Abstract
Background
Synchronous bilateral breast cancers (BC) frequently share the same estrogen receptor (ER) status, yet may differ in other histopathologic features. We sought to examine concordance rates of Oncotype DX (RS) testing in women with synchronous bilateral ER-positive BC.
Methods
Institutional databases were reviewed to identify patients with synchronous (within 6mos) bilateral primary invasive BC and multiple RS. RS were stratified by risk group (RS<18, low; RS 18–30, intermediate; RS≥31, high) and considered discordant if they reflected different risk groups.
Results
From 2005–2014, 115 patients presented with synchronous bilateral invasive BC; 43 (37%) had 2 RS available. Median patient age was 60yrs (42–84); median tumor size was 1.2cm (0.5–3.7), all cases were HER2 negative and node negative. Of 86 RS, 63 (73%) were low risk, 20 (23%) intermediate risk, and 3 (3%) high risk. RS were concordant in 29 (67%) patients. Patients with concordant RS were older (62yrs versus 56yrs) and had median levels of PR expression that were higher and more similar—80% and 85% for bilateral cancers, respectively—as compared to 55% and 75% for bilateral cancers in discordant cases. Discordant RS led to a treatment change in 8/14 (57%) cases.
Conclusions
Among women with synchronous bilateral ER+ HER2- breast cancer, RS were concordant in 67% of cases. Concordance rates may be higher in older women or among those with comparable levels of PR expression. These data suggest that testing of both tumors should be considered in patients who are candidates for adjuvant chemotherapy.
Keywords: bilateral breast cancer, Oncotype DX
INTRODUCTION
The 21-gene recurrence score, Oncotype DX (Genomic Health, Redwood City, CA) is widely used to assess risk of recurrence and benefit from chemotherapy in women with node-negative, estrogen receptor-positive breast cancer.1,2 The 21-gene assay, which measures the relative levels of expression of genes associated with proliferation, invasion, HER2, and estrogen receptor, provides a single recurrence score which corresponds to a point estimate of the 10-year risk of distant recurrence with a 95% confidence interval.3 The prognostic value of this assay has been demonstrated in clinical trials of both node-negative and node-positive breast cancer, and numerous studies have shown that the Oncotype DX recurrence score (RS) affects clinical decision making.4-6
The incidence of synchronous bilateral invasive breast cancer is reported to range from 0.8%–3.0%; largely reflecting differing definitions used for synchronous diagnoses, with cutoffs ranging from within 3–12 months of initial diagnosis.7,8 Currently, there are no consistent evidence-based guidelines for the management of synchronous bilateral breast cancer, and treatment decisions are often made based on the primary tumor with the most high-risk features. However there is often considerable similarity in standard histopathologic features and hormone receptor status, making it difficult to differentiate how long-term prognosis is impacted by the presence of two primary tumors. The aim of this study was to determine the concordance rate for RS testing in women with synchronous bilateral primary breast cancer, and to identify clinical and pathologic features predictive of discordant recurrence scores in this population.
METHODS
Prospective institutional databases were retrospectively reviewed to identify patients presenting to Memorial Sloan Kettering Cancer Center (MSKCC) with synchronous bilateral invasive breast cancer from January 2005 to June 2014. This time period was chosen as RS testing for patients with estrogen receptor-positive, HER2 negative, node-negative breast cancer was incorporated into standard clinical practice at our institution in 2005. Estrogen receptor positivity was defined as greater than or equal to 1% nuclear staining. Bilateral invasive diagnoses were considered synchronous if they occurred within 6 months of each other. Patients with a personal history of breast cancer, those with node-positive disease, and those with only one RS were excluded. This study was approved by the institutional review board.
Patient, tumor, and treatment characteristics were abstracted from the medical record. RS were categorized by risk group as originally described: RS<18, low risk; RS 18–30, intermediate risk; and RS≥31, high risk.3 Patients were considered to have discordant RS if the bilateral tumor RS reflected different risk groups. Comparisons were made between patients/tumors with concordant and discordant risk scores. Due to limited sample size, all analyses are descriptive.
