Abstract
Cell-cell communication is critical to coordinate the activity and behavior of a multicellular organism. The cells of the immune system not only must communicate with similar cells, but also with many other cell types in the body. Therefore, the cells of the immune system have evolved multiple ways to communicate. Exosomes and tunneling nanotubes (TNTs) are two means of communication used by immune cells that contribute to immune functions. Exosomes are small membrane vesicles secreted by most cell types that can mediate intercellular communication and in the immune system they are proposed to play a role in antigen presentation and modulation of gene expression. TNTs are membranous structures that mediate direct cell-cell contact over several cell diameters in length (and possibly longer) and facilitate the interaction and/or the transfer of signals, material and other cellular organelles between connected cells. Recent studies have revealed additional, but sometimes conflicting, structural and functional features of both exosomes and TNTs. Despite the new and exciting information in exosome and TNT composition, origin and in vitro function, biologically significant functions are still being investigated and determined. In this review, we discuss the current field regarding exosomes and TNTs in immune cells providing evaluation and perspectives of the current literature.
Keywords: Tunneling nanotubes, cell-cell communication, multivesicular bodies, exosomes
INTRODUCTION
Everyday humans use a variety of means of communication that are critical for our survival and our ability to function as a community. Analogous to this, a similar practice also occurs on the microscale of the organism, where cells must communicate with each other to function as an intact organism. Cell-cell communication is important in every aspect of the human body whether it is between neurons of the brain, synchronous beating of heart cells, or uniform contraction of muscles. Our complex immune system also relies on communication for crucial immune cell functions including cell survival, maturation, migration, signaling, and most importantly, coordinating inflammatory responses. In the course of any infection, cells of the immune system must interact with each other for the proper immune response to be activated. Even in the absence of infection, immune cells must communicate to perform normal maintenance functions. Historically, research in communication between immune cells has focused on soluble means of communications (e.g. cytokines). This term refers to a cell secreting a factor, which another cell recognizes and then responds. Soluble factors allow for global communication between cells and for gradients of signal to form. For example, the generation of a fever is a response to soluble communication, as is migration or chemotaxis of neutrophils to a site of injury or infection. The characteristics of soluble factors are extremely important and lead to effective cellular communication throughout the body. However, not all communication can be attributed to soluble means. Another means of cell-cell communication occurs when cells are in physical contact with each other and molecules on the surface of each cell interact leading to a response in one or both of the cells. A well-studied example of communication by direct cell contact is the immune synapse, which forms by direct contact between a T cell and an antigen presenting cell (Davis & Dustin 2004), or the interaction that occurs when immune cells must leave the circulation and migrate across the endothelium of the blood vessel (Nourshargh & Alon 2014). Gap junctions also contribute to contact-dependent communication allowing for trafficking of small molecules between cells (Saez et al 2003).Yet these types of interaction are usually of short range. Between contact dependent communication and secreted factors both short range direct and long range global communication patterns are met. However, there are additional alternative means of communication that allow for some aspects of direct cell communication to span longer distances. The focus of this review will be on two alternative means of communication between immune cells: exosomes and tunneling nanotubes (TNTs).
EXOSOMES
In addition to classical cytokine and chemokine-based signaling, immune cells are known to communicate through secreted extracellular vesicles and, in particular, endosomally-derived exosomes. These are small membrane bound vesicles secreted into the extracellular environment, which can carry a variety of different molecules (Johnstone et al 1987, Ratajczak et al 2006, Tian et al 2010). Immune cells can generate exosomes as well as take up exosomes produced by other cell types (Mittelbrunn et al 2011). The last decade has revealed a plethora of functions for exosomes in the immune system, nervous system, stem cells and cancer cells.
Structure
Exosomes were first identified by several groups using pulse-chase and electron microscopy experiments in reticulocytes (Harding et al 1983, Johnstone et al 1987, Pan et al 1985). In general, exosomes are small round shaped membrane-bound vesicles with sizes ranging between 40 and 100 nm in diameter (Conde-Vancells et al 2008, Raposo et al 1996, Raposo & Stoorvogel 2013). Because of their small size, exosome structure is not readily observed using the light microscope but when examined by electron microscopy, exosomes appear as flattened spheres surrounded by a lipid bi-layer (Figure 1). These characteristics are consistent with the sizes and morphology of internal vesicles in the multivesicular endocytic compartment, or MVB, from which exosomes originate (Raposo et al 1996). Secreted exosomes also float on sucrose gradients with a density ranging between 1.13 g/ml to 1.19 g/ml (Escola et al 1998, Raposo et al 1996, Thery et al 1999). This provides criterion to differentiate exosomes from membrane vesicles and protein aggregates released by apoptotic cells in addition to their structure and protein composition (Thery et al 2001). Secretion of exosomes is constitutive in many cell types including EBV-transformed B cells (Raposo et al 1996) and immature dendritic cells (DCs) (Thery et al 1999). However, exosome secretion is a regulated process in other hematopoietic cell types including mast cells and T-cells (Raposo et al 1997). When regulated, MVBs fuse with the plasma membrane following activation in a Ca2+-dependent manner (Blott & Griffiths 2002). Interestingly, T-cells seem to switch between constitutive release and regulated release depending on stimulation or activation (Blanchard et al 2002).
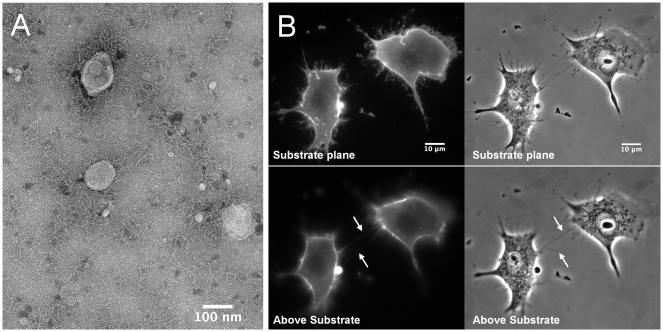
Figure 1. Macrophage exosomes and TNTs.
(A) Transmission electron microscopy of exosomes purified from microglia, mononuclear phagocytes of the CNS. Scale bar indicates 100nm. Figure courtesy of SJ Coniglio in the JE Segall lab and G Perumal in the Analytical Imaging Facility at Albert Einstein College of Medicine (B) Representative images of macrophage/monocyte TNTs. RAW/LR5 cells are stained with membrane dye FM1-43FX and imaged live. Upper panels show substrate planes, lower panels show above substrate planes where TNTs are indicated with arrows. Scale bars indicate 10μm. Figure courtesy of S Hanna in the D Cox lab at Albert Einstein College of Medicine.
Exosomes have been isolated from various sources, in large part from tissue culture media in vitro but also in vivo from circulation. The common method utilized by most groups to purify exosomes is through a series of centrifugation steps to remove cellular organelles and other debris, followed by ultracentrifugation to pellet exosomes (Davis et al 1986, Raposo et al 1996, Thery et al 2006). Sucrose gradients are then used to separate proteins from lipid-containing membrane vesicles (Escola et al 1998, Raposo et al 1996, Thery et al 2006, Thery et al 2009). More recently, polymer-based or immuno-capture methods have been used as fast and simple procedures for exosome purification that do not require ultracentrifugation. Despite the purification method, purified exosomes are further confirmed using multiple techniques including western blot, microscopy and proteomic analysis to characterize their morphology, composition and physical features. Commonly used markers for exosome purification in protein detection methods include tetraspanins CD9, and CD63, which are found to be associated and enriched in intracellular vesicles within MVBs (Escola et al 1998). Recently, the International Society for Extracellular Vesicles (ISEV) has proposed a series of criteria to define minimal characterization of extracellular vesicles, particularly exosomes. Based on the ISEV categories, three or more specific proteins should be present on vesicles to be properly referred to as exosomes including tetraspanins, integrins, adhesion molecules and others (Lotvall et al 2014). However, a detailed comparison is still needed to determine if the different methods purification precipitate different amounts or types of vesicles. Differences in these methods may contribute to potential variations between studies.
Biogenesis/formation
Based on proteomic analyses, exosomes were surprisingly found to lack proteins from the nucleus, mitochondria, endoplasmic reticulum or the golgi apparatus (Raposo et al 1996, Thery et al 2001, Thery et al 1999). Several studies on exosomes from immune cells, including DCs, T-cells, and B-cells support the fact that exosomes are not derived from plasma membrane fragments (Blanchard et al 2002, Clayton et al 2001, Raposo et al 1996, Thery et al 2001). The presence of MVB markers including CD63 and major histocompatibility complex (MHC) class II support the endosomal origin of exosomes (Kleijmeer et al 1996, Thery et al 2001). Extensive protein analyses of exosomes secreted by DCs, lymphocytes, and other cellular sources have further revealed that MVBs represent a specific subcellular compartment to which a specific subset of cellular proteins is targeted (Mathivanan & Simpson 2009, Thery et al 2001, Thery et al 1999, Wubbolts et al 2003). In addition, other proteins typically associated with the endocytic pathway such as annexin II, Rab5 and Rab7 were present in exosomes (Gruenberg & Maxfield 1995). These results overall strengthen the argument that exosomes originate from the endosomal pathway. However, only a subset of endosomal/lysosomal proteins is present in exosomes suggesting specific targeting of proteins (Thery et al 2001, Thery et al 1999). Exclusion of proteins from exosomes also appears to occur during their formation. For example, exosomes derived from B-cells, DCs, or mast cells lack typical endocytic pathway targeting protein the invariant chain CD74 (Escola et al 1998, Raposo et al 1997, Zitvogel et al 1998). The LAMP2 lysosomal marker is also absent in exosomes derived from B-cells (Escola et al 1998). These examples suggest active exclusion of proteins from exosomes. However, this is may not be a general property of exosomes since LAMP2 has been detected in DC derived exosomes (Zitvogel et al 1998). The presence of LAMP1 in exosomes is more controversial where it has been detected in EBV-transformed B cells using immune-electron microscopy (Raposo et al 1996, Raposo et al 1997) but not using western blot (Escola et al 1998) or FACS analysis (Vincent-Schneider et al 2002). The difference in the presence of certain endocytic markers in exosomes can be attributed to the fact that targeting proteins to the internal vesicles of MVBs is variable in different cell types. These proteins can either be cell-type specific as described above, or more common proteins. In general, common proteins include chaperones, tetraspanins, adhesion molecules, Rab proteins, and enzymes as well as cytoskeletal proteins (Fevrier & Raposo 2004) (Figure 2).
