Abstract
Introduction
Moraxella catarrhalis is a prominent pathogen that causes acute otitis media in children and lower respiratory tract infections in adults, resulting in a significant socioeconomic burden on healthcare systems globally. No vaccine is currently available for M. catarrhalis. Promising M. catarrhalis target antigens have been characterized in animal models and should soon enter human clinical trials.
Areas covered
This review discusses the detailed features and research status of current candidate target antigens for an M. catarrhalis vaccine. The approaches for assessing M. catarrhalis vaccine efficacy are also discussed.
Expert opinion
Targeting the key molecules contributing to serum resistance may be a viable strategy to identify effective vaccine targets among M. catarrhalis antigens. Elucidating the role and mechanisms of the serum and mucosal immune responses to M. catarrhalis is significant for vaccine target selection, testing and evaluation. Developing animal models closely simulating M. catarrhalis-caused human respiratory diseases is of great benefit in better understanding pathogenesis and evaluating vaccine efficacy. Carrying out clinical trials will be a landmark in the progress of M. catarrhalis vaccine research. Combined multicomponent vaccines will be a focus of future M. catarrhalis vaccine studies.
Keywords: adhesin, adjuvant, antigen, chronic obstructive pulmonary disease, clinical trial, conserved, epitope, immunogenicity, mucosal immune response, nasopharyngeal colonization, nutrient acquisition, opsonophagocytosis, otitis media, outer membrane protein, serum resistance, virulence
1. Introduction
Moraxella catarrhalis is a Gram-negative, aerobic diplococcus frequently found as a common inhabitant of the upper respiratory tract of humans [1]. Formerly known as Micrococcus catarrhalis, Neisseria catarrhalis and Branhamella catarrhalis, M. catarrhalis has been recognized as an important human-restricted mucosal pathogen causing respiratory tract infections during the last 3 – 4 decades [2,3]. M. catarrhalis is a common cause of otitis media in infants and children, accounting for 15 – 20% of acute otitis media (AOM) episodes [2,3]. M. catarrhalis is also an important cause of lower respiratory tract infections in adults, particularly those with chronic obstructive pulmonary disease (COPD). In addition, M. catarrhalis sometimes causes sinusitis, pneumonia, bacteremia and meningitis in children and adults and other invasive infections in the elderly and immune-compromised individuals [2,3].
Until recently, M. catarrhalis has been ranked as the third most common cause of AOM after Streptococcus pneumoniae (Spn) and nontypeable Haemophilus influenzae (NTHi) in children and adults [4]. Our group has been tracking the otopathogen mix of young children since 1996 [4-6], and in 2014 we found that M. catarrhalis had overtaken Spn and NTHi to become the most frequent cause of episodic and recurrent AOM in children [7]. AOM is the most common infectious disease causing parents to seek medical care for their child and to receive antibiotics. AOM often recurs, and the impact of AOM on health systems is particularly significant due to the high number of AOM cases, which require many medical visits as well as repeated antipyretic and antibiotic prescriptions [8]. There are estimated 107 million – 142 million M. catarrhalis-caused AOM cases each year in children and adults worldwide [8]. In the United States alone, the economic burden of otitis media exceeded US$5.3 billion to $6 billion annually during 1998 – 2008 in medical treatment, surgical management, and loss of income for working parents [9]. M. catarrhalis is responsible for approximately 10% of exacerbations of COPD in adults annually in the United States [10]. COPD is the third leading cause of death in the United States, affecting at least 24 million people and costing $50 billion in healthcare expenses each year [11]. COPD is considered a major unmet medical need that is increasing in prevalence throughout the world [11].
M. catarrhalis produces beta-lactamase, rendering it resistant to the recommended first-line antibiotics to treat children with AOM. Therefore, there are pressing needs for an M. catarrhalis vaccine. M. catarrhalis vaccine development is currently moving from antigen target identification to clinical trial. A number of M. catarrhalis antigens have shown excellent immunogenicity, eliciting functional antibodies and producing protective responses in animal models. However, none of these antigens has been tested in humans to date. Therefore, introducing M. catarrhalis vaccine testing into clinical trials is a main goal for the near future of M. catarrhalis vaccine research efforts.
2. M. catarrhalis vaccine candidates
The characteristics of an effective vaccine antigen target are i) expression of surface epitopes; ii) conservation among strains; iii) expression in vivo at sites of pathogenesis; iv) immunogenicity; v) induction of a protective immune response [12]. The following section will review the current research status of the potential M. catarrhalis vaccine antigens. These antigens are grouped with regard to their functions in bacterial pathogenesis, metabolism and molecular composition and discussed by focusing on the required features, as summarized in Table 1.
Table 1.
Potential vaccine antigens for M. catarrhalis.
| Antigen | Molecular mass (kDa) |
Putative function and other features |
Conservation and expression on the surface |
Immune responses observed |
Protection responses in animal models |
Ref. | |
|---|---|---|---|---|---|---|---|
| Naturally induced antibodies in humans |
Functional antibodies |
||||||
| Adhesive proteins | |||||||
| OMP CD | 46 | Adhesin and porin | Highly conserved at protein level; containing abundantly expressed epitopes on the bacterial surface |
Serum and mucosal antibodies in children and adults |
Bactericidal anti- bodies detected in guinea pig and mouse anti-sera |
Enhanced lung clearance of bacteria in immunized mice |
[13-23] |
| MID/Hag | 200 | Adhesin, hemagglutinin and autotransporter |
Containing surface- exposed and conserved epitopes |
Serum and mucosal antibodies in children and adults |
NA | Enhanced lung clearance of bacteria in immunized mice |
[24-34] |
| McaP | 66 | Adhesin and autotransporter; showing phospholipase B and esterase activity |
Highly conserved at protein level; expressing surface-exposed epitopes |
Serum antibodies in children |
Anti-sera inhibit the adherence of the bacteria to human epithelial cells |
NA | [35,36] |
| Mch/Mha proteins |
191 (MchA1), 183 (MchA2) and 78 (MchB) |
Exoprotein, transporter and FHA-like protein |
Conserved at protein level; expressed in the outer membrane |
Serum antibodies to MhaC in children |
NA | NA | [37-39] |
| Pilin | 16.7 (PilA1a), 15.4 (PilA1b) and 17.0 (PilA2) |
Major protein subunit of TFP; involved in natural genetic transformation, biofilm formation, and adherence of the bacteria to human epithelial cells |
Conserved at protein level |
Serum antibodies in children in this study |
NA | NA | [40-42] |
| UspA1 and UspA2 |
83 (UspA1) and 60 (UspA2) |
Adhesins and autotransporters; involved in serum resistance and other virulence mechanisms |
Heterogeneous at protein level |
Serum and mucosal antibodies in children and adults |
Bactericidal anti- bodies detected in mouse and guinea pig anti-sera |
Enhanced lung clearance of bacteria in immunized mice |
[22,31,33,43-52] |
| Proteins involved in nutrient acquisition | |||||||
| OppA | 74 | Mediating the uptake of peptides and fitness of the organism in the respiratory tract |
Highly conserved at gene level |
Serum antibodies in children in this study and in adults in other studies |
NA | Enhanced lung clearance of bacteria in immunized mice |
[11,53] |
| TbpB | 80 | Transferrin receptor for iron acquisition |
Expressing antigenically conserved epitopes; but showing heterogeneity at protein level |
Serum antibodies in children and adults |
Bactericidal anti- bodies detected in guinea pig anti-sera |
NA | [54-60] |
| LbpB | 84 | Lactoferrin receptor for iron acquisition |
Relatively conserved at protein level; but antigenically heteroge- nous among different strains |
Serum antibodies in adults |
Bactericidal anti- bodies detected in guinea pig anti- sera |
NA | [57,60-62] |
| CopB | 81 | Involved in the utilization of iron and the serum resistance |
Well conserved at gene level |
Serum and mucosal antibodies in children and adults |
NA | Enhanced lung clearance of bacteria in immunized mice |
[31,33,63-66] |
| OMP E | 50 | Porin; involved in binding and transporting fatty acids |
Highly conserved at gene level |
Serum and mucosal antibodies in adults |
NA | NA | [67-69] |
| Other OMPs | |||||||
| Msp22, -75 and -78 |
22, 75 and 78 | Heme-dependent peroxidase activity for Msp22. Functions are unknown for Msp75 and Msp78 |
Highly conserved at protein level |
Serum and mucosal antibodies in children and adults |
NA | Enhanced lung clearance of bacteria in Msp22-immunized mice |
[70-73] |
| OMP G1a | 16 | Surface lipoprotein. Functions are unknown |
Highly conserved at protein level |
Serum and mucosal antibodies in children and adults |
NA | NA | [74-78] |
| M35 | 36 | Necessary for the uptake of important energy sources |
Highly conserved at gene level |
NA | Opsonic but not bactericidal anti- bodies detected in mouse anti-sera |
Enhanced lung clearance of bacteria in immunized mice |
[79-81] |
| Non-protein outer membrane components | |||||||
| LOS | 2.5 – 4 | Inducing inflammatory responses; involved in adherence of the bacteria to human epithelial cells and serum resistance |
Relatively conserved; expressed in the outer membrane |
Serum and mucosal antibodies in adults |
Bactericidal anti- bodies detected in rabbit and mouse anti-sera |
Enhanced lung clearance of bacteria in immunized mice |
[2,3,22,33,82-96] |
OMP: Outer membrane protein; MID/Hag: Moraxella IgD binding protein/hemagglutinin; McaP: M. catarrhalis-adherence protein; Mha: M. catarrhalis filamentous hemagglutinin adhesin-like protein; Mch: M. catarrhalis hemagglutinin-like protein; TFP: Type IV pili; UspA: Ubiquitous surface protein A; OppA: Oligopeptide permease protein A; TbpB: Transferrin-binding protein B; LbpB: Lactoferrin-binding protein B; CopB: Catarrhalis outer membrane protein B; Msp: Moraxella surface protein; LOS: Lipooligosaccharide; NA: Not available.
