Abstract
Introduction
Survival of patients after cytoreductive surgery (CRS) and heated intraperitoneal chemotherapy for appendiceal neoplasms is projected by conventional overall survival (OS) curves that do not address the survival time a patient has already accrued. We sought to study the conditional survival (CS) after CRS, contingent on patients surviving a fixed duration of time after surgery.
Methods
A retrospective analysis of 493 appendiceal cancer patients from a prospective database was performed. OS was calculated for patients who achieved a complete CRS. CS was estimated based on Kaplan–Meier curves to determine what the patient’s long-term survival (3-, 5-, 7-, or 10-year) would be if they were alive at 1, 2, or 3 years from surgery.
Results
OS at 5 and 10 years for 137 low-grade patients with complete resections was 83.3 and 74.2 %, respectively. For low-grade patients still alive at 3 years, 5- and 10-year CS was 93.4 and 83.2 %, respectively. For the 35 high-grade patients with complete CRS who survived to 3 years, CS at 10 years was 41.7 %, while their 10-year conventional OS was 24.6 %.
Conclusions
Conventional analysis underestimates OS due to unpredictable variations in tumor biology. When adjusted for time already elapsed since surgery, improvements in survival estimates are more pronounced with high-grade tumors. CS outcomes can be used in determining the optimal frequency of long-term follow-up of these patients.
Cytoreductive surgery (CRS) with heated intraperitoneal chemotherapy (HIPEC) is an established modality for the treatment of peritoneal dissemination of a variety of epithelial primaries. Survival after CRS/HIPEC for appendiceal neoplasms with peritoneal surface disease (PSD) is multifactorial and often depends on tumor biology, volume of disease at presentation, completeness of CRS, and patients’ functional status and comorbidities.1–4
Despite our best efforts to predict survival outcomes for patients with low- (LGA) and high-grade appendiceal (HGA) primaries who have achieved a complete macroscopic CRS, these outcomes inevitably suffer from variability in tumor biology, which cannot be addressed by actuarial survival curves calculated from the time of diagnosis or initial treatment. These conventional estimates fail to take into consideration the duration of time that a patient has already survived since surgery.
On the other hand, conditional survival (CS) is the survival estimated with the condition that patients have already survived for a certain period of time after surgery, potentially allowing for variations in tumor biology to declare themselves.
Herein, we sought to study if CS is a better tool to predict the long-term survival of patients with appendiceal primaries who undergo CRS/HIPEC. Furthermore, we aimed to define whether CS outcomes can be used in determining the optimal frequency of long-term follow-up of patients with appendiceal primaries who have achieved a complete CRS.
METHODS
A retrospective analysis of a prospectively maintained database of 1149 CRS/HIPEC procedures from 1991 to 2014 was performed. Eligibility criteria for CRS/HIPEC included histologic or cytological diagnosis of peritoneal carcinomatosis, complete recovery from prior systemic chemotherapy, resectable or resected primary lesion, PSD amenable to debulking, and lack of extra-abdominal disease. Institutional Review Board approval was obtained. Data abstracted from the database included patient age, race, sex, Eastern Cooperative Oncology Group (ECOG) performance status, type of primary malignancy, co-morbidities, and R resection status.
CRS involved resection of organs and peritoneum involved by tumor. A right hemicolectomy with associated lymphadenectomy was routinely performed for high-grade lesions. Low-grade lesions underwent a right hemicolec-tomy only when the disease in the right colon itself did not allow for a complete cytoreduction. After CRS was completed, peritoneal perfusion was accomplished via the closed technique using two 22F inflow catheters and two 32F outflow catheters placed percutaneously into the abdominal cavity. A perfusion circuit was established with approximately 3 L of crystalloid solution. Flow rates of approximately 1 L/min were maintained at a maximum inflow temperature of 43 °C. The majority of the patients were perfused with mitomycin C 40 mg for 2 h, whereas a smaller group was perfused with oxaliplatin (200 mg/m2) within the context of an ongoing prospectively randomized clinical trial. Details of the surgical procedure have been described by our group in previous publications.3
R0 and R1 resections were grouped together as complete cytoreductions. Cytoreductions with residual macroscopic disease were characterized as R2 (R2a ≤ 5 mm, R2b >5 mm and ≤2 cm, R2c >2 cm) and were excluded, allowing only patients who underwent a complete R0/R1 macroscopic CRS/HIPEC to be included in the analysis. Excluded patients were also those who had appendiceal tumors with neuroendocrine differentiation, goblet cell carcinoids, or indeterminate pathology.