RESULTS
From January 2005 to June 2014, 115 patients presented with synchronous bilateral primary invasive breast cancer; 43 (37%) of whom met eligibility criteria and had 2 RS available. Patient and bilateral breast tumor characteristics are shown in Table 1. Median patient age was 60 years (range 42–84 years), and median tumor size was 1.2 cm for both primary tumors, with minor differences in the range (0.6–3.7 and 0.5–3.1, respectively). The majority of cases represented invasive ductal carcinomas with a similar distribution of progesterone receptor positivity, nuclear grade, and histologic grade between bilateral breast primaries. All cases were node negative; however, lymphovascular invasion was identified in 7% and 21% of bilateral tumors, respectively. Among 86 primaries, 63 (73%) were categorized as low risk, 20 (23%) were categorized as intermediate risk, and 3 (4%) were categorized as high risk by RS testing. There was no difference in median RS between the two groups of primary (bilateral) tumors (Table 1).
TABLE 1.
Patient and tumor characteristics (n=86)
| Right breast n=43 n (%) |
Left breast n=43 n (%) |
|
|---|---|---|
| Age, years, median (range) | 60 (42–84) | 60 (42–84) |
| Tumor size, cm (range) | 1.2 (0.6–3.7) | 1.2 (0.5–3.1) |
| ER-expression, %, median (range) | 95 (40–100) | 95 (50–100) |
| PR-expression, %, median (range) | 80 (0–95) | 80 (0–100) |
| Oncotype DX recurrence score,, median (range) | 15 (0–35) | 12 (2–29) |
| Subtype | ||
| ER+/PR+/HER2− | 40 (93%) | 41 (95%) |
| ER+/PR−/HER2− | 3 (7%) | 2 (5%) |
| Histology | ||
| IDC | 33 (77%) | 35 (81%) |
| ILC | 6 (14%) | 5 (12%) |
| Other* | 4 (9%) | 3 (7%) |
| Nuclear grade | ||
| 1 | 6 (14%) | 8 (19%) |
| 2 | 24 (56%) | 21 (49%) |
| 3 | 2 (5%) | 6 (14%) |
| Missing | 10 (23%) | 8 (19%) |
| Histologic grade | ||
| 1 | 5 (12%) | 6 (14%) |
| 2 | 13 (30%) | 16 (37%) |
| 3 | 19 (44%) | 18 (42%) |
| Missing | 6 (14%) | 3 (7%) |
| Multifocality | ||
| Yes | 34 (79%) | 31 (72%) |
| No | 9 (21%) | 12 (28%) |
| LVI | ||
| Present | 3 (7%) | 9 (21%) |
| Absent | 36 (84%) | 32 (74%) |
| Missing | 4 (9%) | 2 (5%) |
| Oncotype DX risk | ||
| Low | 27 (63%) | 36 (84%) |
| Intermediate | 13 (30%) | 7 (16%) |
| High | 3 (7%) | 0 (0%) |
tubular and mucinous carcinomas
ER, estrogen receptor; PR, progesterone receptor; IDC, invasive ductal carcinoma; ILC; invasive lobular carcinoma; LVI, lymphovascular invasion
RS were concordant in 29 patients (67%). Table 2 illustrates the concordance rates for each risk group. There was no significant association between any clinical or histopathologic feature and the likelihood of having concordant or discordant recurrence scores; however, patients with concordant RS were older (62 years versus 56 years) and had median levels of progesterone receptor expression that were higher and more similar when compared to those with discordant scores (Table 3).
TABLE 2.
Oncotype DX concordance by risk group
| Oncotype DX risk right breast | Oncotype DX risk left breast | |
|---|---|---|
| Low (n=36) | Intermediate (n=7) | |
| Low (n=27) | 25 | 2 |
| Intermediate (n=13) | 9 | 4 |
| High (n=3) | 2 | 1 |
TABLE 3.
