Abstract
Objective
To validate clinical indices of lupus nephritis (LN) activity and damage when used in children against the criterion standard of kidney biopsy findings.
Methods
In 83 children requiring kidney biopsy the SLE Disease Activity Index Renal Domain (SLEDAI-R); British Isles Lupus Assessment Group index Renal Domain (BILAG-R), Systemic Lupus International Collaborating Clinics Renal Activity (SLICC-RAS) and Damage Index Renal Domain (SDI-R) were measured. Fixed effect and logistic models were done to predict International Society of Nephrology/Renal Pathology Society (ISN/RPS) class; low/moderate vs. high LN-activity [NIH Activity Index (NIH-AI) score: ≤ 10 vs. > 10; Tubulointerstitial Activity Index (TIAI) score: ≤ 5 vs. > 5) or the absence vs. presence of LN chronicity [NIH Chronicity Index (NIH-CI) score: 0 vs. ≥ 1].
Results
There were 10, 50 and 23 patients with class I/II, III/IV and V, respectively. Scores of the clinical indices did not differentiate among patients by ISN/RPS class. The SLEDAI-R and SLICC-RAS but not the BILAG-R differed with LN-activity status defined by NIH-AI scores, while only the SLEDAI-R scores differed between LN-activity status based on TIAI scores. The sensitivity and specificity of the SDI-R to capture LN chronicity was 23.5% and 91.7%, respectively. Despite designed to measure LN-activity, SLICC-RAS and SLEDAI-R scores significantly differed with LN chronicity status.
Conclusion
Current clinical indices of LN fail to discriminate ISN/RPS Class in children. Despite its shortcomings, the SLEDAI-R appears to best for measuring LN activity in a clinical setting. The SDI-R is a poor correlate of LN chronicity.
Key Terms: Lupus nephritis, SLE, Children, Biomarkers
INTRODUCTION
Kidney biopsies are performed in systemic lupus erythematosus (SLE) to establish the diagnosis of lupus nephritis (LN) (1). Correlates of active inflammatory and chronic degenerative changes as seen on kidney biopsy assist with clinical decision making on the types and intensity of treatments in an attempt to protect long-term kidney function. Conversely, kidney biopsies are not used for routine monitoring of LN (2, 3), given cost and invasiveness. Instead laboratory testing, i.e. urinalysis and sediment, complement levels, anti-double stranded DNA antibody levels, timed proteinuria, protein: creatinine ratio (P/C ratio), blood pressure and glomerular filtration rate are performed for monitoring the course of LN in the clinical setting. Several clinical indices have been developed for adults with LN. These include the renal domain scores of the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI-R) and of the British Isles Lupus Assessment Group index (BILAG-R) (4) as well as the Systemic Lupus International Collaborating Clinics Renal Activity Score (SLICC-RAS) (5). Further, the SLICC Damage Index Renal Domain Score (SDI-R) is used to monitor and quantify kidney damage with LN.
Despite widespread use, these indices have not been well validated against the criterion standard, i.e. histological correlates of LN activity and chronicity as well as seen on kidney biopsy in pediatric populations with LN (6–9). This seems important given known differences in renal parameters between children and adults.
The objective of this study was to validate clinical indices of LN activity and damage when used in children against the criterion standard, i.e. findings on kidney biopsy.
MATERIALS & METHODS
Patients
Study participants were children who fulfilled the 1997 American College of Rheumatology (ACR) classification criteria for systemic lupus erythematosus (SLE) (10) prior to 18 years of age and required a kidney biopsy for diagnosis or follow-up of LN. For each patient, clinical and standard of care laboratory information including the findings on kidney biopsy were collected at the time of kidney biopsy. LN clinical indices were compared against criterion standards for LN activity and LN damage. The institutional review boards of the 13 participating pediatric rheumatology centers approved this cross-sectional study. All parents gave informed consent and, as appropriate, participants gave assent prior to the study procedures.
Clinical indices
The SLEDAI-R score (range 0–16; 0 = inactive LN) represents the sum of the renal items of the SLEDAI-2K. If present, each of the four SLEDAI-R items receives a score of 4: proteinuria of > 0.5 gram/day, hematuria and pyuria (both > 5 cells/high power field), and cellular casts (4). Besides the items included in the SLEDAI-R, the renal domain of the BILAG Index (BILAG-R) contains the following items: accelerated hypertension, nephrotic syndrome; serum creatinine concentrations, creatinine clearance, and signs of active LN on kidney biopsy in the preceding 3-month period. Based on the degree of abnormalities of the BILAG-R items an alphabetical BILAG-R score (A – E) can be deduced. For this study the alphabetical scores were converted into numerical scores (A = 12; B = 8; C = 1; D and E = 0) as previously suggested (4, 11). The scores of the BILAG-R range from 0 to 12 with higher scores signifying a more urgent need for therapeutic interventions. The SLICC-RAS considers various degrees of hematuria, pyuria and proteinuria in its summary score, all scored on Likert scales (5).