2.1 Adhesive proteins
Adhesive proteins are outer membrane proteins (OMPs) assisting in the binding of the bacterium to the corresponding host receptors on the human respiratory epithelial cell surface. Targeting the adhesive proteins as vaccine antigens is sought as a method to block the adherence of the bacteria, which is believed to be the first step in pathogenesis.
2.1.1 OMP CD
OMP CD is a heat-modifiable protein with a predicted molecular mass of 46 kDa but appears as 60 kDa when completely denatured in SDS–PAGE [13]. Due to structural similarity, OMP CD is predicted to function as a porin. OMP CD also functions as an adhesin because it specifically binds both human nasal and middle ear mucins [14] and human lung epithelial (A549) cells [15]. OMP CD contains abundantly expressed epitopes on the M. catarrhalis surface, and the protein was found to be highly conserved among 51 strains of M. catarrhalis tested [13,16]. Two separate epitopes that are exposed on the M. catarrhalis surface might contribute to adhesion [17]. Furthermore, this protein appears to be expressed constitutively at a constant level.
OMP CD is immunogenic. Separate mucosal and systemic immunizations with OMP CD both induced immunoglobulin (Ig)G and IgA immune responses in mice [18,19]. IgG antibody to OMP CD has been detected in serum of children with otitis media with effusion [20] and in the convalescent sera of children with otitis media [21]. Serum and sputum IgG, IgA and IgM have also been detected in both healthy adults and COPD patients [17]. OMP CD antibodies can often be found among COPD patients who cleared the organism [22]. However, it is not known whether these natural antibodies can decrease nasopharyngeal (NP) or lung infection in humans. OMP CD has been shown to elicit functional antibodies in animal models. Serum antibodies to OMP CD from guinea pigs and mice demonstrate complement-mediated bactericidal activity [21]. Mouse serum antibodies to OMP CD can inhibit the binding of OMP CD to human mucin [23]. Separate mucosal and systemic immunization with recombinant or native OMP CD enhances bacterial clearance from the lungs of mice challenged by M. catarrhalis [19,23]. Immunization with OMP CD also enhances bacterial lung clearance in mice challenged by both homologous and heterologous M. catarrhalis isolates [23]. Current data suggest that OMP CD should be tested in clinical trials.
2.1.2 Moraxella IgD binding protein/hemagglutinin
Moraxella IgD binding protein (MID) is an OMP that mediates human erythrocyte agglutination; therefore, it is also named as hemagglutinin (Hag) [24,25]. It has a molecular weight of 200 kDa and binds serum IgD but not any other immunoglobulins [26] in a nonimmune manner [25]. MID is also an adhesin and mediates adherence to primary cultures of human middle ear epithelial cells [27]. MID adherence properties have been shown in several cell lines [25,27]. The adhesive domain is located at the sequence MID764 – 913 of the protein, which is a conserved region among strains. MID is expressed on the surface of most M. catarrhalis strains. Considering its huge size and diversity of its sequence among different strains, it is more likely that conserved fragments of MID, such as MID706-1194, which has 88.1% sequence identity among seven strains, could be considered as a vaccine candidate. MID706-1194 elicits a robust naturally acquired antibody responses in COPD patients who acquired and cleared M. catarrhalis [28]. MID contains surface-exposed and conserved epitopes among clinical isolates [27]; however, its expression undergoes phase variation [29]. However, low MID-expressing isolates may account for only a minor MID-expressing population [29].
Serum IgG, IgM and IgA to multiple recombinant MID fragments have been detected in young children from birth to 2 years old [30]. Salivary IgA against MID has also been identified in young patients with acute respiratory tract infection 1 – 24 months old [31]. Naturally acquired serum IgG to MID has been detected in healthy adults, and human serum antibodies to MID exhibit bactericidal activity [32]. By using recombinant MID fragments or purified outer membranes, serum IgG and sputum IgA against MID have been detected in COPD patients [28,33]. Immunization with a truncated MID has shown protective response in a mouse lung clearance model [34]. The collected data suggest that MID meets the vaccine antigen requirements and may be tested in clinical trials.
2.1.3 M. catarrhalis-adherence protein
M. catarrhalis-adherence protein (McaP) is another adhesin and also an autotransporter with phospholipase B and esterase activities [35]. It is a heat-modifiable protein with predicted molecular mass of 66 kDa as a mature form after the cleavage of a signal sequence; however, McaP appears as a 62 kDa protein after complete denaturation on SDS–PAGE [35,36]. McaP contributes to the adherence of M. catarrhalis to human epithelial cells with its esterase activity [35]. M. catarrhalis O35E mutants lacking McaP show considerably decreased adherence to several human cell lines [35]. McaP is highly conserved. The amino acid sequences of McaP were 98 – 100% identical among 8 studied strains and it was expressed in a panel of all 16 isolates tested [35,36]. Surface-exposed epitopes have been found on both the passenger domain and the transporter molecule, which are the two major structural and functional subunits of McaP [35,36].
Studies show that McaP induces functional antibodies, which inhibit the adherence of bacteria to human epithelial cells [35]. Mouse immune serum against a truncated McaP 51–650 inhibits adherence of both M. catarrhalis and recombinant E. coli expressing McaP to human respiratory epithelial cells [35,36]. Further investigations are necessary to expand our view of the immunogenicity of McaP and its various antigen regions and their protective immune responses in animal models and humans.
2.1.4 M. catarrhalis filamentous hemagglutinin adhesion-like protein/M. catarrhalis hemagglutinin-like protein
These proteins include M. catarrhalis filamentous hemagglutinin adhesin-like protein (Mha)B1 and MhaB2 (exoproteins), and MhaC (transporter) [37], which are also termed M. catarrhalis hemagglutinin-like protein (Mch)A1 and MchA2 for the secreted proteins and MchB for the transporter [38]. They are encoded by a locus of three genes that belong to the two-partner secretion (TPS) systems previously described in various other pathogens, including the better-characterized filamentous hemagglutinin adhesin (FHA) in Bordetella pertussis [39]. Therefore, they are also named as FHA-like proteins [37]. MchA1, MchA2 and MchB have estimated molecular masses of 191, 183 and 78 kDa, respectively [38]. MchA1 and MchA2 are 74% identical and 79% similar at the amino acid level over their entire sequences, but the level of similarity decreases to 41% in the divergent C-terminal regions [38]. MchA1 and MchA2 are localized in the outer membrane of M. catarrhalis and MchB is involved in the extracellular transport of these proteins [37]. MchA and MchB proteins show adhesive functions and are specifically involved in M. catarrhalis attachment to human epithelial cells [37,38]. The expression of these proteins in the outer membrane was found to be conserved among a panel of six M. catarrhalis clinical isolates [38]. These antigens were found to be expressed in the outer membrane of 63% of 16 isolates tested in another analysis [37]. There are no detailed data available to show the immunologic properties of these proteins thus far. Further studies are needed to shed more light on the immunogenicity, functional antibody responses and protective immune responses for these proteins to be evaluated as vaccine antigen targets.
2.1.5 Pilin
Pilin is the major protein subunit of type IV pili (TFP), which are filamentous surface appendages of M. catarrhalis [40]. Pilin is composed of two major subgroups of protein, PilA clade 1 that can be subdivided into PilA1a and PilA1b, and PilA clade 2 (PilA2). PilA1a, PilA1b and PilA2 have a calculated molecular weight of 16.7, 15.4 and 17.0 kDa, respectively, constituting 32.1, 10.4 and 57.5% of 106 tested clinical isolates, respectively [41]. By forming TFP, pilin is essential for natural genetic transformation and can also enhance biofilm formation in a continuous-flow chamber model [40,42]. Pilin also contributes to the adherence of M. catarrhalis to human epithelial cells cultured in vitro and NP colonization of M. catarrhalis in a chinchilla model [42]. Pilin clades exhibit a high degree of homology in the N-terminal PilA region, but a divergence at the C terminus. Unlike many other pathogens that express heterogeneous TFP, all M. catarrhalis isolates express one of two major clades of TFP, and these M. catarrhalis surface structures are both homogeneous in nature and highly conserved [41].
There is no available data measuring immune responses to pilin in either animal models or humans although all of the above findings support that pilin may be a potential vaccine antigen target. Further studies are warranted to delineate the immunologic characteristics and potential protective responses that pilin could provide for a vaccine.
2.1.6 Ubiquitous surface protein A1 and A2
Ubiquitous surface protein (Usp)A1 and UspA2 were listed as top antigens for M. catarrhalis vaccine candidates in the past. However, recent observations indicate that UspA1 and UspA2 have diverse sequences with varied structures resulting in different phenotypes and divergent functions in interacting with host targets among strains and clinical isolates. Therefore, these proteins are no longer high in terms of priority in the list of vaccine targets. UspA1 and UspA2 are heat-modifiable proteins with predicted molecular weight of 83 and 60 kDa, respectively, but they appear 130 kDa (UspA1) and above 200 kDa (UspA2) after denaturation in SDS–PAGE [43]. UspAs are adhesins and autotransporters with an oligomeric coiled-coil structure [44]. They also play a role in serum resistance [45,46] and other virulence mechanisms.
