Abstract
Pediatric high-grade gliomas (pHGGs) occur with strikingly different frequencies in infratentorial and supratentorial regions. Although histologically these malignancies appear similar, they represent distinct diseases. Recent genomic studies have identified histone K27M H3.3/H3.1 mutations in the majority of brainstem pHGGs; these mutations are rarely encountered in pHGGs that arise in the cerebral cortex. Previous research in brainstem pHGGs suggests a restricted permeability of the blood-brain-barrier (BBB). In this work, we use dynamic contrast-enhanced (DCE) MRI to evaluate BBB permeability in a genetic mouse model of pHGG as a function of location (cortex vs. brainstem, n = 8 mice/group) and histone mutation (mutant H3.3K27M vs. wild-type H3.3, n = 8 mice/group). The pHGG models are induced either in the brainstem or the cerebral cortex and are driven by PDGF signaling and p53 loss with either H3.3K27M or wild-type H3.3. T2-weighted MRI was used to determine tumor location/extent followed by 4D DCE-MRI for estimating the rate constant (Ktrans) for tracer exchange across the barrier. BBB permeability was 67% higher in cortical pHGGs relative to brainstem pHGGs (t-test, p=0.012) but was not significantly affected by the expression of mutant H3.3K27M versus wild-type H3.3 (t-test, p=0.78). Although mice became symptomatic at approximately the same time, the mean volume of cortical tumors was 3.6 times higher than the mean volume of brainstem tumors. The difference between the mean volume of gliomas with wild-type and mutant H3.3 was insignificant. Mean Ktrans was significantly correlated to glioma volume. These results present a possible explanation for the poor response of brainstem pHGGs to systemic therapy. Our findings illustrate a potential role played by the microenvironment in shaping tumor growth and BBB permeability.
Keywords: high-grade glioma, blood-brain-barrier, perfusion, permeability, MRI, DIPG, K27M
I. INTRODUCTION
Pediatric central nervous system (CNS) tumors account for approximately 7% of all primary CNS malignancies diagnosed in the United States [1]. High-grade gliomas (HGGs) represent the most aggressive sub-group with a prevalence rate of 8–12% and a 5-year survival rate of 15–35% [2]. Children with brainstem HGGs, the majority of which arise in the pons and are referred to as diffuse intrinsic pontine gliomas (DIPG), have a poor prognosis with median survival of less than one year from diagnosis [3]. These tumors are inaccessible to surgical resection and show limited or no response to chemotherapy [4]; the standard of care is focal radiation providing only temporary relief. In the last three decades, clinical trials have failed to identify a single drug that can significantly prolong survival. The location of these tumors poses a particularly difficult challenge for histopathologic examinations, and the scarcity of biopsy samples has historically restricted the number of studies in this disease entity.
Recent evidence suggests that the mechanism of high-grade gliomagenesis in children is region-specific [5]. Brainstem and midline pHGGs have a high occurrence of specific and mutually exclusive K27M mutations in the histone H3 family (H3F3A and HIST1H3B), and less frequently in genes involved in chromatin remodeling (ATRX-DAXX). In contrast, cortical HGGs rarely harbor K27M histone mutations, and instead, can harbor G34R/V mutations in H3F3A, which frequently co-occurs with mutations in ATRX-DAXX [6, 7]. Brainstem and cortical pHGGs also harbor distinct differences in DNA copy-number, DNA methylation, and gene-expression profiles when compared to each other and to their adult analogues [5, 8–10].
Although the role of the newly discovered histone mutations in gliomagenesis is still under intense investigation, recent studies suggest that H3F3A K27M mutations are associated with worse prognosis and decreased survival [11]. These histone mutants have been shown to globally decrease trimethylation of Lys27 on most H3s, working as potent inhibitors of EZH2, leading to global epigenetic and transcriptomal changes [6, 12, 13].
Brainstem pHGGs are diagnosed based on MRI findings and a combination of neurological symptoms [14]. Given the critical importance of in vivo imaging in the diagnosis of pHGGs, several research groups have investigated the feasibility of using different MR pulse sequences as a potential prognostic marker for disease progression [15–18]. As pediatric brainstem HGGs show limited enhancement in contrast-enhanced T1-weighted MRI, and due to the high-frequency of H3.3K27M mutations in this disease group, we hypothesized that both tumor location and H3.3K27M status may influence BBB permeability. In this study, we used dynamic contrast-enhanced (DCE) MRI to evaluate the permeability of the blood-brain-barrier of a genetically engineered mouse model of pHGG as a function of location (cerebral cortex vs. brainstem) and H3.3K27M mutation status (mutant vs. wild-type).
II. MATERIALS AND METHODS
II.A. RCAS/TVA model of pediatric high-grade glioma
All animal studies were approved by the Duke University Institutional Animal Care and Use Committee. The RCAS/TVA system was used to generate a pHGG mouse model expressing either the mutated (H3.3K27M) or wild-type (H3.3WT) histone H3.3 [19]. Post-embryonic day 3 neonates were injected with 1 µL (1×105 cells/µL) of virus expressing DF1 cells divided equally into thirds in the following combinations:
RCAS-PDGF-B + RCAS-Cre + RCAS-H3.3WT (wild-type histone)
RCAS-PDGF-B + RCAS-Cre + RCAS-H3.3K27M (mutant histone)
Neonates were anesthetized on ice and cells were injected in the cortex (n = 16) or brainstem (n = 16) as described in Barton et al [20]. Each group was further subdivided in wild-type (n = 8) or mutant (n = 8) histone sub-groups. Mice were monitored closely for signs of tumor development (lethargy, head tilt, increased head size). On the appearance of glioma symptoms, mice underwent T2-weighted and DCE-MRI. Following the MR study, the animals were euthanized by isoflurane overdose, brains were excised, formalin-fixed for 24 hrs, and embedded in paraffin for histological analysis.
