Abstract
Advances in the study of brain development over the last decades, especially recent findings regarding the evolutionary expansion of the human neocortex, and large-scale analyses of the proteome/transcriptome in the human brain, have offered novel insights into the molecular mechanisms guiding neural maturation, and the pathophysiology of multiple forms of neurological disorders. As a preamble to reviews of this issue, we provide an overview of the cellular, molecular and genetic bases of brain development with an emphasis on the major mechanisms associated with landmarks of normal neural development in the embryonic stage and early postnatal life, including neural stem/progenitor cell proliferation, cortical neuronal migration, evolution and folding of the cerebral cortex, synaptogenesis and neural circuit development, gliogenesis and myelination. We will only briefly depict developmental disorders that result from perturbations of these cellular or molecular mechanisms, and the most common perinatal brain injuries that could disturb normal brain development.
Keywords: Neurodevelopment, Cerebral cortex, Progenitor cell, Neuronal maturation, Gliogenesis, Synaptogenesis, Neurodevelopmental disorders
Introduction
Human brain development starts with neurulation from the ectoderm of the embryo and it takes, on average, 20 to 25 years to mature. This protracted process is presented as both physical and experience-based maturation. Building this most complex and highly organized organ involves the generation of a wide variety of specialized neural and non-neural cell types that must be produced in the correct numbers, at appropriate locations and with the right timing. Additionally, accurate connections between neurons and efficient communication between distinct cell populations are crucial for the brain to exert centralized control for behaviour, perception and higher cognitive function.
Brain develops in an intricately orchestrated sequence of stages. Neural tube, the origin of the entire central nervous system (CNS), is formed at approximately 3–4 weeks of gestation and followed by massive cell proliferation, migration and brain expansion in size, complexity and surface area (gyrification). Neurogenesis and formation of the general architecture of brain regions are largely complete at birth, while maturation of the two principal glial cells (astrocytes and oligodendrocytes), synaptogenesis and synapse pruning, and myelination represent postnatal brain growth (Giedd, 1999). Furthermore, brain constantly changes at the level of connectivity throughout the life span with environmental influences. At the cellular level, both neurons and astrocytes/oligodendrocytes are derived from the common multipotent neuroepithelial cells that line the cerebral ventricles (Davis and Temple, 1994). It is now known that neurogenesis precedes gliogenesis, which is the results of an inherent, exquisitely timed mechanism regulated by complex interactions between intrinsic factors (for example, gene epigenetic modifications) and extrinsic cues (secreted or contact-mediated factors) (Rowitch and Kriegstein, 2010). Adequate neurogenesis and timely switch of developmental program of progenitor domains to gliogenesis are critical for proper neural circuit formation and normal brain function. Disruptions in any of the mechanisms may lead to disorganization and eventually, dysfunction of the CNS.
As a preamble to the reviews of this issue, we will provide an overview of the processes that orchestrate the successive steps of normal cortical brain development, including progenitor division and production in coordination with appropriate layer neuron production, neuron migration and maturation, synaptogenesis and at last gliogenesis. We will only briefly discuss how alterations of such mechanisms can be related to some of the brain development disorders. Neurodevelopmental diseases will be reviewed in detail in the other articles of this issue.
Morphogenesis of the cerebral cortex
The cerebral cortex, also called the neocortex in mammals to distinguish it from the more ancient paleo-cortex and archicortex, differentiates in the dorsal telencephalon, the most anterior region of the embryonic brain (Rubenstein and Beachy, 1998). The neocortex is the seat of the higher cognitive functions and has a very complex cytoarchitecture, including projection neurons born in the same area and interneurons originating in ventral telencephalic regions (Molyneaux et al., 2007). Cortical projection neurons are organized into six layers that constitute a laminar structure called the cortical plate. Layer neurons are distinguished by the expression of specific combinations of molecular markers and distinct axonal projections. Neurons occupying the deep layers (V and VI) are predominantly composed of corticofugal neurons that project to subcortical areas, such as the thalamus, brain stem and spinal cord. In contrast, the superficial layers (IV to II) are composed of intracortical neurons that project locally or to the contralateral hemisphere (Greig et al., 2013). All these neurons are excitatory glutamatergic neurons and derive from the germinative zones of the developing neocortex: the ventricular zone (VZ), which lines the ventricle, and the subventricular zone (SVZ), which develops from the VZ and is juxtaposed to its basal surface (Angevine and Sidman, 1961; Anthony et al., 2004; Molyneaux et al., 2007; Rakic, 2009). The first phase of neurogenesis occurs in the VZ and generates pioneer neurons, including Cajal-Retzius cells that populate the pre-plate (Meyer et al., 1998). A second phase, resulting in a much more important output of neurons, principally occurs in the SVZ and gives rise to projection neurons. These primary neurons split the pre-plate into the marginal zone or layer I, and the subplate (Super et al., 1998; Olson, 2014), an intermediary structure in which neurons receive important informative cues during their migration to their destination layer (Molnar and Clowry, 2012; Molyneaux et al., 2007). Sequentially arising projection neurons migrate to the cortical plate in an inside-out manner, with the youngest upper-layer neurons migrating over deeper neurons born earlier (Molyneaux et al., 2007; Caviness et al., 2008). While the laminar structure of the cortical plate has been relatively well conserved, its size has expanded remarkably with respect to both volume and surface, in particular in humans and other primates, along with the evolutionary complexification of cognitive function. Because the ventricle has not increased to a matching extent, this expansion imposes the folding of the cortical plate and the formation of convolutions or gyri (Fietz et al., 2012; Borrell and Gotz, 2014; Sun and Hevner, 2014). Many genetic and functional studies in animal models, especially in the mouse, have provided valuable insights into the cellular and molecular mechanisms that underlie the complex development of the cerebral cortex (Hansen et al., 2010; Fietz et al., 2012; Betizeau et al., 2013; Gao et al., 2014; Florio et al., 2015). Nonetheless, understanding how these mechanisms have evolved and adapted to allow this increase in cortical size remains a major challenge in neurodevelopmental biology.
Neurogenic phases in the developing neocortex
Neurogenic phases and transitions in progenitor subtypes
In the beginning, the neural tube corresponds to a neuroepithelium, made up of highly polarized cells called neuroepithelial cells (NECs) (Lui et al., 2011; Paridaen et al., 2013). In the developing cortex, NECs extend long process throughout the entire cortical wall. Their apical and basal end feet are attached at the ventricular surface and the pial lamina, respectively (Lui et al., 2011; Borrell and Gotz, 2014; Florio and Huttner, 2014). Cadherins and catenins form adherens junctions that mediate cell-cell adhesion (Elias et al., 2007). NECs divide symmetrically to self-renew and to generate an adequate pool of founder progenitors (Figure 1b). This initial proliferative phase affects both lateral and radial extension and has a significant impact on the final surface area and thickness of the neocortex (Florio and Huttner, 2014; Sun and Hevner, 2014; Dehay et al., 2015).
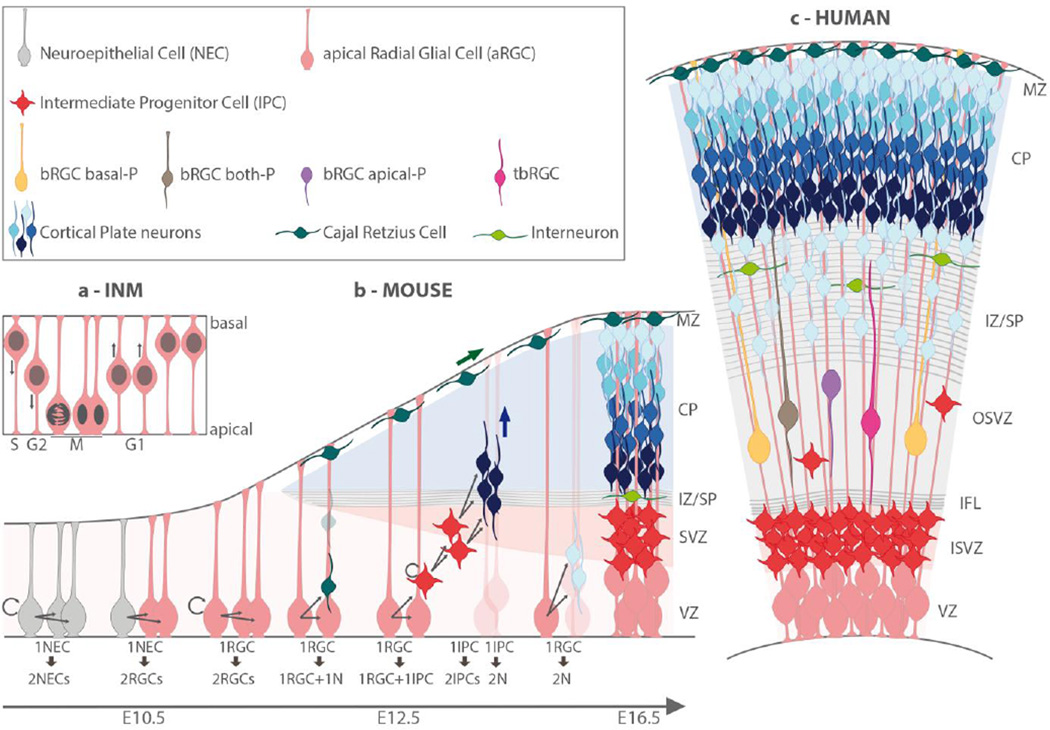
Figure 1. Neural progenitor subtypes and neurogenic phases during cerebral cortex development in mice and humans.
a) Interkinetic nucleus movement (INM) in progenitor cells of the ventricular zone (VZ) is coordinated with the cell cycle phases; b) Schematic presentation of the sequential steps of neurogenesis in the mouse: neuroepithelial cells (NECs, grey) self-renew by symmetric division, then turn into apical radial glial cells (aRGCs, pink) that divide either symmetrically to self-renew, or asymmetrically to give rise first to primary neurons including Cajal-Retzius cells (dark green) (direct neurogenesis) which migrate to the cortical surface to form the marginal zone (MZ), and to intermediate progenitor cells (IPC) at E12.5 and onwards; IPCs populate the sub-ventricular zone (SVZ) and generate cortical layer neurons (dark to light blue), which migrate along the basal process of aRGCs through the intermediate zone or subplate(IZ/SP) towards their destined layer. At later stages, aRGCs can undergo final symmetric divisions generating two neurons, c) schematic presentation of the human foetal cortex at a developmental stage close to 15 PCW; the major differences with the mouse embryonic developing cortex are 1) the SVZ is split into inner (ISVZ) and outer (OSVZ) regions by the inner fiber layer (IFL); 2) the remarkable expansion of the IZ/SP, where migrating cortical layer neurons receive input from thalamic afferent axons and interneurons (light green) originating from sub-cortical structures. Of note, the OSVZ comprises IPCs and further progenitor subtypes with characteristics of radial glial cells and named basal RGCs (bRGCs). Four bRGCs subtypes are distinguished according to their cell processes: b-RGC basal-P (yellow) with a basal process attached to the basal lamina, b-RGC apical-P (purple) with an apical process, b-RGC both-P (color?) with an apical process and a basal process attached to the basal lamina; tbRGC (color?), a transient bRGC with short apical and basal processes.
At the onset of neurogenesis, NECs turn into apical radial glial cells (aRGCs) (Gotz and Huttner, 2005; Kriegstein et al., 2006). aRGCs maintain neuroepithelial features with adherens junctions and apico-basal polarity with long basal process that span the entire thickness of the cortex (Figure 1b). They can be distinguished by their higher expression of the transcription factor Pax6 and by the expression of glial markers, such as GLAST and BLBP (Asami et al., 2011). They can self-renew by symmetric divisions but primarily undergo asymmetric neurogenic divisions, which produce a new aRGC and either a neuron (direct neurogenesis), or an intermediary type of progenitor cell, called IPC that then gives rise to neurons (indirect neurogenesis) (Hartfuss et al., 2001; Campbell and Gotz, 2002; Noctor et al., 2004; Kriegstein et al., 2006). Ultimately, during the last stages of neuron production, aRGCs undergo a terminal symmetric division, giving birth to two neurons (Noctor et al., 2004) (Figure 1b). The prevalence of indirect neurogenic divisions increases markedly as neurogenesis progresses. Recent data from the mouse have shown that each aRGC can give rise to 8–9 neurons stochastically distributed throughout the different layers (Gao et al., 2014). This implies that RGCs are subject to a succession of states, corresponding to a progressive restriction of competence and to changes in their ability to generate distinct neuronal subtypes as a function of time and/or cell division, an idea supported by progenitor transplantation studies (Desai and McConnell, 2000). IPCs are transiently amplifying progenitors characterized by the expression of the transcription factor Tbr2 and by a multipolar morphology. They delaminate from the VZ to settle in the SVZ, where they divide symmetrically to self-renew before undergoing a terminal division that gives rise to two neurons (Figure 1 b) (Noctor et al., 2004; Sessa et al., 2010).
To recapitulate, the final neuronal output is sequentially impacted by the size of the initial pool of founder progenitors (NECs), by the progressive switch from symmetric autoreplicative to neurogenic divisions, and finally by the duration of the neurogenic phase, which, interestingly, presents significant variations between species (Borrell and Calegari, 2014; Sun and Hevner, 2014; Dehay et al., 2015). In the mouse, SVZ progenitors, principally comprising IPCs, undergo at most two rounds of divisions (Noctor et al., 2004), whereas they undergo significantly more rounds in humans and other primates (Fietz et al., 2010; Martinez-Cerdeno et al., 2012; Betizeau et al., 2013; Stahl et al., 2013). This is a major difference observed between lissencephalic species such as rodents, and gyrencephalic species, such as humans and other large primates. In addition, other features differentiate the structure and cell content of the SVZ in lissencephalic and gyrencephalic species (Figure 1b and 1c). First, the primate SVZ is subdivided into two layers by a filamentous structure (the IFL, for inner fiber layer): the inner SVZ (ISVZ), which is juxtaposed to the VZ, and the more basal or outer SVZ (OSVZ). Second, while the ISVZ contains only TBR2+ IPCs, such as in the mouse SVZ, the OSVZ contains additional types of progenitors that are similar to aRGCs, and called bRGCs for basal RGCs; these are distinguished by the presence of only an apical or a basal process or both (Betizeau et al., 2013; LaMonica et al., 2013) (Figure 1c). bRGCs are originally generated from aRGCs by asymmetric divisions most frequently characterized by an horizontal or sometimes by an oblique division plane (Hansen et al., 2010; Lui et al., 2011; Shitamukai and Matsuzaki, 2012). They can in turn give rise to new bRGCs and IPCs as well. Contrasting with the scarcity of bRGCs in the mouse (Shitamukai and Matsuzaki, 2012), the striking expansion of bRGCs, and subsequently of IPCs, is a major hallmark of the primate OSVZ and is now commonly considered as one of the predominant events underlying the evolutionary increase in neuronal output and the related size expansion of the cerebral cortex. In line with this, the different morphotypes of bRGCs have recently been shown to present different rates of proliferation and changes in their relative distribution have been correlated to waves of neurogenesis (Betizeau et al., 2013; Dehay et al., 2015).
