Abstract
Axon‐like neuritogenesis in neuroblastoma (NG108‐15) cells and primary cerebellar granular neurons is furthered by the presence of ganglioside GM1. We describe here that galectin‐1 (Gal‐1), a homobivalent endogenous lectin, is an effector by cross‐linking the ganglioside and its associated glycoprotein α5β1‐integrin. The thereby triggered signaling cascade involves autophosphorylation of focal adhesion kinase and activation of phospholipase Cγ and phosphoinositide‐3 kinase. This leads to a transient increase in the intracellular Ca2+ concentration by opening of TRPC5 channels, which belong to the signal transduction‐gated cation channels. Controls with GM1‐defective cells (NG‐CR72 and neurons from ganglio‐series KO mice) were retarded in axonal growth, underscoring the relevance of GM1 as functional counterreceptor for Gal‐1. The lectin's presence was detected in the NG108‐15 cells, suggesting an autocrine mechanism of action, and in astrocytes in situ. Gal‐1, as cross‐linking lectin, can thus translate metabolic conversion of ganglioside GD1a to GM1 by neuraminidase action into axon growth.
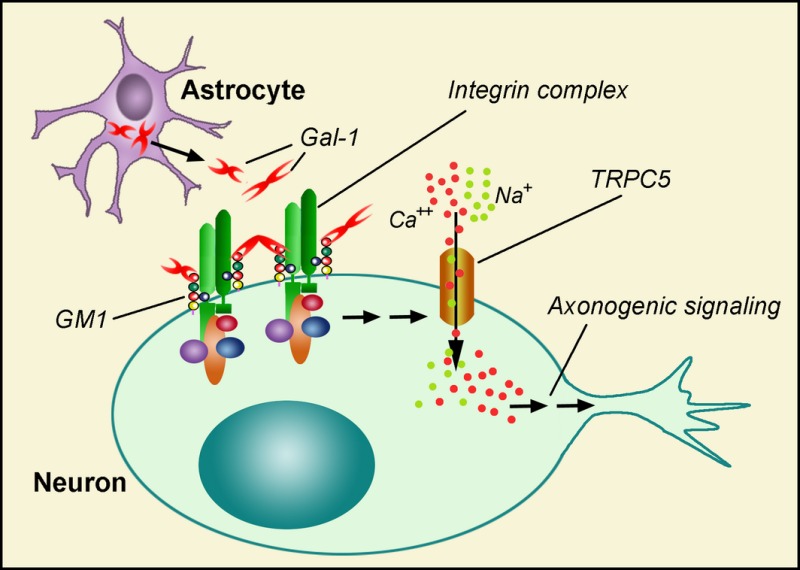
Galectin‐1 (Gal‐1) was shown an effector of axonogenesis in cerebellar granule neurons (CGNs) and NG108‐15 cells by cross‐linking GM1 ganglioside and its associated glycoprotein α5β1‐integrin. The resulting signaling led to a transient increase in intracellular Ca2+ by opening TRPC5 channels. CGNs deficient in GM1 showed retarded axonogenesis, underscoring the relevance of GM1 as functional counterreceptor for Gal‐1 in this process. This Gal‐1/GM1‐induced signaling was manifest only at the earliest, initiating stage of axon development.
Keywords: axon growth, Ca2+ level, ganglioside, integrin, lectin, neuron
Abbreviations used
- [Ca2+]i
intracellular calcium
- BBG
bovine brain gangliosides
- CGN
cerebellar granule neuron
- Ctx‐B
cholera toxin B‐subunit
- FAK
focal adhesion kinase
- FBS
fetal bovine serum
- Gal‐1
galectin‐1
- GFAP
glial fibrillary acidic protein
- HPTLC
high‐performance thin‐layer chromatography
- MAb
monoclonal Ab
- N'ase
neuraminidase
- PI(3)K
phosphoinositide‐3 kinase
- PLC
phospholipase C
- RA
retinoic acid
- TRPC5
transient receptor potential (canonical subgroup 5)
Structural versatility and readily accessible cell surface presentation predestine glycans for roles in cellular sociology, the core of the concept of the sugar code (Gabius 2009, 2015; Solís et al. 2015). In the central nervous system, gangliosides are the predominant form of sialyglycoconjugates, products of intricate biosynthesis and substrates in dynamic remodeling (Ando 1983; Prinetti et al. 2007; Yu et al. 2008; Ledeen and Wu 2009, 2015; Sturgill et al. 2012; Schengrund 2015). In this respect, enzymatic desialylation of gangliosides GD1a/b and GT1b to yield GM1 by a neuraminidase (N'ase) has been established as a molecular switch for neuronal differentiation and neurite outgrowth (Pitto et al. 1989; Wu and Ledeen 1991; Kopitz et al. 1994; Ledeen et al. 1998; Hasegawa et al. 2000; Proshin et al. 2002; Kappagantula et al. 2014). Using a multivalent tool for cross‐linking the generated ganglioside GM1, i.e. the pentameric cholera toxin B‐subunit (Ctx‐B), outgrowth of axon‐like neurites could be triggered, in neuroblastoma cell lines and primary neurons (Milani et al. 1992; Carlson et al. 1994; Wu et al. 1996; Fang et al. 2000). The Ctx‐B‐dependent cross‐linking of GM1 into aggregates was assumed to start a signaling cascade. The molecular nature of its effector had been revealed as a member of the transient receptor potential [canonical] subfamily within the signal transduction‐gated cation channels, i.e. TPRC5, which underlies transient increases of [Ca2+]i in neuroblastoma (NG108‐15) cells and primary cerebellar granular neurons (CGNs) (Wu et al. 2007). In detail, the initial protein (Ctx‐B)–glycan recognition accounts for GM1‐mediated cross‐linking of the α5β1‐integrin (with which GM1 is associated) that in turn triggered downstream activation of autophosphorylation of focal adhesion kinase (FAK) and then activation of phospholipase C (PLCγ) and phosphoinositide‐3 kinase (PI(3)K) (Wu et al. 2007). These experiments with a bacterial sugar receptor lead to the question whether a tissue lectin can qualify as a physiological mimetic of Ctx‐B. Of note in this respect, metabolic precursors of ganglioside GM1 (GD1a, GT1b) are known to engage in contact with myelin‐associated glycoprotein (a lectin known as siglec‐4), an interaction inhibiting axon outgrowth that is abolished by the N'ase‐induced conversion of GD1a and GT1b to GM1 (Collins et al. 1997; Vyas et al. 2005; Quarles 2007).
Several lines of evidence direct interest to members of the family of galectins (β‐galactoside‐specific proteins sharing the β‐sandwich fold and a sequence signature with a Trp in the center (Hirabayashi 1997; Kaltner and Gabius 2012; André et al. 2015), especially homodimeric galectin‐1 (Gal‐1): (i) galectin activity has been found in neuroblastoma (N‐18) cells and brain extracts by lactose‐inhibitable hemagglutination (Teichberg et al. 1975; Simpson et al. 1977; Eisenbarth et al. 1978; Kobiler et al. 1978), (ii) β‐galactoside‐binding capacity has histochemically been revealed in human peripheral nerves and cerebellum (Gabius et al. 1988; Bardosi et al. 1990; Kuchler et al. 1990), (iii) immunohistochemical detection in rat dorsal root ganglion neurons and cerebellum has been reported for Gal‐1 (Regan et al. 1986; Kuchler et al. 1989), (iv) Gal‐1 is a binding partner of the GM1 pentasaccharide on neuroblastoma (SK‐N‐MC) cell surfaces, in solution and in model (Kopitz et al. 1998; Siebert et al. 2003; Majewski et al. 2015) and (v) Gal‐1, as lectin, is active in axon regeneration in vitro (Horie et al. 1999; Kopitz et al. 2004). Proceeding from this experimental basis, we tested the hypothesis whether Gal‐1 elicits axon‐like neuritogenesis in vitro, using as controls a GM1‐negative cell line and primary cultures from mice deficient in GM1 synthesis.
Materials and methods
Galectin‐1 and its antibody
Bacterial extracts containing Gal‐1 were obtained after recombinant production, and the lectin was purified by affinity chromatography on lactosylated Sepharose 4B, prepared by divinyl sulfone activation and ligand coupling, as crucial step as described (André et al. 2007). Analysis of product quality by mass spectrometric fingerprinting of tryptic digests, of purity by one‐ and two‐dimensional gel electrophoresis and gel filtration and of activity by hemagglutination and anoikis induction was performed using optimized protocols (Gabius et al. 1984; André et al. 2007). The lectin was labeled using the N‐hydroxysuccinimide derivative of biotin (Sigma, Munich, Germany) under activity‐preserving conditions (Gabius et al. 1992), and its activity was ascertained by solid‐phase and cell assays (André et al. 2003; Amano et al. 2012). Polyclonal antibodies were raised in rabbits, and the immunoglobulin G preparation was systematically tested against other members of this lectin family (i.e. Gal‐2, ‐3, ‐4, ‐7, ‐8 and ‐9) by ELISAs and western blotting to exclude cross‐reactivity, followed by removal of cross‐reactive antibodies by affinity chromatography‐based depletion, using the respective galectin as bead‐immobilized ligand (Kaltner et al. 2002, 2011).
Cell culture and neuritogenesis
NG108‐15 (neuroblastoma × glioma hybrid) and NG‐CR72 [mutant of NG108‐15 lacking GM1 synthase (Wu et al. 2001)] cells were routinely cultured in Dulbecco's modified Eagle's medium (DMEM) with 5% or 10% fetal bovine serum (FBS). For neuritogenesis experiments, they were grown in DMEM with N2 supplement and treated with Clostridium perfringens N'ase (0.2 unit/mL) alone, N'ase plus Gal‐1 (5 μg/mL), Ctx‐B (5 μg/mL) or anti‐Gal‐1 antibody (10 μg/mL). NG‐CR72 cells were also treated with retinoic acid (20 μM). For testing involvement of PLC, PI(3)K and TRPC5 channels in Gal‐1‐induced neuritogenesis, pharmacological blockers U73122 (PLC, 1–5 μM), wortmannin (PI(3)K, 1–5 μM), LY294002 (PI(3)K, 1–5 μM) and SK&F 96365 (TRP channel, 10–50 μM) were co‐applied. After 72 h, neuritogenesis was quantified as percentage of cells bearing neurites (Wu et al. 1998). Neurites were defined as processes of length more than 2× the soma diameter. The induced neurites were characterized by immunocytochemical (IC) staining for axonal and dendritic marker proteins: mouse monoclonal Ab (MAb) against p‐NF‐H (SMI‐31) and SV2 were used for identifying axon‐like processes, while rabbit anti‐MAP2 antibody was used for dendrite‐like processes (Wu et al. 1998).