Clinical measures of kidney damage are the Systemic Lupus International Collaborating Clinics-Damage Index (SDI-R, score = 0 to maximum of 3) with scores given for the presence of at least 6 months of a 50% reduction in normal creatinine clearance, daily proteinuria exceeding 3.5 grams, and the need for renal replacement therapy (12). We also collected data on using the Chronic Kidney Disease (CKD) classification system, which is a generic measure of kidney function based on glomerular filtration rate (GFR) (13). CKD Stage 1 reflects GFR of at least 90 ml/min/1.73m2; Stage 2 is a GFR between 60 and 89 ml/min/1.73m2; Stage 3 between 30 and 59 ml/min/1.73m2, Stage 4 between 15 and 29 ml/min/1.73m2, and Stage 5 less than 15 ml/min/1.73m2.
Criterion standard – kidney biopsy findings
The International Society of Nephrology and the Renal Pathology Society (ISN/RPS) developed a framework for the classification of LN (14). Features from active inflammation is traditionally quantified using the NIH Activity Index (NIH-AI; score range: 0 – 24; 0 = no active features) (7). The NIH-AI score is based on the proportion of the kidney biopsy showing histological features indicative of active inflammation with LN: endocapillary hypercellularity with/without leukocyte infiltration and substantial luminal reduction, karyorrhexis (fibrinoid necrosis), rupture of glomerular basement membrane, fibrocellular crescents, subendothelial deposits identifiable by light microscopy (wire loops), and intraluminal immune aggregates (hyaline thrombi) (7). Recently, the Tubulointerstitial Activity Index (TIAI; score range: 0– 21; 0 = no active features) has been developed to provide more detailed information about inflammatory changes in the kidney interstitium (8). The TIAI score considers tubular cell pyknosis, nuclear activation, necrosis, flattening, macrophages in the tubular lumens, epithelial cells in the tubular lumens, and interstitial inflammation (8, 15).
Permanent kidney damage as seen on kidney biopsy is commonly quantified using the NIH Chronicity Index (NIH-CI; score range: 0 –12; 0 = no chronicity) (7). The NIH-CI reflects features of damage in LN: glomerular sclerosis (segmental or global), fibrous adhesions or fibrous crescents, interstitial fibrosis, and tubular atrophy (7).
Interpretation of kidney biopsy specimens
All kidney biopsy slides were digitalized and scanned (ScanScope XT system by Aperio) for interpretation by one pediatric nephropathologist (DW), in order to minimize inter-observer variation (16). Biopsy slides included stains for hematoxylin and eosin, trichrome, and immunofluorescence with C3, C4, C5, C1q, IgG, IgM, and IgA. Electron microscopy pictures were collected and used as a means to facilitate the interpretation according to the ISN/RPS classification. The nephropathologist identified the ISN/RPS Class and summary scores of the NIH-AI, NIH-CI and TIAI for the biopsy specimens.
Statistical analysis
Descriptive statistics included means and standard deviations (SD) or standard errors (SE), medians and interquartile ranges, for numerical variables, and frequencies for categorical variables respectively. Spearman correlation coefficients (r) were used to assess relationships between the scores of clinical LN indices (SLEDAI-R, BILAG-R, SLICC-RAS, SDI-R) and histological indices (NIH-AI, TIAI, NIH-CI, ISN/RPS class), Values of r can be interpreted as follows: unrelated: r < |0.2|; weakly related: |0.2| ≤ r < |0.4|; and moderately related: |0.4| ≤ r < |0.6|. Next, we compared clinical LN activity (SLEDAI-R, BILAG-R, SLICC-RAS) and LN damage measures (SDI-R, CKD stage) for their differences in means between high vs. low/moderate histological LN activity (defined as NIH AI > 10 vs. ≤ 10, or TIAI score > 5 vs. ≤ 5), the presence vs. absences of LN chronicity (defined as NIH CI ≥ 1 vs. 0), and ISN/RPS by classes using fixed effect models, after considering both unadjusted and adjusted methods; in the latter, differences in demographic variables between groups, such as age and race/ethnicity, were used as adjusting covariates. Multiple comparisons between two or more groups were adjusted using a Tukey’s method. In addition, we used non-parametric methods such as Wilcoxon rank sum and Kruskal Wallis tests were used to confirm results from the parametric fixed effect models (data not shown). Since findings from different methods were all consistent, only the results from unadjusted fixed models are presented.