Characteristics of concordant and discordant cases
| Concordant Oncotype DX (n=29) |
Discordant Oncotype DX (n=14) |
|
|---|---|---|
| Median (range) | Median (range) | |
| Age (years) | 62 (48–84) | 56 (42–72) |
| Tumor 1 size (cm) | 1.2 (0.6–3.3) | 1.2 (0.6–3.7) |
| Tumor 2 size (cm) | 1.2 (0.5–2.2) | 1.1 (0.8–3.1) |
| ER-expression 1 (%) | 98 (40–100) | 95 (70–100) |
| ER-expression 2 (%) | 95 (60–100) | 96 (50–100) |
| PR-expression 1 (%) | 85 (0–99) | 55 (0–98) |
| PR-expression 2 (%) | 80 (0–100) | 75 (0–95) |
| n (%) | n (%) | |
| Subtype | ||
| ER+/PR+/HER2− | 24 (84%) | 12 (86%) |
| ER+/PR−/HER2− | 5 (17%) | 2 (14%) |
| Histology | ||
| Concordant | 21 (72%) | 10 (71%) |
| Discordant | 8 (28%) | 4 (29%) |
| Nuclear grade | ||
| Concordant | 12 (41%) | 4 (29%) |
| Discordant | 10 (35%) | 4 (29%) |
| Missing* | 7 (24%) | 6 (42%) |
| Histologic grade | ||
| Concordant | 11 (38%) | 8 (58%) |
| Discordant | 13 (45%) | 3 (21%) |
| Missing* | 5 (17%) | 3 (21%) |
due to invasive lobular carcinoma and lack of reporting in those subtypes
ER, estrogen receptor; PR, progesterone receptor
RS were ordered at the same time in 34 (79%) patients and in a staged manner in the remaining 9 patients, with no difference in rates of discordance between the two groups (Table 3). The median time between RS testing for those performed in a staged manner was 31 days. Among the 14 patients with discordant scores, 3 had testing performed in a staged manner, and the second score reflected a higher risk group in all 3 cases, leading to a treatment change (recommendation for chemotherapy) in 2 of 3 cases. The time interval between testing in these 3 cases was 3 weeks, 3 weeks, and 10 weeks, respectively.. Overall, treatment recommendations were affected by the higher of the two RS in 8 of 14 (57%) patients with discordant risk group scores (Table 4) or 18.6% of the study population (8/43).
TABLE 4.
Oncotype scores among those with discordant risk-group scores
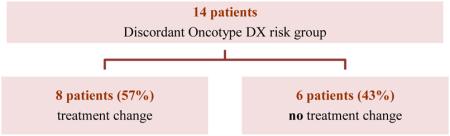
| |||
|---|---|---|---|
| Oncotype DX Recurrence Score |
Predicted 10yr Risk of Recurrence, % |
Risk Group | Recommended Treatment Change |
| 13 22 |
8 14 |
Low Intermediate |
No |
| 2 34 |
4 23 |
Low High |
Yes |
| 32 24 |
21 15 |
High Intermediate |
No |
| 18 and 8 10 |
8 7 |
Intermediate Low |
Yes |
| 35 13 |
24 8 |
High Low |
No |
| 3 19 |
4 12 |
Low Intermediate |
No |
| 11 22 |
7 14 |
Low Intermediate |
Yes |
| 20 0 |
13 3 |
Intermediate high |
yes |
| 13 19 |
9 | Low Intermediate |
Yes |
| 21 12 |
14 8 |
Intermediate Low |
Yes |
| 18 11 |
11 7 |
Intermediate Low |
Yes |
| 19 4 |
12 4 |
Intermediate Low |
Yes |
| 19 10 |
12 7 |
Intermediate Low |
No |
| 18 12 |
12 8 |
Intermediate Low |
No |
DISCUSSION
There are no consistent evidence-based guidelines for the management of synchronous bilateral breast cancer, and treatment decisions are often made based on the primary tumor with the most high-risk features (i.e., hormone receptor status, HER2 status, size, grade, LVI, and others). In the setting of bilateral ER positive, HER2 negative, node-negative breast cancer, this distinction may be difficult as the tumors may appear quite similar by standard histopathologic criteria. There is now a large body of data supporting the use of the 21-gene RS to predict benefit from chemotherapy in the setting of unilateral disease1-6; however, published data regarding the RS in the setting of multiple synchronous breast cancers are limited to a single study by Toole et al demonstrating that 2 of 4 (50%) of patients with multiple primary tumors in different breasts, and 4 of 18 (22%) of patients with multiple primaries in the same breast, had RS differences that led to changes in management.9 Here we demonstrate that RS were discordant in 33% of women with estrogen receptor-positive, HER2 negative, node-negative, synchronous bilateral invasive breast cancer, leading to a change in treatment in 57% of these patients. To our knowledge, this is the only study to date examining concordance rates specifically in this population.