Primary cultures of cerebellar granular neurons were performed as described previously (Wu et al. 1996, 2004, 2007). Cells were prepared from cerebella of 7‐day‐old normal and ganglio‐series null [GM2/GD2 synthase knockout, B4galnt1 (−/−)] pups (C57BL/6 background). On the second day in vitro (2 DIV) before sprouting of neurites, the medium was replaced by DMEM containing N2 supplement and 1% FBS containing Gal‐1 (10 μg/mL), Ctx‐B (5 μg/mL) or anti‐Gal‐1 (10 μg/mL), in the presence or absence of blockers listed above. After 24 h of treatment, photomicrographs were taken, and the longest neurite (axon) in each CGN was measured with UltraView software (PerkinElmer, Wellesley, CA, USA). Axonal property of these neurites was confirmed with IC staining by SMI‐31 MAb (see above).
Binding of Gal‐1 to ganglioside GM1
Lipid extraction of NG108‐15 and NG‐CR72 cells was carried out with chloroform–methanol–water (5 : 5 : 1), and following centrifugation, portions of the supernatants were applied to a silica gel high‐performance thin‐layer chromatography (HPTLC) plate. Bovine brain ganglioside mixture (BBG) was applied in parallel as control. Separation was effected with chloroform/methanol/aqueous KCl (2 M) (50/40/10, v/v/v). The plate was treated with N'ase (1 U/mL) in acetate buffer (50 mM, pH 5.3) at 37°C for 2 h that converted gangliotetraose gangliosides to GM1 and then incubated with biotinylated Gal‐1 (10 μg/mL) in phosphate‐buffered saline (PBS)‐2% bovine serum albumin (BSA) at 20‐24 °C for 1 h, followed by immersion in a solution of avidin‐horseradish peroxidase (HRP) (1 : 100) at 20‐24 °C 1 h. Finally, Gal‐1‐reactive bands were visualized on Blue BIO film using enhanced chemiluminescence (ECL) reagent.
To reveal Gal‐1 reactivity to GM1 on the cell surface, NG108‐15 and NG‐CR72 cells were treated with trypsin (2.5 mg/mL) in culture at 37°C for 30 min. Cells were fixed in cold paraformaldehyde (2% in PBS) and treated with N'ase (1 U/mL, pH 5.3, 37°C, 1.5 h), followed by incubation with solution containing biotinylated Gal‐1 (10 μg/mL, 20‐24 °C, 1 h) and streptavidin‐FITC (1 : 100, 20‐24 °C, 1 h) in PBS‐2% BSA. Parallel staining using FITC‐labeled Ctx‐B (5 μg/mL) was also carried out. Additionally, cells were pretreated (20‐24 °C, 1 h) with Ctx‐B (10 μg/mL) or anti‐GM1 antibody (1 : 100; gift of Dr R. L. Schnaar) in PBS‐2% BSA prior to incubation with solutions containing biotinylated Gal‐1 and avidin/streptavidin‐FITC. Signals were semi‐quantified according to fluorescence intensity on the cell surface that was classified into three groups (none, low and high).
TRPC5 Knockdown by shRNA
To test involvement of TRPC5 channels in the Gal‐1‐dependent effect, a mixture of four pRS plasmids containing sequences encoding short hairpin RNA (shRNA; OriGene, Rockville, MD, USA) to silence TRPC5‐specific mRNA was transfected into both NG108‐15 cells and normal CGNs with Lipofectamine 2000 (Invitrogen, Grand Island, NY, USA) following the manufacturer's protocol. Negative control was the plasmid containing nonsense shRNA. Sequences of TRPC5 shRNA were (1) GCATTACTCACGCCATCCGCAAGGAGGT, (2) TCTACCTGGCAACTATTTCCTTGAAGATC, (3) AAGAAGCCTCTCCACCAGCAGTGCTGATT and (4) AAGTCAGATGAACCTTGGCGAGGTAGAGC. For CGNs, transfection was done in freshly prepared cells before seeding. Forty‐eight hours after transfection, TRPC5‐specific mRNA expression was examined by RT‐PCR as previously described (Wu et al. 2007), using forward primer TTCTCTTTATCTACTGCC and reverse primer TGGAGCRAAYTTCCAYTC (R = G or A and Y = C or T). TRPC5 protein expression was checked with immunoblot (IB) and IC, using goat anti‐TRPC5 antibody plus second antibody labeled with HRP as previously reported (Wu et al. 2007), see below. Neuritogenesis assays in shRNA‐treated cells were performed as above.
Activation of integrin‐controlled signaling molecules
The changes induced by Gal‐1 treatment involving activation (tyrosine‐phosphorylation) of α5β1‐integrin and signaling molecules including FAK, PLCγ, PI(3)K were tested by immunoprecipitation (IP) and IB carried out as before (Wu et al. 2007). Briefly, NG108‐15 cells were treated with N'ase and reacted with Gal‐1 (10 μg/mL, up to 20 min), followed by extraction with M‐PER reagent. Lysate containing ~ 100 μg protein was subjected to polyacrylamide gel (7%) electrophoresis, proteins were electrotransferred to a polyvinylidene difluoride membrane, and the latter was probed with goat anti‐FAKpy397 (1 : 100) or rabbit anti‐p85 PI(3)Kpy508 (1 : 200) antibody, as described (Wu et al. 2007). Immunoblot using anti‐actin MAb was also carried out as loading control. Following reaction of second antibody labeled with HRP, bands were visualized on Blue BIO film using ECL reagent. In addition, lysate (~ 300 μg protein) was immunoprecipitated with 50 μL of agarose beads presenting PT66 MAb against phosphorylated tyrosine, and the IP product was subjected to IB analysis using goat anti‐β1‐integrin (1 : 250) or rabbit anti‐PLCγ (1 : 200) antibody. Double IC staining was performed to ascertain tyrosine phosphorylation of the above‐mentioned proteins and their co‐localization with Gal‐1/GM1 in intact cells as previously described (Wu et al. 2007). Briefly, NG108‐15 cells and mouse CGNs (2 DIV) grown on coverslips were pretreated with N'ase (0.5 U/mL) in culture and exposed to solution containing biotinylated Gal‐1 (10 μg/mL) in ice‐cold Ca2+‐free Hank's solution for 15 min, followed by an incubation period of 30 min at 37°C. They were fixed with 2% paraformaldehyde and immunostained as previously described (Wu et al. 1998, 2007). Primary antibodies included rabbit anti‐β1‐integrin (1 : 100), mouse anti‐FAKpy396 (1 : 200) and rabbit anti‐P85 PI(3)Kpy508 (1 : 200); corresponding second antibodies were labeled with Texas red, and their solution was mixed with avidin‐FITC (1 : 300). Documentation of fluorescence distribution was recorded with a two‐photon confocal microscope (Bio‐Rad, Hercules, CA, USA).
Intracellular calcium ([Ca2+]i) measurement
[Ca2+]i was determined with fura‐2‐based fluorescence spectrometry as previously described (Fang et al. 2000, 2002; Wu et al. 2007). NG108‐15 and NG‐CR‐72 cells were treated with N'ase (2 U/mL, 2 h) and then loaded with fura‐2 AM (5 μM, 30 min) followed by [Ca2+]i measurement in suspension in a physiological saline solution consisting of 140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 10 mM glucose, 0.25 mM sulfinpyrazone and 1% BSA. [Ca2+]i levels were monitored as fluorescence ratio between the two excitation wavelengths (R340/380) for 600–800 s, during which Ca2+ (5 mM), Ctx‐B (5 μg/mL), Gal‐1 (10 μg/mL) and ATP (2 mM) were applied at designated times. When pharmacological inhibitors were used, they were applied to cells in suspension in physiological saline solution containing 2 mM Ca2+ for 15–30 min prior to [Ca2+]i determination. [Ca2+]i measurements were also carried out in NG108‐15 cells transfected with control and TRPC5‐specific shRNA plasmids.
Detection of Gal‐1
To determine whether Gal‐1 was present in the culture medium, undifferentiated NG108‐15 cells and CGNs were grown in DMEM with N2 supplement and 1% FBS for 48 h followed by centrifugation at 4000 rpm for 15 min. Proteins in the supernatant were precipitated with 25% ice‐cold trichloroacetic acid (v/v) for 30 min and pelleted by centrifugation. The protein pellet was washed with ice‐cold (96%) ethanol once and then dissolved in sample buffer with or without 5% (v/v) β‐mercaptoethanol. Aliquots containing ~ 30 μg protein were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis on 10% polyacrylamide gels under reducing (4% β‐mercaptoethanol) or non‐reducing conditions. After electrophoretic transfer to a polyvinylidene difluoride membrane, the lectin was visualized on Blue BIO film by processing with rabbit anti‐Gal‐1 antibody (10 μg/mL) followed by goat anti‐rabbit IgG linked with HRP (1 : 200) and finally ECL reagent.
To determine the source of Gal‐1, IC was performed on undifferentiated NG108‐15 cells and primary CGNs that were grown on coverslips and fixed with 2% paraformaldehyde in PBS. After treatment with 0.5% Triton X‐100 at 20‐24 °C for 30 min, the cells were incubated overnight at 4°C with rabbit anti‐Gal‐1 antibody (1 : 100) in PBS containing 2% BSA and 0.1% Triton X‐100. This was followed by incubation with FITC‐labeled goat anti‐rabbit IgG (1 : 300) for 2 h at 20‐24 °C. In the case of CGNs, antibodies against marker proteins for neurons (NeuN, mouse MAb, 1 : 500) and astrocytes (glial fibrillary acidic protein (GFAP), mouse MAb, 1 : 200) were co‐stained with anti‐Gal‐1 antibody, the corresponding second Abs labeled with Texas red.
