Abstract
Functional reaching is impaired in dystonia. Here, we analyze upper extremity kinematics to quantify timing and coordination abnormalities during unimanual reach-to-grasp movements in individuals with childhood-onset unilateral wrist dystonia. Kinematics were measured during movements of both upper limbs in a patient group (n = 11, age = 17.5 ± 5 years), and a typically developing control group (n = 9, age = 16.6 ± 5 years). Hand aperture was computed to study the coordination of reach and grasp. Time-varying joint synergies within one upper limb were calculated using a novel technique based on principal component analysis to study intra-limb coordination.
In the non-dominant arm, results indicate reduced coordination between reach and grasp in patients who could not lift the grasped object compared to those who could lift it. Lifters exhibit incoordination in distal upper extremity joints later in the movement and non-lifters lacked coordination throughout the movement and in the whole upper limb. The amount of atypical coordination correlates with dystonia severity in patients. Reduced coordination during movement may reflect deficits in the execution of simultaneous movements, motor planning, or muscle activation. Rehabilitation efforts can focus on particular time points when kinematic patterns deviate abnormally to improve functional reaching in individuals with childhood-onset dystonia.
Index Terms: Cerebral palsy, coordination, dystonia, kinematics, reach-to-grasp
I. Introduction
The ability to reach and grasp an object is not present at birth. It is a skill that develops largely over the first year of life [1], continues to develop through childhood [2–5], and grows into a highly stereotyped movement by adulthood [6, 7]. Control and coordination of the reach-to-grasp movement involves multiple neural structures, including various cortical areas, basal ganglia, cerebellum, brainstem, and spinal cord. Consequently, injury to any of these areas or their networks can have detrimental effects on coordinated movement.
One debilitating movement disorder that can occur after childhood brain injury is dystonia. Dystonia is characterized by involuntary sustained or intermittent muscle contractions that cause twisting and repetitive movements, abnormal postures, or both [8]. The vast majority of patients with dystonia acquired before the age of two years are diagnosed as also having cerebral palsy. Although there can be some recovery and reorganization in the motor system after early brain injury [9, 10], persisting impairments of arm and hand control are common in individuals with childhood-onset dystonia. The loss of the ability to reach and grasp efficiently can lead to functional deficits in daily tasks of self-care, including personal hygiene and grooming, dressing and undressing, and feeding. Focused rehabilitation programs are required to improve reach and grasp function in this population. Yet it is unclear which aspects of the reach-to-grasp movement to train.
Muscle activation patterns in children with dystonia have been shown to be less coordinated than in typically developing children, with excessive antagonist muscle activation [11] in addition to overflow of activation to non-essential muscles [12]. This lack of focused muscle activation leads to the disordered movement typical of dystonia. Accordingly, previous kinematic studies incorporating children with dystonia have demonstrated increased variability in arm movements [4, 13], increased duration of reaching [12] and grasping [4], and increased movements of the head and trunk during reaching [4] compared with controls. However, it is not known whether the timing and coordination of kinematic trajectories between different degrees of freedom during movement is preserved in the disorder.
The objective of this study was to quantify abnormalities of the reach-to-grasp movement in individuals with unilateral dystonia to better understand how it affects the timing and coordination of multiple degrees of freedom during movement, and correlate the results with dystonia severity. Current methods to summarize upper extremity kinematics compare single joint trajectories from patients to those from non-impaired groups [14–16] but do not account for temporal coupling between multiple upper extremity joints. Since functional reaching depends on concurrent movement of many joints rather than isolated joint movement, we sought to use an analytical method that would consider all degrees of freedom during reaching together in one analysis. In this study, we investigated the timing and magnitude of hand opening during reach, and introduced a novel method of analysis to study abnormalities in time-varying kinematic synergies between 7 degrees of freedom within one upper limb during reach. We hypothesized that there is an abnormality of temporal coupling in movement associated with dystonia leading to a lack of coordination between reach and grasp, and also between upper extremity degrees of freedom during reach. Identifying specific points in time during functional reach that are particularly uncoordinated can further knowledge of the causes of disordered movement in dystonia as well as suggest targets for physical therapy.
II. Material and methods
A. Participants
Twenty children and young adults participated in this study. Subject characteristics are provided in Table I. Eleven participants (17.5 ± 5.0 years, 3 female, 2 right-handed) were included in the dystonia group (DYS) based on the presence of dystonia in one wrist starting before the age of 13 years, assessed by K.E.A. using the Hypertonia Assessment Tool [17]. All participants in the DYS group were diagnosed with hemiplegic cerebral palsy (CP) by their physiatrist or neurologist prior to enrollment in the study. Eight participants in the DYS group (indicated in Table I) also had concurrent spasticity in their non-dominant, or more affected arm. The dominant arm of the individuals in the DYS group was considered to be the arm less affected by the neurological injury. Nine participants (16.6 ± 4.9 years, 8 female, 8 right-handed) were included in the typically developing group (TD) for comparison based on the absence of any neurological findings on examination by K.E.A. All subjects were in good general health and could understand and follow all instructions.
Table I.
Characteristics of 11 individuals in the DYS group and a summary of the TD group. A subset of 5 individuals in the DYS group was unable to lift the rod after reaching to grasp with the non-dominant hand.