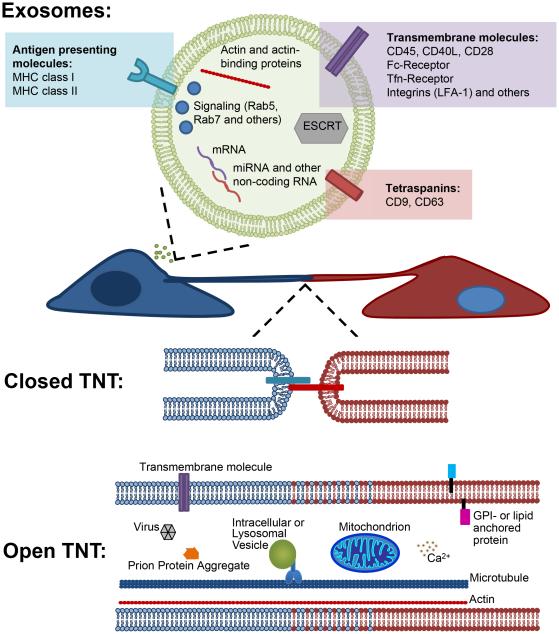
Figure 2. Schematic representation of two different modes of immune cell communication: Exosomes and TNTs.
(Upper panel). Exosomes are lipid bi-layer membrane vesicles that contain similar membrane composition of the cell producing them and contain the cytosol of the producing cell containing various cytosolic signaling molecules such as Rab GTPases as well as cytoskeleton components including actin and actin binding proteins and nucleic acids. (Lower panels) Tunneling Nanotubes are long thin structures that connect two neighboring cells. These structures can mediate direct membrane cell-cell interaction (closed ended) as well as the transfer of various material and cellular organelles including lysosomes and mitochondrial transfer (open ended).
While the mechanism of protein sorting into exosomes is still unclear, some studies suggest a possible role of ubiquitination of cytosolic domains in targeting selected proteins to exosomes similar to that seen in protein targeting to MVBs (Hicke 2001, Katzmann et al 2001). Blanchard et al. have shown that T-cell derived exosomes contained the ubiquitin-ligase c-CBL (Blanchard et al 2002). Protein ubiquitination of membrane proteins results in successive interactions with multiple protein complexes known as ESCRT (endosomal sorting complex required for transport) that is involved in targeting these proteins to the intraluminal vesicles of MVBs (reviewed in (Babst 2005, Michelet et al 2010)). While ubiquitinated proteins were detected in DC-derived exosomes, these proteins were found to be soluble and hence independent of the ESCRT machinery (Buschow et al 2005), which suggests an alternative mechanism targeting membrane proteins to exosomes. Bushow et al. suggested that targeting MHC class II molecules to DC exosomes following activation by T cells is not due to ubiquitination but rather occurs through the transfer and sequestration of MHC II into an intracellular compartment that contains tetraspanin CD9. This compartment was further identified as MVBs based on size and relative content using electron microscopy (Buschow et al 2009). Overall, these studies support the role of MVBs in the generation of exosomes.
Regardless of the mechanism of specific protein targeting, the orientation of exosomal membrane proteins depends on vesicle budding from the endosome/MVB. It is believed that exosomes form by inward budding from the limiting membrane of endosomes to form the internal vesicles of MVBs (van Deurs et al 1993). One function of MVBs is to mediate degradation by fusion with lysosomes, however, when MVBs fuse with the plasma membrane they release their internal vesicles, as exosomes, into the extracellular environment. This method of exosome generation results in the presence of cytoplasmic contents in the lumen and, upon release, exposes the extracellular domains of transmembrane proteins to the extracellular space. This membrane orientation of exosomes is supported by studies using antibodies against the extracellular domains of transmembrane proteins showing that they can be detected on the outside of intact exosomes, including MHC complex II molecules, CD9 and αMβ2 integrin (Raposo et al 1996, Thery et al 1999). Antibodies against cytoplasmic proteins, on the other hand, such as HSC70 and annexin II are not detected in whole-mounts of exosomes (Thery et al 1999). In addition, intact exosomes have also been purified using antibody-coated beads against MHC class II (Clayton et al 2001) and CD63 (Vincent-Schneider et al 2002). These results are consistent with the suggested inward budding model and membrane orientation during exosome biogenesis.
In summary, this mechanism of exosome formation explains the inclusion of cytoplasmic proteins in the exosome lumen and the presence of receptors and other transmembrane proteins with their ligand/receptor binding sites exposed to the extracellular milieu.
Function
The physical properties and composition of exosomes, as discussed above, make them an optimal means of intercellular communication. Many studies have aimed at elucidating how various exosome components affect recipient cells, as summarized in Table 1. As shown in Figure 2, exosomes contain a combination of ligands and receptors that could concurrently interact with multiple cell-surface receptors allowing for typical contact dependent cellular activation to occur as well as mediating exchange of both membrane and cytosolic components without cells actually being in close range. For example, secreted exosomes that contain MHC class II dimers bound to antigenic peptides could potentially elicit the activation of the adaptive immune response. For example, antigen-presenting cells (APCs) release exosomes bearing MHC class II/antigen complexes that activate primed CD4+ and CD8+ T cells through specific TCR-peptide interactions (Admyre et al 2006, Arnold & Mannie 1999, Patel et al 1999). In addition, DCs also become more efficient at stimulating T cells by acquiring exosomes from other DCs (Bedford et al 1999, Knight et al 1998, Vallhov et al 2015) (Table 1).
Table 1.
Exosome functions in Immune Cells
| Cell type-derived exosomes |
Function in Target cell(s) | Reference |
|---|---|---|
| APC | CD4+, CD8+ T cell- activation |
Arnold and Mannie 1999; Patel et al. 1999; Admyre et al. 2006 |
| Dendritic Cell | DC becoming more efficient in stimulating T- cells |
Knight et al. 1998; Vallhov et al. 2015 |
| Dendritic Cell | T cell mediated anti- tumor response by inducing CD8+ T-cells |
Zitvogel et al. 1998 |
| Activated B cell | Stimulate proliferation of primed CD4+ T cells |
Raposo et al. 1996; Munstasell et al. 2007 |
| Mast cell | Stimulate T cells | Vncent-Schneider et al. 2002 |
| Pathogen-infected cell | Stimulate CD4+ and CD8+ |
Bhatnagar and Schorey 2007; Walker et al. 2009 |
| B cell | Alter gene expression in monocytes |
Pegtel et al. 2010 |
| Dendritic Cell | Target DCs or T cell gene expression promoting inflammation |
O’Connell et al. 2007; Turner et al. 2011; Huffaker et al. 2012 |
| Monocyte/macrophage | Inducing inflammation and affecting tumor cell gene expression |
Vigorito et al. 2013; Akexander et al. 2015 |
| Monocyte/macrophage | Immunosuppression of DCs |
Alexander et al. 2015 |
| Tumor cell | Decrease CD4+ and CD8+ T cell proliferation |
Andreola et al. 2002; Taylor et al. 2003; Huber et al. 2005 |
| Tumor cell | Anti-proliferative effect on NKs |
Liu et al. 2006; Clayton et al. 2007,2008 |
| Leishmania major | Inhibition of myeloid cell function and response |
Silverman et al. 2010 |
The generation of an efficient immune response also requires antigen-specific interactions between B and T cells. Exosomes from activated B cells contain peptide-MHC class II complexes that can directly stimulate primed, but not naïve CD4+ T cells stimulating T-cell proliferation in vitro (Muntasell et al 2007, Raposo et al 1996). In contrast, indirect activation of naïve T lymphocytes occurs after DCs capture the released exosomes and then present the exosomal MHC-peptide complexes to specific T cells (Muntasell et al 2007, Thery et al 2002, Vallhov et al 2015). This is proposed to be due to the activated confirmation of αMβ2-integin (also known as LFA-1) on exosomes, which interacts with its corresponding ligand ICAM-1 expressed on the surface of primed and not on naïve T cells (Nolte-'t Hoen et al 2009) as well as on DCs (Segura et al 2007). In addition, other studies reported that mast cell-derived exosomes containing MHC class II molecules could also stimulate T-cells (Vincent-Schneider et al 2002). Interestingly, a recent study has reported that exosomes derived from microglia/macrophages resident in the CNS are involved in spreading pro-inflammatory signals, altering neuronal function and therefore contributing to pathogenesis of multiple sclerosis (MS) (Carandini et al 2015). Pathogen-infected cells can also secrete exosomes that carry antigens from pathogens and stimulate specific CD4+ and CD8+ T-cell responses (Bhatnagar & Schorey 2007, Walker et al 2009). More interestingly, DCs exposed to tumor peptides generated exosomes that were able to induce a T-cell-mediated anti-tumor immune response in an in vivo study (Zitvogel et al 1998). However, the extent of T-cell activation by exosomes depends on the physiological state of the cells secreting them. For instance, exosomes from mature DCs are more efficient at stimulating T-cell activation than those from immature DCs both in vitro and in vivo (Admyre et al 2006, Montecalvo et al 2008, Segura et al 2007). It is also possible that exosomes produced in peripheral tissues could sensitize immature DCs before they migrate to the lymph nodes (Thery et al 1999). Consequently, the proposed role for exosomes in immune response is spreading antigen-specific MHC complexes to activate T-cells directly or indirectly by increasing the number of antigen presenting cells resulting in the amplification of the immune response.