Developmental change of Gal‐1 expression in mouse cerebellum
Cerebella were collected from neonatal normal and ganglio‐series‐deficient mice at the age of 7–9 days. They were fixed in 4% paraformaldehyde and immersed in 30% sucrose for > 48 h; 15 μm sagittal cryosections were immersed overnight at 4°C in a solution containing rabbit anti‐Gal‐1 (1 : 100), together with mouse anti‐GFAP (1 : 200) or mouse anti‐p‐NF‐H (1 : 1000), PBS‐2% BSA‐0.1% Triton X‐100. After thorough washing with PBS containing 0.01% Triton X‐100, slides were immersed for 2 h at 22‐24 °C in a solution containing fluorescent indicator‐labeled second antibody (1 : 200); Hoechst 33 342 dye (2 μg/mL) for nuclear staining was added during the last 30 min. The signal distribution was recorded with a two‐photon confocal microscope as done for signal‐cascade analysis.
Data analysis
For the quantification of neurite outgrowth in NG108‐15 cells, statistical significance was analyzed by Student's t‐test (two‐tailed) from three independent experiments (n = 3), each involving 200–300 cells counted.
Axon formation in primary CGN cultures was analyzed in a total of ~ 300 cells from three individual experiments. Data were statistically analyzed by one‐way anova with Dunnett's post‐test, using GraphPad Prism software (San Diego, CA, USA).
The extent of Gal‐1 binding was analyzed by semi‐quantitative classification into three groups (none, low and high). More than 200 cells in each treatment group were processed in one experiment, and data are the average of three independent experiments.
Results
Binding of Gal‐1 to ganglioside GM1 and to enzymatically treated neuroblastoma cells
Because NG108‐15 and the NG‐CR72 mutant cells differ in display of gangliosides, Gal‐1 reactivity to these two types of cell should be different. In the first step, to ascertain reactivity of Gal‐1 to GM1 and absence of binding to glycolipids of NG‐CR72 cells, gangliosides in the lipid extracts were separated by HPTLC and treated with N'ase and each HPTLC plate then probed with labeled Gal‐1. The mixture of bovine brain gangliosides (BBG) used as control ascertained enzyme activity to convert gangliosides' glycan chains to the GM1 pentasaccharide and its reactivity to Gal‐1 (Fig. 1a). In the case of NG108‐15 cells, this revealed robust Gal‐1 binding to GM1 and to the GD1a locus due to N'ase‐dependent conversion of the latter to GM1. Lipid extract from the mutant NG‐CR72 cells, on the other hand, expectedly showed no Gal‐1‐binding consonant with the absence of a‐series gangliosides (GM1, GD1a)(Fig. 1a). Cell binding, documented by fluorescence microscopy, was rather low without enzyme treatment but significantly enhanced in the case of NG108‐15 cells by N'ase‐induced desialylation; this was also seen with the GM1‐specific probe Ctx‐B (Fig. 1b). Assessed semi‐quantitatively, low‐level binding of Gal‐1 to NG108‐15 cells was in part sensitive to trypsin treatment, pointing to some reactivity to glycoproteins or to relevance of (glyco)proteins as ganglioside‐presenting scaffold (Fig. 1c). In stark contrast, N'ase unmasked prominent binding sites, whose accessibility could be reduced by the presence of Ctx‐B or anti‐GM1 antibody (Fig. 1c). Tested as control, Ctx‐B binding to NG108‐15 cells was not sensitive to trypsinization but markedly increased upon making the GM1 pentasaccharide available; in comparison, Ctx‐B binding to NG‐CR72 cells was very low (Fig. 1c). Obviously, ganglioside GM1 is made available by N'ase treatment. It is the main binding partner of Gal‐1 in the NG108‐15 cells, prompting us to proceed to examine whether it is a functional counterreceptor. Toward this end, TRPC5‐mediated Ca2+ influx was tested.
Figure 1.
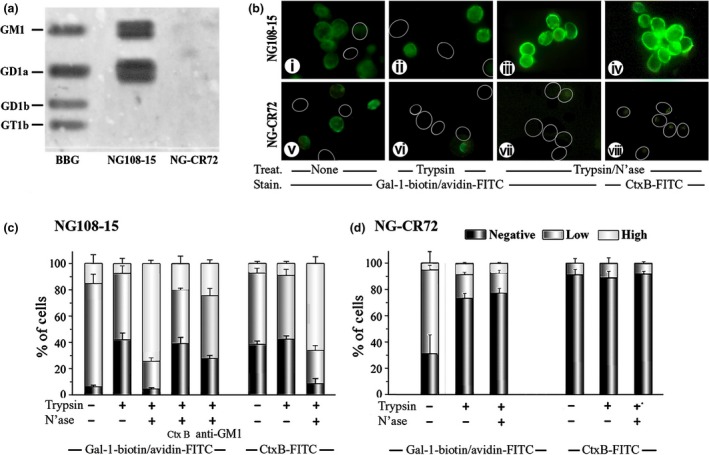
Binding of galectin‐1 (Gal‐1) to ganglioside GM1 and to cell surfaces. (a) Documentation of Gal‐1 binding to gangliosides after high‐performance thin‐layer chromatography (HPTLC). Lipids extracted from NG108‐15 and NG‐CR72 cells, together with bovine brain gangliosides (BBG), were separated by HPTLC. Following treatment with N'ase that converted di‐ and trisialylated gangliosides to GM1, the plate was successively probed with biotinylated Gal‐1 and avidin‐horseradish peroxidase (HRP) to demonstrate interaction of Gal‐1 with GM1; this also reveals the presence of a‐series gangliotetraoses in NG108‐15 but not NG‐CR72 cells. (b) Documentation of Gal‐1 binding to intact cells. NG108‐15 (i‐iv) and NG‐CR72 (v‐viii) cells treated with trypsin alone (ii and vi) or trypsin/N'ase (iii, iv, vii and viii) were stained with Gal‐1‐biotin/avidin‐FITC (i‐iii, v‐vii) and with Ctx‐B‐FITC (iv and viii). Circles = unstained cells. (c) Extent of Gal‐1 binding in semi‐quantitative categories. In addition to results in panel B, Ctx‐B and anti‐GM1 were tested as inhibitors of Gal‐1 binding to NG108‐15 cells.
Gal‐1 induces Ca2+ influx via TRPC5 channels
Using NG108‐15 cells, Gal‐1 was first shown to be without effect on [Ca2+]i if no Ca2+ was added to the medium (Fig. 2a). Mobilization of intracellular Ca2+ as response to Gal‐1 could thus be excluded. In the presence of 5 mM Ca2+ in the medium, a concentration‐dependent increase in extent of transient elevation of [Ca2+]i was measured (Fig. 2a). A mechanistic similarity was inferred for Gal‐1 and Ctx‐B by consecutive exposure of these cells to these two proteins and by recording a diminished response after the second treatment irrespective of the order of treatment (Fig. 2b). In contrast to the GM1‐presenting cells, the mutant cells were not responsive, in line with the concept of GM1 dependence (Fig. 2c). In order to exclude the possibility for impaired Ca2+ import, ATP, an inducer of TRPC5‐mediated Ca2+ influx (Shimizu et al. 2006), was tested and shown to trigger an amplitude of the same size as was typical for NG108‐15 cells and Gal‐1 treatment (Fig. 2c). Having herewith documented a Gal‐1‐dependent increase in [Ca2+]i, the hypothesis of its definitive relationship to TRPC5 channels was examined by silencing channel expression using shRNA.
Figure 2.
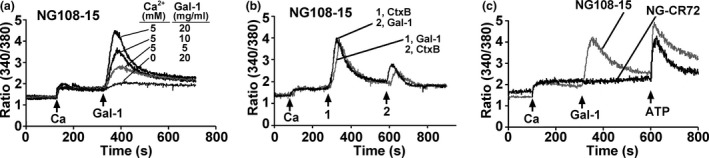
Induction of Ca2+ influx by galectin‐1 (Gal‐1)/GM1 interaction. NG108‐15 and NG‐CR72 cells were treated with N'ase and loaded with fura‐2 AM. They were suspended in Ca2+‐free physiological saline solution and used for [Ca2+]i measurements. Ca2+ (5 mM), Gal‐1, Ctx‐B and ATP were applied as indicated (arrows). (a) Dependence on Gal‐1 concentration and on the presence of extracellular Ca2+ indicating that Gal‐1 induced Ca2+ influx. (b) Comparison of Gal‐1 and Ctx‐B in triggering Ca2+ influx. (c) Response of GM1‐deficient NG‐CR72 cells to ATP but not Gal‐1.
Down‐regulation of respective expression levels was verified by RT‐PCR, IB and IC (Fig. 3a–c), and the transfected cells became unresponsive to Gal‐1 presence (Fig. 3d). The concept of mechanistic similarity of Ctx‐B and Gal‐1 as molecular effectors for transient [Ca2+]i increase was further pursued by determining the effect of the PLC blocker U73122 and the PI(3)K inhibitors LY294002 and wortmannin. All three compounds abolished the response to Gal‐1 (Fig. 3e). This reactivity intimated involvement of β1‐integrin‐dependent signaling and tyrosine phosphorylation.
Figure 3.
