Abstract
NMDA receptor (NMDAR) hypofunction in Parvalbumin-expressing (PV+) inhibitory neurons (IN) may contribute to symptoms in patients with schizophrenia (SZ). This hypothesis was inspired by studies in humans involving NMDAR antagonists that trigger SZ symptoms. Animal models of SZ using neuropharmacology and genetic knockouts have successfully replicated some of the key observations in human subjects involving alteration of gamma band oscillations (GBO) observed in EEG and MEG signals. However, it remains to be seen if NMDAR hypofunction in PV+ neurons is fundamental to the phenotype observed in these models. In this review, we discuss some of the key computational models of GBO and their predictions in the context of NMDAR hypofunction in INs. While PV+ INs have been the main focus of SZ studies in animal models, we also discuss the implications of NMDAR hypofunction in other types of INs using computational models for GBO modulation in the visual cortex.
Keywords: schizophrenia, gamma oscillations, NMDA hypofunction, parvalbumin, inhibition, computational models
Introduction
Schizophrenia (SZ) is a mental disorder that afflicts about 1% of the population and is manifested as mild or debilitating episodes of hallucinations and delusions, and cognitive deficits. Sensory-triggered hallucinations, disordered thoughts and delusions are termed positive symptoms and respond better to medication. Social withdrawal, lack of motivation and flat expressions form the negative symptoms and respond poorly to medication. Alterations in fundamental brain processes of perception as well as executive function are thought to underlie these outcomes in SZ patients (1, 2). Unlike conditions such as Alzheimer's (3), the disease does not involve major neuronal degeneration, although subtle deficits in certain neuronal populations have been described [(4-6), but see (7)]. Prior to clinical assessment, Schizophrenia thus remains a difficult to detect and poorly understood brain disorder.
Diagnostically, several functional and behavioral measures using electro-encephalograms (EEG), magneto-encephalograms (MEG) and functional magnetic resonance imaging (fMRI) are being developed to identify the SZ population from control. For example, sustained oscillations in these signals, that reflect coordinated activity of neural populations, are identified as an increase in power in a narrow band of frequency and are compared between control and SZ patients in terms of both their strength/amplitude/power and frequency. The narrowband power is compared in various behavioral states: stimulus- or task-driven state when the subject is actively processing a stimulus and/or performing a cognitive task, and baseline or resting at other times (Supplement 1). Since resting state in rodents is ill defined, we refer to both as baseline in the review of animal models. The stimulus/task-driven power is compared both in terms of its evoked component as well as induced (2) (Figure S1 and text in Supplement 1). This review focuses mainly on observations of induced narrowband power in the Gamma range (30-80 Hz), commonly referred to as Gamma Band Oscillations (GBO); GBO are of interest because they have been implicated in synchronization of neural ensembles during working memory, feature binding, dynamic routing of information and attention (8, 9). The review first summarizes the observations on GBO abnormalities in patients and the related data in a class of animal models of SZ. It then discusses several computational models of induced GBO for mechanistic insights. Finally, the review discusses the implications of recent findings about the microstructure of the local cortical inhibitory circuits for the computational models and ultimately the animal models of the disease.
Abnormal GBO in schizophrenia patients
Abnormalities in the strength of GBO power in EEG and MEG have been consistently observed in studies with SZ patients (2, 10). GBO power has been reported to be both higher and lower compared with control subjects depending on the task and brain state.
Task dependence
GBO are reduced in SZ patients during sensory processing and working memory [(2, 11-14); but see (15, 16)]. In addition, the severity of the positive or negative symptoms co-varies with alteration of GBO power: a pattern of enhanced GBO emerges in patients with more severe positive symptoms (11, 15, 17, 18), but a clear pattern does not emerge in cases where GBO is reduced in SZ patients (15, 16). The differences that are reported are significant for groups but have low predictive power on individuals.