Patients with complete CRS were further classified, based on pathology, as LGA and HGA tumors. This classification was based on the two-tier Wake Forest Classification system previously described by Bradley et al.5 More specifically, low-grade patients included those with disseminated peritoneal adenomucinosis (DPAM), well-differentiated mucinous carcinomatosis, peritoneal mucinous carcinomatosis with intermediate or discordant features (PMCA-I/D), and well-differentiated variants of mucinous adenocarcinoma or low-grade appendiceal mucinous neoplasms. High-grade tumors included moderately or poorly differentiated adenocarcinoma, PMCA, and cases with signet-ring cell components. In our experience, there was no reproducible difference in survival between DPAM and PMCA-I, while both exhibited the same incidence of parenchymal (beyond the serosa) organ invasion.
Statistical Analysis
Summary statistics, including medians and ranges for continuous measures, and frequencies and proportions for categorical data, were generated for all study measures. The Kaplan–Meier method was used to estimate survival and standard errors, and the log-rank statistic for the χ2 approximation was used to test study groups for statistically significant differences. CS estimates were created after removing subjects who had not yet survived to the time point of interest or had died prior to the cut point.
RESULTS
From 1991 to 2014, a total of 493 patients with appendiceal neoplasms underwent CRS/HIPEC in our institution. For the reasons indicated above, 80 (16.2 %) patients were initially excluded, leaving 413 (83.8 %) patients for further analysis. LGA and HGA primaries were 301 (72.9 %) and 112 (27.1 %), respectively. Among low-grade patients, 137 (45.5 %) had a complete CRS, while 164 (54.5 %) had R2 resections. For patients in the HGA group, complete CRS was achieved in 35 (31.3 %) patients, while 77 (68.7 %) patients underwent R2 resections. The demographic and clinicopathological characteristics of the LGA and HGA cohorts are demonstrated in Table 1.
TABLE 1.
Demographic and clincopathological characteristics
| Characteristic | All patients (n = 413) |
|---|---|
| Median age (n = 413): years (range) | 53 (22–87) |
| Male n (%) | 194 (46.9) |
| Race (n = 411): n (%) | |
| White | 355 (86.37) |
| Black | 42 (10.22) |
| Other | 14 (3.41) |
| Heart disease (n = 406): n (%) | 37 (9.11) |
| Lung disease (n = 407): n (%) | 16 (3.93) |
| Diabetes mellitus (n = 407): n(%) | 34 (8.35) |
| Median BMI (n = 391): kg/m2 (range) | 26.5 (17.9–63.3) |
| Smoking status (n = 402): n (%) | |
| No | 295 (73.4) |
| ECOG performance status (n = 406): n (%) | |
| 0 | 213 (52.5) |
| 1 | 144 (35.5) |
| ≤ 2 | 49 (12.1) |
| Lymph node status (n = 252) | |
| LGA (node positive/negative) | 164 (19/145) |
| HGA (node positive/negative) | 88 (53/35) |
| Median number of organs of resected n (range) | 3 (0–9) |
| Colon/rectal resection: n (%) | 269 (65.1) |
| Grade | |
| Low | 302 |
| High | 112 |
| PCI (n = 204): mean (SD) | 15.2 (8.6) |
| Resection status (n = 412): n (%) | |
| R0/1 | 172 (41.8) |
| R2a | 133 (32.3) |
| R2b | 73 (17.2) |
| R2c | 34 (8.25) |
| Prior surgical score (n = 405): n (%) | |
| 0 | 74 (18.1) |
| 1 | 144 (35.3) |
| 2 | 176 (43.1) |
| 3 | 14 (3.4) |
PCI peritoneal cancer index
Conditional Survival (CS) for Low-Grade Tumors
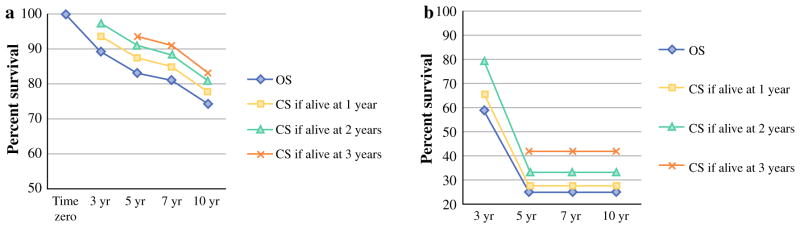
OS for patients undergoing a complete macroscopic CRS (n = 137) was 89.2 % and 74.2 % at 3 and 10 years, respectively. For 105 of the LGA patients who survived to 1 year after surgery, the 3-, 5-, and 10-year CS estimates were 93.5, 87.3, and 77.8 %, respectively. As patients survived longer from surgery, CS estimates showed meaningful improvement, with the 10-year CS estimates for patients surviving to 2 (n = 89) and 3 (n = 68) years after surgery being 81.2 and 83.2 %, respectively. This translates into a relative increase in survival estimates by 9.4 and 12.1 %, respectively (Table 2).