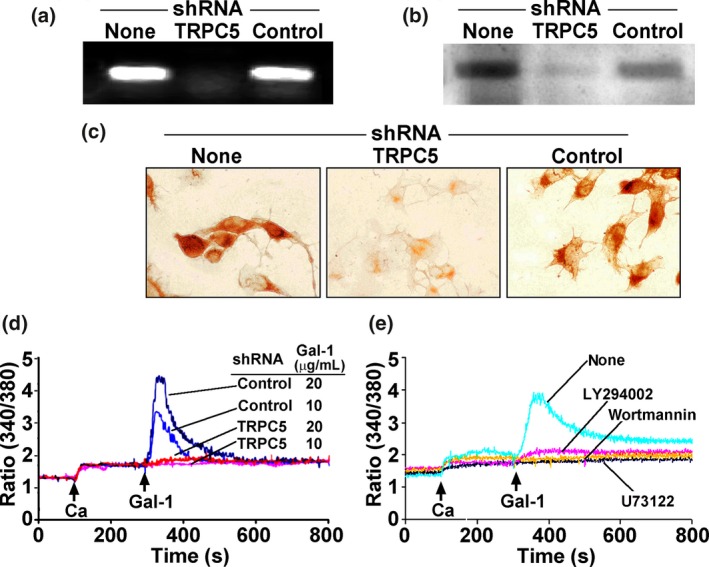
Involvement of TRPC5 in Ca2+ influx. NG108‐15 cells were transfected with TRPC5‐specific shRNA or control shRNA for 48 hr. Suppression of TRPC5 was demonstrated with RT‐PCR (a), immunoblotting (b), and immunocytochemical staining (c). (d) [Ca2+]i measurement showing inhibition of galectin‐1 (Gal‐1)‐induced [Ca2+]i elevation by TRPC5‐specific shRNA but not control shRNA. (e) Cells were treated with the phospholipase C (PLC) inhibitor U73122 (1 μM), the PI(3)K inhibitors LY294002 (5 μM) and wortmannin (2 μM), showing inhibition of Gal‐1‐induced Ca2+ response.
Activation of β1‐integrin signaling by Gal‐1
Using phosphotyrosine site‐specific antibodies (i.e. anti‐FAKpy397 and anti‐p85 PI(3)Kpy508) and the phosphotyrosine‐specific monoclonal antibody PT66 in combination with immunodetection of β1‐integrin and PLCγ respective, probing was performed. In each case, a Gal‐1‐dependent signal increase was detected (Fig. 4b). Fittingly, IC visualization of the phosphorylation was possible, together with Gal‐1 binding at sites of sprouting when incubating cells at 37°C (Fig. 4c). Of physiological relevance, similar data were obtained in primary cultures of CGNs treated with Gal‐1 at 2DIV (Fig. 5). These observations suggested a role of Gal‐1 in neuritogenesis.
Figure 4.
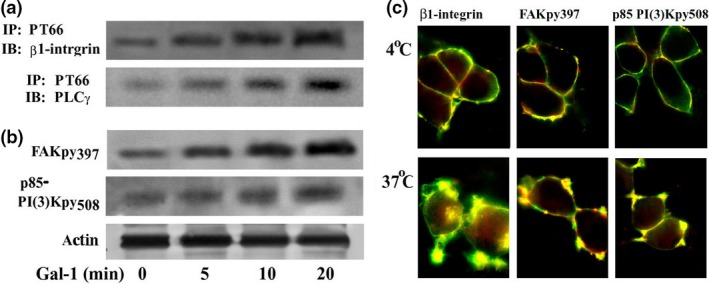
Activation of integrin signaling by galectin‐1 (Gal‐1) binding to NG108‐15 cells. (a) NG108‐15 cells treated with N'ase were incubated with Gal‐1 for up to 20 min. Lysate was immunoprecipitated using PT66 mAb for phosphorylated tyrosine and probed with antibody to β1‐integrin or phospholipase C (PLC)γ after blotting. (b) The same lysate was also directly blotted and probed with antibody to tyrosine‐phosphorylated focal adhesion kinase (FAK)py397, p85 PI(3)Kpy508, or actin (loading control). Increased tyrosine phosphorylation indicates activation of these proteins. (c) Cells were incubated with biotinylated Gal‐1 at 4 and 37°C for 30 min. After fixation, cells were stained with avidin‐FITC (green) together with antibodies to integrin, FAKpy397, or p85 PI(3)Kpy508, and a Texas red‐labeled antibody (red). The photomicrographs show co‐localization of Gal‐1 with these signaling proteins in membrane areas of sprouting after 37°C incubation.
Figure 5.
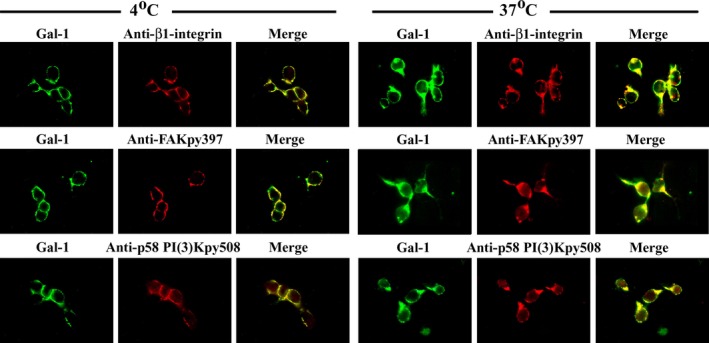
Activation of integrin signaling by galectin‐1 (Gal‐1)/GM1 interaction in primary cerebellar granule neuron (CGN)s. Primary cultures of CGNs were treated with Gal‐1 and then immunostained with the indicated antibodies at 2DIV, as in Fig. 4c. The results show co‐localization of Gal‐1 with β1‐integrin, focal adhesion kinase (FAK)py397 and p85 PI(3)Kpy508, in sprouting regions of the membrane after 37°C incubation.
Induction of axon‐like neurites by Gal‐1
To assess an impact of Gal‐1 on neuritogenesis, cells of NG108‐15 and its GM1‐deficient mutant were exposed to Gal‐1 in the presence of N'ase. Of note, the enzymatic desialylation already had a positive effect on the NG108‐15 cells, increasing the percentage of neurite‐bearing cells from 15.5% to 34.2% (Fig. 6a (i and ii) and b). No such effect was seen in the case of the mutant cells (Fig. 6 a (v and vi)). External presence of Gal‐1 led to a further increase in this parameter (to 49.4%), whereas mutant cells reacted with inhibition of cell proliferation but not neurite growth (Fig. 6 a (iii and vii)). Anti‐Gal‐1‐specific antibody blocked the effect, and the mutant cells were confirmed to be capable of neurite extension in response to retinoic acid treatment (Fig. 6 a (iv and viii)). Statistical significance was reached for differences upon treatment with N'ase, Gal‐1 and Ctx‐B, with the anti‐Gal‐1‐specific antibody as well as with the three signaling inhibitors and the TRP channel blocker SK&F96365 (Fig. 6b). Immunocytochemical monitoring showed neurites to be positive for axon‐specific but not dendrite‐specific markers (Fig. 6c). The functional correlation between TRPC5 presence and neurite outgrowth was further substantiated by testing transfected cells using shRNA (Fig. 6d).
Figure 6.
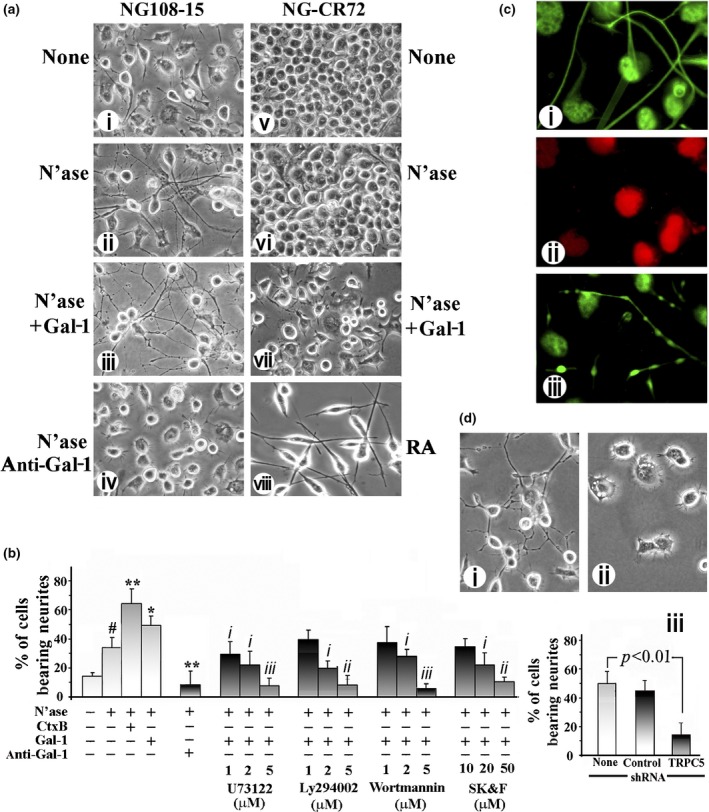
Induction of axon‐like neurites by galectin‐1 (Gal‐1) in NG108‐15 cells. (a) NG108‐15 (i–iv) and NG‐CR72 (v–viii) cells were treated with N'ase (ii and vi) or N'ase + Gal‐1 (iii and vii). NG108‐15 cells were also treated with N'ase + anti‐Gal‐1 antibody (iv), while NG‐CR72 cells were exposed to retinoic acid (RA) (viii). Morphology of neurite outgrowth was recorded 72 h later, revealing neuritogenesis in NG108‐15 cells induced by N'ase, its increase by Gal‐1 and inhibition by anti‐Gal‐1 antibody. NG‐CR72 cells failed to respond to these treatments, neurite growth induced by RA serving as positive control. (b) Quantification of neurite outgrowth in NG108‐15 cells. Data are mean ± SEM, n ≥ 3. Statistical significance was analyzed by Student's t‐test (two‐tailed), # p < 0.05, compared with cells with no treatment; * and **p < 0.05 and 0.01, respectively, compared with N'ase alone; i, ii and iii, p < 0.05, 0.01, and 0.001, respectively, inhibition compared with N'ase +Gal‐1. (c) Immunocharacterization of Gal‐1‐induced neurites, showing positivity for axonal markers pNF‐H (i) and SV2 (iii) and negativity for the dendritic marker MAP2 (ii). Panels i and ii are same field. (d) NG108‐15 cells transfected with TRPC5‐specific shRNA for 24 h were treated with N'ase + Gal‐1 for another 24 h. (i) and (ii) Neuritogenic morphology of cells treated with control shRNA and TRPC5‐specific shRNA, respectively. (iii) Quantitative analysis, showing inhibition of neurite growth by TRPC5‐specific shRNA. Data are mean ± SEM, n = 3. Statistical significance was analyzed by Student's t‐test (two‐tailed).