Recent findings have detected the presence of genetic material in secreted exosomes and in particular mRNA and miRNA, known regulators of protein expression. Genetic material was first detected in mast cell-derived exosomes, which could be transferred between different cells (Valadi et al 2007). Incorporation of genetic material into exosomes appears to be a selective process similar to the targeting of proteins because some groups have suggested that not all mRNAs present in the cell end up in the generated exosomes (Valadi et al 2007). This finding has increased interest in the field and has opened up new avenues in understanding mechanisms of mRNA targeting and the potential function of RNA transfer. Recent studies have observed the presence of miRNAs in immune cell-derived exosomes suggesting the possibility of transfer of miRNAs function. For example, EBV-infected B cell exosomes once captured by monocytes alter the expression of target genes in the host monocytes (Pegtel et al 2010). Furthermore, both DCs and T cells are able to secrete and take up exosomes with different miRNAs depending on the maturation state of DCs and upon specific MHC-T cell receptor interactions (Mittelbrunn et al 2011, Montecalvo et al 2012). A recent study has shown that miRNAs miR-155 and miR-146a, which are critical in regulating inflammatory responses, are released within exosomes from DCs (Huffaker et al 2012, O'Connell et al 2007, Turner et al 2011). Once taken up by other recipient DCs, miR-155 containing exosomes can mediate target gene expression promoting an inflammatory response to endotoxin. One study done on monocytes/macrophages detected miR-155 in exosomes in response to specific inflammatory stimuli, which could affect other target cells including tumor cells in addition to immune cells (Alexander et al 2015, Vigorito et al 2013). Therefore, exosomes provide a means of miRNA transfer between immune cells resulting in the regulation of gene expression and the generation of proper inflammatory responses.
It should be noted that in some cases exosomes can have immunosuppressive effects. For example, exosomal miR-146a mediates an immunosuppressive effect in recipient DCs in response to endotoxin (Alexander et al 2015). Interestingly, the same study discovered that exosomal miR-155 and miR-146a contained within different exosomes are exchanged between immune cells in vivo, where miR-155 enhanced while miR-146a inhibited endotoxin-induced inflammation in mice (Alexander et al 2015). Moreover, multiple studies have found that tumor-derived exosomes bearing Fas ligand and tumor-necrosis factor-related ligands could result in decreased proliferation of CD4+ and CD8+ T lymphocytes leading ultimately to Fas-mediated apoptosis (Andreola et al 2002, Clayton et al 2007, Huber et al 2005, Taylor et al 2003). Other studies have shown that tumor-derived exosomes had inhibitory effects on natural killer (NK) cells. This occurs primarily by blocking IL-2-mediated activation of NK cells where tumor-derived exosomes contained membrane-associated TGF-beta(1) which contributes to the anti-proliferative effects on NK cells as well as the down-regulation of the NK cell activating receptor (NKG2D) (Clayton et al 2008, Clayton et al 2007, Liu et al 2006). In addition, parasites such as Leishmania major produce exosomes that may also generate an immunosuppressive response and contribute to increase resistance by modulating myeloid cell function and responses to infection (Silverman et al 2010). Therefore, the general belief in the field is that exosomes are involved in pleiotropic functions between immune cells themselves or with target cells depending on the source of these vesicles as well as the various molecules they carry (Romagnoli et al 2014). However, more research needs to be done to elucidate the potential roles of exosomes in cell-cell communication in vivo.
Overall, exosomes represent a very important alternative means of intercellular communication within the immune system that could ultimately result in a coordinate an inflammatory response over long distances. The various pro- and anti-inflammatory roles of exosomes have led many to propose a potential use for exosomes as biomarkers in disease diagnosis as well as a means to target cell functions (Vigorito et al 2013, Wei et al 2013).
TUNNELING NANOTUBES (TNTs)
Mechanisms of intercellular communication mediated by direct cell contact are important for many immunological processes including the formation of the immune synapse between T-cells and antigen-presenting cells (Davis & Dustin 2004). However, it is now clear that contact-dependent communication is not always restricted to immediately adjacent cells. TNTs are thin membranous structures that allow the transfer of signals through membrane receptor-mediated signaling or the transfer of material such as vesicles, organelles, proteins, or signaling molecules. These structures were first described in cultured rat pheochromocytoma PC12 cells and have since been described in numerous cells types including almost all immune cells (Rustom et al 2004).
Structure
TNTs are long thin F-actin-based membranous channels connecting cells. These structures typically range between 50-200nm in diameter but can reach up to 800nm in thickness. All TNTs contain actin, but the thicker ones also contain microtubules, which accounts for the increase in diameter (Onfelt et al 2006, Rustom et al 2004). Unlike thin actin containing filopodia, TNTs are often not attached to the substrate and mediate connections between two different cells. TNT lengths vary dramatically and can reach up to several cell diameters long (Figure 1). Additionally, cells can form multiple TNTs with connections to different cells allowing for the formation of networks of cells (Watkins & Salter 2005). These connections are highly fragile and sensitive to light exposure, shearing force and chemical fixation (Rustom et al 2004). Moreover, there is a lack of known markers making these structures difficult to study. It is important to note that TNTs can be visualized after chemical fixation, but many structures are destroyed during the process. Therefore, live cell quantitation measurements are preferred, but some gentle fixation methods have been used (Hase et al 2009, Schiller et al 2013). Currently the term TNT is being used as a generalized term for thin membranous structures. These structures are sometimes referred to as membranous nanotubes, although TNT has become the more common nomenclature. Some earlier papers have employed a stricter criterion for the determination of TNTs making the requirement that these structures are tubular and allow for cytoplasmic interactions (open-ended). However, there is also evidence of close-ended structure meeting the structural requirements of TNTs (Figure 2). Furthermore, these close-ended structures can be electrically coupled by gap junctions providing one of the known functions of nanotubes (Abounit & Zurzolo 2012, Chauveau et al 2010, Sowinski et al 2008, Wang et al 2010). Therefore, the authors suggest that the following structural requirements be used to identify TNTs: (1) they are not attached to the substrate, (2) they attach two cells, and (3) they contain actin. These strict requirements for TNTs help to differentiate them from other actin containing structures such as retraction fibers or filopodia. Unfortunately, the requirement that TNTs attach two cells (#2) restricts the field and causes studies on TNT formation to be more difficult to perform. These additional requirements in defining TNTs has led to some discrepancies in the literature. For instance, one of the first mentioned examples of structures resembling TNTs in B-cells actually refers to the protrusions as cytoneme-like and is commonly cited as the reference for B-cell TNTs (Gupta & DeFranco 2003). While this paper does not show the protrusions making connections between B cells or other cell types, further work confirmed that B cells do indeed form TNTs that meet the current structural requirements (Onfelt et al 2004, Rainy et al 2013, Xu et al 2009). Recently, some studies have differentiated TNTs into two subsets and quantify structures that connect cells as TNTs and structures similar in appearance to TNTs but do not connect cells as membrane protrusions to decrease confusion and will hopefully allow for better formation studies in the future (Hase et al 2009).
Using the criteria of cell-cell connection as a major condition for determining TNTs, it is known that TNTs can connect similar types of cells (homotypic) or connect different cell types (heterotypic). However, a majority of studies on TNT formation use homotypic TNTs as a model, while functional studies use both homotypic and heterotypic TNTs. Also, while rarely discussed, the contribution of membrane from each connecting cell to the resulting TNT also varies and can consist of membrane from either cell or a mixture of both. A common way to visualize these structures is in live cultures using either a membrane dye added to the cultures such as FM1-43 or fluorescently labeled wheat germ agglutinin (WGA), or genetically expressing a fluorescent membrane marker (Chauveau et al 2010, Hase et al 2009, Kimura et al 2013) (Figure 1). When using a membrane dye, it is impossible to distinguish the membrane composition of TNTs. Only with the use of co-culture of cells genetically expressing two different membrane localizing fluorescent molecules or labeled with two different fluorescent dyes can membrane composition be determined (Kimura et al 2013). Currently, structural differences in TNTs do not align with particular cell types. However, this may change as more information is gathered about TNT composition.