Brain-state dependence
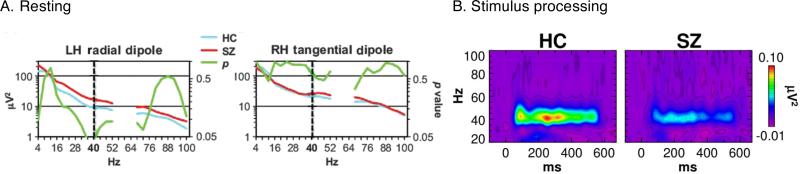
GBO modification in SZ patients depends on the brain state during which activity is monitored. GBO are weakened during sensory processing across multiple modalities (12, 15, 18, 19). For the same group of patients, GBO activity was higher than control subjects before presentation of sensory stimulation (20) (Figure 1A), or baseline, but lower during sensory processing, or stimulus-driven state (17) (Figure 1B). It should be noted that the increase of baseline GBO was significant only at 40 Hz, the frequency of the steady-state stimulation. In addition, the increase was not significant across all electrodes (Figure 1A). However, others studies have reported a decrease in baseline GBO (21). Reconciling GBO changes across studies will require separating the effect in stimulus-locked, or evoked, EEG signal vs. induced part of the signal (22). Each component reflects different aspects of information processing in the cortex: Evoked GBO reflects bottom-up sensory transmission, whereas induced GBO represent the emergent dynamics within cortical networks.
Figure 1. Brain-state dependent modulation of GBO in SZ.
EEG signals recorded during resting state (A) and stimulus-processing (B) states in SZ patients and healthy controls (HC). Stimulus-processing state data were recorded during periodic auditory stimulation at 20 Hz. (A) Time-averaged power in different frequency bands in the EEG signal (blue and red). Also shown is the p-value (green). (B) Power in different frequency bands in the EEG signal as a function of time. (Adapted from (17, 20))
Animal models of SZ
The development of animal models has been confined to measurable symptoms of the disease such as altered sensory processing, locomotion and social behavior. These models have explored several hypotheses about deficits in specific neurotransmission systems that could form the basis of the modified neural processing in SZ patients. Dopamine system has been implicated because antipsychotic drugs target mainly dopamine D2 receptors (23, 24). However, results suggested that glutamate might also be involved (25); low doses of ketamine or phencyclidine, antagonists for glutamate receptor N-methyl-D-aspartate (NMDA), produce a syndrome similar to a psychotic episode in healthy humans (26-34). The main mechanism behind the psychotic effects of these antagonists is thought to be the disinhibition of excitatory circuits in brain (35-37). Postmortem studies in the prefrontal cortex in SZ patients, on the other hand, have shown deficits in the neurotransmitter gamma-amino butyric acid (GABA) that is released by a majority of inhibitory interneurons (IN) in the brain (1, 38-42).
These findings have inspired the hypothesis that disruption of NMDAR neurotransmission in INs leads to abnormalities in the GABA neurotransmission machinery in SZ. Indeed, postmortem evidence in the anterior cingulate cortex suggests that the density of GABAergic INs that express the NMDAR NR2A subunit is decreased in SZ patients in general (43); in particular, the glutamatergic innervation of subsets of GABAergic cells might be differentially altered (44). This has been recently explored in both pharmacological and genetic animal studies (Supplement 1). In addition to behavioral performance, changes in GBO have been observed during baseline and tasks in both surface EEG recordings as well as local field potentials (LFP), a surface-localized “depth” EEG using penetration micro-electrodes.
Abnormal GBOs in animal models of NMDAR hypofunction
Abnormal GBO are observed in pharmacological as well as genetic NMDAR hypofunction models of SZ (45, 46), which are summarized in the following text.
Pharmacological models
In vitro, acute treatment with ketamine, a nonspecific NMDAR antagonist, decreases the strength of kainate-induced GBO in medial entorhinal cortex (47). However, the same treatment increases the kainate-induced GBO in the primary auditory cortex (48). In vivo, chronic treatment with NMDAR antagonists reduces the strength of hippocampal GBO activity (49). However, acute systemic treatment with NMDAR antagonists causes a significant increase in GBO power in the hippocampus and frontal cortex both before and during stimulus processing (49-52). Increase in baseline GBO power in vivo in response to acute treatment with several NMDAR antagonists has been seen in multiple studies in rodents (53-60). Pharmacological models thus demonstrate a mixed dependence of GBO on brain state as well as frequency and duration of drug treatment; the latter requires careful further investigation since differences in this factor can translate to immediate (GABAergic disinhibition) vs. long-term (compensatory homeostatic mechanisms) modification of the cortical network with unique downstream effects on GBO.