The respective correlation with GM1 presence was delineated by axonogenic experiments using primary cultures of CGN from 7‐day‐old normal and ganglio‐series KO mice. When applying Gal‐1 on 2DIV, before outgrowth of processes occurred, and monitoring formation of axons (longest process positive for pNF‐H) on 3DIV (24 h after starting the treatment), spontaneous growth of axons was stimulated (Fig. 7 a (i and iii) vs. Fig 7 a (ii and iv)). Average axon length increased from 199 ± 6.3 μm in controls to 303 ± 7.1 μm in Gal‐1‐treated cultures, comparable to the effect of Ctx‐B (at 286 ± 7.0 μm) (Fig. 7b and f). In contrast, spontaneous axon growth in cultures from the KO mice lacking GM1 was significantly lower (at 132 ± 3.4 μm) (Fig. 7a (v), c and f) and unaffected by Gal‐1 (Fig. 7a (vi) and c), suggesting critical importance of ganglioside GM1 in axonogenesis. As seen in the cell line, axon outgrowth was significantly reduced by signaling inhibitors (average length shortened to 92–123 μm) (Fig. 7d and f) and TRPC5 knockdown (average length shortened to 111 ± 4.0 μm for specific shRNA vs. 198 ± 5.6 μm for control shRNA) (Fig. 7a (vii) vs. Fig. 7a (viii), Fig. 7e and f). Of note, the effect of addition of a Gal‐1‐specific antibody also reached the level of statistical significance for the wild‐type but, expectedly, not mutant CGNs (Fig. 7f). This had similarly been seen in the cell systems (Fig. 6a (iv), b). This result indicates in situ presence of Gal‐1.
Figure 7.
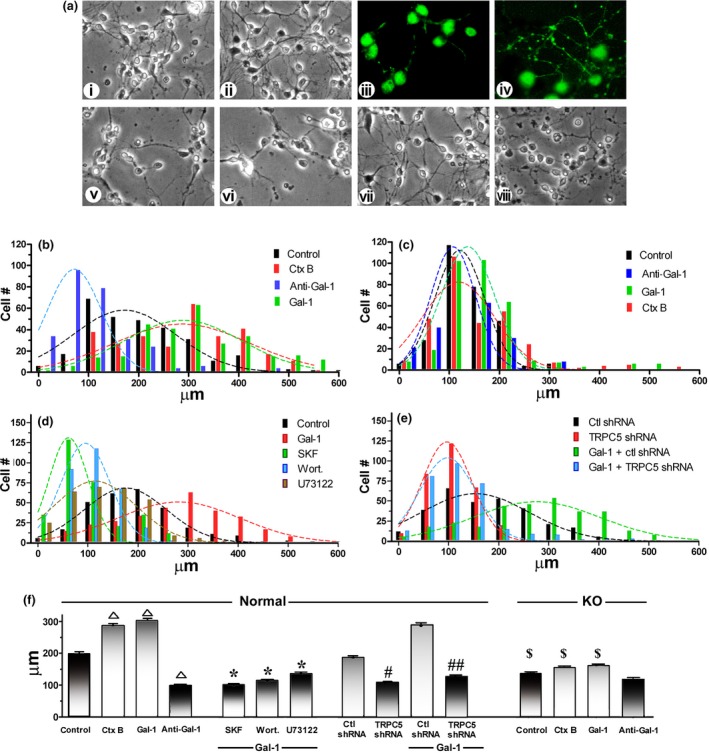
Effect of galectin‐1 (Gal‐1) on axon formation in primary cerebellar granule neuron (CGN) cultures. CGNs prepared from normal and GM1‐deficient (KO) mice were treated with Gal‐1 or Ctx‐B together with the indicated reagents at 2 DIV; some normal CGNs were transfected with TRPC5‐specific or control shRNA at 1 DIV. Axon growth was analyzed at 3 DIV. (a) Morphological comparison of normal (i–iv) and KO (v and vi) CGNs treated with Gal‐1 (ii, iv and vi) or without Gal‐1 (i, iii and v). (iii, iv) Immunocytostaining for the axonal marker pNF‐H. Gal‐1 accelerated axon growth in normal but not KO CGNs; in the latter cells, spontaneous axon growth was retarded, compared with normal cells (v vs. i). Axon formation was retarded in normal CGNs by TRPC5‐specific (viii) but not control (vii) shRNA. (b‐e) Axon length analysis via histograms with Gaussian curves. (f) Average axon length (± SEM) of each group. SKF = SK&F96365; wort = wortmannin; one‐way anova with Dunnett's post‐test was performed: Δp<0.01, compared with control (Gal‐1, Ctx‐B‐ untreated); *p < 0.01, compared with Gal‐1 treated; # p < 0.05, ## p < 0.01, compared with control (ctl) shRNA; $ p < 0.05, compared with normal CGNs with same treatment.
Presence of Gal‐1 in medium of NG108‐15 cells and CGNs in vitro and in vivo
Conditioned media of cell cultures were processed by IP, and the obtained material was probed by IB. The typical 14‐kDa band for the lectin was detected under reducing conditions, together with immunoreactive material in the high‐molecular‐weight range (Fig. 8a). When run under non‐reducing conditions, Gal‐1, which contains six sulfhydryl groups reactive for disulfide bridging, was exclusively detected in the high‐molecular‐weight range (not shown). The neuroblastoma cells (Fig. 8b) and cerebellar cells positive for GFAP (astrocytes) but not NeuN (neurons) (Fig. 8c) were stained for Gal‐1. In vivo, Gal‐1 was detected in the cerebellum of neonatal mice (8–10 days old), prominently in GFAP‐positive cells in the inner granular layer and white matter (Fig. 9a). Examining serial sections by pNF‐H staining detected delayed sprouting of axons (climbing fibers) from inner granular neurons in KO mice (Fig. 9b). In direct comparison, KO mice reached the stage of full extension of the climbing fibers of 9‐day‐old control mice after 10 days.
Figure 8.
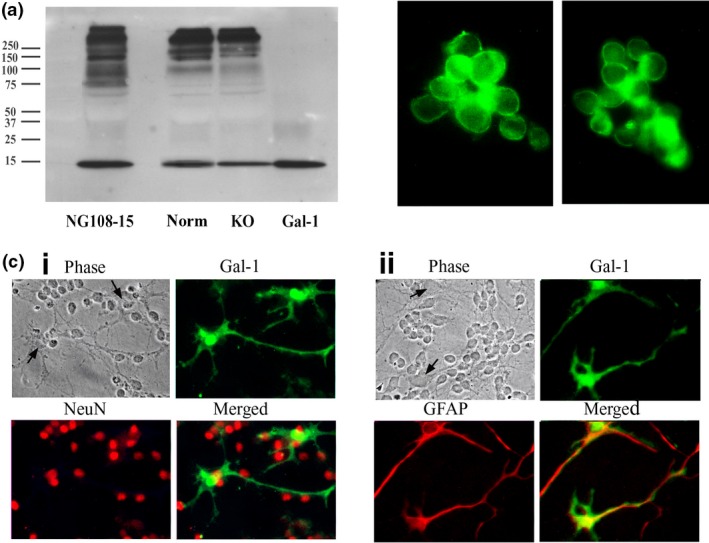
Detection of endogenous galectin‐1 (Gal‐1) in cultures. (a) Proteins precipitated from culture medium of NG108‐15 cells and cerebellar granule neuron (CGN) cultures of normal (Norm) and KO mice were separated via electrophoresis under reducing conditions, blotted and probed with anti‐Gal‐1 antibody, showing presence of Gal‐1 (14 kDi) in all samples. Gal‐1 = recombinant Gal‐1 standard. (b) Immunostaining of NG108‐15 cells with anti‐Gal‐1 antibody, showing presence of Gal‐1. (c) Immunostaining of Gal‐1 in CGN cultures, showing presence of Gal‐1 in glial fibrillary acidic protein (GFAP)‐positive astrocytes (ii), but not in NeuN‐positive neurons (i). Arrows in phase contrast images show bodies of astrocytes.
Figure 9.
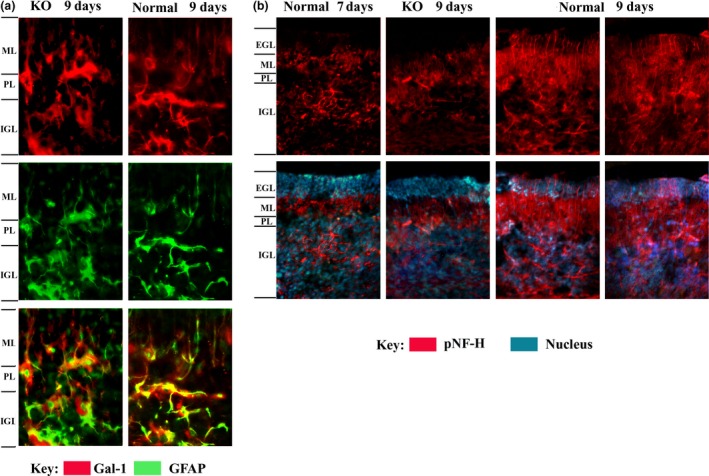
Comparison of galectin‐1 (Gal‐1) expression and axon growth in developing cerebellum of normal and KO mice (7–9 days old). (a) Presence of Gal‐1 in the region between Purkinje layer (PL) and inner granular layer (IGL) and between PL and molecular layer (ML). Gal‐1 is present in glial fibrillary acidic protein (GFAP)‐positive astrocytes in both 9‐day‐old normal and KO cerebellum. Amplification = 400x. (b) Extension of pNF‐H‐positive axons (mossy fibers) from IGL into extra granular layer (EGL). Axons grow through the PL and ML into the EGL from day 7 to 9 in normal cerebellum; growth is retarded in KO cerebellum. Amplification = 200x.
Discussion
Galectin‐1 and ganglioside GM1 had been independently implicated as effectors of neuronal differentiation, often in coordination with N'ase as effector for GM1 elevation. The lectin was shown to enhance axonal regeneration following peripheral nerve transection, such regrowth being blocked in the presence of anti‐Gal‐1 antibody (Horie et al. 1999). Axon regeneration was also observed, when a defect in synthesis of another glycan ligand for Gal‐1, i.e. N‐acetyllactosamine repeats (Merkle and Cummings 1988), was ameliorated (Henion et al. 2005). As counterreceptor for the α5β1‐integrin, Gal‐1 is known to exert α5β1‐integrin‐dependent growth control in carcinoma cells via caspase‐8 or the expression of the cyclin‐dependent kinase inhibitor p27 (Fischer et al. 2005; Sanchez‐Ruderisch et al. 2011; Amano et al. 2012). Interestingly, presence of Gal‐3, another family member with structurally different molecular design, blocks this activity by competition for binding, as is the case for neuroblastoma cells (Kopitz et al. 2001; Sanchez‐Ruderisch et al. 2010).