Mechanisms controlling the production of neocortical progenitors
The interkinetic nucleus movement (INM)
The nucleus of the VZ progenitor cells undergo interkinetic movement, which extends through the entire cytoplasm in NECs, but spans only the VZ in RGCs. This cell cycle-related nuclear movement, called interkinetic nuclear movement (INM), creates a pseudo-stratified structure, in which the positions of asynchronized nuclei are dependent on cell cycle phase: nuclei are located at the basal end of the cell during S phase, undertake basal to apical migration towards the ventricular surface during G2, undergo M phase at the ventricular surface and then migrate back to the basal side during G1 (Taverna and Huttner, 2010; Kosodo et al., 2011) (Figure 1a). The exquisite coordination between INM and cell cycle phases plays an essential role in the homoeostasis of the progenitor pool (Schenk et al., 2009; Taverna and Huttner, 2010). The impairment of this process can lead to the loss of progenitors, due to abnormal abventricular mitosis and the consequent apoptosis or exit from the cell cycle. Interactions of motor protein complexes with microtubules play an important role in INM: Dynein, Lis1 and Nde1 are involved in basal to apical migration, while myosin II is involved in apical to basal migration. The disruption of Lis1 function indeed results in the accumulation of heterotopic multipolar progenitors in the VZ of embryonic rat brains, along with a loss of progenitors (Tsai et al., 2005; Tsai et al., 2007).
Variations in the balance between these progenitor pools have been observed between species and have been hypothesized to account for the variations in final neuronal output and the size of the cerebral cortex. These variations imply that mechanisms influencing this balance have been adjusted during evolution to generate gyrencephalic brains (Dehay et al 2015; Sun and Hevner, 2014).
Symmetric versus asymmetric divisions
The balance between symmetric and asymmetric divisions also has a high impact on the neuronal output. As previously discussed, symmetric divisions are important for the production of an adequate pool of founder progenitors or NECs, whose progeny will ultimately give rise to all cortical projection neurons. Hence, any alteration of this pool, even moderate, has a significant impact on the final neuronal output. On the other hand, asymmetric divisions are important to maintain the proper distribution of the progenitor pools, including aRGCs, bRGCs and IPCs, in coordination with the sequential generation of neurons populating the six layers of the cortical plate (LaMonica et al., 2012; Betizeau et al., 2013; Sun and Hevner, 2014; Dehay et al., 2015).
During symmetric NEC divisions, the cleavage plane is oriented vertically and perpendicular to the ventricular surface. The mitotic spindle is formed horizontally parallel to the ventricular surface and both sides of the spindle present equivalent volumes. Such a spatial organization requires 1) proper centrosome (microtubule organizing center) duplication and assembly; and 2) very well-orchestrated interactions between microtubules and various factors, such as Planar Cell Polarity (PCP) components, LGN, and Inscuteable (Konno et al., 2008; Peyre et al., 2011; Postiglione et al., 2011; Delaunay et al., 2014). Alterations in the centrosome cycle affect spindle positioning, which is detrimental to division symmetry and can drive cells to prematurely exit the cell cycle and differentiate, or even to undergo apoptosis (Kimura et al., 2013). Another important characteristic of symmetric division is, by definition, the equal repartition of cell components necessary for the maintenance of polarity and proliferation, such as Par3/Par6 and proteins that interact with them (Costa et al., 2008; Bultje et al., 2009). Likewise, the basal process, necessary for the maintenance of progenitor fate in the VZ, is equally split between the two daughter cells or rapidly regenerated in one of them (Kosodo and Huttner, 2009; Hansen et al., 2010). Indeed, signals mediated by the meninges and Cajal-Retzius cells through contacts with the basal membrane of NECs and RGCs participate in the activation of proliferation (Hartfuss et al., 2003). Such activation is also promoted by Notch signalling (Gaiano et al., 2000; Gaiano and Fishell, 2002), which is sustained by high Par3 expression (Bultje et al., 2009). The Notch pathway activates Hes genes, which in turn repress neurogenic genes, such as neurogenins, and thus inhibit differentiative divisions. Hence, the unequal repartition of Par3 during asymmetric divisions results in cell cycle exit and differentiation of the daughter cell inheriting the lower amount of Par3 (Bultje et al., 2009; Dong et al., 2012). Notch signalling is further modulated by other pathways induced by factors secreted by the choroid plexus into the cerebrospinal fluid and known to play an important role in the control of progenitor amplification such as Igf2 (Lehtinen et al., 2011), FGFs (Yoon et al., 2004; Rash et al., 2013), Wnts (Buchman et al., 2011; Delaunay et al., 2014), and Shh, which acts on the primary cilium of NECs and RGCs (Willaredt et al., 2008). Glycogen synthetase-kinase 3 (GSK3), which acts as an inhibitor of neural progenitor proliferation, appears to be a key integrative component at the centre of the complex interplay between all these signaling pathways, the net effect of which is to maintain GSK3 in an inactivated state (Kim et al., 2009; Hur and Zhou, 2010). Despite the lack of experimental evidence, we can reasonably suppose that changes observed in the composition of the cerebrospinal fluid during brain development can modulate this interplay and eventually influence the transition from NECs to RGCs. In addition, the proliferation of neural progenitors appears to be influenced by extrinsic factors, such as the Vaso-Intestinal-Peptide (VIP) (Passemard et al., 2011), and by miRNAs (Arcila et al., 2014; Ghosh et al., 2014).
Alterations to these mechanisms controlling cell proliferation induce two main types of brain pathogenesis, which have been included into the Group I of brain malformations (Barkovich et al, 2012): 1) Microcephalies, which are underpinned by a loss of progenitors due to impaired proliferation and/or cell death, are characterized by a decrease in brain size of at least 3 SD, and are associated with mutations of genes promoting proliferation, such as MCPH genes; 2) Macrocephalies, which are characterized by a larger brain and are associated with over proliferation of neural progenitor cells (Table 1).
Table 1. Classification of cerebral malformations according to cell type origins and morphogenic defects.
Cerebral malformations have been classified into three main groups, according to their cellular origin (Barkovich et al, 2012). Each group has been further subdivided into sub-groups based on distinct morphogenic defects. The principal genes involved in each disorder are reported (Barkovich et al., 2012; Mirzaa et al., 2014). Several genes, such as LIS1, MCPH2 (WDR62), RELN, are involved in more than one morphogenic defect, due to their pleiotropic functions.
| Cell type | Cellular function | Classification | Morphogenic defects | Genes |
|---|---|---|---|---|
|
Neural progenitors |
Decreased
proliferation Increased apoptosis Increased proliferation Decreased apoptosis |
GROUP I |
I.A and III.D :
Microcephalies I.B : Megalocephalies |
MCPH1-12, LIS1, NDE1, TBR2 FGFR3, PTEN, GLI3, PTCH1 |
| Neurons | Abnormal migration | GROUP II |
II.A : Heterotopia II.B : Lissencephalies II.C : Subcortical heterotopia sublobar dysplasia II.D : Cobblestone malformations |
FLNA, RELN LIS1, TUB1A, TUBB2B, TUBB3, CDK5, DYNC1H1, WDR62, RELN DCX, LIS1, NDEL1, TUBA1, TUBB2, ACTG1, ACTB,TUBG1, KIF2A, DYNC1H1 GPR56, FKTN |
|
Heterogenous origins |
Abnormal postmigrational development |
GROUP III |
III.A:
Polymicrogyria Schizencephaly III.C : Focal cortical dysplasias III.D : Postmigrational microcephaly |
TBR2, PAX6, NDE1,
EMX2 PTEN CASK, FOXG1, TCF4 |
Neuronal migration
As previously discussed, projection neurons are born in the SVZ and then migrate through the intermediate zone or subplate to reach their layer of destination in the cortical plate. Neuronal migration is a multifaceted process and relies on a large variety of cellular functions intervening in cell shape, polarity and motility. Time-lapse analyses have revealed several shape transitions in neurons on their way to final position. Newborn neurons leaving the SVZ transiently adopt a multipolar morphology, and then a bipolar morphology characterized by leading and trailing processes that neurons maintain during migration (Gressens, 2006; Marin et al., 2006). Of note, this migration proceeds along the basal process of RGCs, and thus underpins the formation of radial units of migrating neurons. This radial organization appears to be a major process for radial extension of the cerebral cortex (Rakic, 2009).
All these processes are governed by a sophisticated interplay between intrinsic and extrinsic instructive cues (Figure 2).
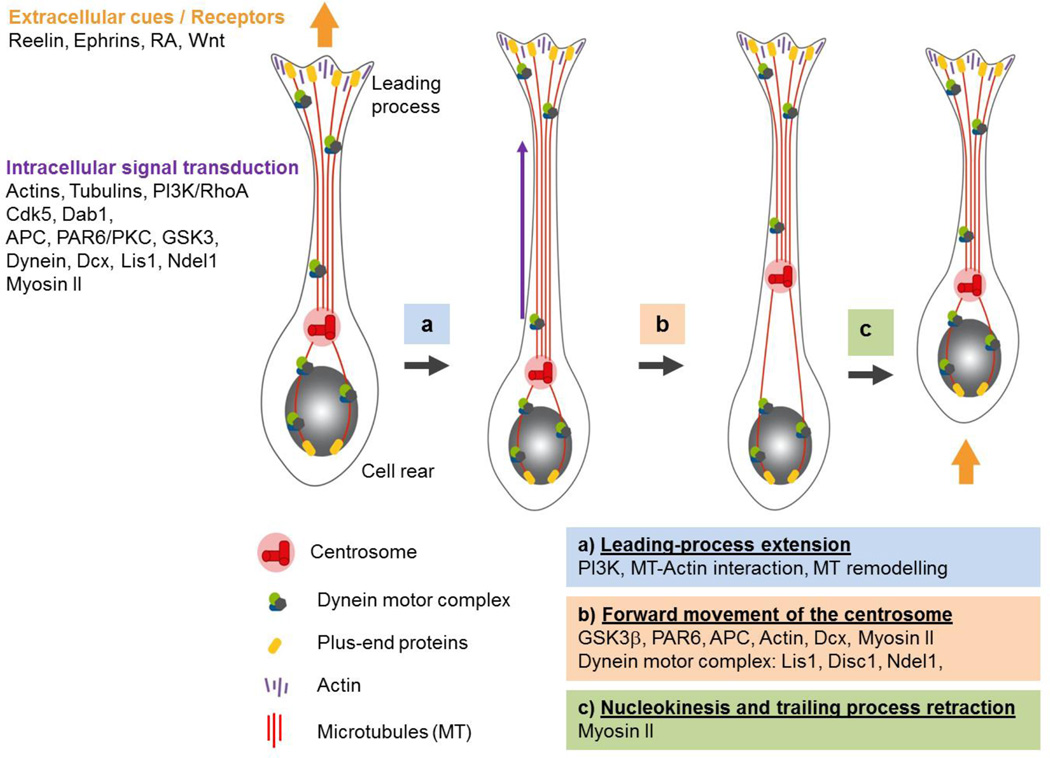
Figure 2. Steps in neuronal migration and molecules involved.
Extracellular cues, mediated by components of the extra-cellular matrix, such as cadherins, or by membrane receptor activation by Reelin, Ephrins, Wnts and RA promote the various steps involved in neuronal migration. a) Polarized extension of the leading process: RhoA inhibition by PI3K enhances leading process outgrowth, microtubules plus ends (yellow) are recruited by cortical actins (purple); in the intermediate segment of the leading process, microtubules are remodelled and extended (purple arrow) upon Dab1 and Cdk5 signalling. b) Forward movement of the centrosome (red rods) requires Par6/PKC and GSK3 activity. c) Nucleokinesis, which pulls the nucleus towards the trailing process, and retraction of the cell rear process rely on the motor protein complex (green) formed by dynein and interacting proteins, Lis1, Ndel1, Disc1 and Dcx, as well on Mysosin II interactions with actin cytoskeleton in front and at the rear of the nucleus. Yellow arrows indicate pulling forces at the cell front and rear.
Intrinsic cues
Cytoskeleton components play a major role in the cellular functions required for cell migration, and most importantly, they are selectively implied in a very highly coordinated manner during the two main phases of cell movement associated with migration: the movement of the centrosome in the leading process, followed by the nuclear movement (nucleokinesis) towards the centrosome (Kriegstein and Noctor, 2004; Tsai and Gleeson, 2005). Of note, nucleokinesis accompanying neuronal migration shares many aspects with INM in progenitors (Figure 2).
The extension of the leading process results from protrusion forces exerted by actin polymerization and microtubules growth. In addition, as during INM, microtubules interact with motor proteins such as Lis1/Ndel1/dynein, which play an important role to ensure coordination between nucleokinesis and centrosome movement (Tsai and Gleeson, 2005; Heng et al., 2009; Solecki et al., 2009). Similarly, proteins involved in cell polarity, such as Par6 and aPKC, and proteins associated with microtubules, such as APC, Dcx and kinesins, are essential for proper migration and cortical morphogenesis (Bai et al., 2003; Reilein and Nelson, 2005; Tsai et al., 2010) (Figure 2). Kinesins participate in the directional and selective transport of cargos, such as vesicles containing neurotrophic factors or presynaptic neurotransmitters (Falnikar et al., 2011; Falnikar and Baas, 2013; Liu et al., 2012). The interactions between these proteins and microtubules are largely regulated by posttranslational modification. In particular, the function of Dcx and kinesins appears to be regulated by phosphorylation by the Cdk5 kinase (Hammond et al., 2004; Nishimura et al., 2014).
Extrinsic cues
During migration, neurons receive important hints from diverse origins. Cajal-Retzius cells settled in the marginal zone secrete reelin, which activates signalling cascades after binding to the VLDLR/ApoER2 receptor expressed at the membrane of the RGC basal process (Cheng et al., 2011) (Figure 2). Reelin signal transduction, which involves DAB1, nectins, affilins, Fyn, and components of the extra-cellular matrix such as cadherins, regulates cytoskeleton organization, nuclear movement and cell shape and adhesion (Beffert et al., 2004; Franco et al., 2011; Gil-Sanz et al., 2013; Forster, 2014). In the reelin deficient Reeler mice, neurons do not reach their prospective final position, which results in a reversed laminar structure of the cortical plate (Landrieu and Goffinet, 1981; Andersen et al., 2002). In humans, Reelin mutations can be associated with lissencephaly, as described in the Norman-Roberts syndrome (Hong et al., 2000; Chang et al., 2007) (Table 1). Migrating neurons express Ephrin-B1, which promotes their radial migration while limits their tangential dispersion by inhibiting neurite extension (Dimidschstein et al., 2013). Interestingly, more selective informative cues can impact the migration of specific classes of neurons. In the absence of retinoic acid (RA), migration of prospective layer V-III neurons that express the RAR403 receptor is retarded. These neurons fail to maintain their fate and instead acquire characteristics of layer II neurons (Choi et al., 2014). This RA effect on the maintenance of neuronal identity and localization relies on the stabilisation of the beta-catenin function and the stimulation of the Wnt signalling, which is necessary for migration process (Ivaniutsin et al., 2009; Morgan-Smith et al., 2014). Furthermore, this effect seems to be more active in the rostral region of the developing cerebral cortex, suggesting that RA can also participate in the regional variations of the neuronal subtype output. These data also indicate that young neurons still possess some plasticity.