Genetic models
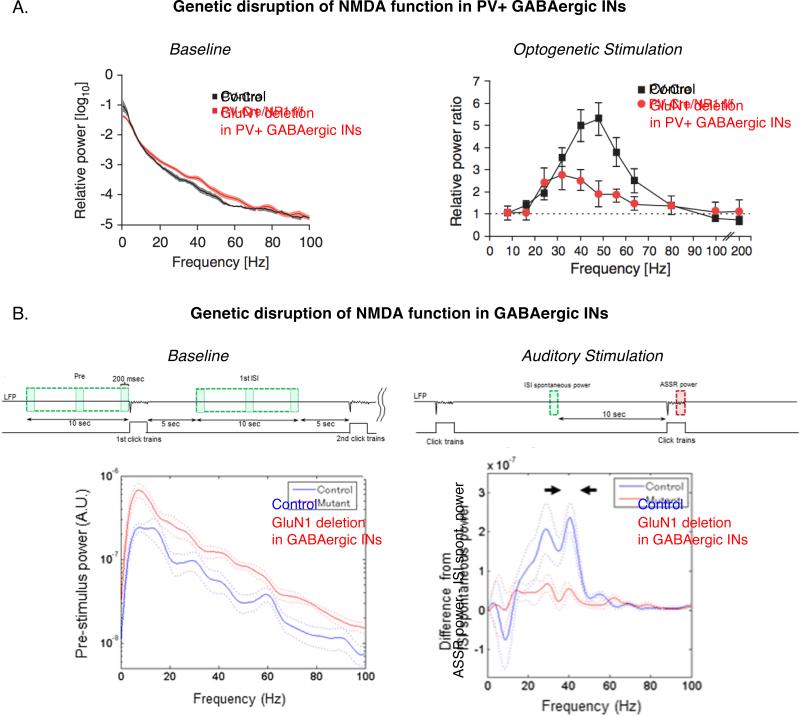
In addition to several SZ-like symptoms, genetic models show abnormalities in GBO power in a brain-state dependent manner (Figure 2). In models involving genetic ablation of NMDARs in PV+ INs, increased GBO power is observed in the baseline LFPs recorded from awake behaving mice, in both the neocortex (61) and the hippocampus (62). The increase in power is broadband, but is shown to be statistically significant in the gamma range of frequencies. In the same model, “stimulus-processing state” GBO induced by optogenetic stimulation have reduced power as compared to control (61). In other models involving hypofunction of NMDARs in GABAergic INs that is not restricted to the PV+, and occurs earlier in development, the baseline LFPs also show a broadband increase in power (63). At the same time, the stimulus-processing state GBO show a decrease in response to auditory stimulation. In general, the genetic models show a consistent dependence of GBO on brain states. One proposed hypothesis for the reduced GBO during sensory processing in both humans and rodent models is that the increased GBO power during baseline period causes a ceiling effect, preventing further GBO recruitment during cognitive tasks (20, 64). However, in the genetic model of NMDAR hypofunction in GABAergic INs not restricted to PV+ (63), the baseline GBO power in the period between repeated presentations of auditory stimuli does not shown any change from control animals; the ceiling effect does not thus explain the entire dataset and requires further investigation in terms of differences in the brain state between the two protocols; a separate definition of baseline vs. resting state in the animal models would be a step in the direction of reconciliation of the data. Caution also needs to be exercised while comparing human and animal studies; GBOs in most animal models are analyzed in LFPs while human studies involve EEG or MEG (Supplement 1).
Figure 2. GBO alteration in genetic NMDAR hypofunction models of SZ.
(A) Power in different frequency bands in the EEG signal recorded from anaesthetized control mice (black) and those with deletion of GluN1 subunit of NMDARs in PV+ GABAergic neurons (red). Left: Baseline, Right: Stimulus-processing state (Adapted from (61))
(B) Power difference in different frequency bands in the EEG signal recorded from control mice (blue) and those with deletion of GluN1 subunit of NMDARs targeting all GABAergic neurons (red). Left: Baseline, Right: Stimulus-processing state (Adapted from (63))
Several hypotheses have been proposed regarding the mechanisms underlying the GBO alterations in both the SZ patients and animal models (2, 10). GABAergic INs are crucial to the generation of oscillations in the cortical population activity and PV+ INs are considered a major contributor to the generation of GBO (65-68). However, unraveling the mechanism of GBO modification in SZ, even for the animal models, is challenging due to at least two key reasons: imprecise understanding of the action of NMDAR antagonists in the pharmacological model and the effects of homeostatic mechanisms during development in the genetic models (Supplement 1). Given these complex issues, computational modeling of GBO is an invaluable tool to explore the various hypotheses regarding the changes in networks that not only modify the GBO, but also ultimately lead to SZ symptoms. GBO computational models have been broadly categorized as those involving IN-IN interactions also termed as ING (Interneuron Network Gamma), or those involving PN-IN interactions, termed as PING (Pyramidal-Interneuron Network Gamma) (Figure S2). They can be further categorized based on the underlying mechanisms and their unique predictions (Table 1). While these models have been previously reviewed in great detail (66), we discuss some of the most relevant ones in the context of the data considered in this review.
