Abstract
Immobilized ions modulate nearby hydrophobic interactions and influence molecular recognition and self-assembly. We simulated disulfide bond-locked double mutants (L17C/L34C) and observed allosteric modulation of the peptide's intra-molecular interactions by the N-terminal tail. We revealed that the non-contacting charged N-terminal residues help the transfer of entropy to the surrounding solvation shell and stabilizing β-hairpin.
Hydrophobic interactions are critical for self-assembly of biomaterial and aggregation-related diseases. Self-assembly of amyloid β (Aβ) monomers into neurotoxic oligomers has been associated with Alzheimer's disease (AD).1-3 Aβ oligomers are rich in β-sheet conformations, especially β-hairpin structures.4, 5 Full-length Aβ peptides Aβ(1–40) contain hydrophilic fragment, Aβ(1–16), and Aβ(17–40) which includes two critical hydrophobic patches, residues 17–21 and 30–35.6 The N-terminal is more soluble and disordered, and is not in the rigid fibril core.7, 8 Consequently, it is generally regarded redundant in formation of Aβ aggregates and largely neglected in previous investigations.9 Obviously, without the highly charged N-terminal Aβ(1–16) fragment, Aβ(17–40) would expose more hydrophobic residues to solvent and become less soluble.10 Aggregation of N-terminal-deleted Aβ such as Aβ(17–40) is highly sensitive to experimental conditions.11, 12 Both Aβ(17–40) and Aβ(17–42) have lower β-sheet content than the longer Aβ(1–40) and Aβ(1–42).11, 12 Mutations in the Aβ N-terminal region (A2V, A2T, H6R, and D7H) affect Aβ aggregation.13-17 Importantly, both experiment and simulations demonstrated that the distinct changes in aggregation propensity of Aβ mutants are closely related to the alteration of the amount of β-hairpin content.17-21 Although this highlights the crucial role of Aβ N-terminal region in tuning Aβ assembly, the underlying molecular mechanism has not been elucidated.
The charged residues can strongly modulate nearby hydrophobic interactions,22 which are the major driving force in amyloid formation.23-25 However, the allosteric effects of Aβ(1–16) on the intra-molecular interactions of Aβ(1–40) appear more complex than expected earlier. Here we designed simulations to delineate the coupling of the N-terminal and β-sheet forming central region of Aβ(1-40). To enrich the sampling of β-sheet conformations, a disulfide bond was introduced at residues 17/34, as commonly used in stabilizing soluble Aβ peptides to characterize Aβ oligomers experimentally.26,27 we used extensive replica-exchange molecular dynamics (REMD) simulations of double mutants of Aβ(1–40) (referred to as Aβ(1–40)cc), Aβ(1–34) (Aβ(1–34)cc), and Aβ(17–40) (Aβ(17–40)cc) in explicit solvent to thoroughly explore the interaction of the freely flying N-terminal with other regions (see method in Supplementary Information).