UspA1 and UspA2 were shown to be antigenically conserved among M. catarrhalis clinical isolates and contain surface-exposed epitopes [47]. However, there are various ‘cassettes’ of peptide sequence in the UspA variants from a variety of independent M. catarrhalis isolates [48]. Modular assortment of unrelated ‘cassettes’ of peptide sequence results in divergence of individual UspA proteins. Exchange of certain variant cassettes accounts for strain-specific differences in UspA protein function and confers differing phenotypes among different M. catarrhalis isolates [48]. UspA1 also has varied adherence activity binding to host epithelial cells due to poorly conserved amino acid sequences in the CEACAM-binding regions among different strains [49]. Another study demonstrates that UspA proteins exchange their functional regions in vivo and this genetic change contributes more heterogeneity in the sequence and function of the protein family than previously believed [50].
Both UspA1 and UspA2 induce naturally acquired antibodies in children and adults [22,31,33]. UspA1- and UspA2-pspecific antibodies from guinea pig and mice also show bactericidal activities [51,52]. Immunization with individual UspA1 and UspA2 proteins enhances bacterial clearance from the lungs in mice challenged with M. catarrhalis [51,52]. In sum, UspA proteins have been extensively characterized and tested and their potential as vaccine antigen targets has diminished over time.
2.2 Proteins involved in nutrient acquisition
2.2.1 Oligopeptide permease protein A
Oligopeptide permease protein A (OppA) is an oligopeptide binding protein of the oligopeptide permease ABC transport system, which mediates the uptake of peptides and fitness of M. catarrhalis in the respiratory tract [11]. OppA is a lipoprotein with a molecular mass of 74 kDa. Although thought to be a soluble periplasmic protein, OppA expresses epitopes on the surface of M. catarrhalis in flow cytometry analysis and in a whole-cell ELISA assay [53]. OppA is pivotal for the ability of M. catarrhalis to persist in the respiratory tract [11]. A nucleotide sequence analysis displays that the gene identity scores range from 98.7 to 100% among 10 sputum isolates from adults with COPD and 10 middle ear fluid isolates from children with otitis media. These data indicate that the oppA gene is highly conserved among M. catarrhalis strains.
Intranasal immunization of OppA induced systemic and mucosal antibodies in mice and resulted in enhanced clearance of M. catarrhalis in a mouse pulmonary clearance model [53]. The collected data suggest that OppA is an immunogenic protein antigen with surface-exposed epitopes inducing protective immune responses. Further studies are warranted to investigate the immune protection response and mucosal immune response in humans to evaluate the vaccine potential of OppA.
2.2.2 Transferrin-binding proteins
Transferrin-binding proteins (Tbps) are receptors for human transferrin in the outer membrane of M. catarrhalis. Two Tbps identified to date are TbpA and TbpB. TbpA has a molecular mass of 115 kDa and is essential for iron acquisition from transferrin [54]. TbpB, previously termed OMP B1 [55], has a molecular mass of 80 kDa [54] and plays only a facilitative role in iron acquisition [56]. TbpA is a TonB-dependent integral membrane protein and TbpB is a peripheral protein attached to the outer membrane via a lipid tail [57]. M. catarrhalis acquires iron via the binding of TbpA and TbpB to transferrin and then transporting iron across the bacterial membrane through a channel formed by TbpA [57]. The tbpA gene is highly conserved, with 98% identity in deduced sequences between two detected M. catarrhalis strains, whereas tbpB gene displays high-level heterogeneity with 63% identity and 70% similarity in deduced sequences between the same strains [54]. Immunoblot analysis demonstrates that epitopes of M. catarrhalis TbpA and TbpB are antigenically conserved and that there is constitutive expression of the tbp genes [54].
Guinea pig anti-TbpB antiserum displays bactericidal activities and kills heterologous strains within the same family, whereas anti-TbpA antiserum is not bactericidal [54]. Serum IgG antibodies directed to TbpB are detected in patients with bronchiectasis persistently colonized with M. catarrhalis, and these antibodies recognize surface-exposed epitopes of TbpB. However, these surface-exposed epitopes of TbpB are heterogeneous among strains of M. catarrhalis [58]. Serum IgG antibodies to TbpB are detected in children with M. catarrhalis-caused otitis media during their convalescent stage [59]. Serum antibodies against TbpB were detected in all 17 convalescent sera from patients with pulmonary infections caused by M. catarrhalis, whereas the antibodies are detected in only 3 of 17 paired sera from the acute phase of infections in the same patients. However, there was considerable antigenic heterogeneity among TbpBs from different strains after analyzing the reactivity of these anti-sera with heterologous strains. Serum antibodies to TbpA were not found in the same study cohort [60]. All these observations suggest that TbpB is expressed and immunogenic in vivo, and elicits serum functional antibodies; however, it has other characteristics that are less favorable for this antigen as a vaccine target.
2.2.3 Lactoferrin-binding proteins
Lactoferrin-binding proteins (Lbps) are receptors for human lactoferrin on the outer membrane of M. catarrhalis. Two members of Lbps, LbpA and LbpB have been identified so far. LbpA and LbpB have molecular masses of approximately 100 and 84 kDa, respectively [61]. LbpA, similar to TbpA, serves as the transmembrane channel for transport of iron across the outer membrane and is essential for iron acquisition from human lactoferrin. LbpB, similar to TbpB, plays an important facilitative role in the iron acquisition process. M. catarrhalis acquires iron from human lactoferrin by the same mechanism as from human transferrin [57]. Two tested M. catarrhalis strains showed 98% identity in deduced sequences of LbpA and 92% identity in deduced sequences of LbpB. The LbpB of a third M. catarrhalis strain exhibited 77% identity and 84% similarity to the other two LbpB proteins [62].
Mouse anti-sera to recombinant LbpA have no bactericidal effect, whereas anti-sera to recombinant LbpB are found to be weakly bactericidal [62]. Serum antibodies against LbpB have been detected in all of 17 convalescent sera tested from patients with pulmonary infections caused by M. catarrhalis, whereas the antibodies were detected in 3 of total 17 paired sera from acute phase of the infections in the same patients. However, antigenic heterogeneity was found among LbpBs from different strains by analyzing the reactivity of these anti-sera with heterologous strains. Serum antibodies to LbpA are not found in the same study cohort [60]. The current data support LbpB to be investigated further for a M. catarrhalis vaccine antigen candidate; however, it has characteristics that are not as favorable for this antigen to become a vaccine target.
2.2.4 Catarrhalis outer membrane protein B/OMP B2
Catarrhalis outer membrane protein B (CopB), also named as OMP B2, is an iron-repressible protein on the outer membrane of M. catarrhalis [63-65]. It has a molecular mass of 81 kDa with a similar amino acid sequence to those of TonB-dependent OMPs of other gram negative bacteria such as Neisseria gonorrhoeae and Neisseria meningitides [65]. CopB exerts a direct or indirect effect on the ability of M. catarrhalis to utilize iron bound to transferrin and lactoferrin although the mechanism is not completely understood [65]. CopB also contributes to the resistance of M. catarrhalis to normal human serum [63]. A Southern blot analysis showed that the genes encoding CopB proteins existed in all of eight tested M. catarrhalis strains and were fairly well conserved [63]. An mAb reactive with CopB was found to bind a majority (70%) of tested M. catarrhalis strains. Moreover, two strains unreactive to the mAb were both demonstrated to possess one or more antigenic determinants that were also present in the CopB protein of a strain, which reacted with the mAb [63]. Serum IgG to CopB was detected in elderly patients with pneumonia [66]. Sputum IgA to CopB was detected in COPD patients [33]. Salivary IgA was observed in children aged 1 – 24 months with acute respiratory tract infections [31]. Passive immunization with an mAb reactive with CopB enhances the clearance of bacteria from the lung of mice challenged with M. catarrhalis [63]. More studies are needed to reveal the protective responses that CopB could induce to be considered as a vaccine antigen target.
2.2.5 OMP E
OMP E, a 50-kDa OMP of M. catarrhalis, is a trimeric porin [67]. It has borderline homology with long-chain fatty acid transporter (FadL) of E. coli, which is responsible for long-chain fatty acid transport, suggesting that OMP E may also be involved in binding and transport of fatty acids [67]. In an analysis of PCR restriction fragment length polymorphisms, the ompE gene was present in all 19 tested strains from diverse geographic and clinical sources and was the same size (1.4 kb) in all the strains [67]. Gene sequence analysis revealed that the highly conserved genes encoding OMP E remained stable among M. catarrhalis strains during colonization of the human respiratory tract [68]. OMP E expresses at least one surface-exposed epitope, which is highly conserved among M. catarrhalis strains and which is located in the amino-terminal 184 amino acids of the molecule [69].
IgA and IgM to OMP E in sputum supernatants and IgG, IgM and IgA to OMP E in sera were detected in patients with chronic bronchitis [69]. The conservation of OMP E and its surface-exposed epitopes supports OMP E to be studied further as a vaccine candidate antigen target.
2.3 Other OMPs
2.3.1 Moraxella surface proteins
Moraxella surface proteins (Msp)s including Msp22, Msp75 and Msp78 were discovered by genome mining [70]. Msp22 is an ~ 22 kDa lipoprotein of 152 amino acids. The msp22 gene is part of gene cluster that includes a coproporphyrinogen III and GTP cyclohydrolase II, suggesting that Msp22 may be involved in transport of iron and other divalent cations across the outer membrane. Msp22 contains the same peptide CXXCH motif as that of cytochrome C, which is involved in heme binding and exhibits heme-dependent peroxidase activity [71]. Msp75 has a molecular mass of a ~ 75 kDa and shares homology (73% identity, 83% similarity) with succinic semialdehyde dehydrogenase of Psychrobacter species and other gram negative bacteria. Msp75 shares homology with a region of the chromosome of Agrobacterium tumefaciens that is associated with virulence [72]. Msp78, which contains a signal sequence, has high levels of similarity and identity to an anaerobically induced nitrate reductase. Homologs of this protein have been identified as OMPs. Msp22, Msp75 and Msp78 all show 97 – 99% amino acid homology among 10 M. catarrhalis strains tested, and are therefore believed as attractive M. catarrhalis vaccine antigens due to the high conservation [70].