TABLE 2.
Conventional and conditional survival estimates for all patients with low- and high-grade tumors and R0/R1 resection
| Survival estimatesa | 3 years | 5 years | 7 years | 10 years |
|---|---|---|---|---|
| Low-grade appendiceal | ||||
| OS (n = 137) | 89.2 (3.0) | 83.3 (4.0) | 81.1 (4.4) | 74.2 (6.2) |
| If alive at 1 year (n = 105) | 93.5 (2.6) | 87.3 (3.8) | 85.1 (4.3) | 77.8 (6.3) |
| If alive at 2 years (n = 89) | 97.5 (1.7) | 91.0 (3.5) | 88.7 (4.1) | 81.2 (6.4) |
| If alive at 3 years (n = 68) | 93.4 (3.2) | 91.0 (3.9) | 83.2 (6.4) | |
| High-grade appendiceal | ||||
| OS (n = 35) | 59.0 (10.5) | 24.6 (11.1) | 24.6 (11.1) | 24.6 (11.1) |
| If alive at 1 year (n = 25) | 65.7 (10.9) | 27.4 (12.2) | 27.4 (12.2) | 27.4 (12.2) |
| If alive at 2 years (n = 19) | 79.3 (10.9) | 33.0 (14.4) | 33.0 (14.4) | 33.0 (14.4) |
| If alive at 3 years (n = 10) | 41.7 (17.3) | 41.7 (17.3) | 41.7 (17.3) | |
All data represent percentage survival (standard error)
CS for High-Grade Tumors
Complete CRS and HIPEC was achieved in 35 (31.3 %) patients with high-grade appendiceal tumors. Conventionally estimated OS was 59 and 24.6 % at 3 and 10 years, respectively. CS at 3 and 10 years for the 25 patients who were still alive at 1 year was 65.7 and 27.4 %, respectively. As patients survived to 2 (n = 19) and 3 (n = 10) years from surgery, their 10-year CS estimates increased to 41.7 % (Table 2). This represents a 34.1 and 69.5 % relative increase in survival estimates compared with conventional survival analysis.
DISCUSSION
CRS/HIPEC is a proven treatment modality in the management of patients with peritoneal dissemination from appendiceal primaries. Survival outcomes are influenced by several prognostic factors, including histologic type and grade, age, performance status, lymph node involvement, perioperative chemotherapy, tumor burden, and completeness of cytoreduction.2–4,6–10
Our institution has recently published a multivariate analysis of factors that affect survival in patients with appendiceal cancer. In this study, 481 procedures in 430 patients undergoing CRS/HIPEC were analyzed, including 317 (77.3 %) LGA and 93 (22.7 %) HGA primaries. Complete CRS was obtained in 211 (44 %) patients. The clinicopathological factors were similar to those presented in the current study, while neuroendocrine appendiceal tumors were not included. Negative predictors of survival in LGA patients included positive nodal status (hazard ratio [HR] 3.6; p = 0.003), incomplete cytoreduction (HR 2.5 for R2a vs. R0/1, 9 for R2b vs. R0/1, and 3.8 for R2c vs. R0/1; p < 0.001), and preoperative chemotherapy (HR 2.2; p = 0.04), while incomplete cytoreduction (HR 3.8 for R2a vs. R0/1, 4.9 for R2b vs. R0/1, and 5.9 for R2c vs. R0/1; p < 0.001) and preoperative chemotherapy (HR 2.5; p = 0.006) were negative predictors of survival in HGA patients.4 In the present study, for analysis we considered only those patients who had a complete CRS and were still alive up to 3 years after CRS/HIPEC.