Since the first publication of a case series of synchronous bilateral breast cancer by Kilgore in 192110, there has been ongoing debate regarding the prognostic significance of bilateral disease. The concept of a time interval to distinguish between synchronous and metachronous bilateral breast cancer was first introduced by Haagensen in 1971.11 He used 6 months as the cutoff for distinguishing these two categories; however, since then, various cut-points have been used to define synchronous bilateral breast cancer, ranging from 0–60 months, making it difficult to draw firm conclusions regarding the impact of bilateral disease on long-term outcomes.12 Hartman et al13 analyzed incidence and mortality rates for bilateral breast cancer in a large nationwide cohort of 123,757 breast cancer patients in Sweden. Compared to women with unilateral disease, women with synchronous bilateral breast cancer (SBBC), defined as a contralateral diagnosis within 3 months, experienced inferior breast cancer specific mortality at 10 years, 45% (95% confidence interval [CI], 41.4–48%) and 33% (95% CI, 32.8–33.5%), respectively, and this difference was strongly influenced by age, whereby a diagnosis of SBBC under 50 years of age was associated with a 120% higher mortality rate when compared to women with unilateral disease. For women with metachronous bilateral breast cancer, the mortality risk was largely dependent on the time interval between the two diagnoses, with those diagnosed more than 10 years later having similar 5-year breast cancer mortality to that of women of the same age with unilateral disease.
A large meta-analysis of 17 studies from 11 different countries, including 8050 synchronous bilateral breast cancers, defined as contralateral diagnosis within 6 months, also reported inferior outcomes for women with SBBC.14 After adjusting for other known prognostic factors, the diagnosis of synchronous bilateral breast cancer was associated with a pooled HR of 1.37 (95% CI, 1.24–1.50; p<0.0001) for breast cancer mortality compared to unilateral disease. However, among the 4 studies included in the meta-analysis that used a matched approach to compare prognosis between SBBC and unilateral breast cancer, no difference in disease-specific survival was observed(15-18), and in the sensitivity analysis of studies that included lymph node status as a covariate, there was also no difference in breast cancer mortality (pooled HR, 1.18; 95% CI, 0.95–1.48).14 The overall estimate of increased breast cancer mortality for patients with SBBC in this dataset does suggest, however, that there is room for improvement in determining appropriate adjuvant treatment for this patient subset.