Table 1.
Computational models of GBO
| Model | NO-ING* | NO-PING** | D-ING | D-PING | ISN-PING | SR-ING | SR-PING |
|---|---|---|---|---|---|---|---|
| GBO Mechanism | Spike synchronization of neuronal oscillators (NO) in an I-I network. | Spike synchronization of neuronal oscillators (NO) in an E-I network. | Oscillation in firing rates induced by conduction delays (D) in an I-I network. | Oscillation in firing rates induced by conduction delays (D) in an E-I network. | Oscillation in firing rates induced by inhibition-stabilized network (ISN) connectivity regime in an E-I network. | Stochastic resonance (SR) - Noise induced sustenance of damped oscillations - in an I-I network. | Stochastic resonance (SR) in an E-I network. |
| [e.g. (84-87)] | [e.g. (70, 72, 88)] | [e.g. (93, 94)] | [e.g. (93, 94)] | [e.g. (81-83)] | [e.g. (112)] | [e.g. (113-116)] |
| Firing pattern | Regular | Regular | Irregular | Irregular | Irregular | Irregular | Irregular |
|---|---|---|---|---|---|---|---|
| GBO Frequency determinant | Decay time constants of IPSCs. | Decay time constants of IPSCs. | Delays in synaptic transmission. | Delays in synaptic transmission, Decay time constants for IPSCs and EPSCs. | Connection strength of E-E, E-I, I-E and I-I, Decay time constants for IPSCs and EPSCs. | Decay time constants for IPSCs. | Decay time constants for IPSCs (113), Connection strength of E-E, E- I, I-E and I-I, Decay time constants for IPSCs and EPSCs (114-116) |
| GBO Power determinant | Excitation to INs | Excitation to INs. | Excitation to INs and ENs. | Excitation to INs. | Noise level in network activity and/or input. | Noise level in network activity and/or input. | |
ING: Interneuron Network Gamma
PING: Pyramidal-Interneuron Network Gamma
Computational models of GBO modulation
Modeling the increase in baseline GBO
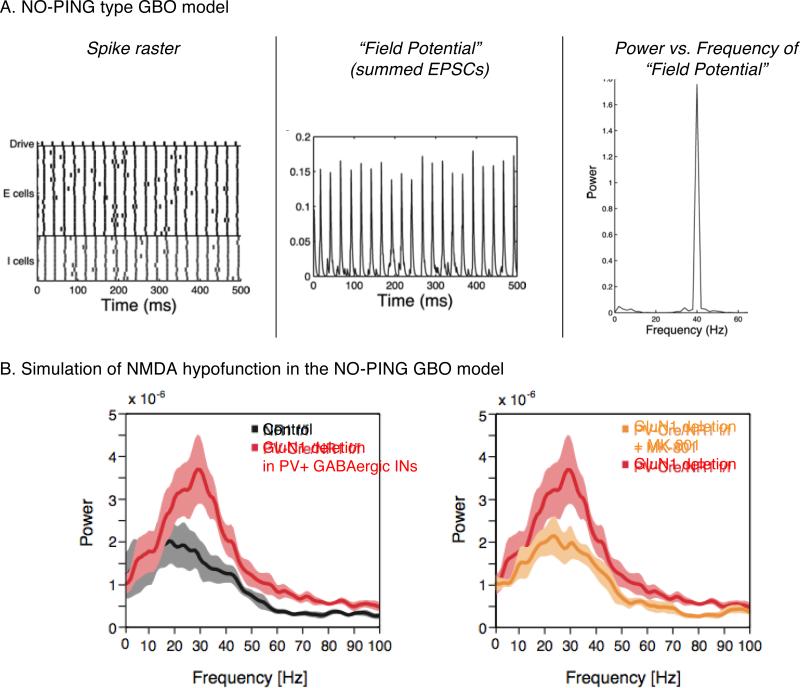
Baseline activity in animal models of NMDAR hypofunction shows a moderate to robust increase in GBO power. Since this is also observed in the genetic knockout model of NMDARs in the PV+ INs, altered PV+ inhibition in the cortical network could have a key role in this effect. This hypothesis was explored in a computational model (61) that is based on the GBO mechanism of synchronization of neural oscillators, which we term here as NO-ING or NO-PING (Neural Oscillator based ING or PING) (Table 1)(69-73). The modified cortical circuit in the NMDAR receptor knockout mice was captured as decreased excitability of fast spiking INs in the computational model (61) (Figure 3A). In the presence of noisy background activity, this modification to the model resulted in reduced spontaneous activity in the PV+ INs during minimal (baseline period) excitation, resulting in increased synchronous activity of excitatory population. Since reduced excitability of the model INs required more synchronized excitation to activate them, it resulted in more synchronous inhibition at gamma frequency and hence an enhancement of GBO (Figure 3B). Other models exhibited stronger GBO with weakened E-to-I connections as a result of NMDAR hypofunction in an E-I network (74, 75).