Formation
Despite the universal requirement for actin polymerization, the mechanism for TNT formation is not completely understood. There have been two widely proposed models for TNT formation: actin-driven protrusion and cell-dislodgment mechanism, both of which are supported by time-lapse recoding studies (Marzo et al 2012, Onfelt et al 2006, Onfelt et al 2004, Rustom et al 2004, Sowinski et al 2008, Watkins & Salter 2005). The actin-driven protrusion mechanism involves one or two protrusive events that connect and eventually fuse with the membrane of the other cell or a protrusion from the other cell (Hase et al 2009, Rustom et al 2004). The cell-dislodgement mechanism involves two cells in close contact allowing membranes to fuse. As the cells migrate away from each other, a TNT is formed composed of membrane originating from either one or both cells involved (Davis & Sowinski 2008, Rustom et al 2004). It has been suggested that the cell dislodgment mechanism may be typical for motile cells which include immune cells (e.g. macrophages or lymphocytes) (Chauveau et al 2010, Zhang & Zhang 2013). It is unclear what induces TNT formation but studies have shown that longer duration of cell-cell contact prior to separation increases TNT formation in T lymphocytes and NK cells (Chauveau et al 2010, Sowinski et al 2011, Sowinski et al 2008). Also in some cases, the number of receptor/ligand interactions can directly influence TNT formation presumably by increasing the initial duration of cell-cell contact (Chauveau et al 2010). Veranic et al. suggested naming TNTs formed by the actin polymerization model as type I and the cell dislodgement model as type II, but this has yet to be adopted by the field (Veranic et al 2008). It is important to note that the two mechanisms are not mutually exclusive and could occur in the same cell type. Furthermore, both mechanisms could potentially be used to form homotypic or heterotypic TNTs that contain membrane from either one cell or a mixture of the two contributing cells. Despite the two possible mechanisms of formation, the field is in agreement that actin polymerization is required for TNT formation. Neutrophils appear to form membrane tubular extensions similar to TNTs. However, this occurs in the presence of staurosporine or cytochalasin D (Galkina et al 2010). Therefore, these structures are truly not TNTs due to the independence of actin polymerization for formation. Currently, no single structure meeting all TNTs requirements has been found in neutrophils, basophils or eosinophils. Using the above criteria for TNT structure and formation it is possible that only a subset of immune cell types form TNTs.
Little has been done to investigate the signaling pathways involved in TNT formation, especially with regard to immune cells. Many studies have employed over-expression of proteins into easily transfectable cells to examine the signaling requirements for TNTs but these do not directly address immune cells. Since actin polymerization is required, it has been proposed that some of the proteins involved in actin polymerization, such as Cdc42, will also be important for nanotube formation. Indeed, Cdc42, Rac1, ezrin and N-WASP are all localized to TNTs, but the requirement of these factors in TNT formation was not assessed (Lachambre et al 2014). Treatment of Jurkat T-cells with the Cdc42 specific inhibitor secramine A blocked TNT formation (Arkwright et al 2010). Using expression of dominant negative constructs in HeLa cells, inhibition of Cdc42 resulted in decreased TNT formation, but Rac1 inhibition showed no effect (Hase et al 2009). It is unknown if the signaling requirements for TNT formation will be different in cells artificially induced to make TNTs verses cells that form them constitutively. For example, DCs form TNTs in response to prion infection and HIV infection, but they also form them in the absence of infection (Eugenin et al 2009, Gousset & Zurzolo 2009, Nikolic et al 2011, Salter & Watkins 2006, Zaccard et al 2015). Also, the signaling requirements for TNT formation may be different depending on the mechanisms of formation. Despite these caveats, some proteins have been identified that regulate TNT formation.
One of the first proteins implicated in TNT formation in immune cells is M-Sec, also known as TNFaip2 (tumor necrosis factor –α-induced protein) (Hase et al 2009). Using RAW264.7 macrophages cells, it was shown that the M-Sec reduction using shRNA significantly decreased TNT formation. Furthermore, overexpression of M-Sec increased formation of TNTs. M-Sec induced TNT formation in HeLa cells is partially dependent on the exocyst complex and requires RalA (Hase et al 2009). This work quantitated both TNTs using cellular connections as a requirement and as membrane protrusions and all data was supported by functional analysis of the TNTs. Further work in HeLa and HEK-293T cells has shown that leuckocyte specific transcript 1, LST1, a highly expressed protein in macrophages and DCs, is also required for M-Sec dependent TNT formation. This study shows that LST1 recruits RalA to the plasma membrane and thus promotes the interaction of RalA with the exocyst complex (Schiller et al 2013). RalA is also known to bind filamin and activate Cdc42, proteins that are involved in actin remodeling. Filamin (an actin cross-linking protein) co-localizes with LST1 and also localizes to TNTs suggesting that filamin may function in this complex. However, overexpression of filamin did not enhance formation of TNTs indicating that while filamin may be involved, it is not a limiting factor in TNT formation (Schiller et al 2013). Interestingly, the exocyst complex is involved in vesicle docking required for membrane recycling and has led to the hypothesis that M-Sec and the exocyst complex are involved in supplying the extra membrane that is required during TNT formation. These results suggest that a large complex is formed uniting both actin polymerization and membrane recruitment at the site of TNT formation.
As mentioned above, TNTs are found in many different types of cells with several structural differences and multiple formation models so it may be possible that different proteins are involved in TNT formation in different cell types. For example, M-Sec is important in macrophage TNT formation, but it did not localize to or increase TNT formation in Jurkat T-cells (Lachambre et al 2014). It is also unclear if the different mechanisms of TNT formation lead to different types of connections. Furthermore, it should be noted that other than blocking actin polymerization, removing the activity of a single protein or modifying a pathway (either by inhibition, shRNA constructs, or dominate negative constructs) does not completely suppress TNT formation suggesting that either multiple pathways are required or other compensatory pathways are involved.
While mechanisms of TNT formation are mentioned above, stimulation or induction of TNT formation requires discussion as well. There seems to be a basal level of TNT formation in many immune cells, for example in macrophages and DCs. However, there are descriptions in the literature of ways to increase the levels of TNTs in cells. Stimulation with pro-inflammatory signals either exogenously or during infection increases TNT formation in macrophages, DCs, T cells, and NK cells (Chauveau et al 2010, Eugenin et al 2009, Gousset & Zurzolo 2009, Mukerji et al 2012, Nikolic et al 2011, Nobile et al 2010, Van Prooyen et al 2010, Xu et al 2009). So far, while stimulation of immune cells increases TNT formation, there does not seem to be dramatic differences between different stimuli. For example, cytokine stimulation of NK cells increases formation of TNTs in NK cells, but there was no significant difference when NK cells were activated by different stimulating cytokines (Chauveau et al 2010). Again it is prudent to mention that the mechanisms by which exogenous stimulation induces nanotubes is unknown and whether the “basal level” of nanotubes observed is in fact due to stimulants and growth factors that occur in normal serum/media.
Function
TNT function can be quite varied although all known functions involve the transfer of cargo or signals from one cell to another (summarized in Table 2). For example, TNTs have been shown to transfer mitochondria or vesicles derived from early endosomes, endoplasmic reticulum, Golgi complex, and lysosomes (Kadiu & Gendelman 2011, Onfelt et al 2006, Xu et al 2009). Plasma membrane can also be transferred via TNTs as well as proteins associated with the plasma membrane (Rainy et al 2013). Finally cytoplasmic molecules, including signaling molecules such as calcium, can also be transferred through TNTs (Gousset & Zurzolo 2009, Rinaldo 2013, Watkins & Salter 2005). Transfer of microRNA through TNTs has been described in cancer cells, but this function has not been attributed to TNTs in immune cells as of yet (Thayanithy et al 2014). The necessity for transfer of a signal or cargo can also be used as a distinguishing characteristic of TNTs. Including this as a criterion would help to differentiate TNTs from other similar structures such as neutrophil membrane tethers and slings, which function in movement and adherence as opposed to communication (Sundd et al 2012). Therefore, the last suggested requirement for a structure to be considered verifiable TNT is transfer of material.
Table 2.
TNT functions in Immune Cells
| Cells | Target | Function | Reference |
|---|---|---|---|
| B cell | NK cell | Transfer of HLA-Cw5 | Onfelt et al. 2004 |
| B cell | T cell | Transfer of H-Ras containing PM patches |
Rainy et al. 2013 |
| B cells | Mesenchymal stromal cell |
Transfer of signal to upregulate cytokines |
Polak et al. 2015 |
| T cell | T cell | Transfer of p8 protein | Van Prooyen et al. 2010 |
| NK cell | Macrophage, B cell |
Target cell can travel back to NK cell to reform immune synapse |
Chauveau et al. 2010 |
| Macrophage | Macrophage | Transfer of DiO labelled, LysoTracker-labeled and MitoTracker-labeled vesicles |
Onfelt et al. 2004; Onfelt et al. 2006 |
| Macrophage | B cell | Transfer of vesicles labeled with lysotracker, Transfer of Nef protein |
Xu et al. 2009 |
| Macrophage | Macrophage | Propagation of calcium signaling, |
Watkins and Salter, 2005; Salter and Watkins, 2006; Hase et al. 2009 |
| Macrophage | Macrophage | Cytoplasmic interaction | Watkins and Salter, 2005 |
| Dendritic Cell |
Dendritic Cell |
Propagation of calcium signaling | Watkins and Salter, 2005 |
| Dendritic Cell |
Neuronal Cell |
Transfer of Alexa-PrPSC, Transfer of LysoTracker-labeled vesicles |
Gousset et al. 2009 |
| Dendritic Cell |
Dendritic Cell |
Transfer of YG latex beads, Transfer of HIV |
Zaccard et al. 2015 |
Despite the number of functions that have been ascribed to TNTs in various immune cells, overarching questions still remain regarding the biological significance of TNTs during immune cell function. Many TNTs have been shown to propagate calcium signals and to transfer mitochondria or other vesicles and organelles, yet the question remains of how relevant these functions are in the immune system. While the field is still struggling to define TNT structure and function, there are several examples where TNTs have provided mechanistic insights into a number of fundamental biological questions.