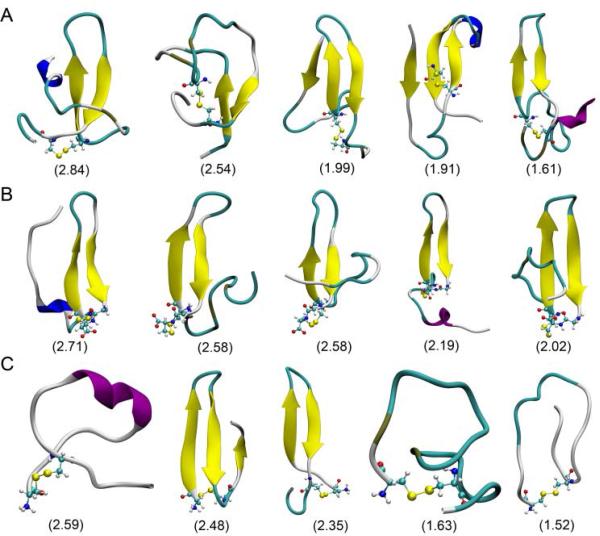
We found that the β-hairpin conformation is stable in both Aβ(1–40)cc and Aβ(1–34)cc but not in Aβ(17–40)cc. Figure 1 shows representative conformations and their populations of the top five clusters of each ensemble. Compared to the Aβ(17–40)cc, both Aβ(1–40)cc and Aβ(1–34)cc ensembles display higher population of β-hairpin structures. The distribution of the first 100 clusters decreases sharply for each system, suggesting that there is no dominant cluster and different Aβ peptides are still polymorphic due to their disordered nature (Figure S4). The constraint of the disulfide bond does not reduce the conformational diversity, as the top 10 clusters of Aβ(1–40)cc only represent 17.2% of its structure ensemble, less than 36.9% in the top 10 cluster of wild-type Aβ(1–40) as investigated in recent REMD simulations.28 The result demonstrates that Aβ(17–40)cc becomes more disordered without the Aβ(1–16) fragment although all three peptides are locked by an identical disulfide bond.
Figure 1.
The representative conformations of the top five clusters of conformational ensemble of Aβ(1–40)cc (A), Aβ(1–34)cc (B), and Aβ(17–40)cc (C). The population (%) of each cluster is shown in parentheses.
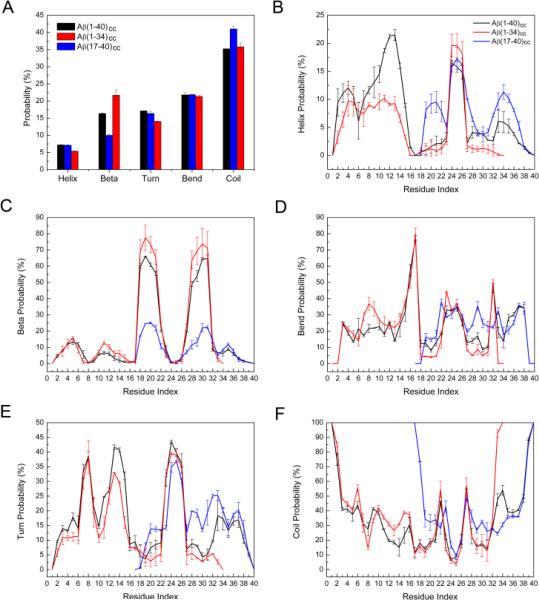
Figure 2A shows the distribution of secondary structures of each system. Random coil structures are still dominant, covering 35.2%, 35.8%, and 41.0% of conformations of Aβ(1–40)cc, Aβ(1–34)cc, and Aβ(17–40)cc, respectively. Comparable amounts of bend and turn structures are displayed by each ensemble (38.9%, 38.2%, and 35.4%). The helix content of all structures varies from 5.4% to 7.2%, and the β-sheet contents are 16.4%, 21.7 and 10.0% for the Aβ(1–40)cc, Aβ(1–34)cc, and Aβ(17–40)cc ensembles, respectively (close to the final result of the cumulative average values shown in Figure S1). Note that the wild-type Aβ generally exhibits a collapsed coil structure in water.29 A previous circular dichroism experiment determined that Aβ(1–40) displays 8.7 % α-helix, 24.0% β-sheet, and 67.3% statistical coil.30 Recent REMD simulations also showed that Aβ(1–40) populates ~25% β-structure, and the most-populated four clusters display β-hairpin motifs.28 The introduction of a disulfide bond decreases the β-sheet propensity of Aβ(1–40)cc, which is expected because only those conformations that satisfy the constraint imposed by the disulfide bond are able to sample β-sheet conformations.
Figure 2.
The average percentage of secondary structure calculated for each system. (B-F) The distribution of secondary structures (helix, β-strand, bend, turn, and coil) per residue calculated for each system. The standard deviations were obtained by averaging the results of 100–150 ns and 150–200 ns.