We also determined for the LN damage status sensitivity, specificity and the positive (LR+) and the negative likelihood ratios (LR−) reflecting LN damage status. LR+ is defined as sensitivity/(1-specificity) and LR− is defined as (1-sensitivity)/specificity. LR +values can be interpreted as: > 10: large, often conclusive increase in the likelihood of “ruling in” the presence of LN chronicity (NIH-CI > 0); 5 – 9.9: moderate increase; and 2 – 4.9: small increase, respectively. In other words, a LR+ of 2 increases the probability for a LN chronicity by 15%, a LR+ of 5 increases it by 30%, and a LR+ of 10 increases it by 45% (17). LR− can be interpreted accordingly for “ruling-out” LN chronicity. All statistical analyses were performed using a SAS 9.4 software (SAS, Cary, NC) package. P-values < 0.05 were considered statistically significant.
RESULTS
Patients
At the time of the study, the mean (SD) age of the patients was 15.5 (2.7) years. A total of 65 patients (77%) were female; 34 (41%) were Caucasian and 30 (36%) were African American (Table 1). The mean extra-renal disease activity as measured by SLEDAI was moderate, with arthritis and mucocutaneous involvement constituting the most common extra-renal manifestations (Supplementary table 1). Proliferative LN (class III and IV) as present in 60% of the patients, while 28% had class V. None of the patients had Class VI but 15% of the patients had CKD stage 3 or higher.
Table 1.
Baseline characteristics of the patients
| Characteristics | Number (%)(N=83) | Mean (Standard deviation) |
|---|---|---|
| Females | 65 (77) | |
| Race | ||
| Caucasian | 34 (41.0) | |
| African-American | 30 (36.1) | |
| Asian with Pacific Islander | 7 (8.4) | |
| American Indian | 1 (1.2) | |
| Mixed | 11 (13.3) | |
| Ethnicity | ||
| Hispanic | 10 (12.1) | |
| Non-Hispanic | 73 (87.9) | |
| Age (in years) | 15.53 (2.71) | |
| Disease duration (in years) | 1.52 (0.96) | |
| Extra-renal SLEDAI* | 8.13 (6.61) | |
| ISN/RPS classŦ | ||
| I | 1 (1.2) | |
| II | 9 (10.8) | |
| III | 15 (18.1) | |
| IV | 35 (42.2) | |
| V | 23 (27.7) | |
| VI | 0 | |
| Biopsy interval (in days)¶ | 83 | 10 (3) |
| NIH-AI† | 71 | 7.82 (6.8) |
| TIAI¶¶ | 55 | 4 (0, 11) |
| NIH-CI# | 70 | 1.77 (1.75) |
| Pyuria (> 5 white blood cells/high power field) | 39 (46.9) | |
| Hematuria (> 5 red blood cells/high power field) | 50 (60.2) | |
| Urine casts | 14 (16.8) | |
| Urine protein/creatinine ratio mg/dL) | 3.4 (3.2) | |
| SDI-R** | 83 | 0.36 (0.61) |
| CKD StageŦŦ | ||
| 1 | 56 (67.5) | |
| 2 | 16 (19.3) | |
| 3 | 6 (7.2) | |
| 4 | 4 (4.8) | |
| 5 | 1 (1.2) | |
| Medications | ||
| Prednisone dose (mg/day) | 34 (19.2) | |
| Hydroxychloroquine | 72 (87) | |
| Mycophenolate mofetil | 31 (37.3) | |
| Azathiopine | 3 (3.6) | |
| Cyclophosphamide | 21 (25.3) | |
| Methylprednisolone pulse | 16 (19.2) | |
| Angiotensin converting enzyme inhibitor | 27 (32.5) | |
| Angiotensin II receptor blocker | 6 (7.2) | |
Extra-renal SLEDAI: Sum of the Systemic Lupus Erythematosus Disease Activity Index except the renal domain score
ISN/RPS: International Society of Nephrology/Renal Pathology Society (ISN/RPS) classification for lupus nephritis
Biopsy interval: interval in days between time of kidney biopsy and measurement of lupus nephritis clinical indices
NIH-AI: National Institutes of Health-Activity Index (score = 0 to maximum of 24). Based on proportion of the kidney biopsy with: endocapillary hypercellularity with/without leukocyte infiltration and substantial luminal reduction, karyorrhexis, rupture of glomerular basement membrane, fibrocellular crescents, wire loops, and hyaline thrombi
TIAI: Tubulointerstitial Activity Index (score = 0 to maximum of 21). Based on: tubular cell pyknosis, nuclear activation, necrosis, flattening, macrophages and epithelial cells in the tubular lumens, and interstitial inflammation
NIH-CI: National Institutes of Health-Chronicity Index (score = 0 to maximum of 12). Based on: glomerular sclerosis, fibrous adhesions or fibrous crescents, interstitial fibrosis, and tubular atrophy
SDI-R: Systemic Lupus International Collaborating Clinics-Damage Index (score = 0 to maximum of 5). Based on glomerular filtration rate (GFR) < 50%, presence of end stage renal disease and proteinuria > 3.5 gram/day
CKD: Chronic Kidney Disease Stage 1: normal glomerular filtration rate (GFR); Stage 2: GFR 60–89 ml/min/1.73m2; Stage 3: GFR 30–59 ml/min/1.73m2; Stage 4: GFR 15–29 ml/min/1.73m2; Stage 5: GFR <15 ml/min/1.73m2
Association between histological and clinical measures of LN-activity
None of the patients had a SLEDAI-R, SLICC-RAS or BILAG-R score of 0 at the time of biopsy. Scores of the SLEDAI-R were moderately and those of the SLICC-RAS and BILAG-R, weakly correlated with the NIH-AI scores (Table 2). TIAI scores were only correlated with the SLEDAI-R scores but not with those of the BILAG-R or SLICC-RAS.