II.B. T2-weighted and dynamic contrast-enhanced MRI
MRI experiments were performed on a 7T small-animal, MRI scanner (Bruker BioSpin MRI GmbH, Ettlingen, Germany). An actively detuned volume excite RF-coil was used in conjunction with a four-element array coil for surface receive.
The location and extent of the tumor were determined in a T2-weighted image acquired with a spin-echo 3D-RARE pulse sequence. The imaging parameters were: TR/TEeff = 6300/40 ms, RARE factor = 8, BW = 50 kHz, 150 µm isotropic voxels, total acquisition time ≈ 28 mins.
The DCE-MRI study protocol has been described in detail elsewhere [21]. Briefly, an interleaved 3D-UTE radial sampling pulse sequence was reconstructed using a sliding-window keyhole approach allowing for the acquisition of 4D datasets. The imaging parameters were: FOV = 20×20×20 mm3, matrix = 128×128×128, TR/TE = 5/0.02 ms, NEX = 1, BW = 100 kHz, α = 10°, and 10 sec temporal resolution. The pre-injection longitudinal relaxation time was measured with the variable flip-angle acquisition [22] using the same radial sampling strategy and identical imaging parameters except α = {2°, 10°}.
Animals were maintained under anesthesia by isoflurane delivery via a nose cone in a custom-made animal positioning system. Body temperature was controlled between 36 °C and 37 °C by circulating warm water. Dynamic imaging was initiated 2 min prior to contrast agent injection and lasted for approximately 20 min post-injection. An automatic syringe pump (KD Scientific Inc., Holliston, MA) was used to administer Gd-DTPA (Magnevist, Schering AG, Berlin, Germany) as a bolus via a 27-gauge tail vein catheter at a dose of 0.5 mmol/kg and flow rate of 2.4 mL/min as described by Loveless et. al [23].
II.C. Image analysis
The time-dependent contrast agent concentration C(t) was calculated using:
| (1) |
where R10 is the native relaxation rate and r1 is the longitudinal relaxivity of Gd-DTPA. The post-injection relaxation rate is found by [24, 25]:
| (2) |
where E10=exp(−TR*R10), S(0)=signal intensity before contrast injection, and S(t)=time-dependent signal intensity.
In the framework of the extended Tofts model [26], the time-dependent contrast agent concentration in the tissue compartment is described by:
| (3) |