A common function of TNTs is the propagation of an electrical signal (Wang et al 2010, Watkins & Salter 2005). Propagation of calcium signals has been seen in many cells types and can be associated with or be independent of gap junctions (Wang & Gerdes 2012, Watkins & Salter 2005). The first report of this in macrophages and DCs was by Watkins and Salter where they found that calcium flux was propagated along TNTs independently of gap junctions or ATP release (Watkins & Salter 2005). Calcium flux is often used as evidence of functional TNTs and propagation of calcium signals was seen to occur only through macrophages connected by TNTs. To conclude that M-Sec was involved in macrophage TNT formation Hase et al., demonstrated that depletion of M-Sec using shRNA resulted in the loss of calcium signal propagation (TNT function) as well as in a decrease in the number of TNTs formed (Hase et al 2009). However, it is unknown why calcium signal propagation in non-neuronal immune cells would be beneficial. In immune cells, there are several signaling pathways that are modulated by voltage-sensitive phosphatases or calcium sensitive protein kinases that could respond to TNT propagation of a calcium signals (Boudsocq et al 2010, Feske 2007). Yet, no work has been done to link calcium signal propagation through TNTs to a specific signaling pathway. This may be a potential line of investigation in the future. One hypothesized consequence of electric coupling through TNTs is synchronized actin-remodeling activity of various cell types during the healing process (Wang & Gerdes 2012). Another hypothesis involves TNTs mediating glial guidance although this would require that the TNTs are electrically coupled to gap junctions (Abounit & Zurzolo 2012).
TNTs have also been suggested to provide cell-contact dependent communication over long distances. This would suggest that a ligand on one cell membrane could interact with its receptor on another cell mediated through a TNT. Evidence supporting such TNT highways comes from studies examining the interaction of receptor/ligand pairs (Chauveau et al 2010). In heterotypic cellular TNTs, for example those connecting NK cells to target P815 cells, enough of a receptor ligand, MICA protein, accumulates at the TNT junction to trigger NK cell activation (Chauveau et al 2010). Furthermore, the signaling adaptor DAP10 accumulated at TNT junctions with MICA along with a large number of tyrosine-phosphorylated proteins indicating a signaling pathway leading to NK cells activation can occur through TNTs. After TNT activation of the NK cell, the target cell then migrated toward the NK cell using the TNT as a tether to form a tight contact leading to cell lysis. Adding to the biological relevance of TNTs, it has been speculated that this mode of NK cell activation and targeted cell lysis may aid in killing particularly motile target cells (e.g., other lymphocytes) that otherwise would escape before effector responses could proceed to completion (Chauveau et al 2010).
Signaling through TNTs has been seen between primary B cell precursor acute lymphoblastic leukemia cells and primary mesenchymal stromal cells although the exact signaling pathway was not investigated. This study compares cytokine production in co-culture conditions with TNTs and in conditions where TNTs are removed either by shaking to mechanically break TNTs or by inhibiting formation of TNTs using inhibitors of actin polymerization (Polak et al 2015). In conditions where TNTs were present, an increase in several pro-survival cytokines such as IP10, IL-8, and MCP-1 was observed. Polak et al suggest that TNT communication modulate the bone marrow microenvironment and allow increased survival for the cancer cells (Polak et al 2015). Another potential biological relevance of TNTs in cancer, while not in immune cells, relates to the transfer of mitochondria through TNTs. Transfer of mitochondria in PC12 cells rescues apoptotic cells suggesting potential roles during survival of tumor cells during chemotherapy (Wang & Gerdes 2015).
One biologically significant role for TNTs that has been extensively studied is TNT-mediated disease transmission including prion transmission in DCs and neuronal cells (Gousset & Zurzolo 2009). This finding transformed the prion field by finally providing a mechanism for the transmission of the infection. Another example of TNT mediated transmission involves HIV. There is substantial evidence that antibody production in B cells is influenced by HIV, specifically mediated by the protein Nef (Xu et al 2009). However, as B cells are not routinely infected by HIV, it was unclear how the viral protein Nef was found to be expressed/localized in B cells. Xu et al. provided the answer by showing that Nef can be transferred through TNTs from macrophages into B cells. This resulted in the inhibition of immunoglobulin G2 (IgG2) and IgA class switching (Xu et al 2009). It is interesting to note that the majority of the established biologically significant contributions of TNTs involve transmission and infection of foreign agents.
Few consequences of TNT functions have yet to be assigned to normal immune surveillance, maintenance, or activation of immune functions. However, two interesting examples exist in DCs. To activate a cytotoxic T-lymphocyte response, DCs must migrate and transport antigen to the draining lymph nodes. It is here that transfer of antigenic information between migratory and lymph node-residing DCs occurs. One method of transfer of antigenic information occurs in a process called cross-dressing, with the acquisition of APC membrane patches (peptide-MHC complexes) by the partner cell. Schiller et al. hypothesized that TNTs would allow cross-dressing between remote DCs and they showed proof of concept where transfer of MHC class I molecules could occur via TNTs in HeLa cells (Schiller et al 2013). TNTs have now also been shown to facilitate intercellular antigen exchange in dendritic cells resulting in the enhancement of antigen-specific T cell responses (Zaccard et al 2015).
Overall, TNTs represent an exciting alternative means of communication allowing for much more than just receptor-meditated signaling to occur. These structures are unique and allow for the transfer of varying and large materials from one cell to another. Open channels could also allow for transfer of more than one signal increasing the potential significance of TNT communication. The questions regarding the biological significance of TNTs will undoubtedly yield much fascinating research in the coming years.
CONCLUSIONS AND PERSPECTIVES
This review has focused on two alternative means of communication between immune cells: exosomes and tunneling nanotubes. Each of these methods of communication, in addition to traditionally studied soluble factors and direct cell-cell contact, has unique properties. It is these unique properties that help address the varying requirements for different types of communication. For example, intercellular communication can occur over a very short range affecting only cells in the immediate vicinity or occur over a long range affecting multiple cells and acting more globally. Therefore, under different circumstances one or more types of communication might be favored over the others based on different requirements needed to accomplish a particular function. Long range communication, for instance, needs signal stability that can survive over time and distance needed for its transmission, a requirement not needed in short distance communication. We can speculate on how each of these alternative means of communication might fulfill different functions in the immune system.
The question remains as to how all these different communication mechanisms function together and what are the benefits of using one versus the other. Traditionally, long range communication has been thought to occur via secreted factors including hormones and cytokines providing systemic communication. However, secreted signals travelling throughout the body are more prone to degradation in addition to potentially causing off-target effects on many different cell types. Exosomes provide another mechanism for long range communication overcoming some of the limitations of secreted factors. For instance, molecules within these membrane-enclosed vesicles are protected from interacting with the extracellular environment. This allows signals that would typically be degraded immediately to persist during transport (e.g. nucleic acids). Another benefit of exosomes is that signaling molecules do not need to be soluble and can be part of the membrane, thus increasing the versatility of the signaling molecules that can participate in long range communication. Additionally, exosomes allow for the aggregation of signaling molecules resulting in a greater strength of signal at long distances than soluble factors. Exosomes can also facilitate membrane exchange between the donor and the recipient cell. This transfer would result in the exchange of information between cells over long distance, such as MHC/antigen complexes being transferred to multiple immune cells. Despite the benefits, it is important to note that unlike secreted factors, exosomes cannot establish gradients and require more material to generate a signal (e.g. additional membrane material required for exosome formation). While these means of communication can also work in short or intermediates ranges, systemic communication may only be achieved by secreted factors and exosomes.
Short range communication can often be mediated by contact dependent communication. This is a direct interaction that does not spread globally. However, contact dependent interaction is restricted by number of cells that can be in direct contact with each other and often involves stationary or co-migratory cells in order to obtain a more prolonged effect. TNTs, on the other hand, can overcome the restriction of direct interaction solely with neighboring cells by allowing contact over larger distances. Additionally, cells can form multiple TNTs with connections to different cells allowing for the formation of networks of cells and the transmission of the signal throughout the network further propagating any signals.
Lastly, while the different forms of cellular interactions mentioned here fulfill different niches in terms of range of communication, it is also important to mention that the type of signal communicated via each method is also different. While these forms of communication can mediate activation of signaling pathways through receptor ligand interactions, exosomes and TNTs also allow for the transfer of other material. However, it is not clear whether the luminal components of exosomes, other than the genetic material, have any function. TNTs allow for the transfer of organelles, cytoplasmic proteins, and plasma membrane proteins as well as directly transferring signaling molecules such as calcium. It should be noted that several studies have demonstrated interplay between the two types of cellular communication. For instance, in mesothelioma cells, exogenous exosomes enhance TNT formation (Thayanithy et al 2014). In addition, studies done on melanoma and leukemia showed that tumor-derived exosomes can promote endothelial cell communication via TNTs which in turn contributes to endothelial angiogenic responses (Hood et al 2009, Mineo et al 2012).