However, the aforementioned prognostic factors generate static survival estimates that rely on information obtained at the time of surgery and do not change over time. These projections may not be as accurate once patients have already survived a certain duration of time after surgery. This is especially true of patients with poor prognostic factors in whom projected survival is worse at the time of initial surgery, with most deaths occurring in the first few years. CS has therefore been proposed as an alternative to assess long-term probability of survival in patients who have already survived for a certain duration of time after surgery.
The first aim of this study was to evaluate if CS produces more accurate survival estimates that can be used in postoperative patient consultation. The second aim was to identify whether these CS estimates can be used to determine the optimal frequency of long-term follow-up of appendiceal primary patients who have achieved a complete CRS.
In the current study, a significant improvement in CS-obtained estimates was observed for both HGA and LGA appendiceal primaries treated with complete CRS/HIPEC.
LGA patients who have already lived to 3 years demonstrated a 12 % relative increase from their traditionally projected 10-year survival, from 74.2 to 83.2 %. In addition, once patients survive to 3 years their survival showed a minimal decline from years 5 to 7, from 93.4 to 91 %, with a late decline at 10 years to 83.1 % (Fig. 1a). This could potentially justify a recommendation that an annual surveillance computed tomography (CT) scan after year 3 is safe and cost effective for the long-term follow-up of LGA patients who had a complete macroscopic CRS. Interestingly, since most of the patients who survive over 10 years after initial treatment continue to survive with minimal decline in their survival curves, it is tempting to consider the likelihood of cure from their disease. However, given the lack of data regarding the cause of death and the slow late decline even at 10 years, we would approach this observation with caution and recommend that annual imaging probably be considered during a patient’s functional lifetime.
FIG. 1.
Conventional and conditional survival curves for (a) LGA R0/R1 patients and (b) HGA R0/R1 patients. LGA low-grade appendiceal, HGA high-grade appendiceal, OS overall survival, CS conditional survival
In the HGA cohort, CS becomes even more important for prognostication. Overall survival (OS) for HGA patients is significantly worse compared with LGA patients, even after a complete CRS (Fig. 1b). However, the effect of accrued survival on OS is very pronounced in the HGA cohort. More specifically, 10-year CS estimates for HGA patients who survive 3 years is 41.7 %, compared with conventional estimate of 24.6 %, representing a relative increase of 69.5 %. Even so, once patients survive over 3 years, their curves plateau; this is likely an indication of a selection mechanism of those patients with favorable tumor biology.
Based on the above data, we currently follow LGA patients who have had a complete CRS/HIPEC with a surveillance CT scan every 6 months for the first 3 years, followed by annual CT scans thereafter. For HGA primaries, we obtain contrast-enhanced CT scans or magnetic resonance imaging (MRI) every 3–6 months until year 5, and every 6 months from that point onwards. In addition, we use CS data to alleviate surveillance anxiety of long-term appendiceal cancer survivors.
This study was limited by being a retrospective, single-institution analysis of data incorporating the learning curve of our institution over a period of several years. Even though selection bias is inevitably part of the reported survival outcomes, these outcomes are in line with what other centers have presented in the literature.2,11,12
CS estimates incorporate the time patients accrue after developing a recurrence. Since recurrence is also part of the reported OS outcomes and not an independent component, we did not analyze these data separately. Additionally, even though the small power of the HGA group might prevent drawing definitive conclusions about long-term HGA outcomes, it is not unreasonable to suggest that until we have an accurate genetic signature of these tumors, CS could be used as an acceptable alternative in the counseling of these patients.