where Cp(t) is the arterial input function (AIF), vp is the fractional volume of the plasma compartment, ve is the fractional volume of the extravascular-extracellular space (EES), and Ktrans (permeability parameter) is the rate constant for the transfer of the contrast agent from plasma to EES measured in ml/s of contrast agent per ml of tissue. This equation can be represented in matrix form and solved on a pixel-by-pixel basis using the linear least-squares method [27]. In the analysis of our dynamic data, the population AIF reported by Loveless et al. [23] was used when solving Eq. (3).
The tumor volume was manually segmented in the T2-weighted image and the mask was imported into the functional parameter maps obtained from DCE-MRI. Difference among group means was analyzed with ANOVA. Student’s t-test was used in comparing the means between cortical and brainstem gliomas or wild-type and mutant gliomas. A p-value of less than 0.05 was deemed to indicate a statistically significant difference. Ktrans values outside the range [0 1] min−1 were omitted from the analysis.
III. RESULTS
III.A. Mouse tumor model shows similar imaging characteristics to human DIPG
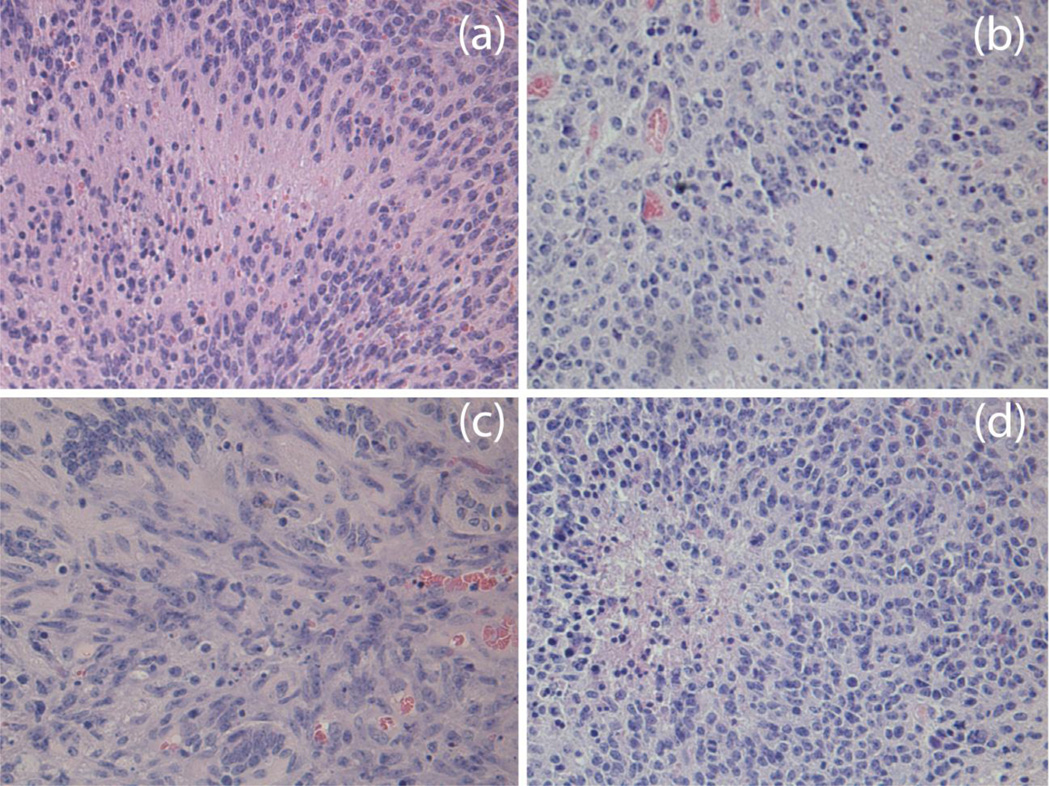
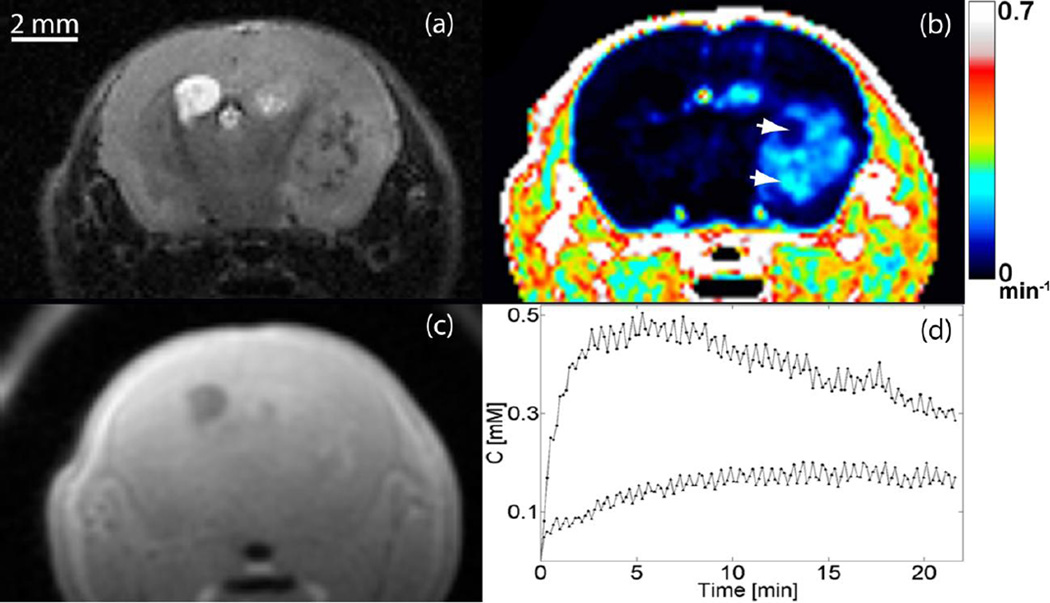
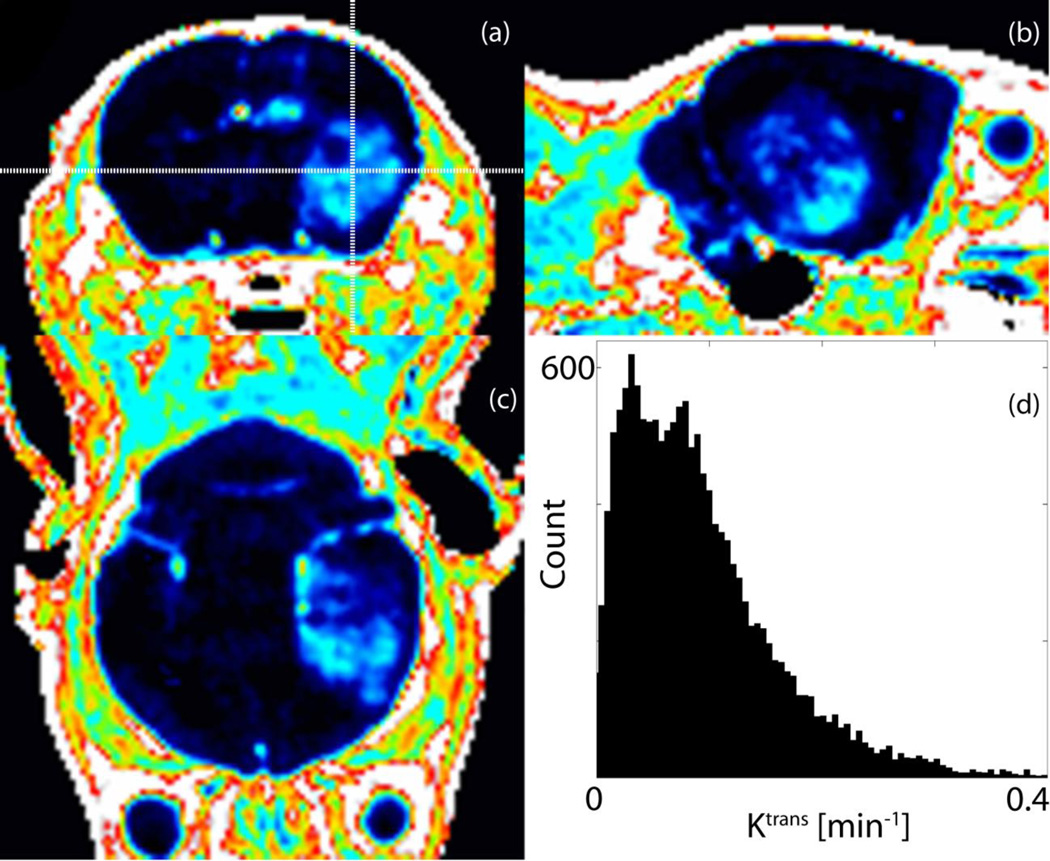
We induced brainstem and cortical gliomas that are driven by PDGF-B, p53 loss and either H3.3K27M or H3.3 wild-type as previously described [28]. All tumors in this work were histologically confirmed to be high-grade gliomas defined by the presence of microvascular proliferation and/or necrosis. Pseudopalisading necrosis and vascular proliferation can be seen in a representative H&E section in Fig. 1. The MR image features of the murine tumors reflect the clinical analogues observed in children [4]: gliomas appear hyperintense on T2-weighted images and hypo/iso-intense on T1-weighted images, as shown in Fig. 2. The pre-injection T1 values in the tumor volume typically were (μ ± σ) 1.54 ± 0.21 s. Figure 2(b) depicts the functional Ktrans map at the same axial location as the slice in Fig. 2(a). Graphs of the temporal evolution of the concentration of the contrast agent at two pixels (arrowheads in Fig. 2(b)) with distinct permeability parameters are given in Fig. 2(d). Figure 3 demonstrates the heterogeneity of BBB permeability in a typical cortical glioma.