| Subject Number | Age (years) | Sex | Dom side | Etiology | BFM score Dom/Non-Dom | MACS level | Can lift with Non-Dom hand? |
|---|---|---|---|---|---|---|---|
| DYS 1 | 8.1 | Male | Left | Left MCA stroke | 2/9 | II | Yes |
| DYS 2 a | 11.2 | Female | Left | Left MCA stroke | 1/12 | I | No |
| DYS 3 a | 13.6 | Male | Left | Left MCA stroke | 2/16 | II | No |
| DYS 4 | 16.8 | Female | Left | Left MCA/ACA stroke | 1/6 | I | Yes |
| DYS 5 a | 17.1 | Male | Left | Left MCA stroke | 1/16 | III | Yes |
| DYS 6 | 18.8 | Female | Left | Internal carotid artery stroke | 0/16 | II | Yes |
| DYS 7 a | 19.1 | Male | Left | Left MCA stroke | 2/12 | II | Yes |
| DYS 8 a | 19.3 | Male | Left | Left MCA stroke | 1/16 | II | No |
| DYS 9 a | 20.2 | Male | Right | Right MCA stroke | 1/16 | II | No |
| DYS 10 a | 23.5 | Male | Left | Traumatic brain injury | 1/16 | II | No |
| DYS 11a | 24.9 | Male | Right | Basilar artery stroke | 1/9 | I | Yes |
| TD (n=9) | 16.6±4.9 | 8F, 1M | 8 R, 1L | - | 1±1/1±1 | - | Yes |
Participants with concurrent spasticity in the non-dominant arm (shoulder, elbow, wrist, or fingers)
DYS = Dystonia; TD = typically developing; Dom = Dominant; Non-Dom = Non-Dominant; BFM = Burke-Fahn-Marsden arm score; MACS = manual ability classification system; MCA = Middle cerebral artery, ACA = Anterior cerebral artery
Adult participants and parents of participating children provided written informed consent before testing. Participating children also provided written informed assent. All procedures were approved by the National Institutes of Health Institutional Review Board and adhered to the ethical guidelines of the Declaration of Helsinki. This study was a part of a larger study including electroencephalography [18], electromyography, sensory testing [19], posturography, magnetic resonance brain imaging, and transcranial magnetic stimulation.
B. Equipment
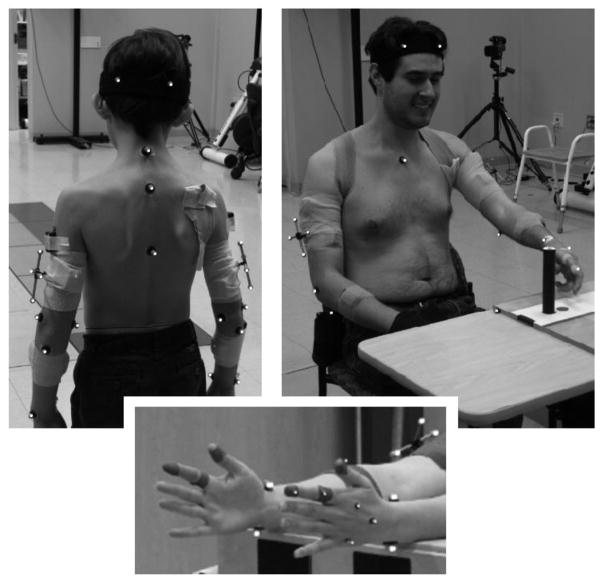
The three-dimensional motion of reflective markers on the upper extremity and trunk was captured using a 10-camera Vicon MX40 system (Vicon Motion Systems, Oxford, UK) at a rate of 120 Hz. All markers are depicted in Fig. 1. Visual3D software (C-Motion, Inc., Germantown, MD, USA) was used to compute joint angles from marker locations. Marker placement and joint coordinate system definitions were based on recommendations of the International Society of Biomechanics [20], with an adaptation for the shoulder joint. In the absence of markers on the clavicle and the scapula, the shoulder joint angles were considered to originate at the glenohumeral joint, which was estimated based on motion recordings of the arm passively moved by an investigator in extension/flexion, abduction/adduction, and circumduction [21].
Fig. 1.
Experimental setting and marker placement.
C. Reach-to-grasp task
Participants were seated comfortably with their feet on the ground or a stool so the hips and knees were flexed at 90 degrees and the ankles were dorsiflexed at 90 degrees. Shoulder straps were used to limit trunk motion during reaching tasks and isolate upper extremity kinematics. A cylindrical rod (height = 6 inch; diameter = 1 inch) was placed in a ½ inch well on a table at elbow height (considered when the shoulder was at 0 degrees flexion and abduction) at midline and just short of full arm extension. A reflective marker was placed on top of the rod to track its movement.
Each task trial began with the subject’s hands placed on the lap. After hearing a bell, the subject reached towards the rod with one hand, grasped the rod, and lifted it. Once the rod was lifted, the subject replaced the rod in the well and returned to the starting position with the hands on the lap. Five trials were attempted for each hand starting with the dominant hand.
D. Analyses
The main kinematic outcomes included shoulder flexion/extension, shoulder abduction/adduction, shoulder internal/external rotation, elbow flexion/extension, forearm pronation/supination, wrist flexion/extension, wrist ulnar/radial deviation, and hand aperture trajectories. Hand aperture was measured as the distance between the marker on the first digit (thumb) nail bed and the marker on the second digit (index finger) which represented the functional tip of the second finger. In some cases in the DYS group, the functional fingertip was not the nail bed, but the proximal interphalangeal joint of the second finger.
For each trial, the timing of three events (start of reach, hand-rod contact time, and rod lift-off time) was noted. The start of reach was the time when the magnitude of the velocity of the marker at the base of the hand exceeded 0.05 m/s. Hand-rod contact time was the time when the minimum of the magnitude of the velocity of the hand marker occurred. If any subject exhibited multiple local minima in hand velocity during reaching, the minimum occurring when the hand was nearest the rod was considered. Lift-off time was the time when the distance along the vertical axis of the marker on the rod exceeded 5 mm. Video recordings of all trials were assessed visually to verify the appropriateness of the three events.
The reach-to-grasp movement was considered to begin at the start of reach and end at hand-rod contact time. The Independent-samples t-test was used to compare reach times between groups for each arm separately. All statistical tests were performed using SPSS Version 21 software (IBM Corporation, Armonk, NY, USA) with a significance level of 0.05. If subjects were unable to lift the rod at all due to deficits in motor control, they were characterized as “non-lifters”. Otherwise, if there was a finite lift-off time, subjects were characterized as “lifters”. The participants’ ability or inability to lift the rod with their non-dominant hand is in Table I. Although non-lifters were unable to lift the rod, they were able to open and close the hand to grasp small objects.
1) Coordination of hand shaping with functional reach
The time to maximum hand aperture during the reach-to-grasp movement and the endpoint hand aperture (hand aperture at hand-rod contact time) were determined for each trial. The mean values over all the trials for each subject and each hand were computed. The mean values were compared across subject groups for each hand separately using the Kruskal-Wallis one-way analysis of variance if there were three subject groups (TD, DYSlifters, DYSnon-lifters) for that hand, and using the Mann-Whitney U-test if there were two subject groups (TD, DYS).