Brain malformations linked to impaired neuronal migration
Genetic alterations affecting genes encoding cytoskeleton components, such as tubulins (Bahi-Buisson et al., 2014; Fallet-Bianco et al., 2014), and proteins of the motor complex, impair neuronal migration and are responsible for various brain morphogenic disorders, in particular lissencephalies (Table 1). More specifically, DCX is involved in X-linked brain disorders, sub-cortical band heterotopia primarily in females and more severe lissencephaly, usually in males. LIS1 haploinsufficiency causes lissencephaly and is involved in Miller-Diecker syndrome (Pilz et al., 1998; Shu et al., 2004; Tsai et al., 2007). DNC1H1 (Dynein) (Poirier et al., 2014, as well as CDK5 (Magen et al., 2015), have also recently been related to lissencephaly). Some types of lissencephalies are associated with microcephalic features (microlissencephalies), as observed in the context of LIS1, NDEL1 and WDR62 (MCPH2) mutations (Fallet-Bianco et al., 2014). These disorders can indeed result from the cumulative effects of impairment of centrosome assembly, coordination of the nucleus and centrosome movement in progenitors and differentiating post-mitotic neurons, which together cause the loss of progenitors and alter neuronal migration. Hence, the discrimination between malformations due to cell proliferation defects or to impaired neuronal migration is not always clear-cut.
Evolution and folding of the cerebral cortex
The size of the cerebral cortex varies markedly among mammalian species. Its evolutionary increase corresponds to the enhanced cognitive function and intelligence, and is associated with the acquisition of folding or gyri, which differentiate primates (gyrencephalic) from rodents (lissencephalic or smooth-surfaced) cortices. As discussed above, cortical growth depends on progenitor expansion, in particular in the OSVZ, and varies regionally, in respect with species cognitive abilities and sensorimotor skills. The mechanisms that underlie this complex control of progenitor expansion in the OSVZ are far from being understood, although recent data have evidenced an important role of extra-cellular matrix components and miRNA (Fietz et al., 2012; Arcila et al., 2014). In addition, variations in TRNP1 expression, a gene expressed at higher levels in self-renewing aRGCs, have been correlated with regional expansion of the ventricular surface of the foetal human brain (Stahl et al., 2013). However, genetic manipulations in mice, which led to over-amplification of VZ progenitors and VZ over-expansion failed to reproduce gyri with proper cortical architectonic (Poschl et al., 2013; Rash et al., 2013). Understanding how regional differences in the proliferation rate of VZ progenitors influence gyrogenesis remains a challenging issue. Gyrogenesis also encompasses further multiple sequential events, including neurogenesis, cell migration, synaptogenesis and gliogenesis. The fact that mutations in cytoskeletal genes that are involved in cell migration, in particular Tubulins, cause lissencephaly illustrates the importance of cell migration processes in gyrus-building; Chang et al., 2007; Spalice et al., 2009; Liu, 2011). Other important cues provided by embryonic thalamic axons affect cortical development in two ways: i) regulation of neurogenesis by stimulating the proliferation of progenitors; ii) forces generated by axon tension may influence gyrogenesis by pulling together interconnected regions of the cortex and may thus participate in the optimization of cortex wiring. In line with this, early focal ablation of thalamic afferent axons causes profound abnormalities in sulci development and orientation (Dehay et al., 1989; Dehay et al., 1996).
Synaptogenesis and neural circuit development
The most remarkable feature of the nervous system is accuracy and efficiency of synaptic connectivity and neural circuitry, which results from formation and maturation of synapses to support general brain function, intricate cognition and behaviours. These processes start with axonal/dendritic outgrowth during or shortly after neuronal migration in the embryo and continue well into adolescence or young adulthood, in phases which include axon pathfinding/target recognition; synapse formation/pre-and postsynaptic specializations; and synapse pruning /stabilization. During foetal life, connections are established and influenced by endogenous, spontaneous patterned activity (e.g. retinal waves). After birth, synapses and neuronal connections are subject to activity-dependent changes induced by their interactions with the environment and by learning, i.e. developmental plasticity.
Axon guidance and pathfinding
At the initial stage, the growth of developing axons depends on intrinsic mechanisms as well as local or long-range environmental guidance. The guidance forces could be attractive or repulsive and thereby, pulling or pushing the growth cone (enlargements on a growing axon’s tip) in the correct direction towards its target (Tessier-Lavigne and Goodman, 1996). The initial axons (pioneering axons) find intermediate targets (guidepost cells) on their way towards final point and are later joined by other axons to turn into tracts (fasciculation). It is now known that chemical cues, for example selected cell-surface adhesion molecules, such as neuronal cell adhesion molecules (NCAM), N-cadherin and integrins, as well as extracellular matrix (ECM) molecules, are primarily responsible for axon guidance and axon fasciculation (Dickson, 2002). Classical guidance proteins include Ephrins, Netrins, Semaphorins, Slits and their receptors, receptor and non-receptor tyrosine kinases, etc (Dickson, 2002; Bashaw and Klein, 2010; Raper and Mason, 2010; Kolodkin and Tessier-Lavigne, 2011). Cue-mediated signalling induces reorganization of the cytoskeleton (actin filaments and microtubules) in growth cones leading to its target-orientated movement. Classical morphogens, including Shh, TGFβ/BMP and Wnt also work in concert as non-conventional axon guidance cues. They could act either as chemoattractants or chemorepulsants depending on the axon target and what axonal receptors they bind to (Charron and Tessier-Lavigne, 2005; Aviles et al., 2013; Yam and Charron, 2013). MicroRNA was recently discovered to serve as another level of regulators for these chemical cues and related signalling pathways (Chiu et al., 2014; Iyer et al., 2014). Axon guidance crossing CNS midline in developing spinal cord, the cortico-spinal tract (CST) and the corpus callosum is guided mainly by short-range cues derived from the midline structure and the neighbouring pioneer axons (Kaprielian et al., 2000; Nishikimi et al., 2013).
Pre-and postsynaptic specializations
Once reaching its final target, the growth cone transforms into a presynaptic axon terminal, on which a nascent presynaptic site forms and is further specialized into functional presynaptic structures for depolarization-evoked neurotransmission. Presynaptic compartments are characterized by the presence of neurotransmitter-filled synaptic vesicles (SV) and the active zones (AZ), patch of plasma membrane where SV dock, fuse and release neurotransmitter triggered by Ca2+ influx through voltage-gated calcium channels. The electro-dense cytomatrix at the AZ membrane (CAZ) is composed of AZ structural molecules (Synapsins, Bassoon and Piccolo, Rim binding protein, etc.) and proteins involved in SV docking, fusion (SNARE complex, Munc18-1, Munc13, Rim, etc) and recycling machinery (Ziv and Garner, 2004; Owald and Sigrist, 2009; Sigrist and Schmitz, 2011). In the excitatory synapses, CAZ align precisely with a similar electron-dense thickening at the postsynaptic membrane, the postsynaptic density (PSD), where the neurotransmitter receptors are enriched. The assembly of CAZ and PSD, as well as the trans-synaptic molecular bridging (cell adhesion molecules, such as N-cadherin, Neurexin/Neuroligin complex, Ephrin-B/EphB complex) is tightly coordinated and developmentally regulated (Fletcher et al., 1994; Li and Sheng, 2003). Structural maturation of presynaptic boutons is represented by the accumulation of SV with consequent formation of SV reserve pool and the increased complexity of CAZ. Functional presynaptic maturation includes changes in the types and subunit composition of voltage-gated calcium channels mediating synaptic neurotransmitter release, increased Ca2+ sensitivity, and changes in the probability of neurotransmitter release (Ziv and Garner, 2004).
In order for efficient reception of presynaptic neurotransmitter signal, the corresponding receptors on the postsynaptic membrane are clustered at sites opposite the presynaptic AZ, the PSD in the case of glutamatergic synapses that reside on dendritic spines and use glutamate as the neurotransmitter. Inhibitory GABAergic synapses are established on dendritic shafts and are formed earlier than excitatory synapses in hippocampal CA1 pyramidal neurons during neuronal development (Tyzio et al., 1999; Khazipov et al., 2001). Similar to CAZ, the PSDs undergo structural and functional maturation over time (Ethell and Pasquale, 2005). The core PSD components are scaffold (Homer/Shank complex and PSD-95 proteins) and cytoskeleton proteins, glutamate receptors and their interacting proteins, protein kinases/phosphotases, and other signalling complexes (Yamauchi, 2002; Okabe, 2007; Sheng and Kim, 2011). The two major classes of glutamate receptors in the synapses are the ionotropic glutamate receptors (NMDA, AMPA and kainate receptors) and metabotropic glutamate receptors. NMDA receptors (NMDARs) are recruited more rapidly and earlier than the AMPA receptors (AMPARs) and kainate receptors, therefore, immature synapses could contain only NMDARs but not AMPARs (silent synapses). During development, synapses become functional and strengthened by activity-dependent insertion of AMPARs. NMDARs are assemblies of obligatory NR1 and modulatory NR2 subunits (NR2A-2D), and sometimes the NR3 subunit. AMPAR channels comprise heterotetramers of subunits GluR1–4. The importance of glutamate receptors in synaptic and neuronal plasticity during development makes them special targets in the pathogenesis of neonatal encephalopathy. The developing brain is associated with enhanced excitability and susceptibility to seizures and epilepsy than the adult brain possibly due to the following findings from studies of developing rat brain: 1). The numbers of brain NMDARs and AMPARs are overshooting during neonatal life (NMDARs peak late in the first postnatal week in many forebrain regions, while AMPARs peak around P10–12) (Jensen, 2002; Jantzie et al., 2015). 2). In the immature brain, neuronal NMDARs contain high levels of the NR2B subunit, which results in longer current decay times and also high levels of NR2D and NR3A that are associated with low magnesium sensitivity of the NMDARs (Cull-Candy et al., 2001; Haberny et al., 2002; Ritter et al., 2002). 3). Ca2+-permeable AMPARs that lack a GluR2 subunit are more abundant in immature brain, and are highly expressed on white matter cells in the first week and cortical neurons during the second week (Pellegrini-Giampietro et al., 1992; Talos et al., 2006a; Talos et al., 2006b). Therefore, age-dependent patterns of neurotransmitter receptor expression correlate with temporal and regional vulnerability to excitotoxic brain damage such as occurs with hypoxia and hypoxia-ischemia (HI) in the perinatal period. Recent studies with human foetal and autopsy tissue have demonstrated similar patterns of AMPAR and NMDAR subunit composition and expression in human brain during the window of vulnerability to white matter or/and grey matter injury in both term and preterm neonates (Talos et al., 2006b; Jantzie et al., 2015). Excitatory synapses are overshooting during development, especially from birth to early childhood, a critical period when activity-dependent synaptogenesis and plasticity is at its peak, while inhibition takes a long time to develop.
A growing body of evidence suggest that an imbalance of excitation/inhibition during neural circuit development is implicated in neonatal hypoxic-ischemic encephalopathy (HIE), seizure and associated later-life epilepsy, and a range of cognitive disorders such as autism, Fragile X syndrome, Rett syndrome, schizophrenia and major depression (Gatto and Broadie, 2010). The molecular mechanisms for establishing and maintaining balanced excitation and inhibition have been the attention of enormous studies and likely involve the synchronized efforts of presynaptic, trans-synaptic, and postsynaptic molecular regulators at genetic, transcriptional, translational and posttranslational levels.
Synapse pruning /stabilization
Until approximately 2 years of age, there is an overproduction of neurons and synapses, particularly in the cortex, which will be eliminated later to allow for strengthening of more productive connections and more efficient neural networks. The pruning of unused or weak synapses continues until late adolescence (about 15 years old) by which time nearly 50% of the synapses are eliminated. Synapse elimination and rearrangement result in the fine-tuning of neuronal circuitry and synapse strengthening in the nervous system. Pruning is influenced by environmental factors and is associated with learning (use it or lose it). Synapse elimination is due to axon pruning with mechanisms of axon degeneration, axon retraction, and axon shedding. On the molecular levels, pruning process is mainly regulated by axon guidance receptors, TGFβ receptors, and death receptors (Vanderhaeghen and Cheng, 2010; Schuldiner and Yaron, 2015). It is interesting that microglia play a major role in synaptic pruning by phagocytosing weak synapses during development and injury to decrease synapse numbers (Paolicelli et al., 2011; Kettenmann et al., 2013; Zhan et al., 2014). According to the most recent studies, deficits in microglia function (lack the chemokine receptor Cx3cr 1) (Zhan et al., 2014) or decrease in spine pruning (impaired mTOR-autophagy mechanism) (Tang et al., 2014) result in excess synapses left in the brain, which may link to autism pathophysiology in human. It is not known whether these two events are associated and whether gene mutations found in autism are involved in regulation of these signalling pathways. In addition, astrocytes also play pivotal roles in synapse formation, maturation and elimination (see below).
The establishment of appropriate neural circuit and plasticity of synaptic transmission depend upon not only pre- and post-synaptic neuronal mechanisms, but also the active involvement of perisynaptic glial cells. Any events that perturb their partnership may impact normal brain development and lead to severe neurological consequences.
Gliogenesis and myelination
Glia constitute the majority of the brain cells (make up at least 50% of the cells in the human brain). It is increasingly recognized that they are not only indispensable for maintaining homeostasis of mature brain function, but are also essential for normal brain development. The two principal macroglial cells, astrocytes and oligodendrocytes (OLGs), originate from the neuroepithelium at the VZ and are produced by the common multipotent progenitors that give rise to neurons before switching developmental programs to gliogenesis (Rowitch, 2004; Rowitch and Kriegstein, 2010). Therefore, glial cell generation starts late in embryonic stage, for example, in mice, cortical neurogenesis begins at about E12, while astrocytes are first detected around E16 and OLGs around birth. Regulation of neuron to glia switch involves complex neuron-glial interactions as well as spatiotemporal interplay of both cell-intrinsic factors and extracellular signals, for example, down regulation of proneural factors/ anti-astrogenesis growth factors and activation of pro-glial transcriptional programs (Rowitch, 2004; Rowitch and Kriegstein, 2010; Bayraktar et al., 2014). Proliferation of both cell types peaks during the first postnatal month in rodents and their mitotic activity is preserved in later life once under deleterious conditions. In human brain, gliogenesis starts around 24 post conception weeks (PCW) and develop till early childhood.
Oligodendrocyte development and myelination
Mouse fate mapping studies demonstrate that oligodendrocyte precursor cells (OPCs) of the forebrain are produced in three successive waves initiating primarily in ventral ventricular regions (medial ganglionic eminence, MGE) at E11.5–12.5 in the embryonic stage. The second and third waves of OPCs production occur in more dorsal regions in lateral ganglionic eminence (LGE) starting at E15.5 and in the cortex at birth or early postnatal stage (Kessaris et al., 2006). Dlx-positive neural stem cells at MGE and LGE preferentially generate GABAergic neurons and OPCs (He et al., 2001; Marshall and Goldman, 2002). As brain matures, OPCs proliferate, migrate dorsally and populate the forebrain before differentiating into premyelinating oligodendrocytes (preOLs, starting E16.5). At their last destination in fiber tracts, the preOLs undergo late proliferation and mature into myelinating oligodendrocytes where they associate with axons and produce myelin sheaths wrapping around the fibers (Figure 3). Myelination provides a salutatory, smooth and fast flow of neural impulses throughout the CNS and allows for more complex brain processes. In humans, OPCs generation starts at gestational age (GA) 13 weeks and perOLs at GA 17–20 weeks (Jakovcevski et al., 2009). In contrast to a highly transient stage of preOLs in the mouse, a minor population of preOLs are present in human cerebral white matter for at least 3 months before further maturation (Back et al., 2001; Back et al., 2002). Myelinating OLGs are one of the last cells to be developed and myelination persists from 32 PCW to adulthood with the peak activity occurring at 6th month to 2 years old. In the postnatal brain, the myelination progresses from dorsal to ventral and from caudal to cephalad with the prefrontal cortex being myelinated last. Oligodendrocyte lineage progression is accompanied by the changes in morphology, motility and a gradual shift of expression of different cell surface antigens that are used as markers for OLG stage-specific maturation. OPCs are simple, often bipolar and express PDGFRa, Olig 1/2, Sox10 and NG2. PreOLs show a more complex form with multiple processes and acquire the O4 or O1 antigens (Figure 3). Mature myelinating OLGs have perpendicular processes as well as long parallel processes following the axonal tracts, and they express myelin basic protein (MBP), APC and myelin proteolipid protein (PLP) (Rowitch and Kriegstein, 2010; Silbereis et al., 2010). OLG specification, proliferation, survival, migration, differentiation and myelination represent independent but intimately related steps in OLG maturation and are regulated by distinct mechanisms (Emery, 2010; Mitew et al., 2014).