Fig 1.
High-grade brainstem and cortex glioma induced with identical genetic drivers: (a) cortical HGG with H3.3WT, (b) cortical HGG with H3.3K27M, (c) brainstem HGG with H3.3WT, and (d) brainstem HGG with H3.3K27M. Tumors appear histologically similar in H&E sections regardless of location and H3.3 mutation status
Fig 2.
Representative MR images of cortical glioma. Tumors appear hyperintense on (a) T2-weighted and (c) hypo/iso-intense on T1-weighted MRI. (b) Ktrans map at same axial location, (d) Concentration of the contrast agent as a function of time at two pixels (arrowheads in panel (b)) with distinct permeability parameters
Fig 3.
Orthogonal views of Ktrans map demonstrate an inhomogeneous spatial distribution of the permeability parameter in (a) axial, (b) sagittal, and (c) coronal planes. (d) Ktrans histogram over tumor volume in a representative cortical glioma.
III.B. BBB permeability is reduced in brainstem gliomas, but is not regulated by H3.3K27M
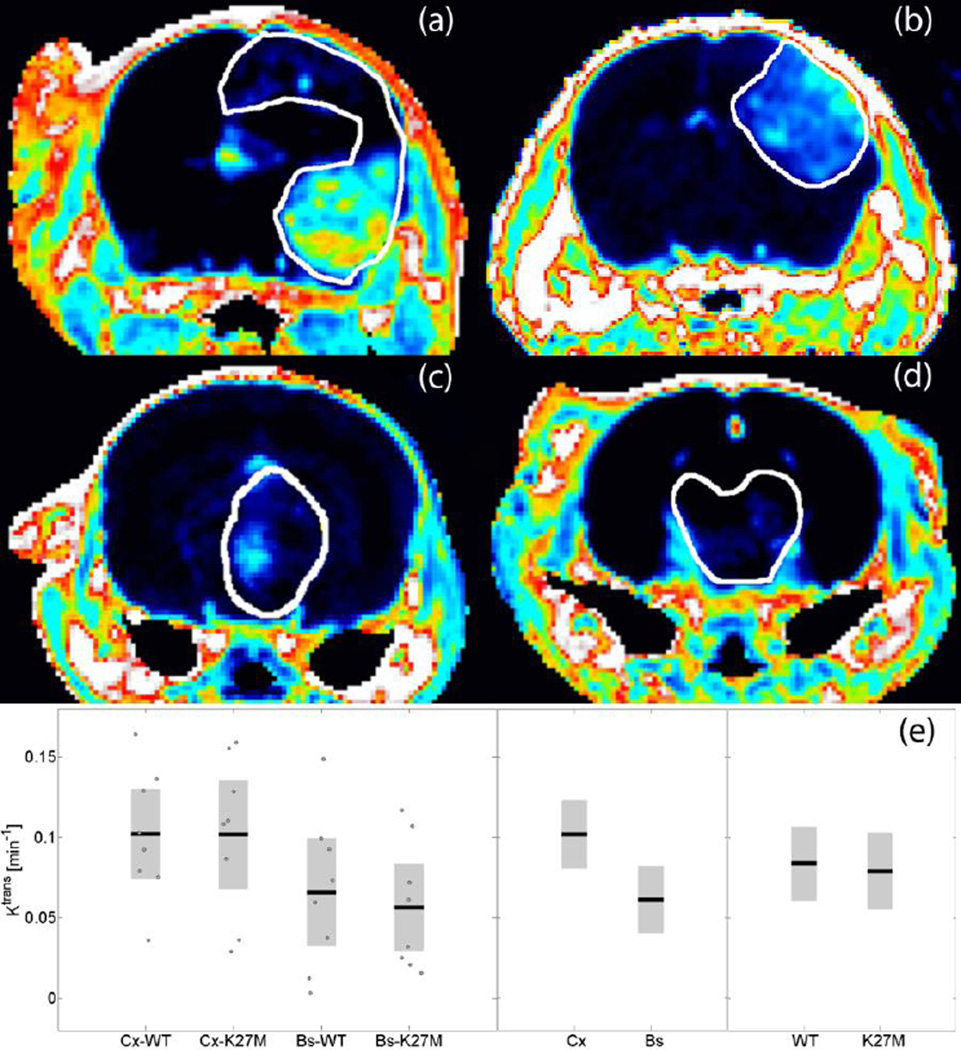
The Ktrans maps from a representative animal in each phenotype group are compared in Fig. 4. The outline of the tumor volume determined from the corresponding T2-weighted image is overlaid for reference. Figure 4(a, b) shows a high-grade glioma with the H3.3WT or H3.3K27M histone mutation originating in the cortex; Fig. 4(c, d) presents the respective brainstem glioma. Notice the inhomogeneous spatial distribution and distinctly higher Ktrans values in the cortical tumors. This heterogeneity is characterized in Fig. 4(e) depicting the mean of the permeability parameter for each group. In cortical HGGs, the mean BBB permeability was 67% higher than in brainstem HGGs (t-test, p=0.012); however we observed that the permeability was not significantly influenced by expression of the H3.3K27M mutation (t-test, p=0.78). These findings suggest BBB permeability is dependent on tumor location, but not histone mutation.
Fig 4.