In this study, we defined bilateral breast cancer as synchronous if the 2 diagnoses occurred within 6 months of each other; yet the majority of cases (39 [91%]) were diagnosed at the same time, and the remaining 4 (9%) were diagnosed within a 3-month window. This observation likely reflects the increased use of breast MRI for extent of disease in patients with newly diagnosed breast cancer during the time period of this study. It is generally assumed that synchronous bilateral breast cancer represents 2 clonally independent malignancies—a hypothesis that has been tested in clonality experiments by several different groups.19-21 However, given the conflicting studies reporting outcome differences for women with unilateral versus bilateral breast cancer, it is plausible that a small proportion of these cases represent early metastatic events.16-18 The ability to develop multiple tumors simultaneously may also be suggestive of an underlying genetic variation or susceptibility to malignancy, and some studies of patients with synchronous bilateral breast cancer have suggested a relationship between family history and bilaterality; however, others have not confirmed this finding.22-24
Multiple primary breast cancers share the same host, the same history in regards to metabolic changes, and the same exposure to external influences, yet there are limited data on how these shared characteristics impact concordance in regard to the molecular portrait of tumors. Buggi et al examined 113 multiple (multicentric and multifocal) breast cancers of the same histologic subtype and found differences in grade (18.8%), estrogen receptor status (4.4%), progesterone receptor status (15.9%), and HER2 status (9.7%) in a minority of cases.25 In an analysis of 12 patients with synchronous bilateral breast cancer Saad et al reported similar histologic subtype in 58% of the cases, and similar estrogen receptor, progesterone receptor, and HER2 status in 67% of cases.26 Similar findings were reported by Hungness et al and Coradini et al.27,28 Although we selected our patients to have bilateral estrogen receptor-positive, HER2 negative, node-negative breast cancer, there was no significant difference in other traditional markers, such as histologic subtype, size, and grade when comparing the bilateral breast tumors, nor did we observe a significant difference in any of these parameters between tumors with concordant and discordant RS. We did observe a trend for patients with concordant RS to be older (62 years of age versus 56 years) and to have median levels of progesterone receptor expression that were higher and more similar between the two primary tumors when compared to those with discordant scores; however, this finding awaits confirmation in a larger dataset (Table 3).
Toole et al also examined factors associated with discordant RS.9 Among 22 patients with either bilateral breast cancer (n=4) or multiple primaries in the same breast (n=18), 8 patients had discordant RS. In the Toole dataset, cases with discordant scores were more likely to differ in tumor histology (p=0.05) and tumor grade (p=0.04) than concordant cases. In patients with concordant scores, tumor histology and grade differed in 18.8% and 6.3% of cases, respectively, as compared to 66.7% and 50% of cases for those with discordant scores. Although not statistically significant, tumors with different RS were also more likely to differ in progesterone receptor status (33.3% vs 18.8%).
In our study, a change in chemotherapy recommendation based on the result of the second RS was made in 8 of 14 cases (57%). In all cases, patients who would have not received chemotherapy based on one tumor score received chemotherapy due to a higher second score. In the cases with discordant RS and no treatment change (6 patients), 4 patients received chemotherapy either due to one of the tumors having a high RS or physician/patient choice, and 2 patients whose bilateral tumors had low and intermediate RS, respectively, received endocrine therapy alone. Changes in treatment recommendations based on discordant RS were also reported by Toole et al where different scores led to a treatment change in 2 of 4 (50%) patients with bilateral disease and 4 of 18 (22%) patients with multiple tumors in the same breast.
In conclusion, Oncotype DX recurrence scores were discordant in 33% of women with estrogen receptor-positive, HER2 negative, node-negative, synchronous bilateral invasive breast cancer; leading to a change in treatment in 57% of these patients. To our knowledge, this is the only study to date examining concordance rates specifically in this population. Given our small sample size, we were unable to find significant clinical or pathologic predictors of discordant results, yet given the high rate of discordance, with the potential for a change in treatment recommendation and the controversy surrounding the prognostic impact of bilateral disease, these data suggest that testing of both tumors should be considered in patients with synchronous bilateral breast cancer who are candidates for adjuvant chemotherapy.
Synopsis.
Oncotype DX testing of synchronous bilateral breast cancers yields different risk group scores in approximately 30% of patients; suggesting that testing of both tumors should be considered in patients who are candidates for adjuvant chemotherapy.