2) Intra-limb coordination during functional reach
Temporal coordination between the 7 degrees of freedom of the upper extremity (shoulder flexion/extension, shoulder abduction/adduction, shoulder internal/external rotation, elbow flexion/extension, forearm pronation/supination, wrist flexion/extension, and wrist ulnar/radial deviation) was computed using principal component analysis (PCA). PCA is a method to represent data in which the correlated degrees of freedom in the original data set are transformed so they can be represented by linearly uncorrelated variables, or principal components [22]. The principal components do not signify joint coordinates, but carry the same information as the original data set in an alternate form that specifies the relative contributions of the original degrees of freedom to different aspects of the movement. The first principal component accounts for the largest fraction of the total variance in the original data, and each component thereafter adds subsequently less. By looking at only the first few principal components that together account for more than 80% of the total variance, prominent features of the kinematic data can be summarized. When this is done across small increments of time throughout the reach-to-grasp movement, a time-varying summary of coordinated kinematics can be achieved. This method does not specify how movement is coordinated neurophysiologically, but rather how the kinematic outcome is coordinated between the different degrees of freedom. As such, coordination in the context of this study is defined as the temporal organization of multiple kinematic trajectories. In order to use this general method to summarize typical kinematic data during the reach-to-grasp movement and determine how atypical movement diverges from typical movement, the following steps were followed.
2a) Data normalization
Kinematic data from the 7 degrees of freedom of the upper extremity were normalized in time and amplitude. Time normalization was accomplished by re-sampling all kinematic data at 100 points spaced evenly between the start time and the time of rod contact. Points were labeled from 0 (movement start) to 1 (rod contact) in increments of 0.01 normalized time units. Amplitude normalization was achieved by first subtracting the minimum value in each trajectory from that trajectory to reduce variability due to differences in initial postures between subjects. Next, data were divided by the largest range of joint angles observed over all subjects and all degrees of freedom during reach. This method preserved relative differences between axes (e.g., wrist ulnar/radial deviation range was typically smaller than shoulder extension/flexion range and would remain proportionally smaller after normalization) and relative differences between subjects (e.g., reduced wrist extension range in a patient would remain proportionally smaller than the typical wrist extension range after normalization). By normalizing the amplitude in this way, minor variations in axes with small ranges of motion would not be overstated.
2b) Creating the “typical movement” filter using PCA
PCA was applied at each 1% of reach time to the 7 degrees of freedom on all the data from the dominant reach of the TD group (5 trials for each of the 9 TD subjects resulting in 45 individual trials in total) to describe typical movement. Data from the dominant hand of the TD group was used since the task in this group represents stable movement patterns learned and practiced in the absence of neurological injury. Three principal components were sufficient to account for a substantial amount of the total variance in the kinematic data (mean and standard deviation over all 1% time intervals of the reach = 84.5% ± 0.03%). Therefore, the first 3 principal components were used to summarize the typical movement pattern. The matrix representing the transformation from the original 7-dimensional kinematic data to the 3-dimensional principal component subspace including only the first 3 principal components (7 × 3 matrix with 7 rows and 3 columns) was then used as a typical movement filter for all data. This was done to assess the extent to which movement trajectories conformed to typical movement patterns. One hundred 7 × 3 typical movement filters were computed (one for each 1% of the movement time) to allow for varying coordination patterns over time. The “princomp” function in Matlab (MathWorks, Natick, MA) was used for PCA analysis. Of note within the “princomp” function is a preliminary step to center the data, or subtract the mean from each degree of freedom, prior to computing the principal components.
2c) Applying the “typical movement” filter to all data
Two transformations were applied to process the data. First, data at each 1% of the reach from the dominant and non-dominant arms of all participants were transformed into the 3-dimensional typical movement space computed from the dominant arm data of the TD group (see step 2b). This transformation was done using data from all trials within one subject group for each arm separately. Prior to this initial transformation into the typical movement space, the mean across trials for each degree of freedom was subtracted from the data, since the typical movement filter was based on centered data. Second, these filtered data were projected back into the 7-dimensional kinematic space using the 3 × 7 transpose of the typical movement filter. In order to compare filtered data to original trajectories, the mean of each degree of freedom was added back to the filtered data. These two transformations are described by the following equations, where Aorig is the original centered data with n trials and 7 degrees of freedom; TMF is the 7×3 typical movement filter; B is the filtered data represented in the 3-dimensional typical movement space; (TMF)T is the 3×7 transpose of the typical movement filter; Afilt is the filtered data represented in the 7-dimensional joint space prior to adding back the mean of each degree of freedom.
(Aorig)(TMF) = B (n×7)(7×3) = (n×3)
(B)(TMF)T = Afilt (n×3)(3×7) = (n×7)
2d) Computing error between original and filtered data
Errors at each 1% of the reach were computed by subtracting filtered trajectories from original trajectories for all degrees of freedom. These error values signified to what extent the movement pattern diverged from the typical movement pattern. Since only the first 3 principal components of the TD data were used in the typical movement filter, non-zero errors were observed not only in the non-dominant reach of the TD group and all movements in the DYS group, but also in the dominant reach of the TD group. A threshold error value was computed as the mean plus 3 standard deviations of the error in the dominant hands of the TD group at each 1% of the reach. If any subject had a mean error over all trials outside of this threshold, their movement was considered atypical at that point in time. This allowed analysis of the timing of abnormalities in movement kinematics in individual participants.
In summary, our kinematic analysis method quantified the extent to which the multiple degrees of freedom in individual reach-to-grasp trials conformed to the time-varying principal component space defined by movement of the dominant arm in the TD group. Since this typical movement space was based on multiple trials from multiple TD subjects, it did not necessarily describe the intra-limb coordination of any one individual, and did not allow for comparison between individuals in each subject group. Rather, it provided a more general summary of dominant arm movement in the entire TD group, against which movement trials of individuals could be compared. Large variability (between- and within-subjects) in the original kinematic trajectories of the TD group at a particular time point would manifest as large variability in the error between the original and filtered TD dominant arm data at that time point. Large error variability would in turn increase the threshold error value that was used to assess the presence of kinematic abnormalities at that time point in individual trials of the non-dominant arm and the DYS group. By this method, judgment of temporal abnormalities was most lenient at time points in which TD subjects exhibited high variability across trials and individuals.