Figure 3. Computational models of changes in baseline GBO.
(A) Spike rasters and “LFPs” in the Neural oscillator-Pyramidal-Inhibitory neuron network Gamma (NO-PING) model.
(B) Simualtion of NMDAR hypofunction in PV+ INs by reducing excitatbility of INs in the NO-PING model shown in (A).
However, these computational models conflict with the observation that, in at least one animal model of NMDAR hypofunction, disinhibition of cortical excitatory neurons is accompanied by reduced neuronal synchrony (76). It should however be noted that in this animal model the hypofunction was not limited to PV+ neurons in the cortex. In addition, as was pointed out earlier, the alterations in NMDAR function in the different animal models occur at different points during development; it is thus not clear how comparable the different animal models are in terms of the GBO observations. A recent in vivo study has revealed that cortical disinhibition achieved by MK-801, an NMDAR antagonist, causes an increase in GBO power but a reduced synchronization in the firing of action potentials in the mPFC of free-moving rats (60). While INs have been shown to be more sensitive to NMDAR antagonists (77), a broad range of cortical INs are expected to be affected in these experiments (60), similar to the genetic knockout model of Belforte et al. (76). This suggests the possibility that the increase in baseline GBO power in the animal models of cortical NMDAR hypofunction could also reflect a robust increase in synaptic inputs due to disinhibition, and not a true increase in synchronization of neural activity, as seen in the computational models (61, 70, 71, 73). Finally, a key issue with the models of GBO that are based on synchronization of neuronal oscillators is that they show a narrow distribution of inter-spike interval (ISI), whereas, experimental data from cortex and the hippocampus suggests a broad distribution of spike ISIs during GBO (78-80).
We recently explored the modulation of GBO power and frequency in a special case of Wilson-Cowan (81) oscillation model based on an Inhibition-Stabilized PING model (ISN-PING) with superlinear inhibition (82, 83). The strong inhibition in this model stabilizes the positive feedback in the population of pyramidal cells. This model replicated a range of observations on GBO modulation in the primate visual cortex (82). The model predicted rate-level oscillations, rather than spike to spike, with a broad distribution of ISI. In this regime, the power of GBO in the model is proportional to the ratio of stimulation to the local excitatory and inhibitory neurons, other factors remaining relatively unchanged; if this ratio increases, power in GBO increase (82, 83). The model predicts an increase in the GBO strength if the key effect of NMDAR hypofunction is captured as reduction in the excitability of INs (83). However, the model predicts a decrease in the GBO strength if the key effect of NMDAR hypofunction is captured as a selective decrease in the strength of excitatory connections to the INs (83).
Finally, it is not known how the cortical circuit re-organizes through compensatory mechanisms in the genetic knockout models. This makes it difficult to accurately model the effects of genetic knockout of NMDARs in PV+ neurons in this as well as other computational models, especially since the alteration occurs at different developmental time points in different animal models. A clean way to test the computational models would be acute suppression of NMDARs selectively in PV+ neurons (thus allowing the circuits to develop normally), which could be a possibility in future.
Modeling the decrease in GBO during stimulus processing
As discussed above, several studies in SZ patients and rodent model of NMDAR hypofunction demonstrate a reduction in GBO strength during sensory stimulus processing (Figures 1 & 2). An underlying cause is thought to be the reduced excitability of PV+ INs, which are crucial to GBO generation. Most computational models show sensitivity of GBO strength to the excitability of INs (Table 1), and predict weaker gamma in response to reduced excitability, such as through NMDAR hypofunction in the INs. Here we discuss the specific predictions of different computational models and how they might be tested in future.