As for GM1, several studies have implicated its involvement in neurite/axon extension (Ledeen and Wu 2015). An early example was prolific neurite outgrowth resulting from GM1 elevation by N'ase application to Neuro2A cells (Wu and Ledeen 1991), Ca2+ influx functioning as a necessary concomitant to axonal—as opposed to dendritic—outgrowth in that system (Wu et al. 1998). Endogenous ganglioside‐specific N'ase was shown to be up‐regulated during Neuro2A cell differentiation (Hasegawa et al. 2000), thereby indicating an intrinsic mechanism for neuronal elevation of GM1. Similar up‐regulation of N'ase had been observed during developmental axonal growth in hippocampal neurons (Abad‐Rodríguez et al. 2001), while axonal specification depended on accumulation of plasma membrane ganglioside‐specific N'ase (Neu3 sialidase) at the tip of the destined neurite (Da Silva et al. 2005). Neu3 activation that accompanied successful regeneration of injured peripheral nerve resulted from activation of p38 MAPK, a reaction that did not occur in retinal axons; that was suggested as the rationale for well‐known failure of CNS axons to regenerate in contrast to the facile regeneration of PNS axons (Kappagantula et al. 2014).
The present data provide a connecting link between these two lines by defining Gal‐1 as elicitor of axonal neuritogenesis dependent on cross‐linking of ganglioside GM1. Testing cell models with GM1 deficiency, Ctx‐B in consecutive incubation steps and anti‐Gal‐1 antibodies substantiated the operativeness of a functional lectin–glycan recognition. It initiated signaling, eventually transiently opening TRPC5 channels and increasing [Ca2+]i. Considering the growing implication of N'ase in axon regeneration after damage (Abad‐Rodríguez et al. 2001; Mountney et al. 2010; Kappagantula et al. 2014), the negative impact of the interaction of GD1a and GT1b with siglec‐4 can be assumed to be switched off by GD1a and GT1b conversion to GM1, while regeneration capacity is then switched on via the Gal‐1/GM1 route. This lectin's cross‐linking capacity, when considered from a therapeutic perspective, can be increased by protein engineering, turning the homodimer into a covalently connected bivalent (tandem‐repeat‐type) variant (Zhang et al. 2015). In view of the functional analogy between Ctx‐B and Gal‐1 in this respect, it is essential to set limits to extrapolations from data with exogenous (bacterial) compounds and to emphasize the apparent significance of the topology of lectin‐counterreceptor aggregates for functional activity (Gabius et al. 2011, 2015). Despite sharing high‐affinity binding to neuroblastoma (SK‐N‐MC) cells and the relevance of microdomain integrity, Ctx‐B was not a negative growth regulator for these cells as Gal‐1 and other homodimeric galectins are (Kopitz et al. 2001, 2010, 2012; André et al. 2005).
Equally important to be noted in the physiological context, GM1 has been implicated in another neuritogenic mechanism. The glycoprotein laminin‐1 here serves as binding partner, and translocation of β1‐integrin in lipid rafts with colocalization with TrkA and induction of MAPK signaling were reported (Ichikawa et al. 2009). Because Gal‐1 is known to be able to bind to glycans of laminin (Zhou and Cummings 1993; Ohannesian et al. 1994, 1995; André et al. 1999), it is an open question whether the lectin may be a part of this molecular interplay. Also, examining the possibility of TRPC5 channel activation and the nature of induced neurites is of interest in further delineating post‐binding events.
It should be noted that Ca2+ influx via TRPC5 channels, triggered by Gal‐1 cross‐linking of integrin‐associated GM1, is likely restricted to the initial phase of axon outgrowth, consistent with the abundant presence of TRPC5 only at an early stage of neuronal differentiation (Wu et al. 2007). This puts in context the earlier observation that Ctx‐B induced Ca2+ influx in CGNs only during the first 7 days in culture, after which Ctx‐B proved inhibitory (Wu et al. 1996). Whereas this TRPC5‐mediated elevation of [Ca2+]i depended on prior elevation of membrane GM1, the latter in turn arose from activation or up‐regulation of plasma membrane ganglioside N'ase. This in turn may have been mediated by another Ca2+ effect, such as that required for regeneration of PNS axons (Kappagantula et al. 2014).
In a broader context, the dynamic remodeling of gangliosides can be functionally relevant along this pathway also for other cell types. Considering the impact of Ctx‐B and GM1 on [Ca2+]i in rat lymphocytes and human T‐lymphocyte (Jurkat) cells (Dixon et al. 1987; Gouy et al. 1994), the concomitant up‐regulation of GM1 presentation by effector T cells and Gal‐1 expression in regulatory T cells upon activation engenders a mechanism of suppressing autoimmune disease state (Wang et al. 2009). If a defect in GM1 generation lets effector T cells evade the regulatory control by Gal‐1, as is the case in an animal model of type I diabetes (Wu et al. 2011), then corresponding therapeutic approaches could restore the intercellular communication.
This evidence for a functional antagonism and the expression of several galectins, as documented for various types of malignancies including brain tumors (Camby et al. 2001; Dawson et al. 2013; Katzenmaier et al. 2014), thus gives direction to a histochemical and functional monitoring of these lectins in situ/in vitro. Of note, respective work has revealed expression of Gal‐4, a tandem‐repeat‐type protein, in cortical and hippocampal neurons, where its positive influence on axon growth is attributed, at least in part, to its role in glycoprotein (L1) routing and clustering (Velasco et al. 2013; Abad‐Rodríguez and Díez‐Revuelta 2015). Put in perspective, these results together with the presented data indicate the potential of these lectins to modulate axon growth along different pathways, warranting further study.
Acknowledgments and conflict of interest disclosure
This study was generously supported by an EC grant (GLYCOPHARM) and by an NIH grant 2 RO1 NS33912 (RWL). Inspiring input by our friend Dr J. Abad‐Rodríguez is gratefully acknowledged.
All experiments were conducted in compliance with the ARRIVE guidelines. The authors have no conflict of interest to declare.
References
- Abad‐Rodríguez J. and Díez‐Revuelta N. (2015) Axon glycoprotein routing in nerve polarity, function, and repair. Trends Biochem. Sci. 40, 385–396. [DOI] [PubMed] [Google Scholar]
- Abad‐Rodríguez J., Piddini E., Hasegawa H., Miyagi T. and Dotti C. G. (2001) Plasma membrane ganglioside sialidase regulates axonal growth and regeneration in hippocampal neurons in culture. J. Neurosci. 21, 8387–8395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano M., Eriksson H., Manning J. C., Detjen K. M., André S., Nishimura S.‐I., Lehtiö J. and Gabius H.‐J. (2012) Tumour suppressor p16INK4a: anoikis‐favouring decrease in N/O‐glycan/cell surface sialylation by down‐regulation of enzymes in sialic acid biosynthesis in tandem in a pancreatic carcinoma model. FEBS J. 279, 4062–4080. [DOI] [PubMed] [Google Scholar]
- Ando S. (1983) Gangliosides in the nervous system. Neurochem. Int. 5, 507–537. [DOI] [PubMed] [Google Scholar]
- André S., Kojima S., Yamazaki N., Fink C., Kaltner H., Kayser K. and Gabius H.‐J. (1999) Galectins‐1 and ‐3 and their ligands in tumor biology. J. Cancer Res. Clin. Oncol. 125, 461–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- André S., Liu B., Gabius H.‐J. and Roy R. (2003) First demonstration of differential inhibition of lectin binding by synthetic tri‐ and tetravalent glycoclusters from cross‐coupling of rigidified 2‐propynyl lactoside. Org. Biomol. Chem. 1, 3909–3916. [DOI] [PubMed] [Google Scholar]
- André S., Kaltner H., Lensch M. et al (2005) Determination of structural and functional overlap/divergence of five proto‐type galectins by analysis of the growth‐regulatory interaction with ganglioside GM1 in silico and in vitro on human neuroblastoma cells. Int. J. Cancer 114, 46–57. [DOI] [PubMed] [Google Scholar]
- André S., Sanchez‐Ruderisch H., Nakagawa H. et al (2007) Tumor suppressor p16INK4a: modulator of glycomic profile and galectin‐1 expression to increase susceptibility to carbohydrate‐dependent induction of anoikis in pancreatic carcinoma cells. FEBS J. 274, 3233–3256. [DOI] [PubMed] [Google Scholar]
- André S., Kaltner H., Manning J. C., Murphy P. V. and Gabius H.‐J. (2015) Lectins: getting familiar with translators of the sugar code. Molecules 20, 1788–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardosi A., Bardosi L., Lindenblatt R. and Gabius H.‐J. (1990) Detection and mapping of endogenous receptors for carrier‐immoblized constituents of glycoconjugates (lectins) by labelled (neo)glycoproteins and by affinity chromatography in human adult mesencephalon, pons, medulla oblongata and cerebellum. Histochemistry 94, 285‐291. [DOI] [PubMed] [Google Scholar]