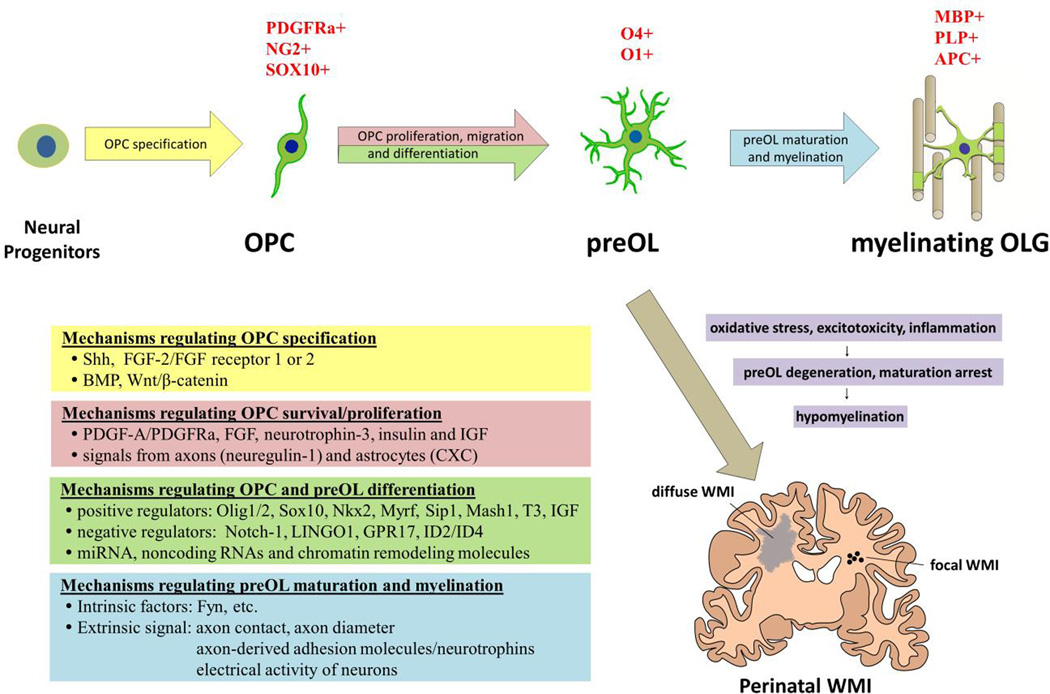
Figure 3. Major developmental phases of oligodendrocyte with the related regulatory mechanisms.
Schematic representation of oligodendrocyte lineage progression from oligodendrocyte precursor cells (OPC) to premyelinating oligodendrocyte (preOL) and myelinating oligodendrocyte (OLG). The characteristic markers of each phase are shown on top of the cells. The mechanisms regulating each step of OLG development are summarized in the left table. PreOLs are selectively vulnerable to oxidative stress, excitotoxicity and inflammation. PreOL cell death and failure of maturation of regenerated preOLs are responsible for focal or diffuse white matter injury (WMI) associated with brain hypoxia-ischemia and infections in preterm and term infants. IGF: insulin-like growth factors; Myrf: myelin regulatory factor; Sip1: Smad interacting protein-1; LINGO1: leucine-rich repeat and immunoglobulin domain containing1; GPR17: G-protein-coupled receptor 17.
Signalling molecules that regulate oligodendrocyte specification
Intensive studies have demonstrated that Shh, Wnt, BMP, Notch and FGF2 signalling pathways are implicated in OLG lineage specification in the embryonic forebrain. In the ventral domains of the forebrain, Shh is required for early phase of OPCs development. In the middle embryonic stage, it is most concentrated in the MGE of the ventral midline of the diencephalon, where OPCs are originated. Molecular mechanisms of Shh induction of OPCs are not very clear. Shh may regulate OPC fate specification by controlling expression of transcription factors, such as Nkx2 and Gsh2, Olig 1/2 and Mash 1, which are important for OPCs development in the forebrain (Alberta et al., 2001; Tekki-Kessaris et al., 2001; Ortega et al., 2013) (Figure 3).
On the other hand, the dorsally located BMP and Wnt/β-catenin pathways antagonize Shh effect and negatively regulate OLG induction. BMP4 is a member of the TGFβ super family of ligands, which promotes astroglial lineage determination at the expense of oligodendrogliogenesis (Gomes et al., 2003; Wu et al., 2012). Wnt/β-catenin pathway components regulate OLG development in a stage-dependent manner where β-catenin activity inhibits OPC generation in mice at early stage and promotes OLG differentiation later on (Dai et al., 2014).
Notch is a transmembrane receptor and generally mediates cell fate decision through interactions between neighbouring cells in a contact-dependent manner. Both loss- and gain-of-function studies have suggested that Notch signalling inhibits neuronal and oligodendrocyte differentiation, while promotes astrocyte differentiation. Expression of Jagged 1, one of the Notch ligands, in neuron-committed intermediate progenitor (IP) cells initially prevents neighbour OPC differentiation through activation of Notch1 receptor and its downstream Hes1/Hes5 protein; and later in development when axons reach their targets and establish synaptic contacts, decline in neuronal agged 1 allows OPC differentiation and maturation (Wang et al., 1998; Louvi and Artavanis-Tsakonas, 2006) (Figure 3).
Signals regulating oligodendrocyte proliferation and differentiation
OPCs are actively proliferating during migration before differentiation into myelinating OLG. Most pro-mitotic signals that promote OPCs proliferation are also pro-survival, but anti-differentiation. Among these, PDGF-A, acting through its receptor PDGFRa associating with members of integrin receptors on OPCs, is one of the most important. OPCs receive PDGF-A secreted from neurons or astrocytes, and once they start to differentiate, they rapidly lose PDGF receptors (Calver et al., 1998; Fruttiger et al., 1999). Other proteins that control OLG proliferation include FGF family members, neurotrophin-3, insulin and insulin-like growth factors. Additionally, communication between OLG and astrocytes (astrocyte-derived chemokine CXC), between OLG and the axons that they ensheath (neuregulin growth factors) also regulate their proliferation and survival to ensure a sufficient number of OLGs are generated to produce myelin (Mitew et al., 2014).
The transition of OPCs proliferation to differentiation seems to be controlled mainly by cell-intrinsic factors while thyroid hormone T3 may serve as an extrinsic co-signal by regulating p53 or p21cip1 (cell cycle inhibitor protein) pathway from results of in vitro experiments (Barres et al., 1994; Tokumoto et al., 2001; Zezula et al., 2001). Although OLG and their progenitors express T3 receptors, the mechanisms for systemic T3 in regulating OLG differentiation remain to be explored in vivo. It is also not clear about the mechanisms responsible for initiating differentiation in vivo. Other proposed positive regulators for OLG differentiation include transcription factors (Olig1/2, Sox10, Nkx2.2, Myrf, Sip1, Mash1, etc), whereas leucine-rich repeat and immunoglobulin domain-containing1 (LINGO1), the G-protein-coupled receptor 17 (GPR17), Hes5 and Id2/Id4 are major negative regulators (Rosenberg et al., 2007; Emery, 2010; Mitew et al., 2014) (Figure 3). It was recently reported that epigenetic factors, such as miRNA (miR-219 and miR-338), noncoding RNAs and chromatin remodeling molecules (histone deacetylases HDACs, DNA methyltransferase Dnmt3a and SWI/SNF enzyme Amarca4/Brg1) are also involved in regulating OLG differentiation (Zuchero and Barres, 2013).
Molecular regulators of myelination
Initiation, progression and repair of myelination are regulated by both intrinsic and extrinsic factors. Fyn, a member of Src family kinases, plays unique roles in CNS myelination (Kramer-Albers and White, 2011). Fyn null mice have decreased MBP expression, significantly less myelin and OLGs in forebrain, but not in the cervical spinal cord, at all developing ages (Umemori et al., 1994; Sperber et al., 2001). Fyn regulates OLG maturation and myelination by four pathways: 1) inhibition of RhoA GTPase activity, to promote OPC survival and process extension (Liang et al., 2004); 2) association with α-tubulin and Tau to regulate OLG microtubule assembly (Klein et al., 2002); 3) through FAK/laminin α2 pathway to drive preOL process outgrowth (Hoshina et al., 2007); 4) Fyn phosphorylation of RNA binding protein hnRNP A2, which stimulates transport and translation of MBP mRNA (White et al., 2008).
Environmental cues, especially the axonal influences play important roles for OLG survival, differentiation, and myelin protein synthesis. Axon contact, axon diameter and axon-derived cell adhesion molecules (for example polysialic acid-neural cell adhesion molecule, PSA-NCAM), and neurotrophins are known to regulate myelination (Rosenberg et al., 2007; Mitew et al., 2014). In addition, electrical activity of neurons, for instance, excitatory neuronal activity may affect OLG development through forming functional synapses with OPCs or preOLs and releasing glutamate or ATP acting on their AMPA receptors or purinergic receptors (Bergles et al., 2000; Wake et al., 2011; Zonouzi et al., 2011). In fact, in the developing brain, axon-OPC synapses are highly vulnerable to white matter injury (WMI), the predominant form of brain injury and the major antecedent to cerebral palsy in preterm infants (Shen et al., 2012). At premature stage (25–37 PCW), white matter preOLs transiently express Ca2+-permeable GluR2-lacking AMPA receptors, which render them selectively susceptible to excitotoxicity under hypoxia-ischemia (Talos et al., 2006). Furthermore, preOLs show greater sensibility to oxidative stress and inflammation, which often go along with HI and infections (Silbereis et al., 2010). PreOLs death and failure of maturation of regenerated preOLs are responsible for myelination insufficiency of cerebral white matter in preterm brains (Buser et al., 2012; Back and Miller, 2014).
Taken together, appropriate axonal myelination involves a protracted but constantly fine-tuned process of OPCs specification, proliferation, migration and differentiation during development. Understanding the genetic mechanisms, extracellular signalling and the intracellular pathways regulating these processes will not only unveil the normal functions of OLG but also inform the development of novel therapeutics for regeneration and repair in immature brain and adult demyelinating diseases as well.
Astrocyte development and function
Many studies have established the functions of astrocytes in the mature brain, which include: provide mechanical and metabolic support; regulate chemical and ionic environment; maintain the blood brain barrier and modulate synaptic transmission. In the setting of brain injury, these cells respond actively by acting as phagocytes or forming scar tissue. Much less is known, however, about their developmental origins and heterogeneity, roles in brain development, and contribution to neurodevelopmental disorders. This is due to a lack of definitive markers to identify neural progenitor cells that give rise to astrocytes and to trace their maturation stages. One consensus is that GFAP cannot serve as a valuable marker for this purpose because GFAP-positive radial glia generate neurons and OLG, and it is increasingly recognized that astrocytes are not one homogenous population.
Astrocyte development
It has been reported that astrocytes and OLGs have different precursor ancestors and originate in mutually exclusive domains of forebrain and spinal cord. Astrogenesis takes place after neurogenesis. The early stage astrocytes are produced by radial glia in the ventricular zone, as the final way out for radial glia, and at later stages by migratory progenitors in SVZ (Rowitch, 2004; Rowitch and Kriegstein, 2010; Bayraktar et al., 2014). In the first three weeks after birth in rodents, cortical astrocytes are mainly generated by division of local differentiated astrocytes, and these cells can integrate functionally into the existing glial network as mature astrocytes and interact properly with synapses and blood vessels (Ge et al., 2012). Spatially, astrocytes are predominantly induced in the dorsal telencephalon regions that normally express Neurogenin 2 (Ngn2) including the cortex where the excitatory pyramidal neurons arise (Rowitch, 2004; Rowitch and Kriegstein, 2010).
Similar to OLG specification, astrocyte cell fate acquisition is controlled by the integrated and well-programmed effects of cell intrinsic and extrinsic signals. Gliogenesis is repressed during neurogenesis, however, at the end of neurogenesis, down-regulation of proneural factors (Ngn1, etc.) and anti-astrogenesis growth factors, for example ERBB2, a member of EGF receptor family, facilitates astrocyte production (Rowitch and Kriegstein, 2010). As described in Figure 4, astrocyte-specific gene transcription is restrained during neurogenesis by epigenetic mechanisms; for example, DNA methyltransferase DNMT1-mediated CpG methylation of the STAT3-binding element in the GFAP promoter prevents STAT3, a direct GFAP transcriptional activator, from binding to its target DNA sequence (Fan, Martinowich et al. 2005). In the meantime, methyl CpG binding proteins, such as MeCP2, coupled with other repressor complexes including histone deacetylase HDAC1, may bind to the promoter leading to chromatin condensation and transcription silencing (Cheng, Lin et al. 2011). During this period, the proneural bHLH transcription factor Ngn1 binds to p300/CBP complex promoting neurogenesis while inhibiting astrocyte differentiation (Sun, Nadal-Vicens et al. 2001). At late-gestation stage, astrogenesis is promoted mainly by three signalling pathways: Notch, BMPs, and the IL-6 family cytokines, which act independently or converge to activate the JAK-STAT3 pathway.