Representative Ktrans maps from each phenotype group: (a) cortical HGG with H3.3WT, (b) cortical HGG with H3.3K27M, (c) brainstem HGG with H3.3WT, and (d) brainstem HGG with H3.3K27M. Notice the heterogeneous spatial distribution and distinctly higher Ktrans values in cortical tumors, (e) Group comparison of mean Ktrans. In cortical (Cx) HGGs the mean BBB permeability was significantly higher than in brainstem (Bs) HGGs (p=0.012) but was not significantly affected by the expression of mutant H3.3K27M versus wild-type H3.3 (p=0.78).
III.C. Cortical HGGs show increased tumor volume independent of H3.3K27M
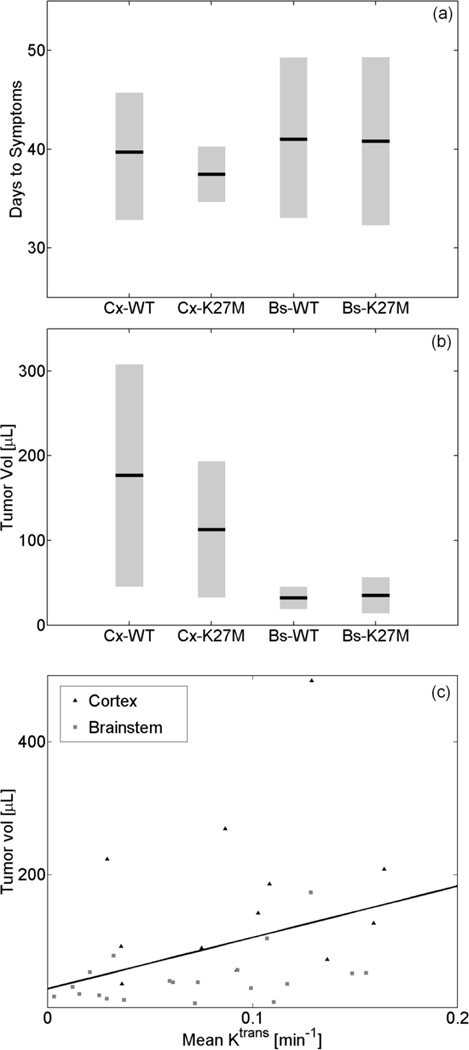
Figure 5(a) plots the time in days since DF1-virus injection until the appearance of glioma symptoms (lethargy, head tilt, and increased head size); the number of days for mice to become symptomatic was not significantly different between the four groups (ANOVA, p=0.89). Although on average it took the same number of days for mice to become symptomatic, the mean volume of the cortical tumors was 3.6 times higher (t-test, p<0.01) than the mean volume of the brainstem tumors. The difference between the mean volume of the gliomas with wild-type or mutated H3.3 histone was not statistically significant (t-test, p=0.46). The observations summarized in Fig. 5(b) imply that the growth rate for tumors in the cortex is significantly higher than for those developing in the brainstem, regardless of H3.3K27M mutation status. Interestingly, we find a significant correlation (p<0.05, p=0.37) between HGG volume and mean of Ktrans as shown in Fig. 5(c).
Fig 5.
(a) Time in days (µ ± sem) since DF1-virus injection until appearance of glioma symptoms; on average it took the same number of days for mice to become symptomatic, (b) The volume of the cortical (Cx) tumors was significantly higher (p<0.01) than the volume of the brainstem (Bs) tumors. In each location (cortex or brainstem) the difference between the mean volume of the gliomas with wild-type (WT) or mutated (K27M) H3.3 histone was not statistically significantly, (c) Tumor volume as a function of the mean of the permeability parameter. Mean Ktrans was significantly correlated (p<0.05, ρ=0. 37) to glioma volume
IV. DISCUSSION
Preclinical models are essential in the study of carcinogenesis in a biologically relevant microenvironment [29]. The advent of genetically-engineered mouse models of high-grade gliomas [19] provides a unique opportunity for studying mechanisms of oncogenesis in vivo. Using the RCAS/tv-a genetically-engineered mouse modeling system, we generate de novo tumors arising from endogenous tissue that closely resemble pediatric high-grade gliomas, both histologically and immunephenotypically. The RCAS system allows for spatiotemporal delivery of oncogenes to specific cell populations in the mouse CNS enabling investigations of regional differences in gliomagenesis. Here, we target nestin-expressing progenitors of the neonatal brainstem or cortex as cells-of-origin, which is consistent with previous studies demonstrating nestin-expressing progenitors as candidate cells-of-origin for DIPG [30]. This system allowed for real-time monitoring of the unperturbed, endogenous BBB permeability in both cortical and brainstem tumor locations of an immunecompetent glioma model. Our previous work has shown this is a relevant model for the recently identified H3.3K27M mutation found in DIPG as expression of H3.3K27M significantly reduces the H3K27me3 mark [12]. We have successfully used this modeling system in preclinical studies, although it remains to be determined whether this approach or the more traditional xenograft approach can successfully predict activity in the clinic for pHGGs [20, 28]. Mouse models may not harbor the full spectrum of genetic alterations found in human disease.
The aim of this work was to determine in vivo the permeability of the blood-brain-barrier in four distinct subgroups of pediatric high-grade gliomas in mice: pHGGs with wild-type vs. mutant histone H3.3 (H3.3K27M) developing in the brainstem vs. the cortex. K27M mutations are highly prevalent in brainstem pHGGs leading to inhibition of the PRC2 complex and a decrease in global H3 trimethylation [12]. These histone mutations occur more commonly in midline tumors, but they have also been identified in the cortex, albeit rarely [31]. In this study we include the K27M mutation in the cortical HGG group to control for any effect on BBB permeability attributed to this mutation as presence of H3K27M mutation has been linked to poor prognosis. Our study provides further insight into DIPG biology by presenting a potential explanation as to why adjuvant therapies have not demonstrated additional efficacy beyond the standard of care of focal radiation.