- Camby I., Belot N., Rorive S. et al (2001) Galectins are differentially expressed in supratentorial pilocytic astrocytomas, astrocytomas, anaplastic astrocytomas and glioblastomas, and significantly modulate tumor astrocyte migration. Brain Pathol. 11, 12–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson R. O., Masco D., Brooker G. and Spiegel S. (1994) Endogenous ganglioside GM1 modulates L‐type calcium channel activity in N18 neuroblastoma cells. J. Neurosci. 14, 2272–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins B. E., Yang L. J., Mukhopadhyay G., Filbin M. T., Kiso M., Hasegawa A. and Schnaar R. L. (1997) Sialic acid specificity of myelin‐associated glycoprotein binding. J. Biol. Chem. 272, 1248–1255. [DOI] [PubMed] [Google Scholar]
- Da Silva J. S., Hasegawa T., Miyagi T., Dotti C. G. and Abad‐Rodríguez J. (2005) Asymmetric membrane ganglioside sialidase activity specifies axonal fate. Nat. Neurosci. 8, 606–615. [DOI] [PubMed] [Google Scholar]
- Dawson H., André S., Karamitopoulou E., Zlobec I. and Gabius H.‐J. (2013) The growing galectin network in colon cancer and clinical relevance of cytoplasmic galectin‐3 reactivity. Anticancer Res. 33, 3053–3059. [PubMed] [Google Scholar]
- Dixon S. J., Stewart D., Grinstein S. and Spiegel S. (1987) Transmembrane signaling by the B subunit of cholera toxin: increased cytoplasmic free calcium in rat lymphocytes. J. Cell Biol. 105, 1153–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbarth G. S., Riffolo R. R. J., Walsh F. S. and Nirenberg M. (1978) Lactose‐sensitive lectin of chick retina and spinal cord. Biochem. Biophys. Res. Commun. 83, 1246–1252. [DOI] [PubMed] [Google Scholar]
- Fang Y., Wu G., Xie X., Lu Z. H. and Ledeen R. W. (2000) Endogenous GM1 ganglioside of the plasma membrane promotes neuritogenesis by two mechanisms. Neurochem. Res. 25, 931–940. [DOI] [PubMed] [Google Scholar]
- Fang Y., Xie X., Ledeen R. W. and Wu G. (2002) Characterization of cholera toxin B subunit‐induced Ca2+ influx in neuroblastoma cells: evidence for a voltage‐independent GM1 ganglioside‐associated Ca2+ channel. J. Neurosci. Res. 69, 669–680. [DOI] [PubMed] [Google Scholar]
- Fischer C., Sanchez‐Ruderisch H., Welzel M. et al (2005) Galectin‐1 interacts with the α5β1 fibronectin receptor to restrict carcinoma cell growth via induction of p21 and p27. J. Biol. Chem. 280, 37266–37277. [DOI] [PubMed] [Google Scholar]
- Gabius H.‐J., ed. (2009) The Sugar Code. Fundamentals of Glycosciences. Wiley‐VCH, Weinheim, Germany. [Google Scholar]
- Gabius H.‐J. (2015) The magic of the sugar code. Trends Biochem. Sci. 40, 341. [DOI] [PubMed] [Google Scholar]
- Gabius H.‐J., Engelhardt R., Rehm S. and Cramer F. (1984) Biochemical characterization of endogenous carbohydrate‐binding proteins from spontaneous murine rhabdomyosarcoma, mammary adenocarcinoma, and ovarian teratoma. J. Natl Cancer Inst. 73, 1349–1357. [PubMed] [Google Scholar]
- Gabius H.‐J., Kohnke B., Hellmann T., Dimitri T. and Bardosi A. (1988) Comparative histochemical and biochemical analysis of endogenous receptors for glycoproteins in human and pig nerve. J. Neurochem. 51, 756–763. [DOI] [PubMed] [Google Scholar]
- Gabius H.‐J., Walzel H., Joshi S. S., Kruip J., Kojima S., Gerke V., Kratzin H. and Gabius S. (1992) The immunomodulatory β‐galactoside‐specific lectin from mistletoe: partial sequence analysis, cell and tissue binding and impact on intracellular biosignaling of monocytic leukemia cells. Anticancer Res. 12, 669–676. [PubMed] [Google Scholar]
- Gabius H.‐J., André S., Jiménez‐Barbero J., Romero A. and Solís D. (2011) From lectin structure to functional glycomics: principles of the sugar code. Trends Biochem. Sci. 36, 298–313. [DOI] [PubMed] [Google Scholar]
- Gabius H.‐J., Kaltner H., Kopitz J. and André S. (2015) The glycobiology of the CD system: a dictionary for translating marker designations into glycan/lectin structure and function. Trends Biochem. Sci. 40, 360–376. [DOI] [PubMed] [Google Scholar]
- Gouy H., Deterre P., Debre P. and Bismuth G. (1994) Cell calcium signaling via GM1 cell surface gangliosides in the human Jurkat T cell line. J. Immunol. 152, 3271–3281. [PubMed] [Google Scholar]
- Hasegawa T., Yamaguchi K., Wada T., Takeda A., Itoyama Y. and Miyagi T. (2000) Molecular cloning of mouse ganglioside sialidase and its increased expression in Neuro2a cell differentiation. J. Biol. Chem. 275, 8007–8015. [DOI] [PubMed] [Google Scholar]
- Henion T. R., Raitcheva D., Grosholz R., Biellmann F., Skarnes W. C., Hennet T. and Schwarting G. A. (2005) β1,3‐N‐Acetylglucosaminyltransferase 1 glycosylation is required for axon pathfinding by olfactory sensory neurons. J. Neurosci. 25, 1894–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirabayashi J., ed. (1997) Recent topics on galectins. Trends Glycosci. Glycotechnol. 9, 1–180. [Google Scholar]
- Horie H., Inagaki Y., Sohma Y. et al (1999) Galectin‐1 regulates initial axonal growth in peripheral nerves after axotomy. J. Neurosci. 19, 9964–9974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa N., Iwabuchi K., Kurihara H. et al (2009) Binding of laminin‐1 to monosialoganglioside GM1 in lipid rafts is crucial for neurite outgrowth. J. Cell Sci. 122, 289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltner H. and Gabius H.‐J. (2012) A toolbox of lectins for translating the sugar code: the galectin network in phylogenesis and tumors. Histol. Histopathol. 27, 397–416. [DOI] [PubMed] [Google Scholar]
- Kaltner H., Seyrek K., Heck A., Sinowatz F. and Gabius H.‐J. (2002) Galectin‐1 and galectin‐3 in fetal development of bovine respiratory and digestive tracts. Comparison of cell type‐specific expression profiles and subcellular localization. Cell Tissue Res. 307, 35–46. [DOI] [PubMed] [Google Scholar]
- Kaltner H., Kübler D., López‐Merino L. et al (2011) Toward comprehensive analysis of the galectin network in chicken: unique diversity of galectin‐3 and comparison of its localization profile in organs of adult animals to the other four members of this lectin family. Anat. Rec. 294, 427–444. [DOI] [PubMed] [Google Scholar]
- Kappagantula S., Andrews M. R., Cheah M., Abad‐Rodríguez J., Dotti C. G. and Fawcett J. W. (2014) Neu3 sialidase‐mediated ganglioside conversion is necessary for axon regeneration and is blocked in CNS axons. J. Neurosci. 34, 2477–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenmaier E.‐M., André S., Kopitz J. and Gabius H.‐J. (2014) Impact of sodium butyrate on the network of adhesion/growth‐regulatory galectins in human colon cancer in vitro . Anticancer Res. 34, 5429–5438. [PubMed] [Google Scholar]
- Kobiler D., Beyer E. C. and Barondes S. H. (1978) Developmentally regulated lectins from chick muscle, brain and liver have similar chemical and immunological properties. Dev. Biol. 64, 265–272. [DOI] [PubMed] [Google Scholar]
- Kopitz J., von Reitzenstein C., Mühl C. and Cantz M. (1994) Role of plasma membrane ganglioside sialidase of human neuroblastoma cells in growth control and differentiation. Biochem. Biophys. Res. Commun. 199, 1188–1193. [DOI] [PubMed] [Google Scholar]
- Kopitz J., von Reitzenstein C., Burchert M., Cantz M. and Gabius H.‐J. (1998) Galectin‐1 is a major receptor for ganglioside GM1, a product of the growth‐controlling activity of a cell surface ganglioside sialidase, on human neuroblastoma cells in culture. J. Biol. Chem. 273, 11205–11211. [DOI] [PubMed] [Google Scholar]
- Kopitz J., von Reitzenstein C., André S., Kaltner H., Uhl J., Ehemann V., Cantz M. and Gabius H.‐J. (2001) Negative regulation of neuroblastoma cell growth by carbohydrate‐dependent surface binding of galectin‐1 and functional divergence from galectin‐3. J. Biol. Chem. 276, 35917–35923. [DOI] [PubMed] [Google Scholar]
- Kopitz J., Russwurm R., Kaltner H., André S., Dotti C. G., Gabius H.‐J. and Abad‐Rodríguez J. (2004) Hippocampal neurons and recombinant galectins as tools for systematic carbohydrate structure‐function studies in neuronal differentiation. Dev. Brain Res. 153, 189–196. [DOI] [PubMed] [Google Scholar]
- Kopitz J., Bergmann M. and Gabius H.‐J. (2010) How adhesion/growth‐regulatory galectins‐1 and ‐3 attain cell specificity: case study defining their target on neuroblastoma cells (SK‐N‐MC) and marked affinity regulation by affecting microdomain organization of the membrane. IUBMB Life 62, 624–628. [DOI] [PubMed] [Google Scholar]
- Kopitz J., Ballikaya S., André S. and Gabius H.‐J. (2012) Ganglioside GM1/galectin‐dependent growth regulation in human neuroblastoma cells: special properties of bivalent galectin‐4 and significance of linker length for ligand selection. Neurochem. Res. 37, 1267–1276. [DOI] [PubMed] [Google Scholar]
- Kuchler S., Joubert R., Avellana‐Adalid V., Caron M., Bladier D., Vincendon G. and Zanetta J.‐P. (1989) Immunohistochemical localization of a beta‐galactoside‐binding lectin in rat central nervous system. II. Light‐ and electron‐microscopical studies in developing cerebellum. Dev. Neurosci. 11, 414–427. [DOI] [PubMed] [Google Scholar]