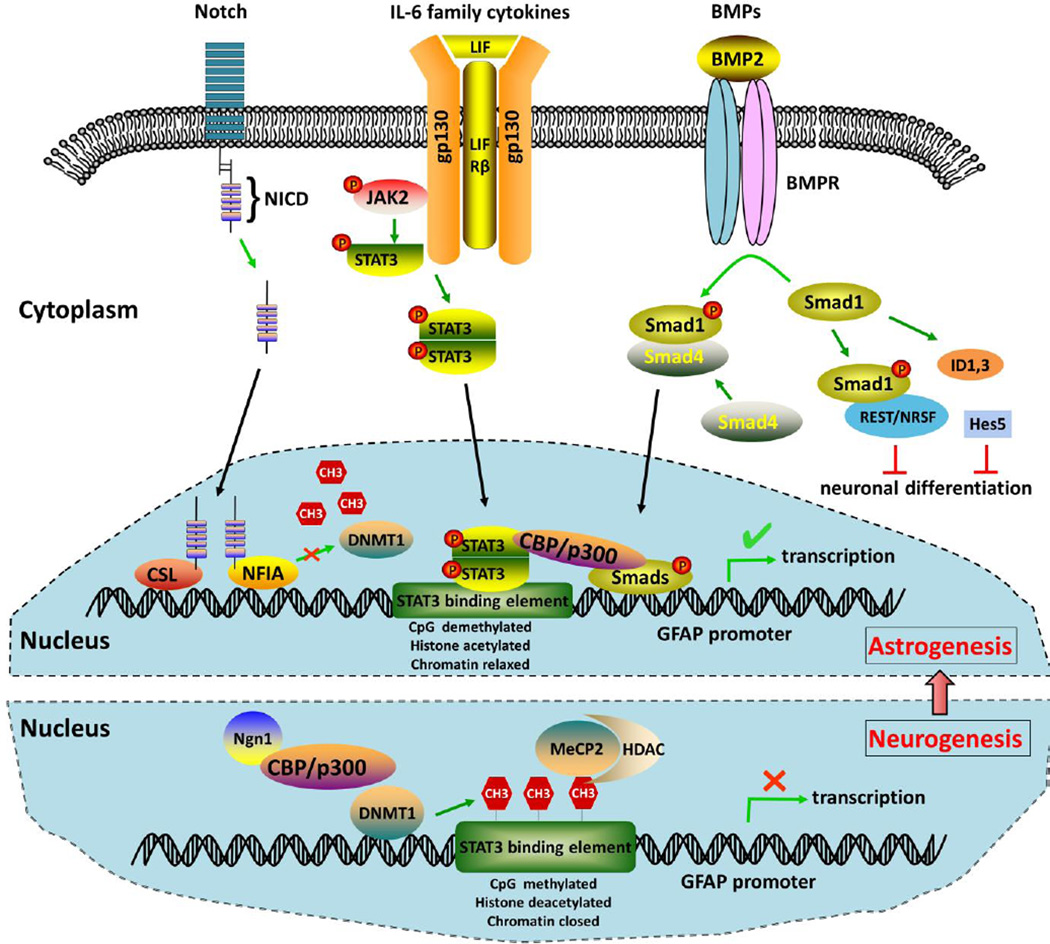
Figure 4. Molecular mechanisms that are important for the switch of neural progenitor cell development from neurogenesis to astrogenesis.
GFAP transcription is inhibited during neurogenesis by epigenetic mechanisms. DNA methyltransferase DNMT1- mediated CpG methylation of the STAT3-binding element in the GFAP promoter prevents STAT3 from binding to its target DNA sequence. Meanwhile, methyl CpG binding protein MeCP2, coupled with other repressor complexes including histone deacetylase HDAC1, may bind to the promoter leading to chromatin condensation and transcription silencing. The proneural bHLH transcription factor Ngn1 binds to CBP/p300 complex promoting neurogenesis while inhibiting astrocyte differentiation. At the onset of astrogenesis, signals from Notch, BMPs, and the IL-6 family cytokines could act independently or converge to activate the JAK-STAT3 pathway to facilitate astrocyte production. Notch receptor is cleaved upon ligand activation and Notch intracellular domain (NICD) is released and translocated into the nucleus, where it binds to CSL and/or NF1A leading to dissociation of DNMT1 from GFAP promoter, allowing for STAT3 binding and GFAP expression. IL-6 family cytokines (CT1, LIF and CNTF) promote astrocyte differentiation via their receptor LIFRβ and gp130-activated JAK2-STAT3 pathway. BMP2, through its receptor (BMPR) and downstream effector Smad1, inhibits neuronal differentiation by upregulation of negative HLH genes (ID1, ID3 and Hes5), or transcriptional repressor REST/NRSF. Additionally, BMP2 and LIF have synergistic effect to activate GFAP promoter through formation of a ternary complex of STAT3/p300/Smad1.
Astrogenic role of Notch is in part mediated by the canonical CSL (CBF1/RBPjk/Suppressor of Hairless/LAG1)-dependent Notch pathway via direct binding of Notch intracellular domain (NICD) with CSL, activating astrocyte gene transcription (Ge et al., 2002). The endogenous CSL is involved in the initiation of astrocyte differentiation. Another study has shown that committed neuronal precursors and young neurons promote astrocyte generation from the neighbouring radial glia by activating Notch signalling, through its target the nuclear factor 1a (NFIA), which results in dissociation of DNMT1 from GFAP promoter allowing for STAT3 binding and upregulation of astrocyte-specific genes (GFAP and S100β). CSL may function upstream of NF1A because it binds to NF1A and NF1A expression is reduced significantly in NPCs from CSL-deficient mouse embryonic stem cells (Figure 4) (Namihira et al., 2009). There is crosstalk between Notch and JAK-STAT signalling whereby Notch effector Hes1 associates with STAT3 and facilitates JAK2-mediated STAT3 phosphorylation in mouse cortical NPCs (Kamakura et al., 2004). It is not clear whether this interplay is implicated in Notch-induced astrocyte differentiation.
BMPs promote astrocyte development at different levels (Figure 4). First of all, they play a role in specification of astrocytes from NPCs during late embryonic development (Rowitch and Kriegstein, 2010). BMP2 can redirect cultured NPC cells from neurogenesis to astrocytogenesis by triggering the expression of negative HLH genes (Ids and Hes5), resulting in the inhibition of proneural bHLHs genes Mash1 and NGN2 (Nakashima et al., 2001). BMP4 directs radial glia cells in vivo to commit to the astrocytic rather than the oligodendroglial lineage (Gomes et al., 2003). Secondly, BMP induces a transcriptional repressor, RE1 silencer of transcription (REST)/neuron-restrictive silencer factor (NRSF), which restricts neuronal differentiation, while helps establish and maintain astrocytic identity from NPCs (Kohyama et al., 2010). The transcriptional up-regulation of REST/NRSF by BMP signaling is mediated by a direct interaction between Notch effector Smad1 and REST/NRSF. Thirdly, BMP2 and the cytokine leukemia inhibitory factor (LIF) were found to act in synergy on fetal neural progenitor cells to induce astrocytes through formation of a ternary complex of STAT3/ p300/Smad1, which leads to transcriptional activation of the GFAP promoter (Nakashima et al., 1999). Lastly, according to a recent study, BMP signalling promotes survival and maturation of immature astrocytes isolated from P7 rat cortices through phosphorylation of smads1/5/8 pathway, but limits their proliferation, suggesting its role in postnatal astrocyte development (Scholze et al., 2014).
In addition, neuron-derived cytokines, for example, the IL-6 family members LIF, cardiotriphin-1 (CT1) and ciliary neurotrophic factor (CNTF) promote astrocyte differentiation in vitro and in vivo via their receptor (LIFR and gp130 subunits)-activated JAK-STAT signalling pathway (Figure 4) (Bonni et al., 1997; Nakashima et al., 1999; Barnabe-Heider et al., 2005).
Roles of astrocytes in brain development and developmental disorders
Astrocytes play a critical role in shaping neural development. In addition to their contribution to neuronal survival/migration and axon guidance, their most important function is participation in synapse formation/maturation/pruning and neural circuit development. By releasing different molecular signals, and through contact-mediated signals, astrocytes have the capacity to control the development of both excitatory and inhibitory synaptogenesis, and probably fine-tune the balance of neuronal excitation and inhibition (Clarke and Barres, 2013). Recent studies with mouse and human glial transcriptomes also suggested the similar function of astrocytes in human brain development (Preuss et al., 2004; Miller et al., 2010). Defects in astrogenesis have been implicated in some forms of neurodevelopmental and psychiatric disorders that are associated with impairment in synapse development and function, such as Rett syndrome, Fragile X syndrome, Down syndrome and autism. Increased astrocyte numbers are seen in Down syndrome, while decreased astrocyte numbers are seen in deeper cortical layers in schizophrenia. Alterations in astrocyte morphology, along with abnormal expression of astrocyte markers (GFAP, AQP4 and CX43) are observed in autistic patients (Molofsky et al., 2012; Sloan and Barres, 2014).
In summary, the advent of new genetic, biochemical and imaging techniques has added further power to address more detailed mechanistic questions, these include, but are not limited to: 1) regional and functional heterogeneity of astrocytes and oligodendrocytes with their developmental origins; 2) the intricate interplay and relative plasticity of neuron/glial cells in normal development and in response to injury or disease; 3) differences across species in glial biology to gain insight into their contribution to human developmental disorders. These questions also apply to microglia, the other group of glia with haematopoietic origin (Kettenmann et al., 2013), but might enter into the brain in early embryonic stage. With increasing studies on microglia development, their functions in the developing brain, such as mediating programmed neuronal death and developmental axon/synapse pruning, are increasingly recognized (Eyo and Dailey, 2013; Bilimoria and Stevens, 2014). However, this raises another level of complexity and more questions regarding how these “main architects” perform and interact tactfully in the big project of building a “perfect” brain model. For example, both astrocytes and microglia have been reported to participate in synapse elimination and subsequent circuit refinement with some shared mechanisms (via complement system) (Corty and Freeman, 2013; Kettenmann et al., 2013), whether they coordinate in these processes with different timing or have individual specific roles, and how neuronal activity regulate their team work or vis-versa, await further exploration. More specific and reliable markers for developing astrocyte and microglia are needed to advance these studies. Overall, these investigations will open new avenues to develop safe and effective therapies specifically for damaged immature brain.
Acknowledgments
We thank Drs Patrick McQuillen and Sowmyalakshmi Rasika for critical reading and valuable comments of the manuscript. We also thank Nathalie Journiac and Fuxin Lu for their assistance on the figures. This work was supported by NINDS grant RO1NS084057 to X.J. J.N. was supported by Inserm, University Paris 7 Denis Diderot, Grace de Monaco and Roger de Spoelberch Foundations, and by ARC (Association pour la Recherche sur le Cancer).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alberta JA, Park SK, Mora J, Yuk D, Pawlitzky I, Iannarelli P, Vartanian T, Stiles CD, Rowitch DH. Sonic hedgehog is required during an early phase of oligodendrocyte development in mammalian brain. Molecular and cellular neurosciences. 2001;18:434–441. doi: 10.1006/mcne.2001.1026. [DOI] [PubMed] [Google Scholar]
- Andersen TE, Finsen B, Goffinet AM, Issinger OG, Boldyreff B. A reeler mutant mouse with a new, spontaneous mutation in the reelin gene. 2002;105:153–156. doi: 10.1016/s0169-328x(02)00389-3. [DOI] [PubMed] [Google Scholar]
- Angevine JB, Jr, Sidman RL. Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. 1961;192:766–768. doi: 10.1038/192766b0. [DOI] [PubMed] [Google Scholar]
- Anthony TE, Klein C, Fishell G, Heintz N. Radial glia serve as neuronal progenitors in all regions of the central nervous system. 2004;41:881–890. doi: 10.1016/s0896-6273(04)00140-0. [DOI] [PubMed] [Google Scholar]
- Arcila ML, Betizeau M, Cambronne XA, Guzman E, Doerflinger N, Bouhallier F, Zhou H, Wu B, Rani N, Bassett DS, Borello U, Huissoud C, Goodman RH, Dehay C, Kosik KS. Novel primate miRNAs coevolved with ancient target genes in germinal zone-specific expression patterns. 2014;81:1255–1262. doi: 10.1016/j.neuron.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asami M, Pilz GA, Ninkovic J, Godinho L, Schroeder T, Huttner WB, Gotz M. The role of Pax6 in regulating the orientation and mode of cell division of progenitors in the mouse cerebral cortex. 2011;138:5067–5078. doi: 10.1242/dev.074591. [DOI] [PubMed] [Google Scholar]
- Aviles EC, Wilson NH, Stoeckli ET. Sonic hedgehog and Wnt: antagonists in morphogenesis but collaborators in axon guidance. Frontiers in cellular neuroscience. 2013;7:86. doi: 10.3389/fncel.2013.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SA, Luo NL, Borenstein NS, Levine JM, Volpe JJ, Kinney HC. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:1302–1312. doi: 10.1523/JNEUROSCI.21-04-01302.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SA, Luo NL, Borenstein NS, Volpe JJ, Kinney HC. Arrested oligodendrocyte lineage progression during human cerebral white matter development: dissociation between the timing of progenitor differentiation and myelinogenesis. Journal of neuropathology and experimental neurology. 2002;61:197–211. doi: 10.1093/jnen/61.2.197. [DOI] [PubMed] [Google Scholar]
- Back SA, Miller SP. Brain injury in premature neonates: A primary cerebral dysmaturation disorder? Annals of neurology. 2014;75:469–486. doi: 10.1002/ana.24132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahi-Buisson N, Poirier K, Fourniol F, Saillour Y, Valence S, Lebrun N, Hully M, Bianco CF, Boddaert N, Elie C, Lascelles K, Souville I, Beldjord C, Chelly J. The wide spectrum of tubulinopathies: what are the key features for the diagnosis? 2014;137:1676–1700. doi: 10.1093/brain/awu082. [DOI] [PubMed] [Google Scholar]
- Bai J, Ramos RL, Ackman JB, Thomas AM, Lee RV, Loturco JJ. RNAi reveals doublecortin is required for radial migration in rat neocortex. 2003;6:1277–1283. doi: 10.1038/nn1153. [DOI] [PubMed] [Google Scholar]
- Barkovich AJ, Guerrini R, Kuzniecky RI, Jackson GD, Dobyns WB. A developmental and genetic classification for malformations of cortical development: update 2012. 2012;135:1348–1369. doi: 10.1093/brain/aws019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnabe-Heider F, Wasylnka JA, Fernandes KJ, Porsche C, Sendtner M, Kaplan DR, Miller FD. Evidence that embryonic neurons regulate the onset of cortical gliogenesis via cardiotrophin-1. Neuron. 2005;48:253–265. doi: 10.1016/j.neuron.2005.08.037. [DOI] [PubMed] [Google Scholar]
- Barres BA, Lazar MA, Raff MC. A novel role for thyroid hormone, glucocorticoids and retinoic acid in timing oligodendrocyte development. Development. 1994;120:1097–1108. doi: 10.1242/dev.120.5.1097. [DOI] [PubMed] [Google Scholar]
- Bashaw GJ, Klein R. Signaling from axon guidance receptors. Cold Spring Harbor perspectives in biology. 2010;2:a001941. doi: 10.1101/cshperspect.a001941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayraktar OA, Fuentealba LC, Alvarez-Buylla A, Rowitch DH. Astrocyte Development and Heterogeneity. Cold Spring Harbor perspectives in biology. 2014;7 doi: 10.1101/cshperspect.a020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beffert U, Weeber EJ, Morfini G, Ko J, Brady ST, Tsai LH, Sweatt JD, Herz J. Reelin and cyclin-dependent kinase 5-dependent signals cooperate in regulating neuronal migration and synaptic transmission. J Neurosci. 2004;24:1897–1906. doi: 10.1523/JNEUROSCI.4084-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergles DE, Roberts JD, Somogyi P, Jahr CE. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 2000;405:187–191. doi: 10.1038/35012083. [DOI] [PubMed] [Google Scholar]
- Betizeau M, Cortay V, Patti D, Pfister S, Gautier E, Bellemin-Menard A, Afanassieff M, Huissoud C, Douglas RJ, Kennedy H, Dehay C. Precursor diversity and complexity of lineage relationships in the outer subventricular zone of the primate. 2013;80:442–457. doi: 10.1016/j.neuron.2013.09.032. [DOI] [PubMed] [Google Scholar]
- Bilimoria PM, Stevens B. Microglia function during brain development: New insights from animal models. Brain research. 2014 doi: 10.1016/j.brainres.2014.11.032. [DOI] [PubMed] [Google Scholar]
- Bonni A, Sun Y, Nadal-Vicens M, Bhatt A, Frank DA, Rozovsky I, Stahl N, Yancopoulos GD, Greenberg ME. Regulation of gliogenesis in the central nervous system by the JAK-STAT signaling pathway. Science. 1997;278:477–483. doi: 10.1126/science.278.5337.477. [DOI] [PubMed] [Google Scholar]