Conventional anatomic MR imaging is the current standard of care for diagnosing and monitoring tumor progression in pediatric patients with HGG. Using the clinically available MR sequences to date, the parameters of tumor size and invasiveness, metastatic lesions, necrosis, hydrocephalus, or edema have not been able to accurately predict overall survival in these patients [32]. However, the addition of spectroscopy, perfusion, and diffusion MRI has recently been used to identify prognostically distinct subgroups [33] and to correlate with survival time [15, 34]. Here we use DCE MRI to identify region specific differences in BBB permeability (cortex vs. brainstem) using genetic mouse modeling. Additionally, we test if the H3.3K27M mutation plays a role in changes in permeability and if DCE MRI can be used to identify mutation status.
In this work, we observe that the permeability of the blood-brain-barrier in a high-grade glioma mouse model is dependent on the location of the tumor, while expression of the H3.3K27M mutation does not significantly alter BBB permeability. Our study demonstrates that cortical HGG blood vessels have a significantly higher permeability (−67% higher) than those developing in the brainstem. Tumor location may play a role in tumor growth rate as cortical HGGs had a significantly larger volume than the brainstem HGGs even though symptoms for both tumor locations appeared at approximately the same time. One caveat for this observation is that the number of nestin-expressing cells infected at the time of tumor initiation in the neonatal cortex vs. the brainstem may be different potentially impacting tumor growth. In contrast, H3.3K27M mutation status did not lead to a significantly different tumor volume.
These results suggest that the reduced permeability of the BBB in brainstem HGGs compared to cortical HGGs is a possible explanation for the lack of response of brainstem HGGs to systemic therapy. The significant correlation between permeability and tumor volume holds both advantages and disadvantages for DIPG treatment. The slower tumor growth rate in the brainstem relative to the cortex may offer more opportunities for therapeutic intervention; however, the critical structures of the brainstem are less tolerant of neoplastic growth in its location relative to the cortex. This is demonstrated in our data as the mice became symptomatic at similar time points, suggesting tumor volume is not a good predictor of survival. Given that our experiments controlled for variability in epigenetic drivers, our findings further illustrate the important role played by the glioma microenvironment in influencing tumor growth and BBB permeability. In summary, DCE-MRI imaging of a genetically-engineered mouse model of pediatric HGGs demonstrates that tumor location plays a significant role in determining BBB permeability. Future work should address whether the extent of the permeability of the BBB influences response to treatment, particularly to therapies (such as bevacizumab) that impact BBB directly.
CONCLUSION
In this work we demonstrate a differential opening of the blood-brain-barrier dependent on HGG location and independent of the newly described epigenetic driver (H3.3K27M). Although the genetic drivers in our tumor model are identical, the response of the microenvironment to the process of tumorigenesis is not the same. Therefore, the local microenvironment in which the tumor cells interact with normal brain may have implications regarding the degree of invasion, drug delivery, and ultimately patient survival. DCE-MRI can measure these effects in vivo, non-destructively and without the use of ionizing radiation, allowing for repeated measurements.
ACKNOWLEDGMENTS
This work was performed at the Duke Center for In Vivo Microscopy, an NIH/NIBIB National Biomedical Technology Resource Center (P41 EB015897 and 1S10OD010683-01). The authors wish to thank Ms. Sally Zimney for the careful editorial assistance. KGH is supported by NINDS R25 NS065731 (PI John Sampson). OJB is a Rory David Deutsch scholar, is supported by the Damon Runyon Cancer Research Foundation, and K02NS086917.
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, Stroup NE, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2006–2010. Neuro-Oncology. 2013;15:1–56. doi: 10.1093/neuonc/not151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fangusaro J. Pediatric High Grade Glioma (HGG): a Review and Update on Tumor Clinical Characteristics and Biology. Frontiers in Oncology. 2012;2 doi: 10.3389/fonc.2012.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen KJ, Heideman RL, Zhou T, Holmes EJ, Lavey RS, Bouffet E, Pollack IF. Temozolomide in the treatment of children with newly diagnosed diffuse intrinsic pontine gliomas: a report from the Children's Oncology Group. Neuro-Oncology. 2011;13:410–416. doi: 10.1093/neuonc/noq205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warren K. Diffuse intrinsic pontine glioma: Poised for progress. Frontiers in Oncology. 2012;2 doi: 10.3389/fonc.2012.