiology reviews. 2011;24(3):490–497. doi: 10.1128/CMR.00001-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.