- Borrell V, Calegari F. Mechanisms of brain evolution: Regulation of neural progenitor cell diversity and cell cycle length. Neurosci Res. 2014;86C:14–24. doi: 10.1016/j.neures.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Borrell V, Gotz M. Role of radial glial cells in cerebral cortex folding. Curr Opin Neurobiol. 2014;27:39–46. doi: 10.1016/j.conb.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Buchman JJ, Durak O, Tsai LH. ASPM regulates Wnt signaling pathway activity in the developing brain. 2011;25:1909–1914. doi: 10.1101/gad.16830211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bultje RS, Castaneda-Castellanos DR, Jan LY, Jan YN, Kriegstein AR, Shi SH. Mammalian Par3 regulates progenitor cell asymmetric division via notch signaling in the developing neocortex. 2009;63:189–202. doi: 10.1016/j.neuron.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buser JR, Maire J, Riddle A, Gong X, Nguyen T, Nelson K, Luo NL, Ren J, Struve J, Sherman LS, Miller SP, Chau V, Hendson G, Ballabh P, Grafe MR, Back SA. Arrested preoligodendrocyte maturation contributes to myelination failure in premature infants. Annals of neurology. 2012;71:93–109. doi: 10.1002/ana.22627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calver AR, Hall AC, Yu WP, Walsh FS, Heath JK, Betsholtz C, Richardson WD. Oligodendrocyte population dynamics and the role of PDGF in vivo. Neuron. 1998;20:869–882. doi: 10.1016/s0896-6273(00)80469-9. [DOI] [PubMed] [Google Scholar]
- Campbell K, Gotz M. Radial glia: multi-purpose cells for vertebrate brain development. Trends Neurosci. 2002;25:235–238. doi: 10.1016/s0166-2236(02)02156-2. [DOI] [PubMed] [Google Scholar]
- Caviness VS, Bhide PG, Nowakowski RS. Histogenetic processes leading to the laminated neocortex: migration is only a part of the story. Dev Neurosci. 2008;30:82–95. doi: 10.1159/000109854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang BS, Duzcan F, Kim S, Cinbis M, Aggarwal A, Apse KA, Ozdel O, Atmaca M, Zencir S, Bagci H, Walsh CA. The role of RELN in lissencephaly and neuropsychiatric disease. 2007;144B:58–63. doi: 10.1002/ajmg.b.30392. [DOI] [PubMed] [Google Scholar]
- Charron F, Tessier-Lavigne M. Novel brain wiring functions for classical morphogens: a role as graded positional cues in axon guidance. Development. 2005;132:2251–2262. doi: 10.1242/dev.01830. [DOI] [PubMed] [Google Scholar]
- Cheng L, Tian Z, Sun R, Wang Z, Shen J, Shan Z, Jin L, Lei L. ApoER2 and VLDLR in the developing human telencephalon. 2011;15:361–367. doi: 10.1016/j.ejpn.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Cheng PY, et al. Interplay between SIN3A and STAT3 mediates chromatin conformational changes and GFAP expression during cellular differentiation. PLoS One. 2011;6:e22018. doi: 10.1371/journal.pone.0022018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu H, Alqadah A, Chang C. The role of microRNAs in regulating neuronal connectivity. Frontiers in cellular neuroscience. 2014;7:283. doi: 10.3389/fncel.2013.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Park S, Sockanathan S. Activated retinoid receptors are required for the migration and fate maintenance of subsets of cortical neurons. 2014;141:1151–1160. doi: 10.1242/dev.104505. [DOI] [PubMed] [Google Scholar]
- Clarke LE, Barres BA. Emerging roles of astrocytes in neural circuit development. Nature reviews. Neuroscience. 2013;14:311–321. doi: 10.1038/nrn3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corty MM, Freeman MR. Cell biology in neuroscience: Architects in neural circuit design: glia control neuron numbers and connectivity. The Journal of cell biology. 2013;203:395–405. doi: 10.1083/jcb.201306099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa MR, Wen G, Lepier A, Schroeder T, Gotz M. Par-complex proteins promote proliferative progenitor divisions in the developing mouse cerebral cortex. Development. 2008;135:11–22. doi: 10.1242/dev.009951. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Current opinion in neurobiology. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- Dai ZM, Sun S, Wang C, Huang H, Hu X, Zhang Z, Lu QR, Qiu M. Stage-specific regulation of oligodendrocyte development by Wnt/beta-catenin signaling. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:8467–8473. doi: 10.1523/JNEUROSCI.0311-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AA, Temple S. A self-renewing multipotential stem cell in embryonic rat cerebral cortex. Nature. 1994;372:263–266. doi: 10.1038/372263a0. [DOI] [PubMed] [Google Scholar]
- Dehay C, Giroud P, Berland M, Killackey HP, Kennedy H. Phenotypic characterisation of respecified visual cortex subsequent to prenatal enucleation in the monkey: development of acetylcholinesterase and cytochrome oxidase patterns. 1996;376:386–402. doi: 10.1002/(SICI)1096-9861(19961216)376:3<386::AID-CNE3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Dehay C, Horsburgh G, Berland M, Killackey H, Kennedy H. Maturation and connectivity of the visual cortex in monkey is altered by prenatal removal of retinal input. 1989;337:265–267. doi: 10.1038/337265a0. [DOI] [PubMed] [Google Scholar]
- Dehay C, Kennedy H, Kosik KS. The Outer Subventricular Zone and Primate-Specific Cortical Complexification. 2015;85:683–694. doi: 10.1016/j.neuron.2014.12.060. [DOI] [PubMed] [Google Scholar]
- Delaunay D, Cortay V, Patti D, Knoblauch K, Dehay C. Mitotic spindle asymmetry: a Wnt/PCP-regulated mechanism generating asymmetrical division in cortical precursors. 2014;6:400–414. doi: 10.1016/j.celrep.2013.12.026. [DOI] [PubMed] [Google Scholar]
- Desai AR, Mcconnell SK. Progressive restriction in fate potential by neural progenitors during cerebral cortical development. 2000;127:2863–2872. doi: 10.1242/dev.127.13.2863. [DOI] [PubMed] [Google Scholar]
- Dickson BJ. Molecular mechanisms of axon guidance. Science. 2002;298:1959–1964. doi: 10.1126/science.1072165. [DOI] [PubMed] [Google Scholar]
- Dimidschstein J, Passante L, Dufour A, Van Den Ameele J, Tiberi L, Hrechdakian T, Adams R, Klein R, Lie DC, Jossin Y, Vanderhaeghen P. Ephrin-B1 controls the columnar distribution of cortical pyramidal neurons by restricting their tangential migration. 2013;79:1123–1135. doi: 10.1016/j.neuron.2013.07.015. [DOI] [PubMed] [Google Scholar]
- Dong Z, Yang N, Yeo SY, Chitnis A, Guo S. Intralineage directional Notch signaling regulates self-renewal and differentiation of asymmetrically dividing radial glia. 2012;74:65–78. doi: 10.1016/j.neuron.2012.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias LA, Wang DD, Kriegstein AR. Gap junction adhesion is necessary for radial migration in the neocortex. 2007;448:901–907. doi: 10.1038/nature06063. [DOI] [PubMed] [Google Scholar]
- Emery B. Regulation of oligodendrocyte differentiation and myelination. Science. 2010;330:779–782. doi: 10.1126/science.1190927. [DOI] [PubMed] [Google Scholar]
- Ethell IM, Pasquale EB. Molecular mechanisms of dendritic spine development and remodeling. Progress in neurobiology. 2005;75:161–205. doi: 10.1016/j.pneurobio.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Eyo UB, Dailey ME. Microglia: key elements in neural development, plasticity, and pathology. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2013;8:494–509. doi: 10.1007/s11481-013-9434-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallet-Bianco C, Laquerriere A, Poirier K, Razavi F, Guimiot F, Dias P, Loeuillet L, Lascelles K, Beldjord C, Carion N, Toussaint A, Revencu N, Addor MC, Lhermitte B, Gonzales M, Martinovich J, Bessieres B, Marcy-Bonniere M, Jossic F, Marcorelles P, Loget P, Chelly J, Bahi-Buisson N. Mutations in tubulin genes are frequent causes of various foetal malformations of cortical development including microlissencephaly. 2014;2:69. doi: 10.1186/2051-5960-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falnikar A, Baas PW. Neuronal migration re-purposes mechanisms of cytokinesis. 2013;12:3577–3578. doi: 10.4161/cc.26821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falnikar A, Tole S, Baas PW. Kinesin-5, a mitotic microtubule-associated motor protein, modulates neuronal migration. 2011;22:1561–1574. doi: 10.1091/mbc.E10-11-0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan G, et al. DNA methylation controls the timing of astrogliogenesis through regulation of JAK-STAT signaling. Development. 2005;132:3345–3356. doi: 10.1242/dev.01912. [DOI] [PubMed] [Google Scholar]
- Fietz SA, Kelava I, Vogt J, Wilsch-Brauninger M, Stenzel D, Fish JL, Corbeil D, Riehn A, Distler W, Nitsch R, Huttner WB. OSVZ progenitors of human and ferret neocortex are epithelial-like and expand by integrin signaling. 2010;13:690–699. doi: 10.1038/nn.2553. [DOI] [PubMed] [Google Scholar]
- Fietz SA, Lachmann R, Brandl H, Kircher M, Samusik N, Schroder R, Lakshmanaperumal N, Henry I, Vogt J, Riehn A, Distler W, Nitsch R, Enard W, Paabo S, Huttner WB. Transcriptomes of germinal zones of human and mouse fetal neocortex suggest a role of extracellular matrix in progenitor self-renewal. 2012;109:11836–11841. doi: 10.1073/pnas.1209647109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher TL, De Camilli P, Banker G. Synaptogenesis in hippocampal cultures: evidence indicating that axons and dendrites become competent to form synapses at different stages of neuronal development. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1994;14:6695–6706. doi: 10.1523/JNEUROSCI.14-11-06695.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florio M, Albert M, Taverna E, Namba T, Brandl H, Lewitus E, Haffner C, Sykes A, Wong FK, Peters J, Guhr E, Klemroth S, Prufer K, Kelso J, Naumann R, Nusslein I, Dahl A, Lachmann R, Paabo S, Huttner WB. Human-specific gene ARHGAP11B promotes basal progenitor amplification and neocortex expansion. 2015 doi: 10.1126/science.aaa1975. [DOI] [PubMed] [Google Scholar]
- Florio M, Huttner WB. Neural progenitors, neurogenesis and the evolution of the neocortex. 2014;141:2182–2194. doi: 10.1242/dev.090571. [DOI] [PubMed] [Google Scholar]
- Forster E. Reelin, neuronal polarity and process orientation of cortical neurons. Neuroscience. 2014;269:102–111. doi: 10.1016/j.neuroscience.2014.03.004. [DOI] [PubMed] [Google Scholar]
- Franco SJ, Martinez-Garay I, Gil-Sanz C, Harkins-Perry SR, Muller U. Reelin regulates cadherin function via Dab1/Rap1 to control neuronal migration and lamination in the neocortex. Neuron. 2011;69:482–497. doi: 10.1016/j.neuron.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruttiger M, Karlsson L, Hall AC, Abramsson A, Calver AR, Bostrom H, Willetts K, Bertold CH, Heath JK, Betsholtz C, Richardson WD. Defective oligodendrocyte development and severe hypomyelination in PDGF-A knockout mice. Development. 1999;126:457–467. doi: 10.1242/dev.126.3.457. [DOI] [PubMed] [Google Scholar]
- Gaiano N, Fishell G. The role of notch in promoting glial and neural stem cell fates. 2002;25:471–490. doi: 10.1146/annurev.neuro.25.030702.130823. [DOI] [PubMed] [Google Scholar]
- Gaiano N, Nye JS, Fishell G. Radial glial identity is promoted by Notch1 signaling in the murine forebrain. 2000;26:395–404. doi: 10.1016/s0896-6273(00)81172-1. [DOI] [PubMed] [Google Scholar]
- Gao P, Postiglione MP, Krieger TG, Hernandez L, Wang C, Han Z, Streicher C, Papusheva E, Insolera R, Chugh K, Kodish O, Huang K, Simons BD, Luo L, Hippenmeyer S, Shi SH. Deterministic progenitor behavior and unitary production of neurons in the neocortex. 2014;159:775–788. doi: 10.1016/j.cell.2014.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto CL, Broadie K. Genetic controls balancing excitatory and inhibitory synaptogenesis in neurodevelopmental disorder models. Frontiers in synaptic neuroscience. 2010;2:4. doi: 10.3389/fnsyn.2010.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge W, et al. Notch signaling promotes astrogliogenesis via direct CSL-mediated glial gene activation. J Neurosci Res. 2002;69:848–860. doi: 10.1002/jnr.10364. [DOI] [PubMed] [Google Scholar]
- Ge WP, et al. Local generation of glia is a major astrocyte source in postnatal cortex. Nature. 2012;484:376–380. doi: 10.1038/nature10959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh T, Aprea J, Nardelli J, Engel H, Selinger C, Mombereau C, Lemonnier T, Moutkine I, Schwendimann L, Dori M, Irinopoulou T, Henrion-Caude A, Benecke AG, Arnold SJ, Gressens P, Calegari F, Groszer M. MicroRNAs establish robustness and adaptability of a critical gene network to regulate progenitor fate decisions during cortical neurogenesis. 2014;7:1779–1788. doi: 10.1016/j.celrep.2014.05.029. [DOI] [PubMed] [Google Scholar]
- Giedd J. Brain development, IX: human brain growth. Am J Psychiatry. 1999;156:4. doi: 10.1176/ajp.156.1.4. [DOI] [PubMed] [Google Scholar]
- Gil-Sanz C, Franco SJ, Martinez-Garay I, Espinosa A, Harkins-Perry S, Muller U. Cajal-Retzius cells instruct neuronal migration by coincidence signaling between secreted and contact-dependent guidance cues. 2013;79:461–477. doi: 10.1016/j.neuron.2013.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes WA, Mehler MF, Kessler JA. Transgenic overexpression of BMP4 increases astroglial and decreases oligodendroglial lineage commitment. Developmental biology. 2003;255:164–177. doi: 10.1016/s0012-1606(02)00037-4. [DOI] [PubMed] [Google Scholar]
- Gotz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005;6:777–788. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- Greig LC, Woodworth MB, Galazo MJ, Padmanabhan H, Macklis JD. Molecular logic of neocortical projection neuron specification, development and diversity. 2013;14:755–769. doi: 10.1038/nrn3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gressens P. Pathogenesis of migration disorders. Curr Opin Neurol. 2006;19:135–140. doi: 10.1097/01.wco.0000218228.73678.e1. [DOI] [PubMed] [Google Scholar]
- Haberny KA, Paule MG, Scallet AC, Sistare FD, Lester DS, Hanig JP, Slikker W., Jr Ontogeny of the N-methyl-D-aspartate (NMDA) receptor system and susceptibility to neurotoxicity. Toxicological sciences : an official journal of the Society of Toxicology. 2002;68:9–17. doi: 10.1093/toxsci/68.1.9. [DOI] [PubMed] [Google Scholar]
- Hammond V, Tsai LH, Tan SS. Control of cortical neuron migration and layering: cell and non cell-autonomous effects of p35. J Neurosci. 2004;24:576–587. doi: 10.1523/JNEUROSCI.4529-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen DV, Lui JH, Parker PR, Kriegstein AR. Neurogenic radial glia in the outer subventricular zone of human neocortex. 2010;464:554–561. doi: 10.1038/nature08845. [DOI] [PubMed] [Google Scholar]
- Hartfuss E, Forster E, Bock HH, Hack MA, Leprince P, Luque JM, Herz J, Frotscher M, Gotz M. Reelin signaling directly affects radial glia morphology and biochemical maturation. Development. 2003;130:4597–4609. doi: 10.1242/dev.00654. [DOI] [PubMed] [Google Scholar]
- Hartfuss E, Galli R, Heins N, Gotz M. Characterization of CNS precursor subtypes and radial glia. Dev Biol. 2001;229:15–30. doi: 10.1006/dbio.2000.9962. [DOI] [PubMed] [Google Scholar]