CONCLUSIONS
Patients with appendiceal primaries who have survived a certain duration of time after complete CRS/HIPEC are better served by conditional as opposed to conventional survival data. This difference is noted for both LGA and HGA patients, with the relative effect of accrued survival time being more pronounced in patients with high-grade tumors. These data can be used to tailor surveillance strategies and ameliorate survivor anxiety.
Acknowledgments
The authors are grateful for the support, in part, of grants from the National Organization for Rare Diseases and the Smith Family Foundation. This study was supported by Wake Forest University Biostatistics shared resource NCI CCSG P30CA012197.
Footnotes
Accepted for oral presentation at: 10th International Symposium on Regional Cancer Therapies, Clearwater, FL Feb 2015.
Accepted for poster presentation at: 68th SSO Annual Cancer Symposium, Houston, TX March 2015.
DISCLOSURE Nothing to disclose.
References
- 1.Youssef H, Newman C, Chandrakumaran K, et al. Operative findings, early complications, and long-term survival in 456 patients with pseudomyxoma peritonei syndrome of appendiceal origin. Dis Colon Rectum. 2011;54:293–9. doi: 10.1007/DCR.0b013e318202f026. [DOI] [PubMed] [Google Scholar]
- 2.Chua TC, Moran BJ, Sugarbaker PH, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol. 2012;30:2449–56. doi: 10.1200/JCO.2011.39.7166. [DOI] [PubMed] [Google Scholar]
- 3.Levine EA, Stewart JH, Shen P, et al. Intraperitoneal chemotherapy for peritoneal surface malignancy: experience with 1,000 patients. J Am Coll Surg. 2014;218:573–85. doi: 10.1016/j.jamcollsurg.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Votanopoulos KI, Russell G, Randle RW, et al. Peritoneal surface disease (PSD) from appendiceal cancer treated with cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC): overview of 481 cases. Ann Surg Oncol. 2015;22:1274–9. doi: 10.1245/s10434-014-4147-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradley RF, Stewart JH, 4th, Russell GB, et al. Pseudomyxoma peritonei of appendiceal origin: a clinicopathologic analysis of 101 patients uniformly treated at a single institution, with literature review. Am J Surg Pathol. 2006;30:551–9. doi: 10.1097/01.pas.0000202039.74837.7d. [DOI] [PubMed] [Google Scholar]
- 6.Winer J, Zenati M, Ramalingam L, et al. Impact of aggressive histology and location of primary tumor on the efficacy of surgical therapy for peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol. 2014;21:1456–62. doi: 10.1245/s10434-013-3328-4. [DOI] [PubMed] [Google Scholar]
- 7.Votanopoulos KI, Newman NA, Russell G, et al. Outcomes of cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) in patients older than 70 years; survival benefit at considerable morbidity and mortality. Ann Surg Oncol. 2013;20:3497–503. doi: 10.1245/s10434-013-3053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halabi HE, Gushchin V, Francis J, et al. Prognostic significance of lymph node metastases in patients with high-grade appendiceal cancer. Ann Surg Oncol. 2012;19:122–5. doi: 10.1245/s10434-011-1903-0. [DOI] [PubMed] [Google Scholar]
- 9.Blackham AU, Swett K, Eng C, et al. Perioperative systemic chemotherapy for appendiceal mucinous carcinoma peritonei treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Surg Oncol. 2014;109(7):740–5. doi: 10.1002/jso.23547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turner KM, Hanna NN, Zhu Y, et al. Assessment of neoadjuvant chemotherapy on operative parameters and outcome in patients with peritoneal dissemination from high-grade appendiceal cancer. Ann Surg Oncol. 2013;20:1068–73. doi: 10.1245/s10434-012-2789-1. [DOI] [PubMed] [Google Scholar]
- 11.Elias D, Gilly F, Quenet F, et al. Pseudomyxoma peritonei: a French multicentric study of 301 patients treated with cytoreductive surgery and intraperitoneal chemotherapy. Eur J Surg Oncol. 2010;36:456–62. doi: 10.1016/j.ejso.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Glehen O, Gilly FN, Boutitie F, et al. Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy: a multi-institutional study of 1,290 patients. Cancer. 2010;116:5608–18. doi: 10.1002/cncr.25356. [DOI] [PubMed] [Google Scholar]