The per residue secondary structure populations for each Aβcc ensemble were also calculated and shown in Figure 2B–2F. Compared with Aβ(1–40)cc, the N-terminal of Aβ(1–34)cc samples less helical structure, whereas Aβ(17–40)cc samples more helical structures in regions involving residues 19–22 and 32–37, and turn structure including residues 19-21 and 28–35. All peptides sample significant β-sheet structures in the 18–22 and 27–32 regions. However, the propensities ranking is Aβ(1–34)cc > Aβ(1–40)cc > Aβ(17–40)cc, indicating that Aβ(17–40)cc samples less β-hairpin conformations. The result is consistent with cluster analysis (Figure 1), highlighting the critical role of the N-terminal in stabilizing the β-hairpin structure.
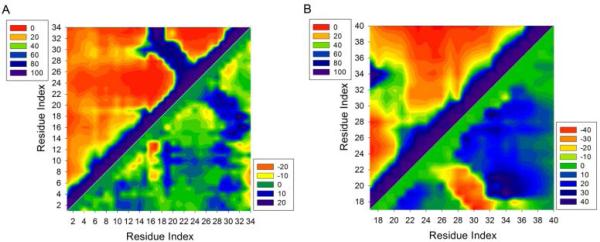
We calculated the contact frequencies to examine how the N-terminal tunes the intra-molecular β-hairpin interactions (Figure S5). Significant contacts in the ring region are constrained by the disulfide bond in both Aβ(1–40)cc and Aβ(1–34)cc. These contacts correspond to the interactions in the β-hairpin region. Contacts in other regions are generally less than 20%, which suggests that the intra-molecular interactions between Aβ(1–16) and the rest of the Aβcc are likely transient and allosterically long-range. The SASA values calculated for each residue confirm that there is no stable association between Aβ(1–16) and other regions of Aβcc peptides as each residue in different systems exposes nearly identical surface area (Figure S6). However, without the Aβ(1– 16) fragment, only weak contacts that potentially sample β-hairpin motif were observed within the ring region in the contact map of Aβ(17–40)cc (Figures 3 and S5).
Figure 3.
Intra-molecular contact maps. Contact frequencies (%) between amino acids in Aβ(1–34)cc (A) and Aβ(17–40)cc (B). Contact difference maps of Aβ(1–34)cc (A) and Aβ(17–40)cc relative to the contacts between the same amino acids in Aβ(1–40)cc are also shown in the same panel (bottom right). A contact occurs if the center of mass of each residue is within 10 Å of the center of mass of another residue.
In addition to the β-hairpin regions, more noticeable long range contacts were identified between Aβ(1–16) and the rest of Aβ(1–34)cc/Aβ(1–40)cc when a cutoff of 10 Å was used to define a contact (Figure S5). Particularly, in the contact map of Aβ(1–34)cc, discernible contacts (~20%) between Aβ(1–16) and regions containing residues 18–20 and 28–32 can be identified (Figure 3), suggesting that Aβ(1–16) transiently fluctuates around the β-hairpin region. The orientations of the Aβ(1–16) fragment in Aβ(1–40)cc and Aβ(1–34)cc can be observed in the representative conformations of each cluster as shown in Figure 1. To identify the dynamic change of Aβ(1–34)cc and Aβ(17–40)cc with regard to the same region in Aβ(1–40)cc, we calculated the contact difference maps (Figure 3). Without the C-terminal residues 35–40, in addition to the increased contacts in the β-hairpin region, Aβ(1–16) fragment fluctuates more frequently around a wide region involving residues 19–33 in Aβ(1–34)cc than in Aβ(1–40)cc (Figure 3A). The N-terminal residues of Aβ(1–34)cc also have long-range contacts, probably because Aβ(1–16) is flexible in aqueous solution. In the absence of residues 1–16, Aβ(17–40)cc displays less frequent contacts in the region locked by the disulfide bond. Consistently, this closed peptide becomes more disordered than in Aβ(1–40)cc.