Both systemic and mucosal immunizations of recombinant Msp22 and Msp75 elicit robust serum and mucosal IgG and IgA antibodies in mice [73]. Both rabbit and mouse anti-sera to recombinant proteins Msp22 and Msp75 recognize corresponding native proteins in multiple strains of M. catarrhalis [73]. Serum IgG and sputum IgA to recombinant Msp22, Msp75 and Msp78 have been detected in COPD patients who acquired and cleared M. catarrhalis [70]. Intranasal immunization with recombinant Msp22 significantly reduced the lung bacterial burden in mice challenged with M. catarrhalis [73]. Further studies are warranted to investigate the functions of Msp22 and the protective immune response in humans, which Msp22 may provide as a vaccine antigen. In addition, further studies should explore the in vivo expression of Msp22 on the surface of M. catarrhalis and the potential protective immune response to Msp75 in animal models and humans.
2.3.2 OMP G1
OMP G1 was first identified as one of the 8 OMP proteins among 50 M. catarrhalis clinical isolates, which constantly appears in SDS–PAGE gel after electrophoresis [74]. A further study found that OMP G1 was composed of two proteins, OMP G1a and OMP G1b [75]. OMP G1a has a calculated molecular mass of 16 kDa but appears as 29 kDa in SDS–PAGE due to the aberrant migration in SDS–PAGE probably caused by the lipidation and the high proline content. OMP G1a is a non-heat-modifiable lipoprotein and both native and recombinant OMP G1a contain covalently bound palmitic acid [75,76]. OMP G1b has a molecular mass of 29 kDa and is a heat-modifiable protein containing an unblocked amino-terminus [75]. The sequence of OMP G1a shares homology with several known and hypothetical copper-binding lipoproteins, such as NlpE (CutF) of E. coli, which plays a role in induction of the two-component signal transduction system by adhesion [77]. No homologous sequence has been found for OMP G1b in Gen Bank. Amino acid sequences of OMP G1a are 90 – 100% identical with 21 of the 24 pairs showing 98% or higher identity among 25 strains tested. Amino acid sequences OMP G1b were 92 – 100% identical with 20 of 24 strains showing a 96% or higher identity among the same panel of 25 strains [75]. OMP G1 proteins were also demonstrated to express determinants that are exposed on the surface of the intact M. catarrhalis bacterium [78].
Serum IgG and sputum IgA to OMP G1a develop in COPD patients following acquisition and clearance of M. catarrhalis [76]. Serum IgG antibodies following natural infection were found to direct predominantly at OMP G1a epitopes that are not exposed on the bacterial surface [76]. Future studies should define surface-exposed epitopes to assess whether the protein can be presented to the host in a form, which elicits protective antibodies. OMP G1b should also be investigated on the immunologic properties to be considered as a potential vaccine antigen target.
2.3.3 M35
M35 is a general porin expressed on the outer membrane of M. catarrhalis with a molecular mass of 36.1 kDa [79]. M35 is necessary for the uptake of important energy sources by M. catarrhalis and is likely an essential functional protein for in vivo colonization [80]. M35 may also be involved in resistance mechanisms against aminopenicillins and general stress responses. DNA sequencing has shown that the M35 gene sequence is highly conserved (99.6 – 100% of nucleotides) among 18 tested M. catarrhalis isolates. Mouse anti-M35 sera bound to whole-cell protein preparations from all 15 tested M. catarrhalis isolates in an immune blot assay [79]. Anti-sera from mice immunized with recombinant M35 were not bactericidal but did enhance opsonic activity. Mucosal immunization with recombinant M35 via intra-Peyer’s patch enhanced bacterial clearance from the lungs of mice challenged with M. catarrhalis [81]. Further studies are necessary to reveal the immune responses and potential protective responses in humans for M35 to be considered as a vaccine candidate target.
2.4 Non-protein outer membrane components
2.4.1 Lipooligosaccharide
Lipooligosaccharide (LOS) is a major virulence factor in the outer membrane of M. catarrhalis. It has a molecular mass of 2.5 – 4 kDa [3] and consists of three major serotypes, A, B and C, based on the different carbohydrate groups added in one of the three carbohydrate branch chains, which are linked to the common polysaccharide inner core [82]. M. catarrhalis is basically classified into three serotypes A, B and C, based on the LOS structures, which account for 61.3, 28.8 and 5.3% of the 302 tested strains, respectively [83]. Serotype A and C LOSs share a common N-acetyl-D-glucosamine residue in their LOS branches and induce antibodies cross-reactive to each other, but less so to serotype B LOS [84]. LOS plays a pivotal role in inducing inflammatory responses [85-87]. LOS is also involved in adherence to human epithelial cells [88] and mediating serum resistance [89]. Studies show that LOS is relatively conserved [2,90].
LOS does not contain epitopes recognized by CD4+ T-cells and induces only low level of non-memory antibody response. Serum IgG and sputum IgA to M. catarrhalis LOS have been detected in COPD patients after clearance of M. catarrhalis [22,33,91]. LOS is detoxified by removal of most lipid A moieties, and then covalently conjugated to protein carriers to form dLOS–protein conjugates. The conjugates derived from serotypes A, B and C all elicit high levels of antigen-specific serum and mucosal antibodies in mice and rabbits [92-94]. Bactericidal antibodies are also detected in both mice and rabbits immunized with dLOS–protein conjugates [92-94]. Moreover, intranasal immunization with dLOS–protein conjugates from serotype A enhances the clearance of M. catarrhalis strains of serotypes A and C but not B from the lungs of mice challenged with either serotype [95]. To circumvent the incomplete coverage of any conjugates from the three LOS serotypes, a mutant LOS is produced from an LOS mutant of serotype A strain O35E (O35Elgt5) with a terminal galactose (Gal) residue removed from the LOS oligosaccharide branch, Gala1-4Galb1-4Glc [82]. The dLOS–protein conjugate from O35Elgt5 exhibits broad spectrum coverage, eliciting high levels of serum IgG with bactericidal activity against all three serotypes of M. catarrhalis [82]. Further investigation on protective effects is required to assess the mutant LOS-based conjugate as a vaccine antigen. Another approach has been attempted by combining the dLOS–protein conjugates from all three serotypes of M. catarrhalis as a vaccine antigen [96]. Intranasal immunization of the combined dLOS–protein conjugates induces significant humoral and cell-mediated immune responses, which enhance the clearance of six strains of all three serotypes of M. catarrhalis from the lungs of mice challenged with the bacteria [96]. Thus, LOS-based conjugates are effective vaccine antigens and deserve to be evaluated further.
2.5 Newly identified potential vaccine antigens
More recently, sustained efforts have achieved progresses in identifying more new M. catarrhalis vaccine antigens such as OlpA [97], ORF113 [98] and liposome-associated OMPs [99]. These antigens have shown the features of a vaccine antigen such as immunogenic, exposed or partially exposed to the surface, and conserved. Some of the antigens play key roles in the pathogenesis of M. catarrhalis infections such as NP colonization and complement resistance [97-99]. Further investigation of these target molecules may prove they are potential M. catarrhalis vaccine candidates.
3. Efficacy evaluation
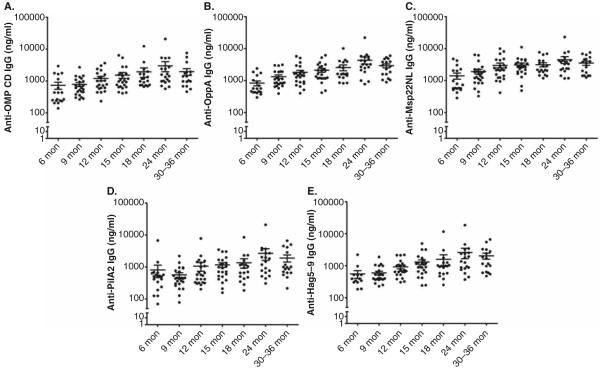
The current approach for assessing the efficacy of M. catarrhalis vaccine antigen targets includes ELISA, bactericidal and opsonophagocytosis assays, adherence inhibition assays, cell-mediated immune assays, and animal models. ELISA is used as a primary method to determine the immunogenicity of vaccine antigen targets. Eliciting effective serum and mucosal antibody responses in the host is required for antigens to be considered for further study. We have selected five M. catarrhalis proteins (OMP CD, OppA, Msp22, Hag and PilA2) as vaccine candidates and detected natural serum IgG responses to these proteins in children with M. catarrhalis NP colonization with or without AOM at ages 6, 9, 12, 15, 18, 24 and 30 – 36 months. Figure 1 shows that all antigens were immunogenic in children at age 6 – 36 months, and there was a rise in antibody concentrations over time as the child ages, although the IgG values are diverse with a spread of >2 log in titer (100 – 20,000 ng/mL). Thus, detecting natural antibody responses by using clinical samples is effective to assess the immunogenicity of M. catarrhalis antigens in targeted populations.
Figure 1.
Natural acquisition of serum IgG to 5 recombinant M. catarrhalis proteins, OMP CD (A), OppA (B), Msp22NL (C), PilA2 (D) and Hag5-9 (E) in children with M. catarrhalis nasopharyngeal (NP) colonization with or without acute otitis media (AOM) at the age of 6 – 36 months. Msp22NL is a recombinant non-lipidated form of Msp22 and Hag5-9 is a truncated MID/Hag spanning the amino acids 385 – 863 of the natural protein. The results are represented by mean ± standard error. n = 17 – 24 for each age time point.