- Kuchler S., Zanetta J.‐P., Vincendon G. and Gabius H.‐J. (1990) Detection of binding sites for biotinylated neoglycoproteins and heparin (endogenous lectins) during cerebellar ontogenesis in the rat. Eur. J. Cell Biol. 52, 87–97. [PubMed] [Google Scholar]
- Ledeen R. W. and Wu G. (2009) Neurobiology meets glycosciences, in The Sugar Code. Fundamentals of glycosciences (Gabius H.‐J., ed.), pp. 495–516. Wiley‐VCH, Weinheim, Germany. [Google Scholar]
- Ledeen R. W. and Wu G. (2015) The multi‐tasked life of GM1 ganglioside, a true factotum of nature. Trends Biochem. Sci. 40, 407–418. [DOI] [PubMed] [Google Scholar]
- Ledeen R. W., Wu G. S., Lu Z.‐H., Kozireski‐Chubak D. and Fang Y. (1998) The role of GM1 and other gangliosides in neuronal differentiation ‐ overview and new findings. Ann. NY Acad. Sci. 845, 341–348. [DOI] [PubMed] [Google Scholar]
- Majewski J., André S., Jones E., Chi E. and Gabius H.‐J. (2015) X‐ray reflectivity and grazing incidence diffraction studies of interaction between human adhesion/growth‐regulatory galectin‐1 and DPPE:GM1 lipid monolayer at the air/water interface. Biochemistry (Moscow) 80, 943–956. [DOI] [PubMed] [Google Scholar]
- Merkle R. K. and Cummings R. D. (1988) Asparagine‐linked oligosaccharides containing poly‐N‐acetyllactosamine chains are preferentially bound by immobilized calf heart agglutinin. J. Biol. Chem. 263, 16143–16149. [PubMed] [Google Scholar]
- Milani D., Minozzi M. C., Petrelli L., Guidolin D., Skaper S. D. and Spoerri P. E. (1992) Interaction of ganglioside GM1 with the B subunit of cholera toxin modulates intracellular free calcium in sensory neurons. J. Neurosci. Res. 33, 466–475. [DOI] [PubMed] [Google Scholar]
- Mountney A., Zahner M. R., Lorenzini I., Oudega M., Schramm L. P. and Schnaar R. L. (2010) Sialidase enhances recovery from spinal cord contusion injury. Proc. Natl Acad. Sci. USA 107, 11561–11566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohannesian D. W., Lotan D. and Lotan R. (1994) Concomitant increases in galectin‐1 and its glycoconjugate ligands (carcinoembryonic antigen, lamp‐1, and lamp‐2) in cultured human colon carcinoma cells by sodium butyrate. Cancer Res. 54, 5992–6000. [PubMed] [Google Scholar]
- Ohannesian D. W., Lotan D., Thomas P., Jessup J. M., Fukuda M., Gabius H.‐J. and Lotan R. (1995) Carcinoembryonic antigen and other glycoconjugates act as ligands for galectin‐3 in human colon carcinoma cells. Cancer Res. 55, 2191–2199. [PubMed] [Google Scholar]
- Pitto M., Chigorno V., Giglioni A., Valsecchi M. and Tettamanti G. (1989) Sialidase in cerebellar granule cells differentiating in culture. J. Neurochem. 53, 1464–1470. [DOI] [PubMed] [Google Scholar]
- Prinetti A., Chigorno V., Mauri L., Loberto N. and Sonnino S. (2007) Modulation of cell functions by glycosphingolipid metabolic remodeling in the plasma membrane. J. Neurochem. 103(Suppl 1), 113–125. [DOI] [PubMed] [Google Scholar]
- Proshin S., Yamaguchi K., Wada T. and Miyagi T. (2002) Modulation of neuritogenesis by ganglioside‐specific sialidase (Neu 3) in human neuroblastoma NB‐1 cells. Neurochem. Res. 27, 841–846. [DOI] [PubMed] [Google Scholar]
- Quarles R. H. (2007) Myelin‐associated glycoprotein (MAG): past, present and beyond. J. Neurochem. 100, 1431–1448. [DOI] [PubMed] [Google Scholar]
- Regan L. J., Dodd J., Barondes S. H. and Jessell T. M. (1986) Selective expression of endogenous lactose‐binding lectins and lactoseries glycoconjugates in subsets of rat sensory neurons. Proc. Natl. Acad. Sci. USA 83, 2248–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez‐Ruderisch H., Fischer C., Detjen K. M., Welzel M., Wimmel A., Manning J. C., André S. and Gabius H.‐J. (2010) Tumor suppressor p16INK4a: downregulation of galectin‐3, an endogenous competitor of the pro‐anoikis effector galectin‐1, in a pancreatic carcinoma model. FEBS J. 277, 3552–3563. [DOI] [PubMed] [Google Scholar]
- Sanchez‐Ruderisch H., Detjen K. M., Welzel M., André S., Fischer C., Gabius H.‐J. and Rosewicz S. (2011) Galectin‐1 sensitizes carcinoma cells to anoikis via the fibronectin receptor α5β1‐integrin. Cell Death Differ. 18, 806–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schengrund C. L. (2015) Gangliosides: glycosphingolipids essential for normal neural development and function. Trends Biochem. Sci. 40, 397–406. [DOI] [PubMed] [Google Scholar]
- Shimizu S., Yoshida T., Wakamori M. et al (2006) Ca2+ ‐calmodulin‐dependent myosin light chain kinase is essential for activation of TRPC5 channels expressed in HEK293 cells. J. Physiol. 570, 219–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert H.‐C., André S., Lu S.‐Y. et al (2003) Unique conformer selection of human growth‐regulatory lectin galectin‐1 for ganglioside GM1 versus bacterial toxins. Biochemistry 42, 14762–14773. [DOI] [PubMed] [Google Scholar]
- Simpson D. L., Thorne D. R. and Loh H. H. (1977) Developmentally regulated lectin in neonatal rat brain. Nature 266, 367–369. [DOI] [PubMed] [Google Scholar]
- Solís D., Bovin N. V., Davis A. P., Jiménez‐Barbero J., Romero A., Roy R., Smetana K., Jr and Gabius H.‐J. (2015) A guide into glycosciences: how chemistry, biochemistry and biology cooperate to crack the sugar code. Biochim. Biophys. Acta 1850, 186–235. [DOI] [PubMed] [Google Scholar]
- Sturgill E. R., Aoki K., Lopez P. H. et al (2012) Biosynthesis of the major brain gangliosides GD1a and GT1b. Glycobiology 22, 1289–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichberg V. I., Silman I., Beitsch D. D. and Resheff G. (1975) A β‐d‐galactoside binding protein from electric organ tissue of Electrophorus electricus . Proc. Natl. Acad. Sci. USA 72, 1383–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco S., Díez‐Revuelta N., Hernández‐Iglesias T., Kaltner H., André S., Gabius H.‐J. and Abad‐Rodríguez J. (2013) Neuronal galectin‐4 is required for axon growth and for the organization of axonal membrane L1 delivery and clustering. J. Neurochem. 125, 49–62. [DOI] [PubMed] [Google Scholar]
- Vyas A. A., Blixt O., Paulson J. C. and Schnaar R. L. (2005) Potent glycan inhibitors of myelin‐associated glycoprotein enhance axon outgrowth in vitro. J. Biol. Chem. 280, 16305–16310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Lu Z.‐H., Gabius H.‐J., Rohowsky‐Kochan C., Ledeen R. W. and Wu G. (2009) Cross‐linking of GM1 ganglioside by galectin‐1 mediates regulatory T cell activity involving TRPC5 channel activation: possible role in suppressing experimental autoimmune encephalomyelitis. J. Immunol. 182, 4036–4045. [DOI] [PubMed] [Google Scholar]
- Wu G. and Ledeen R. W. (1991) Stimulation of neurite outgrowth in neuroblastoma cells by neuraminidase: putative role of GM1 ganglioside in differentiation. J. Neurochem. 56, 95–104. [DOI] [PubMed] [Google Scholar]
- Wu G., Lu Z.‐H., Nakamura K., Spray D. C. and Ledeen R. W. (1996) Trophic effect of cholera toxin B subunit in cultured cerebellar granule neurons: modulation of intracellular calcium by GM1 ganglioside. J. Neurosci. Res. 44, 243–254. [DOI] [PubMed] [Google Scholar]
- Wu G., Fang Y., Lu Z.‐H. and Ledeen R. W. (1998) Induction of axon‐like and dendrite‐like processes in neuroblastoma cells. J. Neurocytol. 27, 1–14. [DOI] [PubMed] [Google Scholar]
- Wu G., Lu Z.‐H., Xie X., Li L. and Ledeen R. W. (2001) Mutant NG108‐15 cells (NG‐CR72) deficient in GM1 synthase respond aberrantly to axonogenic stimuli and are vulnerable to calcium‐induced apoptosis: they are rescued with LIGA‐20. J. Neurochem. 76, 690–702. [DOI] [PubMed] [Google Scholar]
- Wu G., Lu Z.‐H., Xie X. and Ledeen R. W. (2004) Susceptibility of cerebellar granule neurons from GM2/GD2 synthase‐null mice to apoptosis induced by glutamate excitotoxicity and elevated KCl: rescue by GM1 and LIGA20. Glycoconj. J. 21, 305–313. [DOI] [PubMed] [Google Scholar]
- Wu G., Lu Z.‐H., Obukhov A. G., Nowycky M. C. and Ledeen R. W. (2007) Induction of calcium influx through TRPC5 channels by cross‐linking of GM1 ganglioside associated with α5β1 integrin initiates neurite outgrowth. J. Neurosci. 27, 7447–7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G., Lu Z.‐H., Gabius H.‐J., Ledeen R. W. and Bleich D. (2011) Ganglioside GM1 deficiency in effector T cells from NOD mice induces resistance to regulatory T cell suppression. Diabetes 60, 2341–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R. K., Ariga T., Yanagisawa M. and Zeng G. (2008) Ganglioside biosynthesis and degradation in the nervous system in Glycoscience (Fraser‐Reid B. O., Tatsuta K. and Thiem J., eds), pp. 1671–1695. Springer, Berlin‐Heidelberg, Germany. [Google Scholar]
- Zhang S., Moussodia R.‐O., Murzeau C. et al (2015) Dissecting molecular aspects of cell interactions using glycodendrimersomes with programmable glycan presentation and engineered human lectins. Angew. Chem. Int. Ed. 54, 4036–4040. [DOI] [PubMed] [Google Scholar]
- Zhou Q. and Cummings R. D. (1993) L‐14 lectin recognition of laminin and its promotion of in vitro cell adhesion. Arch. Biochem. Biophys. 300, 6–17. [DOI] [PubMed] [Google Scholar]