Acknowledgments
This study was funded in part through NIH/NCI Cancer Center Support Grant No. P30CA008748 and supported in part by the Breast Cancer Alliance.
Footnotes
Conflict of interest/financial disclosures: The authors have no conflicts of interest to declare.
REFERENCES
- 1.Goldstein LJ, Gray R, Badve S, et al. Prognostic utility of the 21-gene assay in hormone receptor-positive operable breast cancer compared with classical clinicopathologic features. J Clin Oncol. 2008;26(25):4063–71. doi: 10.1200/JCO.2007.14.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harris L, Fritsche H, Mennel R, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25(33):5287–312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 3.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351(27):2817–26. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 4.Albain KS, Paik S, van't Veer L. Prediction of adjuvant chemotherapy benefit in endocrine responsive, early breast cancer using multigene assays. Breast. 2009;18(Suppl 3):S141–5. doi: 10.1016/S0960-9776(09)70290-5. [DOI] [PubMed] [Google Scholar]
- 5.Carlson JJ, Roth JA. The impact of the Oncotype Dx breast cancer assay in clinical practice: a systematic review and meta-analysis. Breast Cancer Res Treat. 2013;141(1):13–22. doi: 10.1007/s10549-013-2666-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang G, Shak S, Paik S, et al. Comparison of the prognostic and predictive utilities of the 21-gene Recurrence Score assay and Adjuvant! for women with node-negative, ER-positive breast cancer: results from NSABP B-14 and NSABP B-20. Breast Cancer Res Treat. 2011;127(1):133–42. doi: 10.1007/s10549-010-1331-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gollamudi SV, Gelman RS, Peiro G, et al. Breast-conserving therapy for stage I-II synchronous bilateral breast carcinoma. Cancer. 1997;79(7):1362–9. doi: 10.1002/(sici)1097-0142(19970401)79:7<1362::aid-cncr14>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 8.Kollias J, Ellis IO, Elston CW, et al. Clinical and histological predictors of contralateral breast cancer. Eur J Surg Oncol. 1999;25(6):584–9. doi: 10.1053/ejso.1999.0711. [DOI] [PubMed] [Google Scholar]
- 9.Toole MJ, Kidwell KM, Van Poznak C. Oncotype dx results in multiple primary breast cancers. Breast Cancer (Auckl) 2014;8:1–6. doi: 10.4137/BCBCR.S13727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kilgore AR. The incidence of cancer in the second breast. JAMA. 1921;77:454–7. [Google Scholar]
- 11.Haagensen CD. The physiology of the breast as it concerns the clinician. Am J Obstet Gynecol. 1971;109(2):206–9. doi: 10.1016/0002-9378(71)90868-4. [DOI] [PubMed] [Google Scholar]
- 12.Schwentner L, Wolters R, Wischnewsky M, et al. Survival of patients with bilateral versus unilateral breast cancer and impact of guideline adherent adjuvant treatment: a multi-centre cohort study of 5292 patients. Breast. 2012;21(2):171–7. doi: 10.1016/j.breast.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Hartman M, Czene K, Reilly M, et al. Incidence and prognosis of synchronous and metachronous bilateral breast cancer. J Clin Oncol. 2007;25(27):4210–6. doi: 10.1200/JCO.2006.10.5056. [DOI] [PubMed] [Google Scholar]
- 14.Holm M, Tjonneland A, Balslev E, et al. Prognosis of synchronous bilateral breast cancer: a review and meta-analysis of observational studies. Breast Cancer Res Treat. 2014;146(3):461–75. doi: 10.1007/s10549-014-3045-0. [DOI] [PubMed] [Google Scholar]