Bactericidal assays, opsonophagocytosis assays and adherence assays are widely used in vitro methods to evaluate functional antibodies and may provide information on correlates of protection. Bactericidal and opsonophagocytosis assays examine bacterial surface-exposed antibodies that fix complement on the bacterial cell membrane leading to cell lysis and/or opsonophagocytosis of the bacteria. Adherence assays focus on the inhibitory activity of antibodies against bacterial surface-exposed epitopes that allow the bacteria to adhere to human respiratory tract epithelial cells. Cell-mediated immunity is the primary host response through which vaccines exert antigen-specific defense against the pathogen. The efficacy of M. catarrhalis antigen targets can be evaluated at various stages and aspects of the cell-mediated immune responses such as antigen processing by antigen-presenting cells including dendritic cells and macrophages, and CD4+ T-cell activation (Th-1 and Th-2 cell responses), memory responses of T cells and B cells, and so on.
M. catarrhalis is exclusively a human pathogen and does not cause respiratory infectious diseases in animals. M. catarrhalis is readily cleared within 24 – 48 h after inoculation into mouse lungs 2 and 5 days after inoculation into chinchilla middle ears [90]. Currently, there is not an animal model of M. catarrhalis disease available for vaccine testing. The mouse lung clearance model is broadly used to evaluate in vivo protection response of M. catarrhalis antigens. By systemic and/or intranasal immunization, some M. catarrhalis antigen targets have demonstrated effectiveness in enhancing clearance of bacteria from mouse lungs challenged with M. catarrhalis strains. The chinchilla nasopharynx can be colonized by M. catarrhalis for several weeks, and therefore is another animal model. However, none of the in vitro functional assays, the mouse lung clearance model and the chinchilla NP colonization model demonstrate a definite correlation with protective immune responses in humans. Therefore, animal models for M. catarrhalis diseases such as otitis media and lung infections are highly desired to be developed for M. catarrhalis vaccine target evaluation.
4. Conclusions
M. catarrhalis is an important pathogen causing AOM in children and acute exacerbation of COPD in adults. Elucidation of the roles that the many possible targets play in M. catarrhalis pathogenesis will guide antigen selection and efficacy assessments. A panel of potential vaccine antigens has been identified and tested in animal models or with human samples.
5. Expert opinion
The natural immune responses of some promising M. catarrhalis vaccine protein targets, such as OppA and Msp22, have been investigated by our laboratory recently. A number of OMPs such as MID/Hag, McaP, OMP CD and PilA have been shown to be involved in the adherence of M. catarrhalis to respiratory epithelial cells. The antibodies elicited by some of these molecules have been shown to be functional, blocking the adhesion of M. catarrhalis and therefore protecting against NP colonization and further pathogenesis. Therefore, we envision an M. catarrhalis vaccine that includes three antigens with perhaps two antigens that play a role in M. catarrhalis adherence to human NP and/or lung bronchial epithelial cells. We did not observe a difference in the naturally acquired serum antibody levels to the M. catarrhalis antigens tested among different races and ethnicity, which represents a diverse race and ethnic enrollment in a developed country. However, tests may be required to determine the homogeneity of the responses to these potential antigens especially focusing on some specific high-risk target populations.
By using bioinformatics such as sequence analysis, and structure and function predictions, reverse vaccinology [100] has been applied to identify many M. catarrhalis vaccine candidates. Protein antigens such as OppA and Msp22, -75 and -78, and pili have been identified using this genome-based approach. Subsequently, these antigens have been characterized for their immunogenicity and potential immune protection responses in vitro and in vivo. With much reduced time for antigen identification, reverse vaccinology may be more broadly used for identifying more promising M. catarrhalis protein vaccine candidates in the future. The majority (89%) of M. catarrhalis isolates from patients with lower respiratory tract infections are resistant to complement-mediated killing [101]. Hence, targeting surface molecules involved in serum resistance may help to identify efficacious M. catarrhalis antigens. OMP CD, OMP E, CopB, LOS Pk epitope and UspA proteins have all been found to contribute to serum resistance of M. catarrhalis [102]. Thus, complement-mediated killing of M. catarrhalis may be revived if these antigen targets on the bacterial surface could be blocked by specific serum antibodies through immunization. In vivo expression of antigens such as UspA proteins [103] and MID/Hag [29] is subject to phase variation, which is regulated by phase varions [104]. For vaccine antigen selection, it is necessary to understand what different host conditions modulate phase variation. An antigen constantly and abundantly expressed on the surface of M. catarrhalis without phase variation in both healthy and diseased conditions is a better vaccine candidate. A significant difference in expression of MID/Hag gene and serotype A and B LOS has been observed between M. catarrahlis isolates from children and those from adults with respiratory diseases [105]. The decreased expression of virulence-associated gene MID/Hag and biofilm formation in the M. catarrhalis isolated from adults compared to those from children is suggested as an immune evasion mechanism by the organism [105]. Therefore, the age factor of the target population, children, adults or both, should be evaluated if antigens such as Hag or LOS are selected as vaccine candidates. M. catarrhalis is a mucosal pathogen and the NP and bronchial mucosa is the frontline where M. catarrhalis lands and causes diseases in the human respiratory tract. Induction of a mucosal immune response may be important for host immunity against M. catarrhalis. Current and future M. catarrhalis vaccine studies should place priority on characterizing the role of the mucosal immune response in protection. Developing safe and potent mucosal adjuvants for humans is another important goal for M. catarrhalis vaccine research. The current immunologic data on M. catarrhalis antigens have been mostly collected from animals. Developing an animal model mimicking M. catarrhalis-caused diseases, such as otitis media and lung infections, would be of great value for M. catarrhalis vaccine target assessment. Mice with human immune system components (also termed ‘humanized’ mice) have been developed as in vivo models to study the infections with human pathogens [106]. Humanized mice are explored as hosts for many pathogens that are relevant to human disease but do not infect other animal species. In these models, human immune cells or tissues such as hematopoietic progenitor cells or fetal liver and thymus tissues (bone marrow, liver and thymus ) are transferred into immune-compromised mice such as nonobese diabetic/severe combined immunodeficiency mice [106,107]. The reconstituted mice can produce pathogen-specific human immune responses to varying degrees after infection by human pathogens [106,107]. Immunization of humanized mice with vaccine antigens also induces immunogen-specific human humoral and cell-mediated immune responses [106,108,109]. Therefore, we hope that the lack of an animal model for M. catarrhalis infections will be solved by using humanized mice in the future.
Although natural antibody responses are detected in clinical human samples, vaccination-induced immune responses for M. catarrhalis antigens have not been tested in humans yet. The safety, immunogenicity and antigen-specific protective immune responses of the current potential M. catarrhalis antigens should be evaluated in clinical trials. It is unlikely that an M. catarrhalis vaccine will have only one single antigen component [110]. A combined LOS–protein conjugate vaccine consisting of three serotypes of detoxified LOS has demonstrated broad-spectrum protection against all three serotypes of M. catarrhalis, accounting for > 95% of the total strains and clinical isolates. A combined LOS–protein conjugate vaccine that includes three proteins described in this review that have different functions would represent an attractive vaccine combination product.
Article highlights.
Moraxella catarrhalis is an exclusive human mucosal pathogen that causes respiratory tract infections in children and adults.
The need to target M. catarrhalis with vaccine development is pressing because M. catarrhalis infections cause a heavy socioeconomic burden worldwide and almost all M. catarrhalis are resistant to β-lactam antibiotics.
Thus far, a panel of potential M. catarrhalis vaccine antigens have been identified and characterized.
Developing animal models for M. catarrhalis diseases and uncovering the role and mechanisms of the mucosal immune response are crucial for better evaluating M. catarrhalis vaccine targets.
Clinical trials will be the next milestone for M. catarrhalis vaccine development.
Acknowledgments
D Ren is supported by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under award number R03AI113649. ME Pichichero is supported by the National Institute on Deafness and Other Communication Disorders (NIDCD) of the NIH by award number R0108671.