Table 2.
Spearman correlations between clinical measures of LN-activity and histological measures of LN-activity and chronicity
| SLEDAI-R | BILAG-R | SLICC-RAS | |
|---|---|---|---|
| NIH-AI Score | 0.42 (0.0003) | 0.21 (0.0747) | 0.26 (0.036) |
| TIAI Score | 0.33 (0.014) | 0.13 (0.343) | 0.11 (0.425) |
| NIH-CI Score | 0.32 (0.008) | −0.01 (0.931) | 0.25 (0.05) |
| SDI-R Score | 0.24 (0.027) | 0.07 (0.559) | 0.40 (0.0005) |
| ISN/RPS Class | 0.04 (0.7443) | 0.06 (0.596) | 0.11 (0.3558) |
| CKD stage | 0.30 (0.008) | 0.20 (0.077) | 0.15 (0.196) |
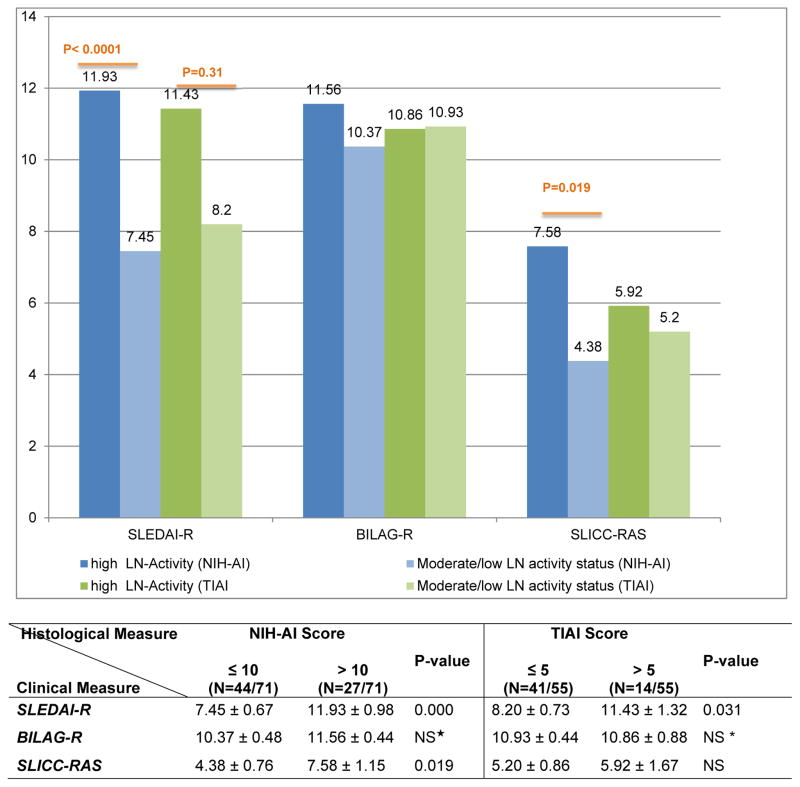
As shown in Figure 1, the mean scores of the SLEDAI-R differed with LN activity status defined by the NIH-AI and TIAI scores, respectively. This was not the case for the BILAG-R scores that did not differ with LN activity status (NIH-AI, TIAI).
Figure 1.
Differences in the scores of clinical indices of LN-activity with high vs. moderate/low LN-activity by kidney biopsy
†Values are means and SE; p-values based on test-tests comparing means of Lupus Nephritis Clinical Indices for low versus high NIH AI Score and TIAI Score, with cut-offs defined in the table
† LN-activity NIH-AI high vs. moderate/low is defined based on NIH-AI scores > 10 vs. ≤ 10
‡ LN-activity TIAI high vs. moderate/low is defined based on TIAI scores > 5 vs. ≤ 5
* Not significant
Associations between treatment with angiotensin-coverting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARB) to NIH AI and TIAI were assessed yet the results were not statistically significant. In particular, for those (N = 27) with ACEI/ARB, 7/27 or 26% have NIH AI score > 10; and for N = 44 who don’t have ACE/ARB, 17/44 or 38.6% have NIH AI score > 10 (p-value = 0.27). Similar results were obtained when comparing TIAI >5 (p-value = 0.95).