In addition, a global score (atypical kinematics score) was derived for each individual to summarize deviations from the typical movement pattern by computing the proportion of abnormalities over each point in time, each degree of freedom, and each trial. This global score is 0 in the absence of any abnormalities, and 1 when the entire set of joint trajectories is abnormal for all trials. The atypical kinematic scores were compared across subject groups for each hand separately using the Kruskal-Wallis one-way analysis of variance if there were three subject groups (TD, DYSlifters, DYSnon-lifters) for that hand, and using the Mann-Whitney U-test if there were two subject groups (TD, DYS). The global atypical kinematic scores were also correlated (Spearman’s rank correlation coefficient) with the arm sub-score of the Burke-Fahn-Marsden dystonia rating scale in the DYS group to explore the relation between dystonia severity and global deficits in functional reaching.
2e) Ten-fold cross-validation of method
Since typical movement patterns were estimated based on one set of data (kinematics of dominant hand reaching in the TD group), there is a risk that the typical movement filter was biased to the particular subjects and trials analyzed. This problem was addressed using cross-validation, which is a computational tool that allows analysis of how well a model created with one set of data (training set) generalizes to another set of data (validation set).
To apply this method to our data set, the order of the 45 trials used to create the typical movement filter (in step 2b) was first randomized to avoid clusters of trials by an individual subject and then split into 10 groups (5 groups with 5 trials and 5 groups with 4 trials). For each of the 10 analyses, 9 groups (training set) were used to compute a typical movement filter. Data from the remaining group (validation set) were then processed as in steps 2c – 2d above. The percentage of atypical kinematics over every 1% of the reach for every degree of freedom and every trial in the validation set was computed. The mean percentage of atypical kinematics for all 10 analyses was 0.59% (range = 0% to 3.98%). This low percentage of errors in the cross-validation procedure argues against substantial bias in the typical movement filter and supports its use in this study.
III. Results
A. General Results
All subjects were able to complete the reach-to-grasp task. However, some trials in the DYS group were discarded from analysis due to marker occlusion during movement, which prevents computation of joint angle trajectories. Reach-to-grasp movement times of a subset of participants from this study (DYS subjects 1, 2, 3, 4, 5, 7, and 8 and all TD subjects) have been presented elsewhere [19]. In the full group of subjects in the DYS group presented in this paper, movement times (mean ± standard deviation) were longer in the DYS group (3.36 ± 0.58 s) compared to the TD group (2.28 ± 0.54 s) on the non-dominant arm (t(18) = 4.286, p < 0.001) and similar between groups on the dominant arm (t(18) = 1.871, p = 0.078; DYS = 2.63 ± 0.73 s; TD = 2.09 ± 0.53 s).
B. Coordination of hand shaping with functional reach
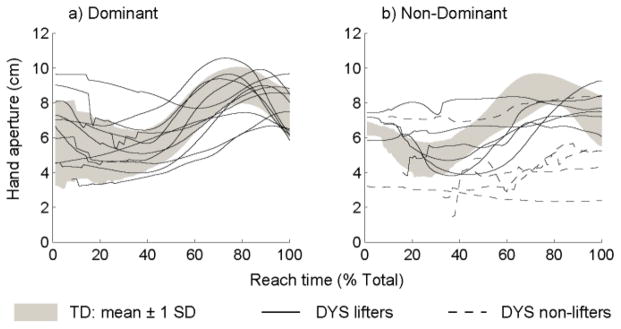
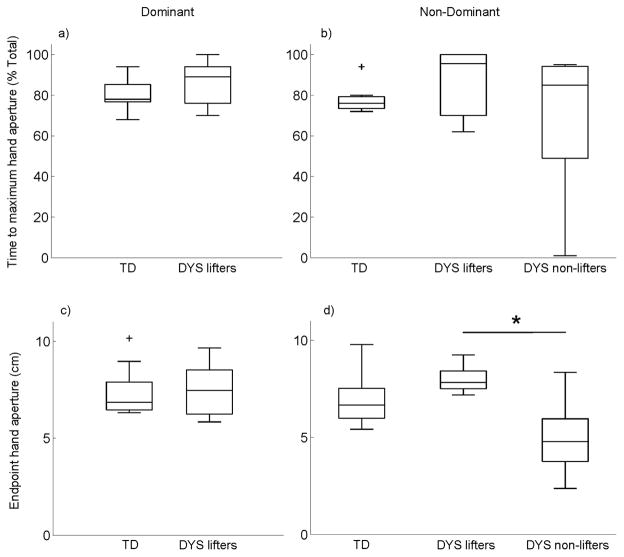
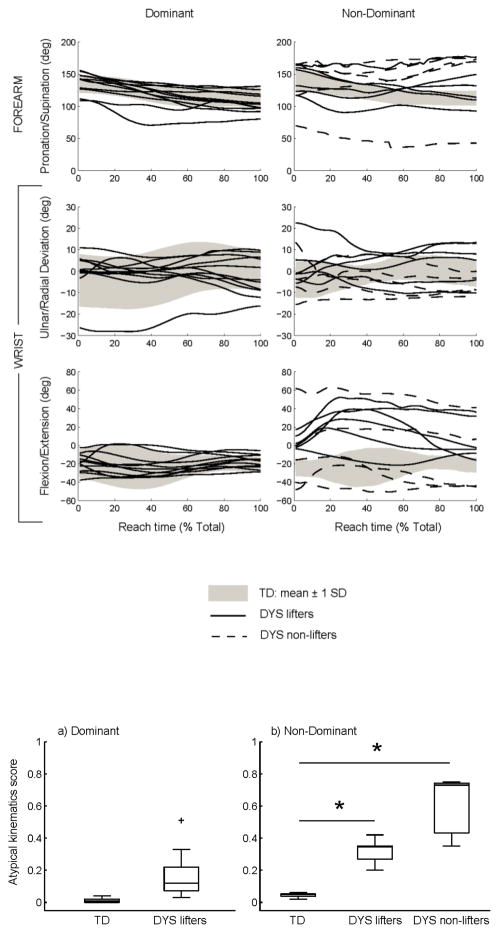
Hand aperture trajectories are shown in Fig. 2 for the dominant (a) and non-dominant (b) hand. Group data for the TD group are represented by the gray shaded areas, and mean trajectories for individuals in the DYS group are shown by solid (lifters) or dashed (non-lifters) lines. Hand opening during reach was stereotyped in the TD group for both hands. However, the DYS group displayed a less consistent pattern in both hands. The DYS hand aperture pattern was especially variable in the non-dominant hand where non-lifters were unable to modulate their hand opening during reach. The time during reach of the maximum hand aperture is shown in Fig. 3 for the dominant hand (a) and the non-dominant hand (b). Despite the apparent variability in the hand aperture traces in Fig. 2, the median times of the maximum hand aperture is statistically similar between groups for the dominant hand (U = 67, p = 0.201) and the non-dominant hand (H(2) = 1.374, p = 0.503).