Footnotes
Declaration of interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Hays JP. The Genus Moraxella. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E, editors. The Prokaryotes. 3rd Springer-Verlag; New York, LLC; New York, NY: 2005. pp. 958–87. [Google Scholar]
- 2.Verduin CM, Hol C, Fleer A, et al. Moraxella catarrhalis: from emerging to established pathogen. Clin Microbiol Rev. 2002;15:125–44. doi: 10.1128/CMR.15.1.125-144.2002. This review comprehensively summarizes various aspects of Moraxella catarrhalis including details in microbiologic features and M. catarrhalis-caused infections. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy TF, Parameswaran GI. Moraxella catarrhalis, a human respiratory tract pathogen. Clin Infect Dis. 2009;49:124–31. doi: 10.1086/599375. This review discusses the M. catarrhalis-caused infectious diseases in details. [DOI] [PubMed] [Google Scholar]
- 4.Casey JR, Kaur R, Friedel VC, et al. Acute otitis media otopathogens during 2008 to 2010 in Rochester, New York. Pediatr Infect Dis J. 2013;32:805–9. doi: 10.1097/INF.0b013e31828d9acc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casey JR, Pichichero ME. Changes in frequency and pathogens causing acute otitis media in 1995-2003. Pediatr Infect Dis J. 2004;23:824–8. doi: 10.1097/01.inf.0000136871.51792.19. [DOI] [PubMed] [Google Scholar]
- 6.Casey JR, Adlowitz DG, Pichichero ME. New patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2010;29:304–9. doi: 10.1097/INF.0b013e3181c1bc48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casey JR, Kauer R, Pichichero ME. Otopathogens Causing Acute Otitis Media in the 13-Valent Pneumococcal Conjugate Vaccine Era. 18th International Symposium on Recent Advances in Otitis Media; National Harbor, MD. 2015. [Google Scholar]
- 8.Monasta L, Ronfani L, Marchetti F, et al. Burden of disease caused by otitis media: systematic review and global estimates. PLoS One. 2012;7:e36226. doi: 10.1371/journal.pone.0036226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casey JR, Pichichero ME. Payment Analysis of Two Diagnosis and Management Approaches of Acute Otitis Media. Clin Pediatr (Phila) 2014;53:865–73. doi: 10.1177/0009922814533592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy TF, Brauer AL, Grant BJ, et al. Moraxella catarrhalis in chronic obstructive pulmonary disease: burden of disease and immune response. Am J Respir Crit Care Med. 2005;172:195–9. doi: 10.1164/rccm.200412-1747OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones MM, Johnson A, Koszelak-Rosenblum M, et al. Role of the Oligopeptide Permease ABC Transporter of Moraxella catarrhalis in Nutrient Acquisition and Persistence in the Respiratory Tract. Infect Immun. 2014;82:4758–66. doi: 10.1128/IAI.02185-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy TF. Vaccine development for non-typeable Haemophilus influenzae and Moraxella catarrhalis: progress and challenges. Expert Rev Vaccines. 2005;4:843–53. doi: 10.1586/14760584.4.6.843. This review covers the main aspects on developing M. catarrhalis vaccines. [DOI] [PubMed] [Google Scholar]
- 13.Sarwar J, Campagnari AA, Kirkham C, et al. Characterization of an antigenically conserved heat-modifiable major outer membrane protein of Branhamella catarrhalis. Infect Immun. 1992;60:804–9. doi: 10.1128/iai.60.3.804-809.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reddy MS, Murphy TF, Faden HS, et al. Middle ear mucin glycoprotein: purification and interaction with nontypable Haemophilus influenzae and Moraxella catarrhalis. Otolaryngol Head Neck Surg. 1997;116:175–80. doi: 10.1016/S0194-59989770321-8. [DOI] [PubMed] [Google Scholar]
- 15.Holm MM, Vanlerberg SL, Foley IM, et al. The Moraxella catarrhalis porin-like outer membrane protein CD is an adhesin for human lung cells. Infect Immun. 2004;72:1906–13. doi: 10.1128/IAI.72.4.1906-1913.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy TF, Kirkham C, Lesse AJ. The major heat-modifiable outer membrane protein CD is highly conserved among strains of Branhamella catarrhalis. Mol Microbiol. 1993;10:87–97. doi: 10.1111/j.1365-2958.1993.tb00906.x. [DOI] [PubMed] [Google Scholar]
- 17.Murphy TF, Kirkham C, DeNardin E, et al. Analysis of antigenic structure and human immune response to outer membrane protein CD of Moraxella catarrhalis. Infect Immun. 1999;67:4578–85. doi: 10.1128/iai.67.9.4578-4585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Becker PD, Bertot GM, Souss D, et al. Intranasal vaccination with recombinant outer membrane protein CD and adamantylamide dipeptide as the mucosal adjuvant enhances pulmonary clearance of Moraxella catarrhalis in an experimental murine model. Infect Immun. 2007;75:1778–84. doi: 10.1128/IAI.01081-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy TF, Kyd JM, John A, et al. Enhancement of pulmonary clearance of Moraxella (Branhamella) catarrhalis following immunization with outer membrane protein CD in a mouse model. J Infect Dis. 1998;178:1667–75. doi: 10.1086/314501. [DOI] [PubMed] [Google Scholar]
- 20.Harabuchi Y, Murakata H, Goh M, et al. Serum antibodies specific to CD outer membrane protein of Moraxella catarrhalis, P6 outer membrane protein of non-typeable Haemophilus influenzae and capsular polysaccharides of Streptococcus pneumoniae in children with otitis media with effusion. Acta Otolaryngol. 1998;118:826–32. doi: 10.1080/00016489850182521. [DOI] [PubMed] [Google Scholar]
- 21.Yang YP, Myers LE, McGuinness U, et al. The major outer membrane protein, CD, extracted from Moraxella (Branhamella) catarrhalis is a potential vaccine antigen that induces bactericidal antibodies. FEMS Immunol Med Microbiol. 1997;17:187–99. doi: 10.1111/j.1574-695X.1997.tb01012.x. [DOI] [PubMed] [Google Scholar]
- 22.Murphy TF, Brauer AL, Aebi C, et al. Identification of surface antigens of Moraxella catarrhalis as targets of human serum antibody responses in chronic obstructive pulmonary disease. Infect Immun. 2005;73:3471–8. doi: 10.1128/IAI.73.6.3471-3478.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu DF, McMichael JC, Baker SM. Moraxella catarrhalis outer membrane protein CD elicits antibodies that inhibit CD binding to human mucin and enhance pulmonary clearance of M. catarrhalis in a mouse model. Infect Immun. 2007;75:2818–25. doi: 10.1128/IAI.00074-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pearson MM, Lafontaine ER, Wagner NJ, et al. A hag mutant of Moraxella catarrhalis strain O35E is deficient in hemagglutination, autoagglutination, and immunoglobulin D-binding activities. Infect Immun. 2002;70:4523–33. doi: 10.1128/IAI.70.8.4523-4533.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forsgren A, Brant M, Karamehmedovic M, et al. The immunoglobulin D-binding protein MID from Moraxella catarrhalis is also an adhesin. Infect Immun. 2003;71:3302–9. doi: 10.1128/IAI.71.6.3302-3309.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forsgren A, Brant M, Mollenkvist A, et al. Isolation and characterization of a novel IgD-binding protein from Moraxella catarrhalis. J Immunol. 2001;167:2112–20. doi: 10.4049/jimmunol.167.4.2112. [DOI] [PubMed] [Google Scholar]
- 27.Bullard B, Lipski SL, Lafontaine ER. Hag directly mediates the adherence of Moraxella catarrhalis to human middle ear cells. Infect Immun. 2005;73:5127–36. doi: 10.1128/IAI.73.8.5127-5136.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LaFontaine ER, Snipes LE, Bullard B, et al. Identification of domains of the Hag/MID surface protein recognized by systemic and mucosal antibodies in adults with chronic obstructive pulmonary disease following clearance of Moraxella catarrhalis. Clin Vaccine Immunol. 2009;16:653–9. doi: 10.1128/CVI.00460-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mollenkvist A, Nordstrom T, Hallden C, et al. The Moraxella catarrhalis immunoglobulin D-binding protein MID has conserved sequences and is regulated by a mechanism corresponding to phase variation. J Bacteriol. 2003;185:2285–95. doi: 10.1128/JB.185.7.2285-2295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verhaegh SJ, de Vogel CP, Riesbeck K, et al. Temporal development of the humoral immune response to surface antigens of Moraxella catarrhalis in young infants. Vaccine. 2011;29:5603–10. doi: 10.1016/j.vaccine.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 31.Meier PS, Freiburghaus S, Martin A, et al. Mucosal immune response to specific outer membrane proteins of Moraxella catarrhalis in young children. Pediatr Infect Dis J. 2003;22:256–62. doi: 10.1097/01.inf.0000054827.86683.bd. [DOI] [PubMed] [Google Scholar]
- 32.Tan TT, Christensen JJ, Dziegiel MH, et al. Comparison of the serological responses to Moraxella catarrhalis immunoglobulin D-binding outer membrane protein and the ubiquitous surface proteins A1 and A2. Infect Immun. 2006;74:6377–86. doi: 10.1128/IAI.00702-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphy TF, Brauer AL, Aebi C, et al. Antigenic specificity of the mucosal antibody response to Moraxella catarrhalis in chronic obstructive pulmonary disease. Infect Immun. 2005;73:8161–6. doi: 10.1128/IAI.73.12.8161-8166.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Forsgren A, Brant M, Riesbeck K. Immunization with the truncated adhesin moraxella catarrhalis immunoglobulin D-binding protein (MID764-913) is protective against M. catarrhalis in a mouse model of pulmonary clearance. J Infect Dis. 2004;190:352–5. doi: 10.1086/422155. [DOI] [PubMed] [Google Scholar]