- He W, et al. Multipotent stem cells from the mouse basal forebrain contribute GABAergic neurons and oligodendrocytes to the cerebral cortex during embryogenesis. J Neurosci. 2001;21:8854–8862. doi: 10.1523/JNEUROSCI.21-22-08854.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng JI, Chariot A, Nguyen L. Molecular layers underlying cytoskeletal remodelling during cortical development. 2009;33:38–47. doi: 10.1016/j.tins.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Hong SE, Shugart YY, Huang DT, Shahwan SA, Grant PE, Hourihane JO, Martin ND, Walsh CA. Autosomal recessive lissencephaly with cerebellar hypoplasia is associated with human RELN mutations. 2000;26:93–96. doi: 10.1038/79246. [DOI] [PubMed] [Google Scholar]
- Hoshina N, Tezuka T, Yokoyama K, Kozuka-Hata H, Oyama M, Yamamoto T. Focal adhesion kinase regulates laminin-induced oligodendroglial process outgrowth. Genes to cells : devoted to molecular & cellular mechanisms. 2007;12:1245–1254. doi: 10.1111/j.1365-2443.2007.01130.x. [DOI] [PubMed] [Google Scholar]
- Hur EM, Zhou FQ. GSK3 signalling in neural development. 2010;11:539–551. doi: 10.1038/nrn2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivaniutsin U, Chen Y, Mason JO, Price DJ, Pratt T. Adenomatous polyposis coli is required for early events in the normal growth and differentiation of the developing cerebral cortex. Neural Dev. 2009;4:3. doi: 10.1186/1749-8104-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer AN, Bellon A, Baudet ML. microRNAs in axon guidance. Frontiers in cellular neuroscience. 2014;8:78. doi: 10.3389/fncel.2014.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakovcevski I, Filipovic R, Mo Z, Rakic S, Zecevic N. Oligodendrocyte development and the onset of myelination in the human fetal brain. Frontiers in neuroanatomy. 2009;3:5. doi: 10.3389/neuro.05.005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzie LL, Talos DM, Jackson MC, Park HK, Graham DA, Lechpammer M, Folkerth RD, Volpe JJ, Jensen FE. Developmental Expression of N-Methyl-d-Aspartate (NMDA) Receptor Subunits in Human White and Gray Matter: Potential Mechanism of Increased Vulnerability in the Immature Brain. Cerebral cortex. 2015;25:482–495. doi: 10.1093/cercor/bht246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen FE. The role of glutamate receptor maturation in perinatal seizures and brain injury. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 2002;20:339–347. doi: 10.1016/s0736-5748(02)00012-6. [DOI] [PubMed] [Google Scholar]
- Kamakura S, Oishi K, Yoshimatsu T, Nakafuku M, Masuyama N, Gotoh Y. Hes binding to STAT3 mediates crosstalk between Notch and JAK-STAT signalling. Nature cell biology. 2004;6:547–554. doi: 10.1038/ncb1138. [DOI] [PubMed] [Google Scholar]
- Kaprielian Z, Imondi R, Runko E. Axon guidance at the midline of the developing CNS. The Anatomical record. 2000;261:176–197. doi: 10.1002/1097-0185(20001015)261:5<176::AID-AR7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Kessaris N, Fogarty M, Iannarelli P, Grist M, Wegner M, Richardson WD. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nature neuroscience. 2006;9:173–179. doi: 10.1038/nn1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H, Kirchhoff F, Verkhratsky A. Microglia: new roles for the synaptic stripper. Neuron. 2013;77:10–18. doi: 10.1016/j.neuron.2012.12.023. [DOI] [PubMed] [Google Scholar]
- Khazipov R, Esclapez M, Caillard O, Bernard C, Khalilov I, Tyzio R, Hirsch J, Dzhala V, Berger B, Ben-Ari Y. Early development of neuronal activity in the primate hippocampus in utero. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:9770–9781. doi: 10.1523/JNEUROSCI.21-24-09770.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WY, Wang X, Wu Y, Doble BW, Patel S, Woodgett JR, Snider WD. GSK-3 is a master regulator of neural progenitor homeostasis. Nat Neurosci. 2009;12:1390–1397. doi: 10.1038/nn.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M, Yoshioka T, Saio M, Banno Y, Nagaoka H, Okano Y. Mitotic catastrophe and cell death induced by depletion of centrosomal proteins. 2013;4:e603. doi: 10.1038/cddis.2013.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura N, Nakashima K, Ueno M, Kiyama H, Taga T. A novel mammalian T-box-containing gene, Tbr2, expressed in mouse developing brain. Brain Res Dev Brain Res. 1999;115:183–193. doi: 10.1016/s0165-3806(99)00064-4. [DOI] [PubMed] [Google Scholar]
- Klein C, Kramer EM, Cardine AM, Schraven B, Brandt R, Trotter J. Process outgrowth of oligodendrocytes is promoted by interaction of fyn kinase with the cytoskeletal protein tau. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:698–707. doi: 10.1523/JNEUROSCI.22-03-00698.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohyama J, Sanosaka T, Tokunaga A, Takatsuka E, Tsujimura K, Okano H, Nakashima K. BMP-induced REST regulates the establishment and maintenance of astrocytic identity. The Journal of cell biology. 2010;189:159–170. doi: 10.1083/jcb.200908048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodkin AL, Tessier-Lavigne M. Mechanisms and molecules of neuronal wiring: a primer. Cold Spring Harbor perspectives in biology. 2011;3 doi: 10.1101/cshperspect.a001727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konno D, Shioi G, Shitamukai A, Mori A, Kiyonari H, Miyata T, Matsuzaki F. Neuroepithelial progenitors undergo LGN-dependent planar divisions to maintain selfrenewability during mammalian neurogenesis. 2008;10:93–101. doi: 10.1038/ncb1673. [DOI] [PubMed] [Google Scholar]
- Kosodo Y, Huttner WB. Basal process and cell divisions of neural progenitors in the developing brain. 2009;51:251–261. doi: 10.1111/j.1440-169X.2009.01101.x. [DOI] [PubMed] [Google Scholar]
- Kosodo Y, Suetsugu T, Suda M, Mimori-Kiyosue Y, Toida K, Baba SA, Kimura A, Matsuzaki F. Regulation of interkinetic nuclear migration by cell cycle-coupled active and passive mechanisms in the developing brain. 2011;30:1690–1704. doi: 10.1038/emboj.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer-Albers EM, White R. From axon-glial signalling to myelination: the integrating role of oligodendroglial Fyn kinase. Cellular and molecular life sciences : CMLS. 2011;68:2003–2012. doi: 10.1007/s00018-010-0616-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein A, Noctor S, Martinez-Cerdeno V. Patterns of neural stem and progenitor cell division may underlie evolutionary cortical expansion. 2006;7:883–890. doi: 10.1038/nrn2008. [DOI] [PubMed] [Google Scholar]
- Kriegstein AR, Noctor SC. Patterns of neuronal migration in the embryonic cortex. Trends Neurosci. 2004;27:392–399. doi: 10.1016/j.tins.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Lamonica BE, Lui JH, Hansen DV, Kriegstein AR. Mitotic spindle orientation predicts outer radial glial cell generation in human neocortex. 2013;4:1665. doi: 10.1038/ncomms2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamonica BE, Lui JH, Wang X, Kriegstein AR. OSVZ progenitors in the human cortex: an updated perspective on neurodevelopmental disease. 2012;22:747–753. doi: 10.1016/j.conb.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrieu P, Goffinet A. Inverted pyramidal neurons and their axons in the neocortex of reeler mutant mice. 1981;218:293–301. doi: 10.1007/BF00210345. [DOI] [PubMed] [Google Scholar]
- Lehtinen MK, Zappaterra MW, Chen X, Yang YJ, Hill AD, Lun M, Maynard T, Gonzalez D, Kim S, Ye P, D'ercole AJ, Wong ET, Lamantia AS, Walsh CA. The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. 2011;69:893–905. doi: 10.1016/j.neuron.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Sheng M. Some assembly required: the development of neuronal synapses. Nat Rev Mol Cell Biol. 2003;4:833–841. doi: 10.1038/nrm1242. [DOI] [PubMed] [Google Scholar]
- Liang X, Draghi NA, Resh MD. Signaling from integrins to Fyn to Rho family GTPases regulates morphologic differentiation of oligodendrocytes. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:7140–7149. doi: 10.1523/JNEUROSCI.5319-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JS. Molecular genetics of neuronal migration disorders. 2011;11:171–178. doi: 10.1007/s11910-010-0176-5. [DOI] [PubMed] [Google Scholar]
- Liu JS, Schubert CR, Fu X, Fourniol FJ, Jaiswal JK, Houdusse A, Stultz CM, Moores CA, Walsh CA. Molecular basis for specific regulation of neuronal kinesin-3 motors by doublecortin family proteins. 2012;47:707–721. doi: 10.1016/j.molcel.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louvi A, Artavanis-Tsakonas S. Notch signalling in vertebrate neural development. Nature reviews. Neuroscience. 2006;7:93–102. doi: 10.1038/nrn1847. [DOI] [PubMed] [Google Scholar]
- Lui JH, Hansen DV, Kriegstein AR. Development and evolution of the human neocortex. 2011;146:18–36. doi: 10.1016/j.cell.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magen D, Ofir A, Berger L, Goldsher D, Eran A, Katib N, Nijem Y, Vlodavsky E, Tzur S, Behar DM, Fellig Y, Mandel H. Autosomal recessive lissencephaly with cerebellar hypoplasia is associated with a loss-of-function mutation in CDK5. 2015;134:305–314. doi: 10.1007/s00439-014-1522-5. [DOI] [PubMed] [Google Scholar]
- Marin O, Valdeolmillos M, Moya F. Neurons in motion: same principles for different shapes? 2006;29:655–661. doi: 10.1016/j.tins.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Marshall CA, Goldman JE. Subpallial dlx2-expressing cells give rise to astrocytes and oligodendrocytes in the cerebral cortex and white matter. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:9821–9830. doi: 10.1523/JNEUROSCI.22-22-09821.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Cerdeno V, Cunningham CL, Camacho J, Antczak JL, Prakash AN, Cziep ME, Walker AI, Noctor SC. Comparative analysis of the subventricular zone in rat, ferret and macaque: evidence for an outer subventricular zone in rodents. PLoS One. 2012;7:e30178. doi: 10.1371/journal.pone.0030178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer G, Soria JM, Martinez-Galan JR, Martin-Clemente B, Fairen A. Different origins and developmental histories of transient neurons in the marginal zone of the fetal and neonatal rat cortex. 1998;397:493–518. [PubMed] [Google Scholar]
- Miller JA, Horvath S, Geschwind DH. Divergence of human and mouse brain transcriptome highlights Alzheimer disease pathways. Proc Natl Acad Sci USA. 2010;107:12698–12703. doi: 10.1073/pnas.0914257107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzaa GM, Paciorkowski AR. Introduction: Brain malformations. Am J Med Genet C Semin Med Genet. 2014;166C:117–123. doi: 10.1002/ajmg.c.31404. [DOI] [PubMed] [Google Scholar]
- Mitew S, Hay CM, Peckham H, Xiao J, Koenning M, Emery B. Mechanisms regulating the development of oligodendrocytes and central nervous system myelin. Neuroscience. 2014;276:29–47. doi: 10.1016/j.neuroscience.2013.11.029. [DOI] [PubMed] [Google Scholar]
- Molnar Z, Clowry G. Cerebral cortical development in rodents and primates. 195:45–70. doi: 10.1016/B978-0-444-53860-4.00003-9. [DOI] [PubMed] [Google Scholar]
- Molofsky AV, Krencik R, Ullian EM, Tsai HH, Deneen B, Richardson WD, Barres BA, Rowitch DH. Astrocytes and disease: a neurodevelopmental perspective. Genes & development. 2012;26:891–907. doi: 10.1101/gad.188326.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Menezes JR, Macklis JD. Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci. 2007;8:427–437. doi: 10.1038/nrn2151. [DOI] [PubMed] [Google Scholar]
- Morgan-Smith M, Wu Y, Zhu X, Pringle J, Snider WD. GSK-3 signaling in developing cortical neurons is essential for radial migration and dendritic orientation. Elife. 2014;3:e02663. doi: 10.7554/eLife.02663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima K, Takizawa T, Ochiai W, Yanagisawa M, Hisatsune T, Nakafuku M, Miyazono K, Kishimoto T, Kageyama R, Taga T. BMP2-mediated alteration in the developmental pathway of fetal mouse brain cells from neurogenesis to astrocytogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:5868–5873. doi: 10.1073/pnas.101109698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima K, et al. Synergistic signaling in fetal brain by STAT3-Smad1 complex bridged by p300. Science. 1999;284:479–482. doi: 10.1126/science.284.5413.479. [DOI] [PubMed] [Google Scholar]
- Namihira M, Kohyama J, Semi K, Sanosaka T, Deneen B, Taga T, Nakashima K. Committed neuronal precursors confer astrocytic potential on residual neural precursor cells. Developmental cell. 2009;16:245–255. doi: 10.1016/j.devcel.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Nishikimi M, Oishi K, Nakajima K. Axon guidance mechanisms for establishment of callosal connections. Neural plasticity. 2013;2013:149060. doi: 10.1155/2013/149060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura YV, Shikanai M, Hoshino M, Ohshima T, Nabeshima Y, Mizutani K, Nagata K, Nakajima K, Kawauchi T. Cdk5 and its substrates, Dcx and p27kip1, regulate cytoplasmic dilation formation and nuclear elongation in migrating neurons. 2014;141:3540–3550. doi: 10.1242/dev.111294. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7:136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- Okabe S. Molecular anatomy of the postsynaptic density. Molecular and cellular neurosciences. 2007;34:503–518. doi: 10.1016/j.mcn.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Olson EC. Analysis of preplate splitting and early cortical development illuminates the biology of neurological disease. 2014;2:121. doi: 10.3389/fped.2014.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega JA, Radonjic NV, Zecevic N. Sonic hedgehog promotes generation and maintenance of human forebrain Olig2 progenitors. Frontiers in cellular neuroscience. 2013;7:254. doi: 10.3389/fncel.2013.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owald D, Sigrist SJ. Assembling the presynaptic active zone. Current opinion in neurobiology. 2009;19:311–318. doi: 10.1016/j.conb.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, Gross CT. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- Paridaen JT, Wilsch-Brauninger M, Huttner WB. Asymmetric inheritance of centrosome-associated primary cilium membrane directs ciliogenesis after cell division. 2013;155:333–344. doi: 10.1016/j.cell.2013.08.060. [DOI] [PubMed] [Google Scholar]
- Passemard S, El Ghouzzi V, Nasser H, Verney C, Vodjdani G, Lacaud A, Lebon S, Laburthe M, Robberecht P, Nardelli J, Mani S, Verloes A, Gressens P, Lelievre V. VIP blockade leads to microcephaly in mice via disruption of Mcph1-Chk1 signaling. J Clin Invest. 2011;121:3071–3087. doi: 10.1172/JCI43824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyre E, Jaouen F, Saadaoui M, Haren L, Merdes A, Durbec P, Morin X. A lateral belt of cortical LGN and NuMA guides mitotic spindle movements and planar division in neuroepithelial cells. 2011;193:141–154. doi: 10.1083/jcb.201101039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilz DT, Matsumoto N, Minnerath S, Mills P, Gleeson JG, Allen KM, Walsh CA, Barkovich AJ, Dobyns WB, Ledbetter DH, Ross ME. LIS1 and XLIS (DCX) mutations cause most classical lissencephaly, but different patterns of malformation. 1998;7:2029–2037. doi: 10.1093/hmg/7.13.2029. [DOI] [PubMed] [Google Scholar]