Fig. 2.
Hand aperture trajectories during reach with the dominant (a) and non-dominant (b) hands.
Fig. 3.
Group comparisons of time to maximum hand aperture (top row) and endpoint hand aperture (bottom row) for the dominant (left column) and non-dominant (right column) hands.
Endpoint hand aperture, which is the hand aperture at the hand-rod contact time, is shown in Fig. 3 for the dominant hand (c) and the non-dominant hand (d). There was no group difference on the dominant hand (U = 46, p = 0.824), but there was a group difference on the non-dominant hand (H(2) = 7.485, p = 0.024). Pairwise comparisons indicated a significantly smaller (p = 0.019) endpoint hand aperture in the non-lifters (mean rank = 5.2) compared to the lifters (mean rank = 15) in the DYS group.
C. Intra-limb coordination during functional reach
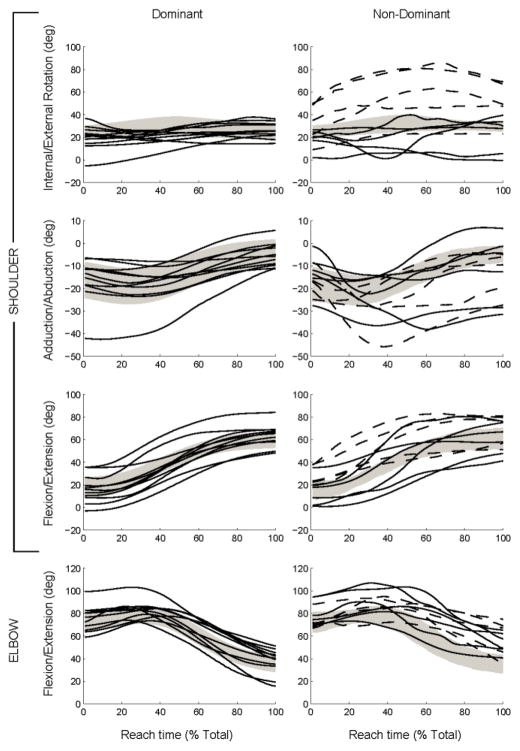
Trajectories of the 7 degrees of freedom in the upper extremity are shown in Fig. 4 for the dominant and non-dominant sides. As in Fig. 2, group data are shown for the TD group by the gray shaded areas, and mean trajectories for individuals in the DYS group are shown by solid (lifters) or dashed (non-lifters) lines. These data are the original kinematic trajectories prior to application of the typical movement filter. The trajectories in the DYS group generally appear similar to those of the TD group for the dominant arm. However, substantial deviations are evident on the non-dominant side. Qualitatively, the non-dominant arm trajectories of the non-lifters deviate from the TD group to a larger extent than those of the lifters.
Fig. 4.
Trajectories of 7 degrees of freedom in the arm during reach.
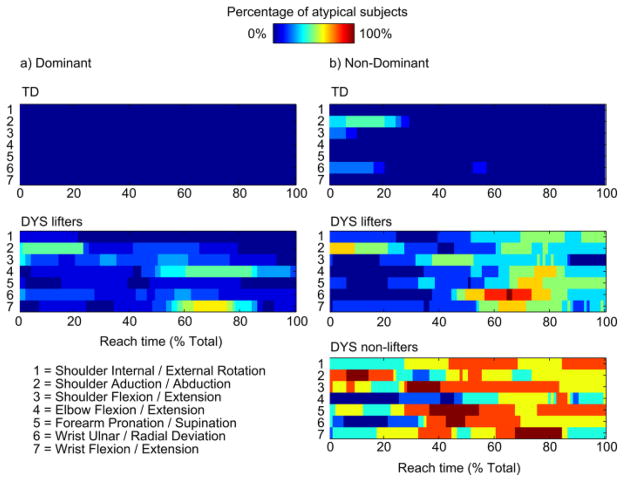
Fig. 5 shows the percentage of subjects in each group with atypical kinematics at every 1% of the reach movement for the dominant (a) and non-dominant (b) arms. These data represent the error between the original kinematics and the data processed (through the typical movement filter) and projected back into the 7-dimenstional kinematic space. There are no errors in the TD group on the dominant side since this data set was used to create the typical movement filter. On the other hand, there are some distributed abnormalities in the DYS group, the most prominent of which are in wrist flexion/extension and elbow flexion/extension around 70 % of the reach time. There are minor abnormalities on the non-dominant side of the TD group, mostly in the first 20 % of the movement, which may indicate variability in initial hand position. The most apparent temporal abnormalities are in the non-dominant arm movements in the DYS group. A substantial percentage of lifters displays kinematic deviations from the typical pattern between 60 – 80 % of the reach in the distal degrees of freedom (elbow, forearm, and wrist). There are kinematic deviations in a considerable number of non-lifters throughout the whole movement and across all degrees of freedom.
Fig. 5.
Percentage of subjects with atypical kinematics at each 1% of reach in the dominant (a) and non-dominant (b) arms.
Fig. 6 presents the global atypical kinematics score for each group from the dominant (a) and non-dominant (b) arm movements. Comparison of groups for each side indicates no group difference on the dominant side (U = 98, p < 0.0001). On the non-dominant side, there was a significant group effect (H(2) = 15.913, p < 0.0001) such that the scores for the lifters (p = 0.036, mean rank = 12.83) and non-lifters (p < 0.0001, mean rank = 17.6) were larger than the TD group (mean rank = 5). There was a strong positive correlation between the atypical kinematics score and the arm sub-score of the Burke-Fahn-Marsden dystonia rating scale (ρ(22) = 0.699, p < 0.0001) indicating that greater kinematic abnormalities were associated with greater severity of dystonia.
Fig. 6.
Group comparisons of the global atypical kinematics score for the reach-to-grasp task with the dominant (a) and non-dominant (b) arms.