- 35.Timpe JM, Holm MM, Vanlerberg SL, et al. Identification of a Moraxella catarrhalis outer membrane protein exhibiting both adhesin and lipolytic activities. Infect Immun. 2003;71:4341–50. doi: 10.1128/IAI.71.8.4341-4350.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lipski SL, Akimana C, Timpe JM, et al. The Moraxella catarrhalis autotransporter McaP is a conserved surface protein that mediates adherence to human epithelial cells through its N-terminal passenger domain. Infect Immun. 2007;75:314–24. doi: 10.1128/IAI.01330-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balder R, Hassel J, Lipski S, et al. Moraxella catarrhalis strain O35E expresses two filamentous hemagglutinin-like proteins that mediate adherence to human epithelial cells. Infect Immun. 2007;75:2765–75. doi: 10.1128/IAI.00079-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Plamondon P, Luke NR, Campagnari AA. Identification of a novel two-partner secretion locus in Moraxella catarrhalis. Infect Immun. 2007;75:2929–36. doi: 10.1128/IAI.00396-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guedin S, Willery E, Tommassen J, et al. Novel topological features of FhaC, the outer membrane transporter involved in the secretion of the Bordetella pertussis filamentous hemagglutinin. J Biol Chem. 2000;275:30202–10. doi: 10.1074/jbc.M005515200. [DOI] [PubMed] [Google Scholar]
- 40.Luke NR, Howlett AJ, Shao J, et al. Expression of type IV pili by Moraxella catarrhalis is essential for natural competence and is affected by iron limitation. Infect Immun. 2004;72:6262–70. doi: 10.1128/IAI.72.11.6262-6270.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luke-Marshall NR, Sauberan SL, Campagnari AA. Comparative analyses of the Moraxella catarrhalis type-IV pilus structural subunit PilA. Gene. 2011;477:19–23. doi: 10.1016/j.gene.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 42.Luke NR, Jurcisek JA, Bakaletz LO, et al. Contribution of Moraxella catarrhalis type IV pili to nasopharyngeal colonization and biofilm formation. Infect Immun. 2007;75:5559–64. doi: 10.1128/IAI.00946-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cope LD, Lafontaine ER, Slaughter CA, et al. Characterization of the Moraxella catarrhalis uspA1 and uspA2 genes and their encoded products. J Bacteriol. 1999;181:4026–34. doi: 10.1128/jb.181.13.4026-4034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoiczyk E, Roggenkamp A, Reichenbecher M, et al. Structure and sequence analysis of Yersinia YadA and Moraxella UspAs reveal a novel class of adhesins. EMBO J. 2000;19:5989–99. doi: 10.1093/emboj/19.22.5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nordstrom T, Blom AM, Forsgren A, et al. The emerging pathogen Moraxella catarrhalis interacts with complement inhibitor C4b binding protein through ubiquitous surface proteins A1 and A2. J Immunol. 2004;173:4598–606. doi: 10.4049/jimmunol.173.7.4598. [DOI] [PubMed] [Google Scholar]
- 46.Nordstrom T, Blom AM, Tan TT, et al. Ionic binding of C3 to the human pathogen Moraxella catarrhalis is a unique mechanism for combating innate immunity. J Immunol. 2005;175:3628–36. doi: 10.4049/jimmunol.175.6.3628. [DOI] [PubMed] [Google Scholar]
- 47.Meier PS, Troller R, Grivea IN, et al. The outer membrane proteins UspA1 and UspA2 of Moraxella catarrhalis are highly conserved in nasopharyngeal isolates from young children. Vaccine. 2002;20:1754–60. doi: 10.1016/s0264-410x(02)00030-0. [DOI] [PubMed] [Google Scholar]
- 48.Brooks MJ, Sedillo JL, Wagner N, et al. Modular arrangement of allelic variants explains the divergence in Moraxella catarrhalis UspA protein function. Infect Immun. 2008;76:5330–40. doi: 10.1128/IAI.00573-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brooks MJ, Sedillo JL, Wagner N, et al. Moraxella catarrhalis binding to host cellular receptors is mediated by sequence-specific determinants not conserved among all UspA1 protein variants. Infect Immun. 2008;76:5322–9. doi: 10.1128/IAI.00572-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hill DJ, Whittles C, Virji M. A novel group of Moraxella catarrhalis UspA proteins mediates cellular adhesion via CEACAMs and vitronectin. PLoS One. 2012;7:e45452. doi: 10.1371/journal.pone.0045452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McMichael JC, Fiske MJ, Fredenburg RA, et al. Isolation and characterization of two proteins from Moraxella catarrhalis that bear a common epitope. Infect Immun. 1998;66:4374–81. doi: 10.1128/iai.66.9.4374-4381.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen D, McMichael JC, VanDerMeid KR, et al. Evaluation of purified UspA from Moraxella catarrhalis as a vaccine in a murine model after active immunization. Infect Immun. 1996;64:1900–5. doi: 10.1128/iai.64.6.1900-1905.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang M, Johnson A, Murphy TF. Characterization and evaluation of the Moraxella catarrhalis oligopeptide permease A as a mucosal vaccine antigen. Infect Immun. 2011;79:846–57. doi: 10.1128/IAI.00314-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Myers LE, Yang YP, Du RP, et al. The transferrin binding protein B of Moraxella catarrhalis elicits bactericidal antibodies and is a potential vaccine antigen. Infect Immun. 1998;66:4183–92. doi: 10.1128/iai.66.9.4183-4192.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luke NR, Russo TA, Luther N, et al. Use of an isogenic mutant constructed in Moraxella catarrhalis To identify a protective epitope of outer membrane protein B1 defined by monoclonal antibody 11C6. Infect Immun. 1999;67:681–7. doi: 10.1128/iai.67.2.681-687.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luke NR, Campagnari AA. Construction and characterization of Moraxella catarrhalis mutants defective in expression of transferrin receptors. Infect Immun. 1999;67:5815–19. doi: 10.1128/iai.67.11.5815-5819.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gray-Owen SD, Schryvers AB. Bacterial transferrin and lactoferrin receptors. Trends Microbiol. 1996;4:185–91. doi: 10.1016/0966-842x(96)10025-1. [DOI] [PubMed] [Google Scholar]
- 58.Sethi S, Hill SL, Murphy TF. Serum antibodies to outer membrane proteins (OMPs) of Moraxella (Branhamella) catarrhalis in patients with bronchiectasis: identification of OMP B1 as an important antigen. Infect Immun. 1995;63:1516–20. doi: 10.1128/iai.63.4.1516-1520.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Campagnari AA, Ducey TF, Rebmann CA. Outer membrane protein B1, an iron-repressible protein conserved in the outer membrane of Moraxella (Branhamella) catarrhalis, binds human transferrin. Infect Immun. 1996;64:3920–4. doi: 10.1128/iai.64.9.3920-3924.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu RH, Bonnah RA, Ainsworth S, et al. Analysis of the immunological responses to transferrin and lactoferrin receptor proteins from Moraxella catarrhalis. Infect Immun. 1999;67:3793–9. doi: 10.1128/iai.67.8.3793-3799.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schryvers AB, Bonnah R, Yu RH, et al. Bacterial lactoferrin receptors. Adv Exp Med Biol. 1998;443:123–33. doi: 10.1007/978-1-4757-9068-9_15. [DOI] [PubMed] [Google Scholar]
- 62.Du RP, Wang Q, Yang YP, et al. Cloning and expression of the Moraxella catarrhalis lactoferrin receptor genes. Infect Immun. 1998;66:3656–65. doi: 10.1128/iai.66.8.3656-3665.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Helminen ME, Maciver I, Latimer JL, et al. A major outer membrane protein of Moraxella catarrhalis is a target for antibodies that enhance pulmonary clearance of the pathogen in an animal model. Infect Immun. 1993;61:2003–10. doi: 10.1128/iai.61.5.2003-2010.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Campagnari AA, Shanks KL, Dyer DW. Growth of Moraxella catarrhalis with human transferrin and lactoferrin: expression of iron-repressible proteins without siderophore production. Infect Immun. 1994;62:4909–14. doi: 10.1128/iai.62.11.4909-4914.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aebi C, Stone B, Beucher M, et al. Expression of the CopB outer membrane protein by Moraxella catarrhalis is regulated by iron and affects iron acquisition from transferrin and lactoferrin. Infect Immun. 1996;64:2024–30. doi: 10.1128/iai.64.6.2024-2030.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Helminen ME, Beach R, Maciver I, et al. Human immune response against outer membrane proteins of Moraxella (Branhamella) catarrhalis determined by immunoblotting and enzyme immunoassay. Clin Diagn Lab Immunol. 1995;2:35–9. doi: 10.1128/cdli.2.1.35-39.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bhushan R, Craigie R, Murphy TF. Molecular cloning and characterization of outer membrane protein E of Moraxella (Branhamella) catarrhalis. J Bacteriol. 1994;176:6636–43. doi: 10.1128/jb.176.21.6636-6643.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martin NL, Rawling EG, Wong RS, et al. Conservation of surface epitopes in Pseudomonas aeruginosa outer membrane porin protein OprF. FEMS Microbiol Lett. 1993;113:261–6. doi: 10.1111/j.1574-6968.1993.tb06524.x. [DOI] [PubMed] [Google Scholar]
- 69.Bhushan R, Kirkham C, Sethi S, et al. Antigenic characterization and analysis of the human immune response to outer membrane protein E of Branhamella catarrhalis. Infect Immun. 1997;65:2668–75. doi: 10.1128/iai.65.7.2668-2675.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ruckdeschel EA, Kirkham C, Lesse AJ, et al. Mining the Moraxella catarrhalis genome: identification of potential vaccine antigens expressed during human infection. Infect Immun. 2008;76:1599–607. doi: 10.1128/IAI.01253-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smidt M, Battig P, Verhaegh SJ, et al. Comprehensive antigen screening identifies Moraxella catarrhalis proteins that induce protection in a mouse pulmonary clearance model. PLoS One. 2013;8:e64422. doi: 10.1371/journal.pone.0064422. This article employs a novel and effective method of ANTIGENome technology for M. catarralis antigen screening. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cullen PA, Lo M, Bulach DM, et al. Construction and evaluation of a plasmid vector for the expression of recombinant lipoproteins in Escherichia coli. Plasmid. 2003;49:18–29. doi: 10.1016/s0147-619x(02)00150-6. [DOI] [PubMed] [Google Scholar]
- 73.Ruckdeschel EA, Brauer AL, Johnson A, et al. Characterization of proteins Msp22 and Msp75 as vaccine antigens of Moraxella catarrhalis. Vaccine. 2009;27:7065–72. doi: 10.1016/j.vaccine.2009.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bartos LC, Murphy TF. Comparison of the outer membrane proteins of 50 strains of Branhamella catarrhalis. J Infect Dis. 1988;158:761–5. doi: 10.1093/infdis/158.4.761. [DOI] [PubMed] [Google Scholar]