- Poirier K, Lebrun N, Broix L, Tian G, Saillour Y, Boscheron C, Parrini E, Valence S, Pierre BS, Oger M, Lacombe D, Genevieve D, Fontana E, Darra F, Cances C, Barth M, Bonneau D, Bernadina BD, N'guyen S, Gitiaux C, Parent P, Des Portes V, Pedespan JM, Legrez V, Castelnau-Ptakine L, Nitschke P, Hieu T, Masson C, Zelenika D, Andrieux A, Francis F, Guerrini R, Cowan NJ, Bahi-Buisson N, Chelly J. Mutations in TUBG1, DYNC1H1, KIF5C and KIF2A cause malformations of cortical development and microcephaly. 2013;45:639–647. doi: 10.1038/ng.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poschl J, Grammel D, Dorostkar MM, Kretzschmar HA, Schuller U. Constitutive activation of beta-catenin in neural progenitors results in disrupted proliferation and migration of neurons within the central nervous system. Dev Biol. 2013;374:319–332. doi: 10.1016/j.ydbio.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Postiglione MP, Juschke C, Xie Y, Haas GA, Charalambous C, Knoblich JA. Mouse inscuteable induces apical-basal spindle orientation to facilitate intermediate progenitor generation in the developing neocortex. 2011;72:269–284. doi: 10.1016/j.neuron.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss TM, Caceres M, Oldham MC, Geschwind DH. Human brain evolution: insights from microarrays. Nature reviews. Genetics. 2004;5:850–860. doi: 10.1038/nrg1469. [DOI] [PubMed] [Google Scholar]
- Rakic P. Evolution of the neocortex: a perspective from developmental biology. Nat Rev Neurosci. 2009;10:724–735. doi: 10.1038/nrn2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper J, Mason C. Cellular strategies of axonal pathfinding. Cold Spring Harbor perspectives in biology. 2010;2:a001933. doi: 10.1101/cshperspect.a001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash BG, Tomasi S, Lim HD, Suh CY, Vaccarino FM. Cortical gyrification induced by fibroblast growth factor 2 in the mouse brain. J Neurosci. 2013;33:10802–10814. doi: 10.1523/JNEUROSCI.3621-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilein A, Nelson WJ. APC is a component of an organizing template for cortical microtubule networks. 2005;7:463–473. doi: 10.1038/ncb1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter LM, Vazquez DM, Meador-Woodruff JH. Ontogeny of ionotropic glutamate receptor subunit expression in the rat hippocampus. Brain research. Developmental brain research. 2002;139:227–236. doi: 10.1016/s0165-3806(02)00572-2. [DOI] [PubMed] [Google Scholar]
- Rosenberg SS, Powell BL, Chan JR. Receiving mixed signals: uncoupling oligodendrocyte differentiation and myelination. Cellular and molecular life sciences : CMLS. 2007;64:3059–3068. doi: 10.1007/s00018-007-7265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowitch DH. Glial specification in the vertebrate neural tube. Nature reviews. Neuroscience. 2004;5:409–419. doi: 10.1038/nrn1389. [DOI] [PubMed] [Google Scholar]
- Rowitch DH, Kriegstein AR. Developmental genetics of vertebrate glial-cell specification. 2010;468:214–222. doi: 10.1038/nature09611. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Beachy PA. Patterning of the embryonic forebrain. Curr Opin Neurobiol. 1998;8:18–26. doi: 10.1016/s0959-4388(98)80004-4. [DOI] [PubMed] [Google Scholar]
- Schenk J, Wilsch-Brauninger M, Calegari F, Huttner WB. Myosin II is required for interkinetic nuclear migration of neural progenitors. 2009;106:16487–16492. doi: 10.1073/pnas.0908928106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholze AR, Foo LC, Mulinyawe S, Barres BA. BMP signaling in astrocytes downregulates EGFR to modulate survival and maturation. PloS one. 2014;9:e110668. doi: 10.1371/journal.pone.0110668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuldiner O, Yaron A. Mechanisms of developmental neurite pruning. Cellular and molecular life sciences : CMLS. 2015;72:101–119. doi: 10.1007/s00018-014-1729-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessa A, Mao CA, Colasante G, Nini A, Klein WH, Broccoli V. Tbr2-positive intermediate (basal) neuronal progenitors safeguard cerebral cortex expansion by controlling amplification of pallial glutamatergic neurons and attraction of subpallial GABAergic interneurons. Genes Dev. 2010;24:1816–1826. doi: 10.1101/gad.575410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Liu XB, Pleasure DE, Deng W. Axon-glia synapses are highly vulnerable to white matter injury in the developing brain. Journal of neuroscience research. 2012;90:105–121. doi: 10.1002/jnr.22722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Kim E. The postsynaptic organization of synapses. Cold Spring Harbor perspectives in biology. 2011;3 doi: 10.1101/cshperspect.a005678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shitamukai A, Matsuzaki F. Control of asymmetric cell division of mammalian neural progenitors. 2012;54:277–286. doi: 10.1111/j.1440-169X.2012.01345.x. [DOI] [PubMed] [Google Scholar]
- Shu T, Ayala R, Nguyen MD, Xie Z, Gleeson JG, Tsai LH. Ndel1 operates in a common pathway with LIS1 and cytoplasmic dynein to regulate cortical neuronal positioning. 2004;44:263–277. doi: 10.1016/j.neuron.2004.09.030. [DOI] [PubMed] [Google Scholar]
- Sigrist SJ, Schmitz D. Structural and functional plasticity of the cytoplasmic active zone. Current opinion in neurobiology. 2011;21:144–150. doi: 10.1016/j.conb.2010.08.012. [DOI] [PubMed] [Google Scholar]
- Silbereis JC, Huang EJ, Back SA, Rowitch DH. Towards improved animal models of neonatal white matter injury associated with cerebral palsy. Disease models & mechanisms. 2010;3:678–688. doi: 10.1242/dmm.002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan SA, Barres BA. Mechanisms of astrocyte development and their contributions to neurodevelopmental disorders. Curr Opin Neurobiol. 2014;27:75–81. doi: 10.1016/j.conb.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solecki DJ. Sticky situations: recent advances in control of cell adhesion during neuronal migration. Curr Opin Neurobiol. 2012;22:791–798. doi: 10.1016/j.conb.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solecki DJ, Trivedi N, Govek EE, Kerekes RA, Gleason SS, Hatten ME. Myosin II motors and F-actin dynamics drive the coordinated movement of the centrosome and soma during CNS glial-guided neuronal migration. 2009;63:63–80. doi: 10.1016/j.neuron.2009.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalice A, Parisi P, Nicita F, Pizzardi G, Del Balzo F, Iannetti P. Neuronal migration disorders: clinical, neuroradiologic and genetics aspects. Acta Paediatr. 2009;98:421–433. doi: 10.1111/j.1651-2227.2008.01160.x. [DOI] [PubMed] [Google Scholar]
- Sperber BR, Boyle-Walsh EA, Engleka MJ, Gadue P, Peterson AC, Stein PL, Scherer SS, Mcmorris FA. A unique role for Fyn in CNS myelination. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:2039–2047. doi: 10.1523/JNEUROSCI.21-06-02039.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl R, Walcher T, De Juan Romero C, Pilz GA, Cappello S, Irmler M, Sanz-Aquela JM, Beckers J, Blum R, Borrell V, Gotz M. Trnp1 regulates expansion and folding of the mammalian cerebral cortex by control of radial glial fate. 2013;153:535–549. doi: 10.1016/j.cell.2013.03.027. [DOI] [PubMed] [Google Scholar]
- Sun T, Hevner RF. Growth and folding of the mammalian cerebral cortex: from molecules to malformations. 2014;15:217–232. doi: 10.1038/nrn3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, et al. Neurogenin promotes neurogenesis and inhibits glial differentiation by independent mechanisms. Cell. 2001;104:365–376. doi: 10.1016/s0092-8674(01)00224-0. [DOI] [PubMed] [Google Scholar]
- Super H, Soriano E, Uylings HB. The functions of the preplate in development and evolution of the neocortex and hippocampus. 1998;27:40–64. doi: 10.1016/s0165-0173(98)00005-8. [DOI] [PubMed] [Google Scholar]
- Talos DM, Fishman RE, Park H, Folkerth RD, Follett PL, Volpe JJ, Jensen FE. Developmental regulation of alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor subunit expression in forebrain and relationship to regional susceptibility to hypoxic/ischemic injuryIRodent cerebral white matter and cortex. The Journal of comparative neurology. 2006a;497:42–60. doi: 10.1002/cne.20972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talos DM, et al. Developmental regulation of alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor subunit expression in forebrain and relationship to regional susceptibility to hypoxic/ischemic injury. II. Human cerebral white matter and cortex. J Comp Neurol. 2006b;497:61–77. doi: 10.1002/cne.20978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G, et al. Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron. 2014;83:1131–1143. doi: 10.1016/j.neuron.2014.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverna E, Huttner WB. Neural progenitor nuclei IN motion. 2010;67:906–914. doi: 10.1016/j.neuron.2010.08.027. [DOI] [PubMed] [Google Scholar]
- Tekki-Kessaris N, Woodruff R, Hall AC, Gaffield W, Kimura S, Stiles CD, Rowitch DH, Richardson WD. Hedgehog-dependent oligodendrocyte lineage specification in the telencephalon. Development. 2001;128:2545–2554. doi: 10.1242/dev.128.13.2545. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- Tokumoto YM, Tang DG, Raff MC. Two molecularly distinct intracellular pathways to oligodendrocyte differentiation: role of a p53 family protein. The EMBO journal. 2001;20:5261–5268. doi: 10.1093/emboj/20.18.5261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai JW, Bremner KH, Vallee RB. Dual subcellular roles for LIS1 and dynein in radial neuronal migration in live brain tissue. 2007;10:970–979. doi: 10.1038/nn1934. [DOI] [PubMed] [Google Scholar]
- Tsai JW, Chen Y, Kriegstein AR, Vallee RB. LIS1 RNA interference blocks neural stem cell division, morphogenesis, and motility at multiple stages. 2005;170:935–945. doi: 10.1083/jcb.200505166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai JW, Lian WN, Kemal S, Kriegstein AR, Vallee RB. Kinesin 3 and cytoplasmic dynein mediate interkinetic nuclear migration in neural stem cells. 2010;13:1463–1471. doi: 10.1038/nn.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai LH, Gleeson JG. Nucleokinesis in neuronal migration. 2005;46:383–388. doi: 10.1016/j.neuron.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Tyzio R, Represa A, Jorquera I, Ben-Ari Y, Gozlan H, Aniksztejn L. The establishment of GABAergic and glutamatergic synapses on CA1 pyramidal neurons is sequential and correlates with the development of the apical dendrite. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:10372–10382. doi: 10.1523/JNEUROSCI.19-23-10372.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemori H, Sato S, Yagi T, Aizawa S, Yamamoto T. Initial events of myelination involve Fyn tyrosine kinase signalling. Nature. 1994;367:572–576. doi: 10.1038/367572a0. [DOI] [PubMed] [Google Scholar]
- Vanderhaeghen P, Cheng HJ. Guidance molecules in axon pruning and cell death. Cold Spring Harbor perspectives in biology. 2010;2:a001859. doi: 10.1101/cshperspect.a001859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake H, Lee PR, Fields RD. Control of local protein synthesis and initial events in myelination by action potentials. Science. 2011;333:1647–1651. doi: 10.1126/science.1206998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, et al. Notch receptor activation inhibits oligodendrocyte differentiation. Neuron. 1998;21:63–75. doi: 10.1016/s0896-6273(00)80515-2. [DOI] [PubMed] [Google Scholar]
- White R, Gonsior C, Kramer-Albers EM, Stohr N, Huttelmaier S, Trotter J. Activation of oligodendroglial Fyn kinase enhances translation of mRNAs transported in hnRNP A2-dependent RNA granules. The Journal of cell biology. 2008;181:579–586. doi: 10.1083/jcb.200706164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willaredt MA, Hasenpusch-Theil K, Gardner HA, Kitanovic I, Hirschfeld-Warneken VC, Gojak CP, Gorgas K, Bradford CL, Spatz J, Wolfl S, Theil T, Tucker KL. A crucial role for primary cilia in cortical morphogenesis. 2008;28:12887–12900. doi: 10.1523/JNEUROSCI.2084-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Hernandez M, Shen S, Sabo JK, Kelkar D, Wang J, O'leary R, Phillips GR, Cate HS, Casaccia P. Differential modulation of the oligodendrocyte transcriptome by sonic hedgehog and bone morphogenetic protein 4 via opposing effects on histone acetylation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:6651–6664. doi: 10.1523/JNEUROSCI.4876-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam PT, Charron F. Signaling mechanisms of non-conventional axon guidance cues: the Shh, BMP and Wnt morphogens. Current opinion in neurobiology. 2013;23:965–973. doi: 10.1016/j.conb.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Yamauchi T. Molecular constituents and phosphorylation-dependent regulation of the post-synaptic density. Mass spectrometry reviews. 2002;21:266–286. doi: 10.1002/mas.10033. [DOI] [PubMed] [Google Scholar]
- Yoon K, Nery S, Rutlin ML, Radtke F, Fishell G, Gaiano N. Fibroblast growth factor receptor signaling promotes radial glial identity and interacts with Notch1 signaling in telencephalic progenitors. 2004;24:9497–9506. doi: 10.1523/JNEUROSCI.0993-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zezula J, Casaccia-Bonnefil P, Ezhevsky SA, Osterhout DJ, Levine JM, Dowdy SF, Chao MV, Koff A. p21cip1 is required for the differentiation of oligodendrocytes independently of cell cycle withdrawal. EMBO reports. 2001;2:27–34. doi: 10.1093/embo-reports/kve008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y, et al. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat Neurosci. 2014;17:400–406. doi: 10.1038/nn.3641. [DOI] [PubMed] [Google Scholar]
- Ziv NE, Garner CC. Cellular and molecular mechanisms of presynaptic assembly. Nature reviews. Neuroscience. 2004;5:385–399. doi: 10.1038/nrn1370. [DOI] [PubMed] [Google Scholar]
- Zonouzi M, Renzi M, Farrant M, Cull-Candy SG. Bidirectional plasticity of calcium-permeable AMPA receptors in oligodendrocyte lineage cells. Nature neuroscience. 2011;14:1430–1438. doi: 10.1038/nn.2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuchero JB, Barres BA. Intrinsic and extrinsic control of oligodendrocyte development. Current opinion in neurobiology. 2013;23:914–920. doi: 10.1016/j.conb.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]