In conclusion, whether mediated by direct contact or through secretion, by exosomes or TNTs, it is well known that cellular communication particularly between immune cells is crucial for multiple cellular processes. Elucidating the mechanisms of these novel and alternative means of communication will no doubt provide critical new insights into both normal physiological and pathological conditions.
Acknowledgements
We thank Sara Donnelly for advice and editorial help. This work was funded by NIH grants R01 GM071828 (DC) and K12GM102779 (KMS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
List of abbreviations
- APC
Antigen presenting cell
- Cdc42
Cell division control protein 42
- DC
Dendritic cell
- EBV
Epstein-Barr virus
- ESCRT
Endosomal sorting complex required for transport
- HIV
Human immunodeficiency virus
- ICAM-1
Intercellular adhesion molecule 1
- IgA
Immunoglobulin A
- IgG2
Immunoglobulin G2
- IL-2
Interleukin 2
- LAMP1
Lysosomal-associated membrane protein-1
- LAMP2
Lysosomal-associated membrane protein-2
- LFA-1
Leukocyte-function associated antigen 1
- LST1
Leukocyte specific transcript 1
- MHC
Major histocompatibility complex
- miRNA
MicroRNA
- MVB
Multivesicular body
- NK
Natural Killer
- NKG2D
Natural-killer group 2, member D
- N-WASP
Neuronal Wiskott-Aldrich syndrome protein
- Rac1
Ras-related C3 botulinum toxin substrate 1
- RalA
Ras-related protein Ral-A
- TCR
T-cell receptor
- TGF
Transforming growth factor
- TNFaip2
Tumor necrosis factor –α-induced protein
- TNTs
Tunneling nanotubes
- WGA
Wheat germ agglutinin
References
- Abounit S, Zurzolo C. Wiring through tunneling nanotubes--from electrical signals to organelle transfer. Journal of cell science. 2012;125:1089–98. doi: 10.1242/jcs.083279. [DOI] [PubMed] [Google Scholar]
- Admyre C, Johansson SM, Paulie S, Gabrielsson S. Direct exosome stimulation of peripheral human T cells detected by ELISPOT. European journal of immunology. 2006;36:1772–81. doi: 10.1002/eji.200535615. [DOI] [PubMed] [Google Scholar]
- Alexander M, Hu R, Runtsch MC, Kagele DA, Mosbruger TL, et al. Exosome-delivered microRNAs modulate the inflammatory response to endotoxin. Nature communications. 2015;6:7321. doi: 10.1038/ncomms8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreola G, Rivoltini L, Castelli C, Huber V, Perego P, et al. Induction of lymphocyte apoptosis by tumor cell secretion of FasL-bearing microvesicles. The Journal of experimental medicine. 2002;195:1303–16. doi: 10.1084/jem.20011624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkwright PD, Luchetti F, Tour J, Roberts C, Ayub R, et al. Fas stimulation of T lymphocytes promotes rapid intercellular exchange of death signals via membrane nanotubes. Cell research. 2010;20:72–88. doi: 10.1038/cr.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold PY, Mannie MD. Vesicles bearing MHC class II molecules mediate transfer of antigen from antigen-presenting cells to CD4+ T cells. European journal of immunology. 1999;29:1363–73. doi: 10.1002/(SICI)1521-4141(199904)29:04<1363::AID-IMMU1363>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Babst M. A protein's final ESCRT. Traffic. 2005;6:2–9. doi: 10.1111/j.1600-0854.2004.00246.x. [DOI] [PubMed] [Google Scholar]
- Bedford P, Garner K, Knight SC. MHC class II molecules transferred between allogeneic dendritic cells stimulate primary mixed leukocyte reactions. International immunology. 1999;11:1739–44. doi: 10.1093/intimm/11.11.1739. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Schorey JS. Exosomes released from infected macrophages contain Mycobacterium avium glycopeptidolipids and are proinflammatory. The Journal of biological chemistry. 2007;282:25779–89. doi: 10.1074/jbc.M702277200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard N, Lankar D, Faure F, Regnault A, Dumont C, et al. TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/zeta complex. Journal of immunology. 2002;168:3235–41. doi: 10.4049/jimmunol.168.7.3235. [DOI] [PubMed] [Google Scholar]
- Blott EJ, Griffiths GM. Secretory lysosomes. Nature reviews. Molecular cell biology. 2002;3:122–31. doi: 10.1038/nrm732. [DOI] [PubMed] [Google Scholar]
- Boudsocq M, Willmann MR, McCormack M, Lee H, Shan L, et al. Differential innate immune signalling via Ca(2+) sensor protein kinases. Nature. 2010;464:418–22. doi: 10.1038/nature08794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschow SI, Liefhebber JM, Wubbolts R, Stoorvogel W. Exosomes contain ubiquitinated proteins. Blood cells, molecules & diseases. 2005;35:398–403. doi: 10.1016/j.bcmd.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Buschow SI, Nolte-'t Hoen EN, van Niel G, Pols MS, ten Broeke T, et al. MHC II in dendritic cells is targeted to lysosomes or T cell-induced exosomes via distinct multivesicular body pathways. Traffic. 2009;10:1528–42. doi: 10.1111/j.1600-0854.2009.00963.x. [DOI] [PubMed] [Google Scholar]
- Carandini T, Colombo F, Finardi A, Casella G, Garzetti L, et al. Microvesicles: What is the Role in Multiple Sclerosis? Frontiers in neurology. 2015;6:111. doi: 10.3389/fneur.2015.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauveau A, Aucher A, Eissmann P, Vivier E, Davis DM. Membrane nanotubes facilitate long-distance interactions between natural killer cells and target cells. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:5545–50. doi: 10.1073/pnas.0910074107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton A, Court J, Navabi H, Adams M, Mason MD, et al. Analysis of antigen presenting cell derived exosomes, based on immuno-magnetic isolation and flow cytometry. Journal of immunological methods. 2001;247:163–74. doi: 10.1016/s0022-1759(00)00321-5. [DOI] [PubMed] [Google Scholar]
- Clayton A, Mitchell JP, Court J, Linnane S, Mason MD, Tabi Z. Human tumor-derived exosomes down-modulate NKG2D expression. Journal of immunology. 2008;180:7249–58. doi: 10.4049/jimmunol.180.11.7249. [DOI] [PubMed] [Google Scholar]
- Clayton A, Mitchell JP, Court J, Mason MD, Tabi Z. Human tumor-derived exosomes selectively impair lymphocyte responses to interleukin-2. Cancer research. 2007;67:7458–66. doi: 10.1158/0008-5472.CAN-06-3456. [DOI] [PubMed] [Google Scholar]
- Conde-Vancells J, Rodriguez-Suarez E, Embade N, Gil D, Matthiesen R, et al. Characterization and comprehensive proteome profiling of exosomes secreted by hepatocytes. Journal of proteome research. 2008;7:5157–66. doi: 10.1021/pr8004887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DM, Dustin ML. What is the importance of the immunological synapse? Trends in immunology. 2004;25:323–7. doi: 10.1016/j.it.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Davis DM, Sowinski S. Membrane nanotubes: dynamic long-distance connections between animal cells. Nature reviews. Molecular cell biology. 2008;9:431–6. doi: 10.1038/nrm2399. [DOI] [PubMed] [Google Scholar]
- Davis JQ, Dansereau D, Johnstone RM, Bennett V. Selective externalization of an ATP-binding protein structurally related to the clathrin-uncoating ATPase/heat shock protein in vesicles containing terminal transferrin receptors during reticulocyte maturation. The Journal of biological chemistry. 1986;261:15368–71. [PubMed] [Google Scholar]
- Escola JM, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. The Journal of biological chemistry. 1998;273:20121–7. doi: 10.1074/jbc.273.32.20121. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, Gaskill PJ, Berman JW. Tunneling nanotubes (TNT): A potential mechanism for intercellular HIV trafficking. Communicative & integrative biology. 2009;2:243–4. doi: 10.4161/cib.2.3.8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feske S. Calcium signalling in lymphocyte activation and disease. Nature reviews. Immunology. 2007;7:690–702. doi: 10.1038/nri2152. [DOI] [PubMed] [Google Scholar]
- Fevrier B, Raposo G. Exosomes: endosomal-derived vesicles shipping extracellular messages. Current opinion in cell biology. 2004;16:415–21. doi: 10.1016/j.ceb.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Galkina SI, Stadnichuk VI, Molotkovsky JG, Romanova JM, Sud'ina GF, Klein T. Microbial alkaloid staurosporine induces formation of nanometer-wide membrane tubular extensions (cytonemes, membrane tethers) in human neutrophils. Cell adhesion & migration. 2010;4:32–8. doi: 10.4161/cam.4.1.10314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gousset K, Zurzolo C. Tunnelling nanotubes: a highway for prion spreading? Prion. 2009;3:94–8. doi: 10.4161/pri.3.2.8917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg J, Maxfield FR. Membrane transport in the endocytic pathway. Current opinion in cell biology. 1995;7:552–63. doi: 10.1016/0955-0674(95)80013-1. [DOI] [PubMed] [Google Scholar]