IV. Discussion
Our results indicate abnormalities of timing and coordination during functional arm movements in individuals with childhood-onset dystonia affecting the upper extremity. Deviations in intra-limb coordination were most obvious in the non-dominant arm and in non-lifters, and may be due both to involuntary muscle contractions and loss of selective motor control related to dystonia. Although the median timing of hand opening during reach was similar between subject groups, higher variability of that outcome and smaller hand aperture at object contact differentiated the non-lifters in the DYS group from all other participants.
Typically, in visually-guided voluntary reach-to-grasp movements, hand opening occurs simultaneously with reaching such that the hand is at its maximum aperture at approximately 75% of the total reach time [7], which is close to what was measured in the TD group. The stereotypical nature of the reach-to-grasp movement has been hypothesized to indicate simultaneous temporal planning of proximal (reach) and distal (grasp) movements [7, 23] as well as motor planning to reduce spatial endpoint errors [24, 25]. The abnormalities of hand aperture among non-lifters with childhood-onset dystonia in this study may therefore indicate difficulty integrating reaching and grasping in parallel, and/or an abnormality of position control.
Although the majority of reaching trials exhibit smooth gradual hand opening that peaks near 75% of the reach time, other hand opening trajectories have been demonstrated in healthy adults [26]. These include a “bumpy” pattern (25.5% of trials) characterized by multiple bursts of hand opening and corresponding zero-crossings of the aperture velocity profile, and a “plateau” pattern (12.8% of trials) where the maximum aperture is reached early in the reach and maintained until hand closure near the end of the trial. The hand opening trajectories from the non-dominant arm of the patients in our study (Fig. 2b) similarly indicate multiple patterns of hand opening that do not fit the standard model. It is conceivable that motor challenges associated with the reach-to-grasp task, especially in the group of non-lifters, may uncover some of the less common hand opening patterns in patients. Instances of delayed opening as well as plateaus in opening are clearly visible in the patients’ non-dominant aperture trajectories. Both of these patterns suggest sequential rather than parallel execution of reach and grasp; in the delayed opening case, reach occurs before hand opening, and in the plateau case, hand opening occurs before reaching. A similar deficit of reach-grasp integration has been shown before in patients with cerebellar damage [27]. Alternatively, the variability in the timing of maximum hand aperture for non-lifters may have been due to an inability to control hand opening at all. This is suggested by the relatively flat hand aperture trajectories and small endpoint apertures of the non-lifters in the dashed hand trajectories of Fig. 2b, which may have contributed to their inability to lift.
The methods developed and used in this study to assess intra-limb coordination were able to distinguish patients from controls as well as the two functional groups of patients. Lifters in the DYS group exhibit deviations from the normal kinematic pattern in the distal upper extremity during the latter half of the reaching movement, especially between 50–80% of the reach time (Fig. 5). The presence of distal abnormalities is expected since all patients had dystonia in the wrist, and provides a confirmation that this analytical method is indeed sensitive to the disorder being studied. Non-lifters in the DYS group deviate from the normal kinematic pattern throughout the reaching movement and in both proximal and distal parts of the upper limb. The unique temporal information provided by this analysis draws attention to the particular times during functional reaching that may require adjustments. Physical and occupational therapy may therefore benefit from a focus on distal movements (forearm and wrist) in the second half of a functional reach. In addition, proximal arm movement may be an important rehabilitation target to improve functional reach in non-lifters. Although atypical kinematics are only presented at the group level in this paper, single subject data can also be compared to normal kinematic patterns during a particular movement. The feasibility and utility of this type of customized assessment of kinematic synergies remains to be tested in future studies.
In addition to the expected deficits in the non-dominant arm, there are milder but noticeable deviations in the dominant, or less affected, arm as well. Since motor control and BFM scores of the dominant arm were generally normal in this subject group, this result suggests there may be a global deficit in motor planning that affects both hands. Similarly, deficits in the dominant arm have been noted in kinematic studies of children with hemiplegia, and interpreted to indicate impaired motor planning [28].
The global atypical kinematics score provides a summary of how much each subject deviated from the normal reach-to-grasp pattern. While this summary score does not provide the detailed temporal information of the time-varying pattern analysis, it describes the general quality of the movement and correlates well with dystonia severity. As such, the atypical kinematics score could be useful to document functional reach ability in patients, much in the same way that the Gait Profile Score (GPS) [29] and Arm Profile Score (APS) [15] were created to summarize gait and arm movement. Although the APS and the atypical kinematics score presented here both aim to summarize arm movement quality, the methods are fundamentally different. In the APS, each degree of freedom for a patient is assessed separately by comparing it to the average for that degree of freedom in a TD group. The method presented here is unique in that divergence from a time-varying pattern across all degrees of freedom is quantified. Consequently, at a basic level, the atypical kinematics score is a comprehensive measure of incoordination, and the APS measures deviations in individual joint axes.
From these data and analysis methods it is not clear to what extent the abnormalities quantified are due to primary deficits in motor control and planning or compensatory effects. In another study analyzing reaching movements of children with hemiplegic CP, abnormalities in proximal joint kinematics during a reach-to-grasp movement were larger than in a reach-to-hit movement that required less distal upper extremity control [30]. Also, treatment of distal muscle impairments with injection of botulium toxin or surgery was shown to improve proximal muscle control during functional tasks [31]. This influential effect of distal arm activity on proximal arm activity suggests that the act of grasping in our study may have caused compensatory proximal muscle activity. Similarly, the early variability associated with differences in initial positions may have triggered abnormalities later in the movement. This may be addressed in the future by standardizing the initial position. Another limitation of the analysis methods used is that abnormalities found in the kinematic synergies are not signed, so in order to know the direction of error (e.g., excess wrist flexion or extension) the unfiltered trajectory (e.g., wrist flexion/extension angle) would have to be checked at the particular time of the abnormality. Finally, this analysis assumes that the typical movement pattern is an important goal or useful comparison for patients. Although achievement of functional goals may be more important than the particular kinematic trajectories used, this analysis allows the identification of when patterns deviate and may be a useful starting point for the training of faster and more efficient functional movements.
All patients were included in our study on the basis of unilateral wrist dystonia acquired during childhood. However, some patients had concurrent spasticity as well as dystonia in other joints, which could also partially contribute to the kinematic abnormalities observed. Further work will be required to determine temporal kinematic features that are specific to dystonia. Nonetheless, the correlation of the BFM arm sub-score and the global atypical kinematics score indicate that the kinematic abnormalities observed were related to dystonia. In future work, the analysis methods presented in this study could also be used to identify timing abnormalities in individuals with other disorders affecting upper extremity movement.