- 75.Adlowitz DG, Hiltke T, Lesse AJ, et al. Identification and characterization of outer membrane proteins G1a and G1b of Moraxella catarrhalis. Vaccine. 2004;22:2533–40. doi: 10.1016/j.vaccine.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 76.Adlowitz DG, Sethi S, Cullen P, et al. Human antibody response to outer membrane protein G1a, a lipoprotein of Moraxella catarrhalis. Infect Immun. 2005;73:6601–7. doi: 10.1128/IAI.73.10.6601-6607.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.DiGiuseppe PA, Silhavy TJ. Signal detection and target gene induction by the CpxRA two-component system. J Bacteriol. 2003;185:2432–40. doi: 10.1128/JB.185.8.2432-2440.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Murphy TF, Bartos LC. Surface-exposed and antigenically conserved determinants of outer membrane proteins of Branhamella catarrhalis. Infect Immun. 1989;57:2938–41. doi: 10.1128/iai.57.10.2938-2941.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Easton DM, Smith A, Gallego SG, et al. Characterization of a novel porin protein from Moraxella catarrhalis and identification of an immunodominant surface loop. J Bacteriol. 2005;187:6528–35. doi: 10.1128/JB.187.18.6528-6535.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Easton DM, Maier E, Benz R, et al. Moraxella catarrhalis M35 is a general porin that is important for growth under nutrient-limiting conditions and in the nasopharynges of mice. J Bacteriol. 2008;190:7994–8002. doi: 10.1128/JB.01039-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Easton DM, Cripps AW, Foxwell AR, et al. Mucosal immunization with the Moraxella Catarrhalis porin m35 induces enhanced bacterial clearance from the lung: a possible role for opsonophagocytosis. Front Immunol. 2011;2:13. doi: 10.3389/fimmu.2011.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ren D, Yu S, Gao S, et al. Mutant lipooligosaccharide-based conjugate vaccine demonstrates a broad-spectrum effectiveness against Moraxella catarrhalis. Vaccine. 2011;29:4210–17. doi: 10.1016/j.vaccine.2011.03.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vaneechoutte M, Verschraegen G, Claeys G, et al. Serological typing of Branhamella catarrhalis strains on the basis of lipopolysaccharide antigens. J Clin Microbiol. 1990;28:182–7. doi: 10.1128/jcm.28.2.182-187.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hu WG, Chen J, McMichael JC, et al. Functional characteristics of a protective monoclonal antibody against serotype A and C lipooligosaccharides from Moraxella catarrhalis. Infect Immun. 2001;69:1358–63. doi: 10.1128/IAI.69.3.1358-1363.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xie H, Gu XX. Moraxella catarrhalis lipooligosaccharide selectively upregulates ICAM-1 expression on human monocytes and stimulates adjacent naive monocytes to produce TNF-alpha through cellular cross-talk. Cell Microbiol. 2008;10:1453–67. doi: 10.1111/j.1462-5822.2008.01138.x. [DOI] [PubMed] [Google Scholar]
- 86.Hassan F, Ren D, Zhang W, et al. Role of c-Jun N-terminal protein kinase 1/2 (JNK1/2) in macrophage-mediated MMP-9 production in response to Moraxella catarrhalis lipooligosaccharide (LOS) PLoS One. 2012;7:e37912. doi: 10.1371/journal.pone.0037912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hassan F, Ren D, Zhang W, et al. Moraxella catarrhalis activates murine macrophages through multiple toll like receptors and has reduced clearance in lungs from TLR4 mutant mice. PLoS One. 2012;7:e37610. doi: 10.1371/journal.pone.0037610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Peng D, Hong W, Choudhury BP, et al. Moraxella catarrhalis bacterium without endotoxin, a potential vaccine candidate. Infect Immun. 2005;73:7569–77. doi: 10.1128/IAI.73.11.7569-7577.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zaleski A, Scheffler NK, Densen P, et al. Lipooligosaccharide P(k) (Galalpha1-4Galbeta1-4Glc) epitope of moraxella catarrhalis is a factor in resistance to bactericidal activity mediated by normal human serum. Infect Immun. 2000;68:5261–8. doi: 10.1128/iai.68.9.5261-5268.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Murphy TF. Branhamella catarrhalis: epidemiology, surface antigenic structure, and immune response. Microbiol Rev. 1996;60:267–79. doi: 10.1128/mr.60.2.267-279.1996. This comprehensive review covers the details of some major M. catarrhalis antigens. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schwingel JM, Edwards KJ, Cox AD, et al. Use of Moraxella catarrhalis lipooligosaccharide mutants to identify specific oligosaccharide epitopes recognized by human serum antibodies. Infect Immun. 2009;77:4548–58. doi: 10.1128/IAI.00294-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gu XX, Chen J, Barenkamp SJ, et al. Synthesis and characterization of lipooligosaccharide-based conjugates as vaccine candidates for Moraxella (Branhamella) catarrhalis. Infect Immun. 1998;66:1891–7. doi: 10.1128/iai.66.5.1891-1897.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yu S, Gu XX. Synthesis and characterization of lipooligosaccharidebased conjugate vaccines for serotype B Moraxella catarrhalis. Infect Immun. 2005;73:2790–6. doi: 10.1128/IAI.73.5.2790-2796.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yu S, Gu XX. Biological and immunological characteristics of lipooligosaccharide-based conjugate vaccines for serotype C Moraxella catarrhalis. Infect Immun. 2007;75:2974–80. doi: 10.1128/IAI.01915-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jiao X, Hirano T, Hou Y, et al. Specific immune responses and enhancement of murine pulmonary clearance of Moraxella catarrhalis by intranasal immunization with a detoxified lipooligosaccharide conjugate vaccine. Infect Immun. 2002;70:5982–9. doi: 10.1128/IAI.70.11.5982-5989.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ren D, Xie H, Zhang W, et al. Intranasal immunization of the combined lipooligosaccharide conjugates protects mice from the challenges with three serotypes of Moraxella catarrhalis. PLoS One. 2011;6:e29553. doi: 10.1371/journal.pone.0029553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brooks MJ, Laurence CA, Hansen EJ, et al. Characterization of the Moraxella catarrhalis opa-like protein, OlpA, reveals a phylogenetically conserved family of outer membrane proteins. J Bacteriol. 2007;189:76–82. doi: 10.1128/JB.00788-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang W, Joslin SN, Pybus C, et al. Identification of an outer membrane lipoprotein involved in nasopharyngeal colonization by Moraxella catarrhalis in an animal model. Infect Immun. 2014;82:2287–99. doi: 10.1128/IAI.01745-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Augustyniak D, Mleczko J, Gutowicz J. The immunogenicity of the liposomeassociated outer membrane proteins (OMPs) of Moraxella catarrhalis. Cell Mol Biol Lett. 2010;15:70–89. doi: 10.2478/s11658-009-0035-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Masignani V, Rappuoli R, Pizza M. Reverse vaccinology: a genome-based approach for vaccine development. Expert Opin Biol Ther. 2002;2:895–905. doi: 10.1517/14712598.2.8.895. [DOI] [PubMed] [Google Scholar]
- 101.Hol C, Verduin CM, Van Dijke EE, et al. Complement resistance is a virulence factor of Branhamella (Moraxella) catarrhalis. FEMS Immunol Med Microbiol. 1995;11:207–11. doi: 10.1111/j.1574-695X.1995.tb00118.x. [DOI] [PubMed] [Google Scholar]
- 102.de Vries SP, Bootsma HJ, Hays JP, et al. Molecular aspects of Moraxella catarrhalis pathogenesis. Microbiol Mol Biol Rev. 2009;73:389–406. doi: 10.1128/MMBR.00007-09. Table of Contents . This review details the molecular pathogenesis of M. catarrhalis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Attia AS, Hansen EJ. A conserved tetranucleotide repeat is necessary for wild-type expression of the Moraxella catarrhalis UspA2 protein. J Bacteriol. 2006;188:7840–52. doi: 10.1128/JB.01204-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Blakeway LV, Power PM, Jen FE, et al. ModM DNA methyltransferase methylome analysis reveals a potential role for Moraxella catarrhalis phasevarions in otitis media. FASEB J. 2014;28:5197–207. doi: 10.1096/fj.14-256578. [DOI] [PubMed] [Google Scholar]
- 105.Verhaegh SJ, Streefland A, Dewnarain JK, et al. Age-related genotypic and phenotypic differences in Moraxella catarrhalis isolates from children and adults presenting with respiratory disease in 2001-2002. Microbiology. 2008;154:1178–84. doi: 10.1099/mic.0.2007/015057-0. [DOI] [PubMed] [Google Scholar]
- 106.Ramer PC, Chijioke O, Meixlsperger S, et al. Mice with human immune system components as in vivo models for infections with human pathogens. Immunol Cell Biol. 2011;89:408–16. doi: 10.1038/icb.2010.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Brainard DM, Seung E, Frahm N, et al. Induction of robust cellular and humoral virus-specific adaptive immune responses in human immunodeficiency virusinfected humanized BLT mice. J Virol. 2009;83:7305–21. doi: 10.1128/JVI.02207-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Biswas S, Chang H, Sarkis PT, et al. Humoral immune responses in humanized BLT mice immunized with West Nile virus and HIV-1 envelope proteins are largely mediated via human CD5+ B cells. Immunology. 2011;134:419–33. doi: 10.1111/j.1365-2567.2011.03501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Spranger S, Frankenberger B, Schendel DJ. NOD/scid IL-2Rg(null) mice: a preclinical model system to evaluate human dendritic cell-based vaccine strategies in vivo. J Transl Med. 2012;10:30. doi: 10.1186/1479-5876-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mawas F, Ho MM, Corbel MJ. Current progress with Moraxella catarrhalis antigens as vaccine candidates. Expert Rev Vaccines. 2009;8:77–90. doi: 10.1586/14760584.8.1.77. This dedicated review comprehensively summarizes the main features of some major M. catarrhalis vaccine antigens. [DOI] [PubMed] [Google Scholar]