- Gupta N, DeFranco AL. Visualizing lipid raft dynamics and early signaling events during antigen receptor-mediated B-lymphocyte activation. Molecular biology of the cell. 2003;14:432–44. doi: 10.1091/mbc.02-05-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. The Journal of cell biology. 1983;97:329–39. doi: 10.1083/jcb.97.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hase K, Kimura S, Takatsu H, Ohmae M, Kawano S, et al. M-Sec promotes membrane nanotube formation by interacting with Ral and the exocyst complex. Nature cell biology. 2009;11:1427–32. doi: 10.1038/ncb1990. [DOI] [PubMed] [Google Scholar]
- Hicke L. A new ticket for entry into budding vesicles-ubiquitin. Cell. 2001;106:527–30. doi: 10.1016/s0092-8674(01)00485-8. [DOI] [PubMed] [Google Scholar]
- Hood JL, Pan H, Lanza GM, Wickline SA, Consortium for Translational Research in Advanced I, Nanomedicine Paracrine induction of endothelium by tumor exosomes. Laboratory investigation; a journal of technical methods and pathology. 2009;89:1317–28. doi: 10.1038/labinvest.2009.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber V, Fais S, Iero M, Lugini L, Canese P, et al. Human colorectal cancer cells induce T-cell death through release of proapoptotic microvesicles: role in immune escape. Gastroenterology. 2005;128:1796–804. doi: 10.1053/j.gastro.2005.03.045. [DOI] [PubMed] [Google Scholar]
- Huffaker TB, Hu R, Runtsch MC, Bake E, Chen X, et al. Epistasis between microRNAs 155 and 146a during T cell-mediated antitumor immunity. Cell reports. 2012;2:1697–709. doi: 10.1016/j.celrep.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) The Journal of biological chemistry. 1987;262:9412–20. [PubMed] [Google Scholar]
- Kadiu I, Gendelman HE. Macrophage bridging conduit trafficking of HIV-1 through the endoplasmic reticulum and Golgi network. Journal of proteome research. 2011;10:3225–38. doi: 10.1021/pr200262q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzmann DJ, Babst M, Emr SD. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell. 2001;106:145–55. doi: 10.1016/s0092-8674(01)00434-2. [DOI] [PubMed] [Google Scholar]
- Kimura S, Hase K, Ohno H. The molecular basis of induction and formation of tunneling nanotubes. Cell and tissue research. 2013;352:67–76. doi: 10.1007/s00441-012-1518-1. [DOI] [PubMed] [Google Scholar]
- Kleijmeer MJ, Raposo G, Geuze HJ. Characterization of MHC Class II Compartments by Immunoelectron Microscopy. Methods. 1996;10:191–207. doi: 10.1006/meth.1996.0095. [DOI] [PubMed] [Google Scholar]
- Knight SC, Iqball S, Roberts MS, Macatonia S, Bedford PA. Transfer of antigen between dendritic cells in the stimulation of primary T cell proliferation. European journal of immunology. 1998;28:1636–44. doi: 10.1002/(SICI)1521-4141(199805)28:05<1636::AID-IMMU1636>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Lachambre S, Chopard C, Beaumelle B. Preliminary characterisation of nanotubes connecting T-cells and their use by HIV-1. Biology of the cell / under the auspices of the European Cell Biology Organization. 2014;106:394–404. doi: 10.1111/boc.201400037. [DOI] [PubMed] [Google Scholar]
- Liu C, Yu S, Zinn K, Wang J, Zhang L, et al. Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function. Journal of immunology. 2006;176:1375–85. doi: 10.4049/jimmunol.176.3.1375. [DOI] [PubMed] [Google Scholar]
- Lotvall J, Hill AF, Hochberg F, Buzas EI, Di Vizio D, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. Journal of extracellular vesicles. 2014;3:26913. doi: 10.3402/jev.v3.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzo L, Gousset K, Zurzolo C. Multifaceted roles of tunneling nanotubes in intercellular communication. Frontiers in physiology. 2012;3:72. doi: 10.3389/fphys.2012.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathivanan S, Simpson RJ. ExoCarta: A compendium of exosomal proteins and RNA. Proteomics. 2009;9:4997–5000. doi: 10.1002/pmic.200900351. [DOI] [PubMed] [Google Scholar]
- Michelet X, Djeddi A, Legouis R. Developmental and cellular functions of the ESCRT machinery in pluricellular organisms. Biology of the cell / under the auspices of the European Cell Biology Organization. 2010;102:191–202. doi: 10.1042/BC20090145. [DOI] [PubMed] [Google Scholar]
- Mineo M, Garfield SH, Taverna S, Flugy A, De Leo G, et al. Exosomes released by K562 chronic myeloid leukemia cells promote angiogenesis in a Src-dependent fashion. Angiogenesis. 2012;15:33–45. doi: 10.1007/s10456-011-9241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelbrunn M, Gutierrez-Vazquez C, Villarroya-Beltri C, Gonzalez S, Sanchez-Cabo F, et al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nature communications. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montecalvo A, Larregina AT, Shufesky WJ, Stolz DB, Sullivan ML, et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012;119:756–66. doi: 10.1182/blood-2011-02-338004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montecalvo A, Shufesky WJ, Stolz DB, Sullivan MG, Wang Z, et al. Exosomes as a short-range mechanism to spread alloantigen between dendritic cells during T cell allorecognition. Journal of immunology. 2008;180:3081–90. doi: 10.4049/jimmunol.180.5.3081. [DOI] [PubMed] [Google Scholar]
- Mukerji J, Olivieri KC, Misra V, Agopian KA, Gabuzda D. Proteomic analysis of HIV-1 Nef cellular binding partners reveals a role for exocyst complex proteins in mediating enhancement of intercellular nanotube formation. Retrovirology. 2012;9:33. doi: 10.1186/1742-4690-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntasell A, Berger AC, Roche PA. T cell-induced secretion of MHC class II-peptide complexes on B cell exosomes. The EMBO journal. 2007;26:4263–72. doi: 10.1038/sj.emboj.7601842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolic DS, Lehmann M, Felts R, Garcia E, Blanchet FP, et al. HIV-1 activates Cdc42 and induces membrane extensions in immature dendritic cells to facilitate cell-to-cell virus propagation. Blood. 2011;118:4841–52. doi: 10.1182/blood-2010-09-305417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobile C, Rudnicka D, Hasan M, Aulner N, Porrot F, et al. HIV-1 Nef inhibits ruffles, induces filopodia, and modulates migration of infected lymphocytes. Journal of virology. 2010;84:2282–93. doi: 10.1128/JVI.02230-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte-'t Hoen EN, Buschow SI, Anderton SM, Stoorvogel W, Wauben MH. Activated T cells recruit exosomes secreted by dendritic cells via LFA-1. Blood. 2009;113:1977–81. doi: 10.1182/blood-2008-08-174094. [DOI] [PubMed] [Google Scholar]
- Nourshargh S, Alon R. Leukocyte migration into inflamed tissues. Immunity. 2014;41:694–707. doi: 10.1016/j.immuni.2014.10.008. [DOI] [PubMed] [Google Scholar]
- O'Connell RM, Taganov KD, Boldin MP, Cheng G, Baltimore D. MicroRNA-155 is induced during the macrophage inflammatory response. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:1604–9. doi: 10.1073/pnas.0610731104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onfelt B, Nedvetzki S, Benninger RK, Purbhoo MA, Sowinski S, et al. Structurally distinct membrane nanotubes between human macrophages support long-distance vesicular traffic or surfing of bacteria. Journal of immunology. 2006;177:8476–83. doi: 10.4049/jimmunol.177.12.8476. [DOI] [PubMed] [Google Scholar]
- Onfelt B, Nedvetzki S, Yanagi K, Davis DM. Cutting edge: Membrane nanotubes connect immune cells. Journal of immunology. 2004;173:1511–3. doi: 10.4049/jimmunol.173.3.1511. [DOI] [PubMed] [Google Scholar]
- Pan BT, Teng K, Wu C, Adam M, Johnstone RM. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. The Journal of cell biology. 1985;101:942–8. doi: 10.1083/jcb.101.3.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel DM, Arnold PY, White GA, Nardella JP, Mannie MD. Class II MHC/peptide complexes are released from APC and are acquired by T cell responders during specific antigen recognition. Journal of immunology. 1999;163:5201–10. [PubMed] [Google Scholar]
- Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MA, Hopmans ES, et al. Functional delivery of viral miRNAs via exosomes. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:6328–33. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak R, de Rooij B, Pieters R, den Boer ML. B-cell precursor acute lymphoblastic leukemia cells use tunneling nanotubes to orchestrate their microenvironment. Blood. 2015 doi: 10.1182/blood-2015-03-634238. [DOI] [PubMed] [Google Scholar]
- Rainy N, Chetrit D, Rouger V, Vernitsky H, Rechavi O, et al. H-Ras transfers from B to T cells via tunneling nanotubes. Cell death & disease. 2013;4:e726. doi: 10.1038/cddis.2013.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, et al. B lymphocytes secrete antigen-presenting vesicles. The Journal of experimental medicine. 1996;183:1161–72. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. The Journal of cell biology. 2013;200:373–83. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G, Tenza D, Mecheri S, Peronet R, Bonnerot C, Desaymard C. Accumulation of major histocompatibility complex class II molecules in mast cell secretory granules and their release upon degranulation. Molecular biology of the cell. 1997;8:2631–45. doi: 10.1091/mbc.8.12.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006;20:1487–95. doi: 10.1038/sj.leu.2404296. [DOI] [PubMed] [Google Scholar]