- 15.de la Rochefordiere A, Asselain B, Scholl S, et al. Simultaneous bilateral breast carcinomas: a retrospective review of 149 cases. Int J Radiat Oncol Biol Phys. 1994;30(1):35–41. doi: 10.1016/0360-3016(94)90516-9. [DOI] [PubMed] [Google Scholar]
- 16.Irvine T, Allen DS, Gillett C, et al. Prognosis of synchronous bilateral breast cancer. Br J Surg. 2009;96(4):376–80. doi: 10.1002/bjs.6553. [DOI] [PubMed] [Google Scholar]
- 17.Nichol AM, Yerushalmi R, Tyldesley S, et al. A case-match study comparing unilateral with synchronous bilateral breast cancer outcomes. J Clin Oncol. 2011;29(36):4763–8. doi: 10.1200/JCO.2011.35.0165. [DOI] [PubMed] [Google Scholar]
- 18.Schmid SM, Pfefferkorn C, Myrick ME, et al. Prognosis of early-stage synchronous bilateral invasive breast cancer. Eur J Surg Oncol. 2011;37(7):623–8. doi: 10.1016/j.ejso.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Imyanitov EN, Suspitsin EN, Grigoriev MY, et al. Concordance of allelic imbalance profiles in synchronous and metachronous bilateral breast carcinomas. Int J Cancer. 2002;100(5):557–64. doi: 10.1002/ijc.10530. [DOI] [PubMed] [Google Scholar]
- 20.Janschek E, Kandioler-Eckersberger D, Ludwig C, et al. Contralateral breast cancer: molecular differentiation between metastasis and second primary cancer. Breast Cancer Res Treat. 2001;67(1):1–8. doi: 10.1023/a:1010661514306. [DOI] [PubMed] [Google Scholar]
- 21.Shibata A, Tsai YC, Press MF, et al. Clonal analysis of bilateral breast cancer. Clin Cancer Res. 1996;2(4):743–8. [PubMed] [Google Scholar]
- 22.Chen JJ, Wang Y, Xue JY, et al. A clinicopathological study of early-stage synchronous bilateral breast cancer: a retrospective evaluation and prospective validation of potential risk factors. PLoS One. 2014;9(4):e95185. doi: 10.1371/journal.pone.0095185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hartman M, Czene K, Reilly M, et al. Genetic implications of bilateral breast cancer: a population based cohort study. Lancet Oncol. 2005;6(6):377–82. doi: 10.1016/S1470-2045(05)70174-1. [DOI] [PubMed] [Google Scholar]
- 24.Jobsen JJ, van der Palen J, Ong F, et al. Synchronous, bilateral breast cancer: prognostic value and incidence. Breast. 2003;12(2):83–8. doi: 10.1016/s0960-9776(02)00278-3. [DOI] [PubMed] [Google Scholar]
- 25.Buggi F, Folli S, Curcio A, et al. Multicentric/multifocal breast cancer with a single histotype: is the biological characterization of all individual foci justified? Ann Oncol. 2012;23(8):2042–6. doi: 10.1093/annonc/mdr570. [DOI] [PubMed] [Google Scholar]
- 26.Saad RS, Denning KL, Finkelstein SD, et al. Diagnostic and prognostic utility of molecular markers in synchronous bilateral breast carcinoma. Mod Pathol. 2008;21(10):1200–7. doi: 10.1038/modpathol.2008.35. [DOI] [PubMed] [Google Scholar]
- 27.Coradini D, Oriana S, Mariani L, et al. Is steroid receptor profile in contralateral breast cancer a marker of independence of the corresponding primary tumour? Eur J Cancer. 1998;34(6):825–30. doi: 10.1016/s0959-8049(97)10121-6. [DOI] [PubMed] [Google Scholar]
- 28.Hungness ES, Safa M, Shaughnessy EA, et al. Bilateral synchronous breast cancer: mode of detection and comparison of histologic features between the 2 breasts. Surgery. 2000;128(4):702–7. doi: 10.1067/msy.2000.108780. [DOI] [PubMed] [Google Scholar]