Relationship between histologically proven LN chronicity and clinical measures of LN damage
SDI-R scores of 1 or higher were more common in patients with vs. without biopsy-proven kidney chronicity (p-value = 0.08). Although the sensitivity of SDI-R to capture LN chronicity (NIH-CI > 0) was only 23%, it was highly specific (91.7%), resulting in a LR+ of 2.8 and a LR− of 0.83. In addition, patients with LN chronicity were more likely to have more severe CKD (stages 3 and 4) than patients with NIH-CI scores of 0 (Table 3). Notably, most children maintained normal GFR even in the presence of LN chronicity.
Table 3.
Comparisons of Lupus Nephritis Clinical Index Scores in Relation to Histological Damage Scores*
| Histological Measure of Damage
|
NIH-CI = 0 (N=36) | NIH-CI > 0 (N=34) | P-value | |
|---|---|---|---|---|
| Clinical Measures of Damage | ||||
| SDI-R† | SDI-R> 0 | 3 (8.3%) | 8 (23.5%) | 0.08 |
| SDI-R = 0 | 30 (91.7%) | 26 (76.5%) | ||
|
| ||||
| CKD Stage | 1 | 26 (69.4%) | 22 (64.7%) | 0.003 |
| 2 | 11 (30.6) | 3 (8.8%) | ||
| 3 | 0 (0%) | 4 (11.8%) | ||
| 4 | 0 (0%) | 5 (14.7%) | ||
Values are n (%) of N patients with available information; P-values are based on contingency table analysis.
SDI was not completed for patients with disease durations for less than 6 months
CKD stages 3 or 4 were only present in patients with NIH-CI scores > 0. Therefore, the presence of CKD stage 3 or worse was 100% sensitive and 73.5% specific for the presence of LN chronicity (LR+: 3.8; LR−: 0). A less stringent cut-off at CKD stage 2 or worse had a sensitivity and specificity of 35% and 70% for the presence of LN chronicity (LR+: 1.5; LR−: 0.94).
Clinical measures of LN activity are associated with histological measures of kidney damage
Although designed to estimate LN activity, the mean scores of the SLEDAI-R and SLICC-RAS but not BILAG-R, were significantly higher in patients with NIH-CI scores > 0 (Table 4). The mean scores of the SLICC-RAS also significantly differed by LN damage status as measured by the SDI-R. The mean scores of the LN clinical indices were generally higher in patients with CKD stage 3–5 than stage 1–2. Adjusting for ACEI or ARB usage did not significantly change these findings.
Table 4.
Mean Scores of Lupus Nephritis Clinical Indices in Relation to Kidney Damage *
| Clinical Measures of Activity
|
SLEDAI-R | BILAG-R | SLICC-RAS | |
|---|---|---|---|---|
| Histological Measure of Damage | ||||
| NIH CI Score | 0 (N=34) | 7.83 ± 0.83 | 10.89 ± 0.50 | 4.26 ± 0.92 |
| > 0 (N=36) | 10.82 ± 0.85 | 10.82 ± 0.51 | 7.69 ± 0.95 | |
| P-value | 0.014 | NS | 0.012 | |
|
| ||||
| SDI-R¶ | 0 (N=68) | 8.32 ± 0.62 | 10.66 ± 0.37 | 5.18 ± 0.64 |
| > 0 (N=14) | 11.14 ± 1.36 | 10.86 ± 0.81 | 11.45 ± 1.52 | |
| P-valueŦ | 0.062 | 0.823 | 0.000 | |
|
| ||||
| Chronic Kidney Disease | Stage 1 (N=54) | 8.00 ± 0.66† | 10.36 ± 0.42 | 5.64 ± 0.78 |
| Stage 2 (N=16) | 8.63 ± 1.22† | 10.81 ± 0.76 | 4.80 ± 1.38 | |
| Stage 3 (N=6) | 12.00 ± 1.99 | 12.00 ± 1.24 | 9.83 ± 2.18 | |
| Stage 4 or 5 (N=5) | 15.20 ± 2.18 | 12.00 ± 1.36 | 10.20 ± 2.39 | |
Values are means (95%CI)
P-values based on t-test comparing means of Lupus Nephritis Clinical Indices for low versus high NIH CI Score, with cut-offs defined in the table
SDI-R: Systemic Lupus International Collaborating Clinics-Damage Index (score = 0 to maximum of 5). Based on glomerular filtration rate (GFR) < 50%, presence of end stage renal disease and proteinuria > 3.5 gram/day
CKD Stage 1: normal GFR; Stage 2: GFR 60–89 ml/min/1.73m2; Stage 3: GFR 30–59 ml/min/1.73m2; Stage 4: GFR 15–29 ml/min/1.73m2; Stage 5: GFR <15 ml/min/1.73m2
Means are statistically different from that of State 4+5, in post hoc tests after adjusting for multiple comparison using a Bonferroni’s method.