Computational models based on synchronization of integrate-and-fire type neurons, which we group and term here as NO-ING or NO-PING (70, 72, 84-88) (Table 1) predict unsustainable GBO when the drive to inhibitory neurons through NMDAR hypofunction is sufficiently reduced. Other models of PING-type GBO exhibit similarly synchronous firing with Hodgkin-Huxley type spiking mechanism in INs (74, 88, 89). One such model predicts stronger GBO with reduced feedback excitation to INs from the local E neurons (74), while another predicts weaker GBO with reduced feedforward excitation to INs (88). Since reduced excitability of INs affects both feedforward and feedback coupling to PV+, it remains to be seen how these effects interact in a computational model of NMDAR hypofunction. One prediction is that GBO could either decrease or increase when the local network is strongly driven by the stimulus, depending on the balance of feedforward vs. feedback excitation to INs. Computational modeling, anatomical evidence, in vitro experiments as well as those using knockout mice shows that gap junctions between GABAergic INs can facilitate GBO, specifically the ING type (90-92). While gap junctions are an important mechanism for synchronizing neuronal activity, the evidence for their disruption in GABAergic neurons in SZ is not clear. We have thus limited this review to examining the connection between oscillatory disruption observed in SZ patients and disruption in chemical synaptic function.
Oscillations in networks can also arise from a mechanism that does not rely on synchronization of individual neural oscillators. In such models, the oscillations are not spike-to-spike, but are observable in the firing rates (93, 94). The mechanism relies on communication delays such as axonal conduction delays and synaptic transmission; the frequency of oscillations is sensitive to these delays. GBO strength in these models, which we term here as D-ING or D-PING (Delay based ING or PING), depends on the drive to the INs; they predict weakened oscillations with reduced drive. This would predict weakened GBO with NMDAR hypofunction in the INs. However, in computational models involving both AMPA and NMDA-mediated excitatory conductances, GBO has been shown to be sensitive to mostly AMPA (and not NMDA)-mediated excitation (93). The high sensitivity of GBO to AMPA receptor hypofunction is explained by the need of fast excitation to IN to sustain oscillations in the computational models of GBO; AMPA sensitivity of GBO has also been experimentally demonstrated in animal models with genetic modification of AMPA receptors in PV+ GABAergic neurons (95).
The ISN-PING model captures a range of observations on GBO modulation in the primate visual cortex (82, 83). If a modification of relative stimulation to local E-I population results in an increase in the net firing rates in the network, it predicts a decrease in GBO power (Figure 4A). When NMDAR hypofunction is modeled as selective decrease in excitation to PV+ neurons that relieves the net inhibition in the local network, the model predicts a decrease in GBO power, as is also observed the genetic models (61) (Figure 4B). However, a recent study with selective NMDAR ablation in pyramidal neurons, in the cortex and hippocampus, also infers a reduction in GBO power (96). While the study reports an increase in the excitability of pyramidal neurons in the animal model, it is not clear if this translates to an increase in spiking response to stimulation. If it does, the computational model provides a mechanism for the observed decrease in GBO power.
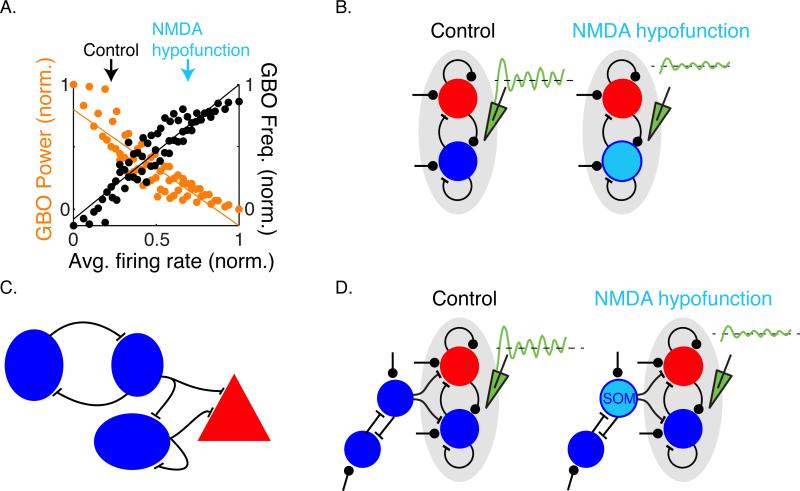
Figure 4. Stimulus induced GBO due to NMDAR hypofunction in an ISN-PING model.