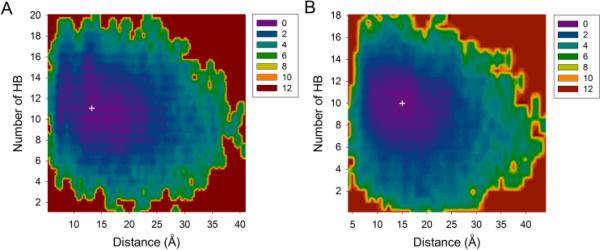
Our analyses revealed that the gain of entropy in the N-terminal compensates for the loss of entropy in the β-hairpin. We examined the potential of mean force (PMF) of each system with respect to the two distances between the N- and β-hairpin parts and the number of backbone hydrogen bonds (the Cα atom of Asp1 and the center of mass of Aβ(17–34) fragment in Figures 4 and the center of mass of Aβ(1–16) and the center of mass of Aβ(17–34) in Figure S7). Both Aβ(1–40)cc and Aβ(1–34)cc display similar free energy landscapes, with the distance restrained to 25 Å and the hydrogen bonds (HB) to a maximum of 16. Only one energy basin was observed in each landscape, corresponding to the most populated distance and number of residues involved in β-hairpin conformation. The energy minimum (ΔG=0 kcal/mol) on each free energy surface suggests that Asp1 is 13 Å and 15 Å away from the center of the β-hairpin of Aβ(1–40)cc and Aβ(1–34)cc, respectively (Figure 4). The most stable conformations of Aβ(1–40)cc and Aβ(1–34)cc involve 11 and 10 backbone HBs. Figure S7 also indicates that when the center of the N-terminal is 10 Å away from the center of the β-hairpin in both systems, the conformations of Aβ(1–40)cc and Aβ(1–34)cc have 10 and 9 HBs in the lowest energy basin. Note that Aβ(1–40)cc can have 18 hydrogen bonds, implying that a total of 9-10 residues could be involved in β-sheet structures. However, although these conformations are accessible, they are not the dominant species with the lowest free energy. Such a result indicates that a balance must be achieved between the gain of enthalpy due to formation of hydrogen bonds and the loss of entropy due to the restriction exerted on the backbone in the extend state of β-sheet conformation. Interestingly, the N-terminal is not fully extended, but moving 10 Å around Aβ(17–34) fragment, partially compensating for the loss of Aβcc entropy.
Figure 4.
Free energy surface of Aβ(1–40)cc (A) and Aβ(1–34)cc (B) at 310 K in terms of the distance between Cα atom of Asp1 and the center of mass of Aβ(17–34) fragment, and the number of backbone hydrogen bonds (HB) corresponding to the number of residues involving the formation of β-hairpin conformation. The free energy values (in kcal/mol) were obtained by ΔG=−kBT(lnPi-lnPmax), where Pi and Pmax are the probability distributions calculated for specific pairs of distance and number of hydrogen bonds. lnPi-lnPmax was used to ensure ΔG=0 for the lowest free energy point (white cross).
The movement of water surrounding the peptides reflects the entropy of the solvent environment due to the fluctuations of the N-terminus. To examine the solvent entropy at the room temperature, we performed additional 400-ns simulations for all three peptide systems. We calculated the number of retained water molecules within 10 Å of the Aβ(17–34) motif of each peptide, and summarized the results in Table S1. The number of the retained water molecules between two adjacent trajectories (100 ps) generally decreases throughout the simulations. Although the difference in the number of water molecules over the last 200 ns is only 3–4 for Aβ(1–40)cc and Aβ(17–34)cc, such result indicates that Aβcc could become more disordered and increasingly disrupt the network of water molecules. We observed that the longer the peptides, the lower the number of retained water molecules, suggesting that the dynamics of the terminals can affect the flux of water molecules. The relatively significant decrease in the number of retained water molecules (~15) in the Aβ(17–40)cc system can be attributed to formation of a new β-hairpin structure during 300–400 ns of the simulation, which transfers larger entropy to the surrounding water clusters.