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sturm D, Witt H, Hovestadt V, Khuong-Quang D-A, Jones David TW, Konermann C, Pfaff E, Tönjes M, Sill M, Bender S, Kool M, Zapatka M, Becker N, Zucknick M, Hielscher T, Liu X-Y, Fontebasso Adam M, Ryzhova M, Albrecht S, Jacob K, Wolter M, Ebinger M, Schuhmann Martin U, van Meter T, Frühwald Michael C, Hauch H, Pekrun A, Radlwimmer B, Niehues T, von Komorowski G, Dürken M, Kulozik Andreas E, Madden J, Donson A, Foreman Nicholas K, Drissi R, Fouladi M, Scheurlen W, von Deimling A, Monoranu C, Roggendorf W, Herold-Mende C, Unterberg A, Kramm Christof M, Felsberg J, Hartmann C, Wiestler B, Wick W, Milde T, Witt O, Lindroth Anders M, Schwartzentruber J, Faury D, Fleming A, Zakrzewska M, Liberski Pawel P, Zakrzewski K, Hauser P, Garami M, Klekner A, Bognar L, Morrissy S, Cavalli F, Taylor Michael D, van Sluis P, Koster J, Versteeg R, Volckmann R, Mikkelsen T, Aldape K, Reifenberger G, Collins VP, Majewski J, Korshunov A, Lichter P, Plass C, Jabado N, Pfister Stefan M. Hotspot Mutations in H3F3A and IDH1 Define Distinct Epigenetic and Biological Subgroups of Glioblastoma. Cancer cell. 2012;22:425–437. doi: 10.1016/j.ccr.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 6.Schwartzentruber J, Korshunov A, Liu X-Y, Jones DTW, Pfaff E, Jacob K, Sturm D, Fontebasso AM, Quang D-AK, Tonjes M, Hovestadt V, Albrecht S, Kool M, Nantel A, Konermann C, Lindroth A, Jager N, Rausch T, Ryzhova M, Korbel JO, Hielscher T, Hauser P, Garami M, Klekner A, Bognar L, Ebinger M, Schuhmann MU, Scheurlen W, Pekrun A, Fruhwald MC, Roggendorf W, Kramm C, Durken M, Atkinson J, Lepage P, Montpetit A, Zakrzewska M, Zakrzewski K, Liberski PP, Dong Z, Siegel P, Kulozik AE, Zapatka M, Guha A, Malkin D, Felsberg J, Reifenberger G, von Deimling A, Ichimura K, Collins VP, Witt H, Milde T, Witt O, Zhang C, Castelo-Branco P, Lichter P, Faury D, Tabori U, Plass C, Majewski J, Pfister SM, Jabado N. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482:226–231. doi: 10.1038/nature10833. [DOI] [PubMed] [Google Scholar]
- 7.Wu G. Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet. 2012;44:251–253. doi: 10.1038/ng.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schiffman JD, Hodgson JG, VandenBerg SR, Flaherty P, Polley M-YC, Yu M, Fisher PG, Rowitch DH, Ford JM, Berger MS, Ji H, Gutmann DH, James CD. Oncogenic BRAF Mutation with CDKN2A Inactivation Is Characteristic of a Subset of Pediatric Malignant Astrocytomas. Cancer Research. 2010;70:512–519. doi: 10.1158/0008-5472.CAN-09-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paugh BS, Broniscer A, Qu C, Miller CP, Zhang J, Tatevossian RG, Olson JM, Geyer JR, Chi SN, da Silva NS, Onar-Thomas A, Baker JN, Gajjar A, Ellison DW, Baker SJ. Genome-Wide Analyses Identify Recurrent Amplifications of Receptor Tyrosine Kinases and Cell-Cycle Regulatory Genes in Diffuse Intrinsic Pontine Glioma. Journal of Clinical Oncology. 2011;29:3999–4006. doi: 10.1200/JCO.2011.35.5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puget S, Philippe C, Bax DA, Job B, Varlet P, Junier M-P, Andreiuolo F, Carvalho D, Reis R, Guerrini-Rousseau L, Roujeau T, Dessen P, Richon C, Lazar V, Le Teuff G, Sainte-Rose C, Geoerger B, Vassal G, Jones C, Grill J. Mesenchymal Transition and PDGFRA Amplification/Mutation Are Key Distinct Oncogenic Events in Pediatric Diffuse Intrinsic Pontine Gliomas. PLoS ONE. 2012;7:e30313. doi: 10.1371/journal.pone.0030313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khuong-Quang D-A, Buczkowicz P, Rakopoulos P, Liu X-Y, Fontebasso A, Bouffet E, Bartels U, Albrecht S, Schwartzentruber J, Letourneau L, Bourgey M, Bourque G, Montpetit A, Bourret G, Lepage P, Fleming A, Lichter P, Kool M, Deimling A, Sturm D, Korshunov A, Faury D, Jones D, Majewski J, Pfister S, Jabado N, Hawkins C. K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol. 2012;124:439–447. doi: 10.1007/s00401-012-0998-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis PW, Muller MM, Koletsky MS, Cordero F, Lin S, Banaszynski LA, Garcia BA, Muir TW, Becher OJ, Allis CD. Inhibition of PRC2 activity by a gain-of-function H3 mutation found in pediatric glioblastoma. Science. 2013;340:857–861. doi: 10.1126/science.1232245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan KM, Fang D, Gan H, Hashizume R, Yu C, Schroeder M, Gupta N, Mueller S, James CD, Jenkins R, Sarkaria J, Zhang Z. The histone H3.3K27M mutation in pediatric glioma reprograms H3K27 methylation and gene expression. Genes & development. 2013;27:985–990. doi: 10.1101/gad.217778.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Epstein FJ, Farmer J-P. Brain-stem glioma growth patterns. Journal of Neurosurgery. 1993;78:408–412. doi: 10.3171/jns.1993.78.3.0408. [DOI] [PubMed] [Google Scholar]
- 15.Hipp SJ, Steffen-Smith E, Hammoud D, Shih JH, Bent R, Warren KE. Predicting outcome of children with diffuse intrinsic pontine gliomas using multiparametric imaging. Neuro-Oncology. 2011;13:904–909. doi: 10.1093/neuonc/nor076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warren KE. NMR Spectroscopy and Pediatric Brain Tumors. The Oncologist. 2004;9:312–318. doi: 10.1634/theoncologist.9-3-312. [DOI] [PubMed] [Google Scholar]
- 17.Sugahara T, Korogi Y, Kochi M, Ikushima I, Hirai T, Okuda T, Shigematsu Y, Liang L, Ge Y, Ushio Y, Takahashi M. Correlation of MR imaging-determined cerebral blood volume maps with histologic and angiographic determination of vascularity of gliomas. American Journal of Roentgenology. 1998;171:1479–1486. doi: 10.2214/ajr.171.6.9843274. [DOI] [PubMed] [Google Scholar]