Acknowledgments
This work was supported by the Intramural Research Programs of the National Institutes of Health Clinical Center and National Institute of Neurological Disorders and Stroke at the National Institutes of Health.
The authors acknowledge the help of Laurie Ohlrich in data collection and the application of clinical rating scales.
Biographies

Sahana N. Kukke earned a B.S. in biomedical engineering (1999, Northwestern University), an M.S. in biomedical engineering (2002, Case Western Reserve University), and a Ph.D. in bioengineering (2009, Stanford University). She is an Assistant Professor of biomedical engineering at the Catholic University of America in Washington, D.C. Her research focuses on sensorimotor control of the upper extremity and how it can be affected by neurological injury. Dr. Kukke is a member of the Society for Neuroscience, the American Academy of Cerebral Palsy and Developmental Medicine, and the Society for the Neural Control of Movement.

Ana Carolina de Campos received B.S. (2005), M.S. (2009) and Ph.D. (2012) degrees in physical therapy from the Federal University of São Carlos (UFSCar), Brazil. From 2011–2012 she was a graduate student with the Human Motor Control Laboratory and from 2013–2014 a post-doctoral fellow with the Functional and Applied Biomechanics Section at the National Institutes of Health. Since 2015 she has been a Professor at UFSCar Physical Therapy Department. Research interests include neurorehabilitation and non-invasive brain mapping in cerebral palsy. Dr. De Campos is a member of the AACPDM and the Latin American Academy for Child Development and Disabilities.

Katharine E. Alter is a Senior Clinician and the Medical Director of Rehabilitation Programs for the Functional and Applied Biomechanics Section in Rehabilitation Medicine at the National Institutes of Health Clinical Center, Bethesda, Maryland. Prior to joining NIH in 2014, she was the Medical Director of Rehabilitation Medicine at Mount Washington Pediatric Hospital, an affiliate of Johns Hopkins and the University of Maryland in the Washington DC area. Dr. Alter’s clinical and investigative interests focus on the assessment of neurologic, musculoskeletal and neuromuscular impairments in children and adults.

Mark Hallett is the Chief of the Human Motor Control Section, National Institute of Neurological Disorders and Stroke, NIH, Bethesda, Maryland. His work mainly deals with principles of motor control and the pathophysiology of movement disorders. Dr. Hallett is past President of the American Association of Neuromuscular and Electrodiagnostic Medicine and the Movement Disorder Society, past Vice-President of the American Academy of Neurology, and is now President of the International Federation of Clinical Neurophysiology.

Diane L. Damiano, PhD PT, is a tenure-track scientist and Chief of the Functional and Applied Biomechanics Section at National Institutes of Health in Bethesda, Maryland. Her research focuses on the use of portable brain imaging during functional movement to elucidate the brain mechanisms underlying normal and impaired coordination and the design and investigation of activity-based rehabilitation programs to promote optimal motor functioning and enhance muscle and neural plasticity in children with cerebral palsy.
Contributor Information
Sahana N. Kukke, National Institutes of Health, Bethesda, Maryland. She is now with the Department of Biomedical Engineering, The Catholic University of America, Washington, D.C
Lindsey A. Curatalo, National Institutes of Health Clinical Center, Bethesda, Maryland. She is now with Ortho Clinical Diagnostics, Rochester, New York
Ana Carolina de Campos, National Institutes of Health, Bethesda, Maryland. She is now with the Department of Physical Therapy at the Federal University of São Carlos.
Mark Hallett, National Institute of Neurological Disorders and Stroke, Bethesda, Maryland.
Katharine E. Alter, National Institutes of Health Clinical Center, Bethesda, Maryland and Mount Washington Pediatric Hospital, Baltimore, Maryland
Diane L. Damiano, National Institutes of Health Clinical Center, Bethesda, Maryland
References
- 1.von Hofsten C, Ronnqvist L. Preparation for grasping an object: a developmental study. J Exp Psychol Hum Percept Perform. 1988 Nov;14:610–21. doi: 10.1037//0096-1523.14.4.610. [DOI] [PubMed] [Google Scholar]
- 2.Kuhtz-Buschbeck JP, Stolze H, Boczek-Funcke A, Johnk K, Heinrichs H, Illert M. Kinematic analysis of prehension movements in children. Behav Brain Res. 1998 Jun;93:131–41. doi: 10.1016/s0166-4328(97)00147-2. [DOI] [PubMed] [Google Scholar]
- 3.Smyth MM, Katamba J, Peacock KA. Development of prehension between 5 and 10 years of age: distance scaling, grip aperture, and sight of the hand. J Mot Behav. 2004 Mar;36:91–103. doi: 10.3200/JMBR.36.1.91-103. [DOI] [PubMed] [Google Scholar]
- 4.Coluccini M, Maini ES, Martelloni C, Sgandurra G, Cioni G. Kinematic characterization of functional reach to grasp in normal and in motor disabled children. Gait Posture. 2007 Apr;25:493–501. doi: 10.1016/j.gaitpost.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 5.Zoia S, Pezzetta E, Blason L, Scabar A, Carrozzi M, Bulgheroni M, et al. A comparison of the reach-to-grasp movement between children and adults: a kinematic study. Dev Neuropsychol. 2006;30:719–38. doi: 10.1207/s15326942dn3002_4. [DOI] [PubMed] [Google Scholar]
- 6.Jeannerod M. The timing of natural prehension movements. J Mot Behav. 1984 Sep;16:235–54. doi: 10.1080/00222895.1984.10735319. [DOI] [PubMed] [Google Scholar]
- 7.Jeannerod M. Intersegmental coordination during reaching at natural objects. In: Long JBA, editor. Attention and performance. IX. Hillsdale, NJ: Erlbaum; 1981. pp. 153–169. [Google Scholar]