Aβ Lys16 contributes to the stability of β-sheet conformation,31 and the N-terminal of Aβ can modulate the aggregation rate and fibril stability at low pH,32 suggesting a synergistic effect of ionic residues: they contribute to the stability of β-sheet conformation and to Aβ peptide assembly. The loss of entropy due to forming β-sheet structures could be compensated by the gain of entropy by the N-terminal, in concert with the charged residues increasing the stability of β-conformations. The free energy change of Aβ(17–34) locked in a β-hairpin conformation is estimated about 9.3 kcal/mol (Supplementary material). Additional entropy is necessary to release the constraints of the locked Aβ(17–34). This entropy compensation can be realized by the movements of the N-terminal residues and solvation water. Aβ(1–16) could become more disordered due to gained entropy. Rebalance of the entropy, as we observed in different Aβcc peptides lengths, could be general in Aβ. For example, the N-terminal of Aβ is the primary metal binding site, and metal binding could decrease entropy and then modulate Aβ self-assembly.35, 36 Other mechanisms may also operate here, like the change of hydrogen bonding structure of hydration shells,33 or electric field effects of conformational dynamics and aggregation behavior of peptides.34
Aβ peptides are intrinsically unstructured, making it challenging to investigate the effects of the N-terminal on the overall misfolding dynamics. Our simulations of different double mutants L17C/L34C Aβ peptides revealed that Aβ(1–40)cc samples significant β-hairpin conformations, consistent with experimental findings that this disulfide bond can trap Aβ in β-hairpin structures. Aβ(1–34)cc and Aβ(1–40)cc demonstrate comparable β-hairpin populations, however, the capability of Aβ(17–40)cc to maintain β-hairpin conformation is surprisingly reduced. Aβ(1–16) is more likely to fluctuate around the other region, and modulate the hydrophobic interactions between residues that form β-hairpin conformations. The fluctuations of Aβ(1–16) fragment increases the entropy of both Aβ(1–40)cc and Aβ(1–34)cc and solvation water molecules, which in turn helps to stabilize the β-hairpin structures. In the absence of this part, Aβ(17–40)cc becomes more disordered and populates less β-hairpin conformations. Our study clearly demonstrated that the fluctuating N-terminal allosterically enhances the β-hairpin stability of the amyloid β peptide in the central amyloidogenic region. While the N-terminal residues can transiently interact with the central regions of Aβ(1–40) through hydrophobic interactions and hydrogen bonding, the most favored distance between the N-terminal fragment and central region is around 10 Å. We found that the transfer of entropy to the surrounding solvation shell is connected to the stabilization of the β-hairpin, helped by nearby fluctuating charged N-terminal residues. Our work provides strong support and explanation to recently observed effects that immobilized charged residues can modulate nearby hydrophobic interactions. The function of entropy compensation in concert with charged residues within Aβ peptides may be a general mechanism regulating the dynamics along misfolding and aggregation pathways.
Supplementary Material
Acknowledgments
This work was supported by NIH contract number HHSN261200800001E. This research was supported (in part) by the Intramural Research Program of the CCR, NCI, NIH. Xu thanks China Scholarship Council (CSC201306065001). MD simulations were performed at the Biowulf PC/Linux cluster at the NIH (http://biowulf.nih.gov/).