- Rinaldo CR. HIV-1 Trans Infection of CD4(+) T Cells by Professional Antigen Presenting Cells. Scientifica. 2013;2013:164203. doi: 10.1155/2013/164203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnoli GG, Zelante BB, Toniolo PA, Migliori IK, Barbuto JA. Dendritic Cell-Derived Exosomes may be a Tool for Cancer Immunotherapy by Converting Tumor Cells into Immunogenic Targets. Frontiers in immunology. 2014;5:692. doi: 10.3389/fimmu.2014.00692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH. Nanotubular highways for intercellular organelle transport. Science. 2004;303:1007–10. doi: 10.1126/science.1093133. [DOI] [PubMed] [Google Scholar]
- Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC. Plasma membrane channels formed by connexins: their regulation and functions. Physiological reviews. 2003;83:1359–400. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- Salter RD, Watkins SC. Dynamic properties of antigen uptake and communication between dendritic cells. Immunologic research. 2006;36:211–20. doi: 10.1385/IR:36:1:211. [DOI] [PubMed] [Google Scholar]
- Schiller C, Diakopoulos KN, Rohwedder I, Kremmer E, von Toerne C, et al. LST1 promotes the assembly of a molecular machinery responsible for tunneling nanotube formation. Journal of cell science. 2013;126:767–77. doi: 10.1242/jcs.114033. [DOI] [PubMed] [Google Scholar]
- Segura E, Guerin C, Hogg N, Amigorena S, Thery C. CD8+ dendritic cells use LFA-1 to capture MHC-peptide complexes from exosomes in vivo. Journal of immunology. 2007;179:1489–96. doi: 10.4049/jimmunol.179.3.1489. [DOI] [PubMed] [Google Scholar]
- Silverman JM, Clos J, Horakova E, Wang AY, Wiesgigl M, et al. Leishmania exosomes modulate innate and adaptive immune responses through effects on monocytes and dendritic cells. Journal of immunology. 2010;185:5011–22. doi: 10.4049/jimmunol.1000541. [DOI] [PubMed] [Google Scholar]
- Sowinski S, Alakoskela JM, Jolly C, Davis DM. Optimized methods for imaging membrane nanotubes between T cells and trafficking of HIV-1. Methods. 2011;53:27–33. doi: 10.1016/j.ymeth.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Sowinski S, Jolly C, Berninghausen O, Purbhoo MA, Chauveau A, et al. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nature cell biology. 2008;10:211–9. doi: 10.1038/ncb1682. [DOI] [PubMed] [Google Scholar]
- Sundd P, Gutierrez E, Koltsova EK, Kuwano Y, Fukuda S, et al. 'Slings' enable neutrophil rolling at high shear. Nature. 2012;488:399–403. doi: 10.1038/nature11248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DD, Gercel-Taylor C, Lyons KS, Stanson J, Whiteside TL. T-cell apoptosis and suppression of T-cell receptor/CD3-zeta by Fas ligand-containing membrane vesicles shed from ovarian tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2003;9:5113–9. [PubMed] [Google Scholar]
- Thayanithy V, Dickson EL, Steer C, Subramanian S, Lou E. Tumor-stromal cross talk: direct cell-to-cell transfer of oncogenic microRNAs via tunneling nanotubes. Translational research : the journal of laboratory and clinical medicine. 2014;164:359–65. doi: 10.1016/j.trsl.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Current protocols in cell biology / editorial board, Juan S. Bonifacino … [et al.] 2006 doi: 10.1002/0471143030.cb0322s30. Chapter 3: Unit 3 22. [DOI] [PubMed] [Google Scholar]
- Thery C, Boussac M, Veron P, Ricciardi-Castagnoli P, Raposo G, et al. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. Journal of immunology. 2001;166:7309–18. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- Thery C, Duban L, Segura E, Veron P, Lantz O, Amigorena S. Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nature immunology. 2002;3:1156–62. doi: 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nature reviews. Immunology. 2009;9:581–93. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- Thery C, Regnault A, Garin J, Wolfers J, Zitvogel L, et al. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. The Journal of cell biology. 1999;147:599–610. doi: 10.1083/jcb.147.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian T, Wang Y, Wang H, Zhu Z, Xiao Z. Visualizing of the cellular uptake and intracellular trafficking of exosomes by live-cell microscopy. Journal of cellular biochemistry. 2010;111:488–96. doi: 10.1002/jcb.22733. [DOI] [PubMed] [Google Scholar]
- Turner ML, Schnorfeil FM, Brocker T. MicroRNAs regulate dendritic cell differentiation and function. Journal of immunology. 2011;187:3911–7. doi: 10.4049/jimmunol.1101137. [DOI] [PubMed] [Google Scholar]
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature cell biology. 2007;9:654–9. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- Vallhov H, Gutzeit C, Hultenby K, Valenta R, Gronlund H, Scheynius A. Dendritic cell-derived exosomes carry the major cat allergen Fel d 1 and induce an allergic immune response. Allergy. 2015 doi: 10.1111/all.12701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deurs B, Holm PK, Kayser L, Sandvig K, Hansen SH. Multivesicular bodies in HEp-2 cells are maturing endosomes. European journal of cell biology. 1993;61:208–24. [PubMed] [Google Scholar]
- Van Prooyen N, Gold H, Andresen V, Schwartz O, Jones K, et al. Human T-cell leukemia virus type 1 p8 protein increases cellular conduits and virus transmission. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:20738–43. doi: 10.1073/pnas.1009635107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veranic P, Lokar M, Schutz GJ, Weghuber J, Wieser S, et al. Different types of cell-to-cell connections mediated by nanotubular structures. Biophysical journal. 2008;95:4416–25. doi: 10.1529/biophysj.108.131375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigorito E, Kohlhaas S, Lu D, Leyland R. miR-155: an ancient regulator of the immune system. Immunological reviews. 2013;253:146–57. doi: 10.1111/imr.12057. [DOI] [PubMed] [Google Scholar]
- Vincent-Schneider H, Stumptner-Cuvelette P, Lankar D, Pain S, Raposo G, et al. Exosomes bearing HLA-DR1 molecules need dendritic cells to efficiently stimulate specific T cells. International immunology. 2002;14:713–22. doi: 10.1093/intimm/dxf048. [DOI] [PubMed] [Google Scholar]
- Walker JD, Maier CL, Pober JS. Cytomegalovirus-infected human endothelial cells can stimulate allogeneic CD4+ memory T cells by releasing antigenic exosomes. Journal of immunology. 2009;182:1548–59. doi: 10.4049/jimmunol.182.3.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Gerdes HH. Long-distance electrical coupling via tunneling nanotubes. Biochimica et biophysica acta. 2012;1818:2082–6. doi: 10.1016/j.bbamem.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Wang X, Gerdes HH. Transfer of mitochondria via tunneling nanotubes rescues apoptotic PC12 cells. Cell death and differentiation. 2015;22:1181–91. doi: 10.1038/cdd.2014.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Veruki ML, Bukoreshtliev NV, Hartveit E, Gerdes HH. Animal cells connected by nanotubes can be electrically coupled through interposed gap-junction channels. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:17194–9. doi: 10.1073/pnas.1006785107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins SC, Salter RD. Functional connectivity between immune cells mediated by tunneling nanotubules. Immunity. 2005;23:309–18. doi: 10.1016/j.immuni.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Wei Y, Nazari-Jahantigh M, Chan L, Zhu M, Heyll K, et al. The microRNA-342-5p fosters inflammatory macrophage activation through an Akt1- and microRNA-155-dependent pathway during atherosclerosis. Circulation. 2013;127:1609–19. doi: 10.1161/CIRCULATIONAHA.112.000736. [DOI] [PubMed] [Google Scholar]
- Wubbolts R, Leckie RS, Veenhuizen PT, Schwarzmann G, Mobius W, et al. Proteomic and biochemical analyses of human B cell-derived exosomes. Potential implications for their function and multivesicular body formation. The Journal of biological chemistry. 2003;278:10963–72. doi: 10.1074/jbc.M207550200. [DOI] [PubMed] [Google Scholar]
- Xu W, Santini PA, Sullivan JS, He B, Shan M, et al. HIV-1 evades virus-specific IgG2 and IgA responses by targeting systemic and intestinal B cells via long-range intercellular conduits. Nature immunology. 2009;10:1008–17. doi: 10.1038/ni.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccard CR, Watkins SC, Kalinski P, Fecek RJ, Yates AL, et al. CD40L induces functional tunneling nanotube networks exclusively in dendritic cells programmed by mediators of type 1 immunity. Journal of immunology. 2015;194:1047–56. doi: 10.4049/jimmunol.1401832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhang Y. Membrane nanotubes: novel communication between distant cells. Science China. Life sciences. 2013;56:994–9. doi: 10.1007/s11427-013-4548-3. [DOI] [PubMed] [Google Scholar]
- Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nature medicine. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]