- 18.Law M, Yang S, Wang H, Babb JS, Johnson G, Cha S, Knopp EA, Zagzag D. Glioma Grading: Sensitivity, Specificity, and Predictive Values of Perfusion MR Imaging and Proton MR Spectroscopic Imaging Compared with Conventional MR Imaging. American Journal of Neuroradiology. 2003;24:1989–1998. [PMC free article] [PubMed] [Google Scholar]
- 19.Becher OJ, Hambardzumyan D, Walker TR, Helmy K, Nazarian J, Albrecht S, Hiner RL, Gall S, Huse JT, Jabado N, MacDonald TJ, Holland EC. Preclinical Evaluation of Radiation and Perifosine in a Genetically and Histologically Accurate Model of Brainstem Glioma. Cancer Research. 2010;70:2548–2557. doi: 10.1158/0008-5472.CAN-09-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barton KL, Misuraca K, Cordero F, Dobrikova E, Min HD, Gromeier M, Kirsch DG, Becher OJ. PD-0332991, a CDK4/6 Inhibitor, Significantly Prolongs Survival in a Genetically Engineered Mouse Model of Brainstem Glioma. PLoS ONE. 2013;8:e77639. doi: 10.1371/journal.pone.0077639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Subashi E, Moding EJ, Cofer GP, MacFall JR, Kirsch DG, Qi Y, Allan Johnson G. A comparison of radial keyhole strategies for high spatial and temporal resolution 4D contrast-enhanced MRI in small animal tumor models. Medical Physics. 2013;40 doi: 10.1118/1.4774050. doi:doi: http://dx.doi.org/10.1118/1.4774050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fram EK, Herfkens RJ, Johnson GA, Glover GH, Karis JP, Shimakawa A, Perkins TG, Pelc NJ. Rapid calculation of T1 using variable flip angle gradient refocused imaging. Magnetic Resonance Imaging. 1987;5:201–208. doi: 10.1016/0730-725x(87)90021-x. doi: http://dx.doi.org/10.1016/0730-725X(87)90021-X. [DOI] [PubMed] [Google Scholar]
- 23.Loveless ME, Halliday J, Liess C, Xu L, Dortch RD, Whisenant J, Waterton JC, Gore JC, Yankeelov TE. A quantitative comparison of the influence of individual versus population-derived vascular input functions on dynamic contrast enhanced-MRI in small animals. Magnetic Resonance in Medicine. 2012;67:226–236. doi: 10.1002/mrm.22988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heilmann M, Kiessling F, Enderlin M, Schad LR. Determination of Pharmacokinetic Parameters in DCE MRI: Consequence of Nonlinearity Between Contrast Agent Concentration and Signal Intensity. Investigative Radiology. 2006;41:536–543. doi: 10.1097/01.rli.0000209607.99200.53. [DOI] [PubMed] [Google Scholar]
- 25.Li K-L, Zhu XP, Waterton J, Jackson A. Improved 3D quantitative mapping of blood volume and endothelial permeability in brain tumors. Journal of Magnetic Resonance Imaging. 2000;12:347–357. doi: 10.1002/1522-2586(200008)12:2<347::aid-jmri19>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 26.Tofts PS. Modeling tracer kinetics in dynamic Gd-DTPA MR imaging. Journal of Magnetic Resonance Imaging. 1997;7:91–101. doi: 10.1002/jmri.1880070113. [DOI] [PubMed] [Google Scholar]
- 27.Murase K. Efficient method for calculating kinetic parameters using T1-weighted dynamic contrast-enhanced magnetic resonance imaging. Magnetic Resonance in Medicine. 2004;51:858–862. doi: 10.1002/mrm.20022. [DOI] [PubMed] [Google Scholar]
- 28.Halvorson KG, Barton KL, Schroeder K, Misuraca KL, Hoeman C, Chung A, Crabtree DM, Cordero FJ, Singh R, Spasojevic I, Berlow N, Pal R, Becher OJ. A high-throughput in vitro drug screen in a genetically engineered mouse model of diffuse intrinsic pontine glioma identifies BMS-754807 as a promising therapeutic agent. PloS one. 2015;10:e0118926. doi: 10.1371/journal.pone.0118926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Becher OJ, Holland EC. Genetically Engineered Models Have Advantages over Xenografts for Preclinical Studies. Cancer Research. 2006;66:3355–3359. doi: 10.1158/0008-5472.CAN-05-3827. [DOI] [PubMed] [Google Scholar]
- 30.Monje M, Mitra SS, Freret ME, Raveh TB, Kim J, Masek M, Attema JL, Li G, Haddix T, Edwards MS, Fisher PG, Weissman IL, Rowitch DH, Vogel H, Wong AJ, Beachy PA. Hedgehog-responsive candidate cell of origin for diffuse intrinsic pontine glioma. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:4453–4458. doi: 10.1073/pnas.1101657108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J, Wu G, Miller C, Tatevossian R, Dalton J, Tang B, Orisme W. Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet. 2013;45:602–612. doi: 10.1038/ng.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hargrave D, Chuang N, Bouffet E. Conventional MRI cannot predict survival in childhood diffuse intrinsic pontine glioma. J Neurooncol. 2008;86:313–319. doi: 10.1007/s11060-007-9473-5. [DOI] [PubMed] [Google Scholar]
- 33.Lober R, Cho Y-J, Tang Y, Barnes P, Edwards M, Vogel H, Fisher P, Monje M, Yeom K. Diffusion-weighted MRI derived apparent diffusion coefficient identifies prognostically distinct subgroups of pediatric diffuse intrinsic pontine glioma. J Neurooncol. 2014;117:175–182. doi: 10.1007/s11060-014-1375-8. [DOI] [PubMed] [Google Scholar]
- 34.McConville P, Hambardzumyan D, Moody JB, Leopold WR, Kreger AR, Woolliscroft MJ, Rehemtulla A, Ross BD, Holland EC. Magnetic Resonance Imaging Determination of Tumor Grade and Early Response to Temozolomide in a Genetically Engineered Mouse Model of Glioma. Clinical Cancer Research. 2007;13:2897–2904. doi: 10.1158/1078-0432.CCR-06-3058. [DOI] [PubMed] [Google Scholar]