Footnotes
† Electronic Supplementary Information (ESI) available: One Table and 10 figures. See DOI:10.1039/x0xx00000x
Notes and References
- 1.Kayed R, Lasagna-Reeves CA. J Alzheimers Dis. 2013;33(Suppl 1):S67–78. doi: 10.3233/JAD-2012-129001. [DOI] [PubMed] [Google Scholar]
- 2.Benilova I, Karran E, De Strooper B. Nature Neuroscience. 2012;15:349–357. doi: 10.1038/nn.3028. [DOI] [PubMed] [Google Scholar]
- 3.Laganowsky A, Liu C, Sawaya MR, Whitelegge JP, Park J, Zhao M, Pensalfini A, Soriaga AB, Landau M, Teng PK, et al. Science. 2012;335:1228–1231. doi: 10.1126/science.1213151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knowles TPJ, Vendruscolo M, Dobson CM. Nature Reviews Molecular Cell Biology. 2014;15:384–396. doi: 10.1038/nrm3810. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed M, Davis J, Aucoin D, Sato T, Ahuja S, Aimoto S, Elliott JI, Van Nostrand WE, Smith SO. Nature Structural & Molecular Biology. 2010;17:561–567. doi: 10.1038/nsmb.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu R, McAllister C, Lyubchenko Y, Sierks MR. Journal of Neuroscience Research. 2004;75:162–171. doi: 10.1002/jnr.10859. [DOI] [PubMed] [Google Scholar]
- 7.Petkova AT, Ishii Y, Balbach JJ, Antzutkin ON, Leapman RD, Delaglio F, Tycko R. Proceedings of the National Academy of Sciences. 2002;99:16742–16747. doi: 10.1073/pnas.262663499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luhrs T, Ritter C, Adrian M, Riek-Loher D, Bohrmann B, Dobeli H, Schubert D, Riek R. Proceedings of the National Academy of Sciences. 2005;102:17342–17347. doi: 10.1073/pnas.0506723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nasica-Labouze J, Nguyen PH, Sterpone F, Berthoumieu O, Buchete N-V, Coté S, De Simone A, Doig AJ, Faller P, Garcia A, et al. Chemical Reviews. 2015;115:3518–3563. doi: 10.1021/cr500638n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dulin F, Léveillé F, Ortega JB, Mornon J-P, Buisson A, Callebaut I, Colloc’h N. FEBS Letters. 2008;582:1865–1870. doi: 10.1016/j.febslet.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Pike CJ, Overman MJ, Cotman CW. Journal of Biological Chemistry. 1995;270:23895–23898. doi: 10.1074/jbc.270.41.23895. [DOI] [PubMed] [Google Scholar]
- 12.Vandersteen A, Hubin E, Sarroukh R, De Baets G, Schymkowitz J, Rousseau F, Subramaniam V, Raussens V, Wenschuh H, Wildemann D, et al. FEBS Letters. 2012;586:4088–4093. doi: 10.1016/j.febslet.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 13.Messa M, Colombo L, del Favero E, Cantu L, Stoilova T, Cagnotto A, Rossi A, Morbin M, Di Fede G, Tagliavini F, et al. J Biol Chem. 2014;289:24143–24152. doi: 10.1074/jbc.M114.576256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benilova I, Gallardo R, Ungureanu AA, Castillo Cano V, Snellinx A, Ramakers M, Bartic C, Rousseau F, Schymkowitz J, De Strooper B. J Biol Chem. 2014;289:30977–30989. doi: 10.1074/jbc.M114.599027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Das P, Murray B, Belfort G. Biophysical Journal. 2015;108:738–747. doi: 10.1016/j.bpj.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bush AI, Chen W-T, Hong C-J, Lin Y-T, Chang W-H, Huang H-T, Liao J-Y, Chang Y-J, Hsieh Y-F, Cheng C-Y, et al. PLoS ONE. 2012;7:e35807. doi: 10.1371/journal.pone.0035807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ono K, Condron MM, Teplow DB. Journal of Biological Chemistry. 