Relationship of ISN/RPS class and LN clinical indices
At the time of kidney biopsy, the mean SLEDAI-R scores with class IV LN were significantly higher than with other classes of LN. In contrast, neither the SLICC-RAS nor the BILAG-R scores differed significantly among the various classes of LN. Notably, the SLEDAI-R and BILAG-R scores for class I/II LN were almost identical to those of class III LN (Table 5). There was a trend towards more clinical LN damage (SDI-R scores > 0) with higher classes of LN (p-value = 0.167) and also a trend of higher CKD stages with higher classes of LN (p-value = 0.072).
Table 5.
Comparison between lupus nephritis clinical index scores in relation to histological classification
| LN Clinical Indices | ISN/RPS Class† | ||||
|---|---|---|---|---|---|
| I/II (N=10) | III (N=15) | IV (N=35) | V (N=23) | ||
| SLEDAI-R* | 6.80 ± 1.43 | 6.40 ± 1.17 | 11.83 ± 0.77 ∫ | 6.55 ± 0.97 | |
| BILAG-R Ŧ | 10.10 ± 0.94 | 10.40 ± 0.77 | 11.54 ± 0.50 | 9.76 ± 0.65 | |
| SLICC-RAS# | 3.11 ± 1.78 | 5.07 ± 1.38 | 7.91 ± 0.94 | 5.29 ± 1.29 | |
| SDI-R>0 | 0% | 6.7% | 25.7% | 18.2% | |
| CKD | Stage 1 (N=54) | 60.0% | 73.3% | 54.3% | 85.7% |
| Stage 2 (N=16) | 40.00% | 20.0% | 17.1% | 14.3% | |
| Stage 3 (N=6) | 0% | 6.7% | 14.3% | 0% | |
| Stage 4 or 5 (N=5) | 0% | 0% | 14.3% | 0% | |
SLEDAI-R: renal domain score of the Systemic Lupus Erythematosus Disease Activity Index. Sum of the SLEDAI item scores for proteinuria > 0.5 gram/day, hematuria and pyuria (both > 5 cells/HPF and cellular casts
BILAG-R: renal domain scores of the British Isles Lupus Assessment Group index. Sum of the BILAG item scores for proteinuria > 0.5 gram/day, hematuria and pyuria (both > 5 cells/HPF), cellular casts, blood pressure, the presence of accelerated hypertension, nephrotic syndrome, raised plasma creatinine concentrations, decreased GFR and for a kidney biopsy showing LN in the preceding 3-month period
SLICC-RAS: Systemic Lupus International Collaborating Clinics-Renal Activity Score. Includes the SLEDAI-R items, except for cellular casts, scored on Likert scales
Values are means ± SE (standard errors), unless stated otherwise
Class IV score was significantly different from I/II, Class III and Class V
DISCUSSION
Kidney biopsies provide information about the degree of inflammation and accrued damage with LN. It is impractical to use repeat kidney biopsies for monitoring LN, especially in the setting of apparent clinical remission and stable kidney function. Hence, surrogate measures of LN activity and LN damage have been proposed and used in clinical and research settings for many years. We present herein the first formal validation of such indices in children with LN. The results suggest that the SLEDAI-R and SLICC-RAS but not the BILAG-R differed with LN-activity status defined by NIH-AI scores, while only the SLEDAI-R scores differed between LN-activity status based on TIAI scores. Despite being specific, the SDI-R lacks sufficient sensitivity to identify true kidney damage.
While our goal was not to suggest that LN clinical indices can replace kidney biopsies, it is important that we evaluate and validate these clinical indices that are commonly used in clinical practice and research. LN clinical indices are comprised of individual laboratory tests, including complement levels, anti-double-stranded DNA, antibodies and proteinuria that have been shown individually to reflect active LN activity as seen on kidney biopsy.
The SLEDAI-R was the only clinical index that differentiated between the degree of both glomerular and tubulointerstitial inflammation as measured by the NIH-AI and TIAI, respectively. Indeed, the SLEDAI-R was superior to the SLICC-RAS and BILAG-R in capturing LN activity. Reasons observed in our data were that blood-pressure measurement and other items considered in the BILAG-R only poorly correlate with LN activity in children. The SLICC-RAS has similar components as the SLEDAI-R, which may explain why the SLICC-RAS performed somewhat better than the BILAG-R in reflecting histological proven LN activity. When considering ease of use, and its known reproducibility, the SLEDAI-R is probably the most appropriate clinical index for measuring LN activity in children with LN.