- 8.Sanger TD, Chen D, Fehlings DL, Hallett M, Lang AE, Mink JW, et al. Definition and classification of hyperkinetic movements in childhood. Mov Disord. 2010 Aug 15;25:1538–49. doi: 10.1002/mds.23088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carr LJ. Development and reorganization of descending motor pathways in children with hemiplegic cerebral palsy. Acta Paediatr Suppl. 1996 Oct;416:53–7. doi: 10.1111/j.1651-2227.1996.tb14278.x. [DOI] [PubMed] [Google Scholar]
- 10.Staudt M, Gerloff C, Grodd W, Holthausen H, Niemann G, Krageloh-Mann I. Reorganization in congenital hemiparesis acquired at different gestational ages. Ann Neurol. 2004 Dec;56:854–63. doi: 10.1002/ana.20297. [DOI] [PubMed] [Google Scholar]
- 11.Kukke SN, Sanger TD. Contributors to excess antagonist activity during movement in children with secondary dystonia due to cerebral palsy. J Neurophysiol. 2011 Feb 16; doi: 10.1152/jn.00998.2009. [DOI] [PubMed] [Google Scholar]
- 12.Casellato C, Zorzi G, Pedrocchi A, Ferrigno G, Nardocci N. Reaching and writing movements: sensitive and reliable tools to measure genetic dystonia in children. J Child Neurol. 2011 Jul;26:822–9. doi: 10.1177/0883073810392997. [DOI] [PubMed] [Google Scholar]
- 13.Sanger TD. Arm trajectories in dyskinetic cerebral palsy have increased random variability. J Child Neurol. 2006 Jul;21:551–7. doi: 10.1177/08830738060210070201. [DOI] [PubMed] [Google Scholar]
- 14.Riad J, Coleman S, Lundh D, Brostrom E. Arm posture score and arm movement during walking: a comprehensive assessment in spastic hemiplegic cerebral palsy. Gait Posture. 2011 Jan;33:48–53. doi: 10.1016/j.gaitpost.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 15.Jaspers E, Feys H, Bruyninckx H, Klingels K, Molenaers G, Desloovere K. The Arm Profile Score: A new summary index to assess upper limb movement pathology. Gait Posture. 2011 Jun;34:227–33. doi: 10.1016/j.gaitpost.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Butler EE, Rose J. The pediatric upper limb motion index and a temporal-spatial logistic regression: quantitative analysis of upper limb movement disorders during the Reach & Grasp Cycle. J Biomech. 2012 Apr 5;45:945–51. doi: 10.1016/j.jbiomech.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jethwa A, Mink J, Macarthur C, Knights S, Fehlings T, Fehlings D. Development of the Hypertonia Assessment Tool (HAT): a discriminative tool for hypertonia in children. Dev Med Child Neurol. 2010 May;52:e83–7. doi: 10.1111/j.1469-8749.2009.03483.x. [DOI] [PubMed] [Google Scholar]
- 18.Kukke SN, de Campos AC, Damiano D, Alter KE, Patronas N, Hallett M. Cortical activation and inter-hemispheric sensorimotor coherence in individuals with arm dystonia due to childhood stroke. Clin Neurophysiol. 2015 Aug;126(8):1589–98. doi: 10.1016/j.clinph.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Campos AC, Kukke SN, Hallett M, Alter KE, Damiano DL. Characteristics of bilateral hand function in individuals with unilateral dystonia due to perinatal stroke: sensory and motor aspects. J Child Neurol. 2014 May;29:623–32. doi: 10.1177/0883073813512523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu G, van der Helm FC, Veeger HE, Makhsous M, Van Roy P, Anglin C, et al. ISB recommendation on definitions of joint coordinate systems of various joints for the reporting of human joint motion--Part II: shoulder, elbow, wrist and hand. J Biomech. 2005 May;38:981–992. doi: 10.1016/j.jbiomech.2004.05.042. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz MH, Rozumalski A. A new method for estimating joint parameters from motion data. J Biomech. 2005 Jan;38:107–16. doi: 10.1016/j.jbiomech.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 22.Hotelling H. Analysis of a complex of statistical variables into principal components. J Educ Psychol. 1933 Sep;24(6):417–441. [Google Scholar]
- 23.Hoff B, Arbib MA. Models of Trajectory Formation and Temporal Interaction of Reach and Grasp. J Mot Behav. 1993 Sep;25:175–192. doi: 10.1080/00222895.1993.9942048. [DOI] [PubMed] [Google Scholar]
- 24.Haggard P, Wing A. Coordinated responses following mechanical perturbation of the arm during prehension. Exp Brain Res. 1995;102:483–94. doi: 10.1007/BF00230652. [DOI] [PubMed] [Google Scholar]
- 25.Alberts JL, Saling M, Stelmach GE. Alterations in transport path differentially affect temporal and spatial movement parameters. Exp Brain Res. 2002 Apr;143:417–25. doi: 10.1007/s00221-002-1005-0. [DOI] [PubMed] [Google Scholar]
- 26.Bongers RM, Zaal FT, Jeannerod M. Hand aperture patterns in prehension. Hum Mov Sci. 2012 Jun;31:487–501. doi: 10.1016/j.humov.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 27.Zackowski KM, Thach WT, Jr, Bastian AJ. Cerebellar subjects show impaired coupling of reach and grasp movements. Exp Brain Res. 2002 Oct;146(4):511–22. doi: 10.1007/s00221-002-1191-9. [DOI] [PubMed] [Google Scholar]
- 28.Hung YC, Henderson ER, Akbasheva F, Valte L, Ke WS, Gordon AM. Planning and coordination of a reach-grasp-eat task in children with hemiplegia. Res Dev Disabil. 2012 Sep-Oct;33:1649–57. doi: 10.1016/j.ridd.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Baker R, McGinley JL, Schwartz MH, Beynon S, Rozumalski A, Graham HK, et al. The gait profile score and movement analysis profile. Gait Posture. 2009 Oct;30:265–9. doi: 10.1016/j.gaitpost.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 30.Domellof E, Rosblad B, Ronnqvist L. Impairment severity selectively affects the control of proximal and distal components of reaching movements in children with hemiplegic cerebral palsy. Dev Med Child Neurol. 2009 Oct;51:807–16. doi: 10.1111/j.1469-8749.2008.03215.x. [DOI] [PubMed] [Google Scholar]
- 31.Fitoussi F, Diop A, Maurel N, Laasel el M, Ilharreborde B, Pennecot GF. Upper limb motion analysis in children with hemiplegic cerebral palsy: proximal kinematic changes after distal botulinum toxin or surgical treatments. J Child Orthop. 2011 Oct;5:363–70. doi: 10.1007/s11832-011-0365-z. [DOI] [PMC free article] [PubMed] [Google Scholar]