2010;285:23186–23197. doi: 10.1074/jbc.M109.086496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viet MH, Nguyen PH, Ngo ST, Li MS, Derreumaux P. ACS Chemical Neuroscience. 2013;4:1446–1457. doi: 10.1021/cn400110d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Viet MH, Nguyen PH, Derreumaux P, Li MS. ACS Chemical Neuroscience. 2014;5:646–657. doi: 10.1021/cn500007j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Truong PM, Viet MH, Nguyen PH, Hu C-K, Li MS. The Journal of Physical Chemistry B. 2014;118:8972–8981. doi: 10.1021/jp503652s. [DOI] [PubMed] [Google Scholar]
- 21.Xu L, Chen Y, Wang X. Proteins: Structure, Function, and Bioinformatics. 2014;82:3286–3297. doi: 10.1002/prot.24669. [DOI] [PubMed] [Google Scholar]
- 22.Ma CD, Wang C, Acevedo-Vélez C, Gellman SH, Abbott NL. Nature. 2015;517:347–350. doi: 10.1038/nature14018. [DOI] [PubMed] [Google Scholar]
- 23.Chong SH, Ham S. Proceedings of the National Academy of Sciences. 2012;109:7636–7641. doi: 10.1073/pnas.1120646109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo Y, Dinkel P, Yu X, Margittai M, Zheng J, Nussinov R, Wei G, Ma B. Chem Commun (Camb) 2013;49:3582–3584. doi: 10.1039/c3cc00241a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma B, Nussinov R. Curr Opin Chem Biol. 2006;10:445–452. doi: 10.1016/j.cbpa.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 26.Yu L, Edalji R, Harlan JE, Holzman TF, Lopez AP, Labkovsky B, Hillen H, Barghorn S, Ebert U, Richardson PL, et al. Biochemistry. 2009;48:1870–1877. doi: 10.1021/bi802046n. [DOI] [PubMed] [Google Scholar]
- 27.Härd T. FEBS Journal. 2011;278:3884–3892. doi: 10.1111/j.1742-4658.2011.08295.x. [DOI] [PubMed] [Google Scholar]
- 28.Rosenman DJ, Connors CR, Chen W, Wang C, García AE. Journal of Molecular Biology. 2013;425:3338–3359. doi: 10.1016/j.jmb.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang S, Iwata K, Lachenmann MJ, Peng JW, Li S, Stimson ER, Lu Y, Felix AM, Maggio JE, Lee JP. J Struct Biol. 2000;130:130–141. doi: 10.1006/jsbi.2000.4288. [DOI] [PubMed] [Google Scholar]
- 30.Ono K, Condron MM, Teplow DB. Proceedings of the National Academy of Sciences. 2009;106:14745–14750. doi: 10.1073/pnas.0905127106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fraser PE, McLachlan DR, Surewicz WK, Mizzen CA, Snow AD, Nguyen JT, Kirschner DA. J Mol Biol. 1994;244:64–73. doi: 10.1006/jmbi.1994.1704. [DOI] [PubMed] [Google Scholar]
- 32.Brännström K, Öhman A, Nilsson L, Pihl M, Sandblad L, Olofsson A. Journal of the American Chemical Society. 2014;136:10956–10964. doi: 10.1021/ja503535m. [DOI] [PubMed] [Google Scholar]
- 33.Verma PK, Lee H, Park J-Y, Lim J-H, Maj M, Choi J-H, Kwak K-W, Cho M. The Journal of Physical Chemistry Letters. 2015;6:2773–2779. doi: 10.1021/acs.jpclett.5b01087. [DOI] [PubMed] [Google Scholar]
- 34.Kelly CM, Northey T, Ryan K, Brooks BR, Kholkin AL, Rodriguez BJ, Buchete NV. Biophysical chemistry. 2015;196:16–24. doi: 10.1016/j.bpc.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller Y, Ma B, Nussinov R. Proc Natl Acad Sci U S A. 2010;107:9490–9495. doi: 10.1073/pnas.0913114107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu L, Shan S, Chen Y, Wang X, Nussinov R, Ma B. Journal of chemical information and modeling. 2015;55:1218–1230. doi: 10.1021/acs.jcim.5b00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.