Among patients with histological damage, we found that the presence of any renal damage captured by the SDI was highly specific but insensitive to the presence of histologically confirmed LN chronicity. Nonetheless, a positive SDI-R score increases the likelihood for the presence of LN chronicity by about 20% and therefore helps to a limited degree to “rule-in” LN-chronicity. Conversely, a SDI-R score of ‘0’ provides little information to help “ruling-out” the presence of histologically-confirmed kidney damage.
Chronic proteinuria is considered in the score of the SDI-R and proteinuria is also a key feature of active LN. Thus it might be expected that the scores of the SLEDAI-R, BILAG-R and SLICC-RAS would be higher in patients with LN chronicity. As done for this study, there is the proviso in the completion of all of these indices that the proteinuria should be considered to be part of active LN. Supported by our findings, it may put patients with proteinuria at risk of unnecessary exposure to immunosuppressives for erroneously presumed active LN and vice versa.
This study is not without its limitations. Despite being the criterion standard for staging and classifying LN, needle kidney biopsies of children have often only provide few glomeruli and little interstitial tissue. This may limit the accuracy of histological interpretation of kidney biopsies (18, 19). However, all histological findings reported in this study were interpreted by a one expert nephropathologist who omitted scoring of histological indices when deemed appropriate due to inferior biopsy quality. We since then asked three other nephropathologists to reinterpret the biopsies and found other raters to provide very similar scoring (unpublished observations). Further, due to the limited number of repeat biopsies (only two patients), we were only able to analyze the data in a cross-sectional fashion. It will be ideal to evaluate the responsiveness of the LN clinical indices to histological changes in repeat kidney biopsies in the future.
In summary, this study stresses the need for improved non-invasive measures of LN activity and LN damage. Although uncommonly performed in pediatrics, serial LN biopsies may prove beneficial to verify response to anti-inflammatory LN therapy. This notion is in line with recent studies in adults that demonstrated ongoing renal inflammation despite normalized LN clinical indices (20). Furthermore, the results of our study emphasize the need for routine access to testing of novel urine biomarkers in LN. There is a large body of evidence supporting the accuracy and even predictive properties of novel urine biomarkers for LN (21). However, none of the proposed LN novel biomarker panels are commercially available at this time or approved for use as companion diagnostics or biomarker in clinical trial of LN. In the meantime, we recommend using the SLEDAI-R to measure LN activity in children with LN.
Supplementary Material
SIGNIFICANCE AND INNOVATION.
We newly provide evidence that the renal domain score of the SLEDAI (SLEDAI-R) is more reflective of histologically confirmed LN activity than the SLICC-RAS and the BILAG-R in children with LN.
The SDI-R scores of 1 or higher are specific for the presence of LN damage but they lack sensitivity.
Most children with LN have normal kidney function despite the presence of active LN, even when renal damage is present.
Acknowledgments
Grant Support: Dr. Brunner is supported by the NIH-grants: U0-AR059509 and U0-AR065098. Dr. Mina is supported by the University of Cincinnati CCTST.
Lukasz Itert, Melanie Hhalol, Kasha Wiley (Cincinnati Children’s Hospital Medical Center, Cincinnati, OH) and Shannen Nelson (Children’s Hospital Los Angeles, CA)
Sheena Kapoor, Nicole Battle (Children’s National Medical Center, Washington, DC)
Lori Ponder (Children’s Healthcare of Atlanta, Emory University, Atlanta, GA)
Michael Miller, MD, Megan Curran MD, Erin Thomas and Alexandra Martyniuk (Lurie Children’s Hospital, Chicago, IL)
Angelynne Sarmiento, David Cabral MD, Kristin Houghton, Kimberly Morishita, Jaime Guzman (BC Children’s Hospital, Vancouver BC)
Rebecca Puplava (University of Chicago Comer Children’s Hospital, Chicago, IL)
References
- 1.Rovin BH, Parikh SV, Alvarado A. The kidney biopsy in lupus nephritis: is it still relevant? Rheum Dis Clin North Am. 2014;40(3):537–52. ix. doi: 10.1016/j.rdc.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Askenazi D, Myones B, Kamdar A, Warren R, Perez M, De Guzman M, et al. Outcomes of children with proliferative lupus nephritis: the role of protocol renal biopsy. Pediatr Nephrol. 2007;22(7):981–6. doi: 10.1007/s00467-007-0447-9. [DOI] [PubMed] [Google Scholar]