(A) Co-variation of average spiking activity and GBO power and frequency in an ISN-PING model (Adapted from (82)). The arrows indicate how GBO power changes in response to changes in the activity of PING network. The variation in activity level and GBO properties is plotted in response to changes in the excitatory drive to the PING network, such as in the case of NMDAR hypofunction.
(B) NMDAR hypofunction in PV+ INs modeled as reduced excitation predicts disinhibition and weaker GBO in the model.
(C) Local inhibition circuit in the cortex (97-99). PV+ INs inhibit each other as well as excitatory neurons. SOM+ INs inhibit both PV+ INs and excitatory neurons. VIP+ INs disinhibition excitatory neurons by inhibiting SOM+ INs.
(D) The ISN-PING model predicts reduced power of stimulus-induced GBO if NMDAR hypofunction in SOM+ INs relieves inhibition of both excitatory neurons and PV+ INs such that the average activity is increased in the network.
Finally, computational models have several limitations in their ability to shed light on underlying mechanisms. They do not address the effects of the cellular/molecular consequences of chronic NMDAR antagonist treatment or ablation of NMDARs in early life, nor the extent to which NMDARs or other receptors are blocked in non-PV+ IN types. Acute NMDAR blockade in adulthood is probably the best understood case in terms of likely cellular changes, with the most potential for successful exploration (computational and otherwise) of modulation of GBO and other network behavior. In addition to more precise characterization of the effect of NMDA hypofunction, predicting the circuit-level disinhibitory effect will involve taking into consideration the additional complexities of connections between the different IN types found in the cortex and hippocampus; some of these have been recently revealed to provide disinhibition to the excitatory neurons. Future efforts in computational modeling will necessitate the evaluation of these disinhibitory mechanisms.
Mechanisms of cortical disinhibition
The reciprocal interaction between the pyramidal neurons and the PV+ INs is the core of the feedback circuit that generates GBOs using the PING mechanism. Inputs to this circuit include thalamo-cortical projections, lateral interactions from neighboring columns and feedback from other areas. Additional cortical circuits involving non-PV INs modulate the gain of the network and the overall level of activity level. For example, a series of studies using optogenetic techniques have uncovered a disinhibitory circuit involving somatostatin positive (SOM+) and vasoactive intestinal polypeptide immunopositive (VIP+) INs (97-99) (Figure 4C). In multiple cortical areas in the mouse, SOM+ INs target both pyramidal and PV+ INs, but silencing SOM+ INs increases the overall excitatory activity in the cortex (100). Integrating these details in different computational models will have unique predictions (Figure 4D; Supplement 1). The predictions related to disinhibition in the cortex due to NMDAR hypofunction in non-PV+ IN types have not been explicitly tested in animal models, but in the final section we briefly discuss the literature that supports a role for alterations in non-PV+ IN types in causing GBO alterations seen in SZ.
NMDAR hypofunction in non-PV neurons: Postmortem studies
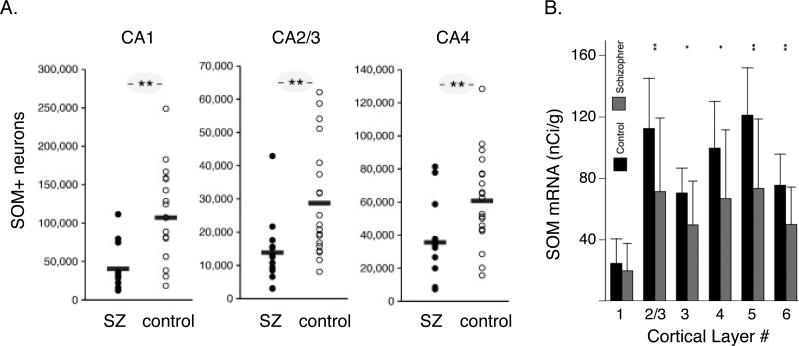
Several lines of experimental evidence suggest that hypofunction of NMDARs is more robust in INs than pyramidal neurons [(22, 77, 101), but see (102)]. Alteration in neurochemical markers of GABA transmission is most prominent in PV+ INs in response to NMDAR hypofunction in the adult cortex (103). However, postmortem studies show that not all classes of cortical INs are affected equally in SZ patients (39, 41, 42, 104). Significant expression deficits in mRNAs associated with GABAergic neurons, including mRNAs of the neuropeptides SOM, Y, and cholecystokinins have been detected in postmortem prefrontal cortical tissues of SZ subjects (39). In hippocampal tissue of SZ subjects, a reduction in the number of PV+ and SOM+ interneurons, and the level of PV+, SOM+ and glutamic acid decarboxylase mRNA expression has been reported (105) (Figure 5A). On the other hand, the levels of calretinin (an interneuron cell-type marker, like PV and SOM, of cortical GABAergic interneurons) are largely unaffected (6, 39, 106). The deficits also show specificity along the cortical laminae; in postmortem studies in prefrontal cortical tissue, SOM mRNA expression is significantly reduced in layers 2 to superficial layer 6 in SZ patients (41) (Figure 5B). Amalgamation of several postmortem studies suggest that SZ patients show alteration of SOM+ inhibitory neurotransmission to the apical dendrites of pyramidal neurons whose cell bodies are present in the deep layers of the DLPFC (107) (38).