- 3.McRae M, Rousseau-Gagnon M, Philibert D, Houde I, Riopel J, Latulippe E, et al. The interpretation of repeat renal biopsies in patients with lupus nephritis. Rheumatology (Oxford) 2013;53(6):1151–2. doi: 10.1093/rheumatology/ket420. [DOI] [PubMed] [Google Scholar]
- 4.Brunner HI, Feldman BM, Bombardier C, Silverman ED. Sensitivity of the Systemic Lupus Erythematosus Disease Activity Index, British Isles Lupus Assessment Group Index, and Systemic Lupus Activity Measure in the evaluation of clinical change in childhood-onset systemic lupus erythematosus. Arthritis Rheum. 1999;42(7):1354–60. doi: 10.1002/1529-0131(199907)42:7<1354::AID-ANR8>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 5.Petri M, Kasitanon N, Lee SS, Link K, Magder L, Bae SC, et al. Systemic lupus international collaborating clinics renal activity/response exercise: development of a renal activity score and renal response index. Arthritis Rheum. 2008;58(6):1784–8. doi: 10.1002/art.23456. [DOI] [PubMed] [Google Scholar]
- 6.Zappitelli M, Duffy CM, Bernard C, Gupta IR. Evaluation of activity, chronicity and tubulointerstitial indices for childhood lupus nephritis. Pediatr Nephrol. 2008;23(1):83–91. doi: 10.1007/s00467-007-0619-7. [DOI] [PubMed] [Google Scholar]
- 7.Austin HA, 3rd, Muenz LR, Joyce KM, Antonovych TA, Kullick ME, Klippel JH, et al. Prognostic factors in lupus nephritis. Contribution of renal histologic data. The American journal of medicine. 1983;75(3):382–91. doi: 10.1016/0002-9343(83)90338-8. [DOI] [PubMed] [Google Scholar]
- 8.Hill GS, Delahousse M, Nochy D, Tomkiewicz E, Remy P, Mignon F, et al. A new morphologic index for the evaluation of renal biopsies in lupus nephritis. Kidney international. 2000;58(3):1160–73. doi: 10.1046/j.1523-1755.2000.00272.x. [DOI] [PubMed] [Google Scholar]
- 9.Marks SD, Sebire NJ, Pilkington C, Tullus K. Clinicopathological correlations of paediatric lupus nephritis. Pediatr Nephrol. 2007;22(1):77–83. doi: 10.1007/s00467-006-0296-y. [DOI] [PubMed] [Google Scholar]
- 10.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis and rheumatism. 1997;40(9):1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 11.Yee C-S, Cresswell L, Farewell V, Rahman A, Teh L-S, Griffiths B, et al. Numerical scoring for the BILAG-2004 index. Rheumatology. 2010;49(9):1665–9. doi: 10.1093/rheumatology/keq026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruce IN, O’Keeffe AG, Farewell V, Hanly JG, Manzi S, Su L, et al. Factors associated with damage accrual in patients with systemic lupus erythematosus: results from the Systemic Lupus International Collaborating Clinics (SLICC) Inception Cohort. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2013-205171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levey AS, Coresh J. Chronic kidney disease. Lancet. 2011;379(9811):165–80. doi: 10.1016/S0140-6736(11)60178-5. [DOI] [PubMed] [Google Scholar]
- 14.Marks SD, Sebire NJ, Tullus K. Classification of pediatric lupus nephritis. Kidney Int. 2007;72(7):897–8. doi: 10.1038/sj.ki.5002497. [DOI] [PubMed] [Google Scholar]
- 15.Hsieh C, Chang A, Brandt D, Guttikonda R, Utset TO, Clark MR. Predicting outcomes of lupus nephritis with tubulointerstitial inflammation and scarring. Arthritis Care Res (Hoboken) 2011;63(6):865–74. doi: 10.1002/acr.20441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grootscholten C, Bajema IM, Florquin S, Steenbergen EJ, Peutz-Kootstra CJ, Goldschmeding R, et al. Interobserver agreement of scoring of histopathological characteristics and classification of lupus nephritis. Nephrol Dial Transplant. 2008;23(1):223–30. doi: 10.1093/ndt/gfm555. [DOI] [PubMed] [Google Scholar]
- 17.McGee S. Simplifying likelihood ratios. Journal of general internal medicine. 2002;17(8):646–9. doi: 10.1046/j.1525-1497.2002.10750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seron D, Moreso F, Fulladosa X, Hueso M, Carrera M, Grinyo JM. Reliability of chronic allograft nephropathy diagnosis in sequential protocol biopsies. Kidney international. 2002;61(2):727–33. doi: 10.1046/j.1523-1755.2002.00174.x. [DOI] [PubMed] [Google Scholar]
- 19.Corwin HL, Schwartz MM, Lewis EJ. The importance of sample size in the interpretation of the renal biopsy. American journal of nephrology. 1988;8(2):85–9. doi: 10.1159/000167563. [DOI] [PubMed] [Google Scholar]
- 20.Zickert A, Sundelin B, Svenungsson E, Gunnarsson I. Role of early repeated renal biopsies in lupus nephritis. Lupus science & medicine. 2014;1(1):e000018. doi: 10.1136/lupus-2014-000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bennett M, Brunner HI. Biomarkers and updates on pediatrics lupus nephritis. Rheumatic diseases clinics of North America. 2013;39(4):833–53. doi: 10.1016/j.rdc.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.