Figure 5. Alteration of non-PV+ inhibition in schizophrenia.
(A) Immunohistochemical estimate of SOM+ neurons in the hippocampus in postmortem SZ subjects (closed circle) and control (open circles) (105).
(B) Laminar expression of the mRNAs for SOM in dorsolateral prefrontal cortex (DLPFC) in postmortem SZ subjects. Mean film optical density (OD) for mRNA expression in each cortical layer between comparison and schizophrenia groups (Adapted from (41)).
NMDAR hypofunction in non-PV neurons: Neuropharmacology & Computational models
In a recent EEG study in rats, several NMDAR antagonists show non-monotonic dose–response effects on cortical GBO power (108). A recent computational study demonstrates that NMDAR manipulation in a single IN population can give rise to a non-monotonic U-shaped dependence of stimulus-induced GBO on NMDAR efficacy (109). On the other hand, at least two local IN populations with either “low” or “high” sensitivity to NMDAR antagonists are crucial to the conceptual model proposed for GBO modulation in response to NMDAR antagonists (108). While PV+ INs are implicated in the GBO generating population (67, 68), the involvement of subpopulations of PV+ INs with differential sensitivity to NMDAR antagonists, or even non-PV+ classes of INs is a possibility (110). In addition to deficits in the GABergic system itself, there are other modulators of inhibition that could play a role in the deficits observed in the disease and need further consideration (Supplement 1).
Conclusions and Future directions
We have examined the empirical data and computational models that support the hypothesis that NMDAR hypofunction in inhibitory neurons, specifically the PV+ ones, may contribute to the observed GBO abnormalities and ultimately the symptoms in patients with SZ. However, possible contribution of other inhibitory systems cannot be ruled out. NMDAR hypofunction in non-PV+ cortical INs, such as SOM+ INs, that exert inhibitory control on cortical activity may also be involved; this finds support in postmortem studies. In addition, recent characterization of SOM+ INs in the rodent somatosensory cortex suggests that they provide differential inhibition to excitatory neurons as well as PV+ INs in a cortical layer specific manner (111). How the disregulation of this sub-system influences the dynamics of the mature cortical circuit both in terms of GBO and otherwise is an important question. In summary, studies focused on the developmental aspects of the complex inhibitory/disinhibtory systems will help elucidate how and when its dysfunction leads to the permanent alterations in cortical circuitry responsible for the observed changes in oscillatory activity in schizophrenia.
Supplementary Material
Acknowledgments
Funding Sources
M.P.J. was supported by NIMH grant T32MH020002, NEI grant K99 EY025026 and the Swartz Foundation. M.M.B was supported by NIMH grants MH091407 and MH094670. T.J.S. was supported by HHMI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 2.Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- 3.Wenk GL. Neuropathologic changes in Alzheimer's disease. The Journal of clinical psychiatry. 2003;64(Suppl 9):7–10. [PubMed] [Google Scholar]
- 4.Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jr., et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- 5.Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 2000;57:237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- 6.Woo TU, Whitehead RE, Melchitzky DS, Lewis DA. A subclass of prefrontal gamma-aminobutyric acid axon terminals are selectively altered in schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:5341–5346. doi: 10.1073/pnas.95.9.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beasley CL, Reynolds GP. Parvalbumin-immunoreactive neurons are reduced in the prefrontal cortex of schizophrenics. Schizophrenia research. 1997;24:349–355. doi: 10.1016/s0920-9964(96)00122-3. [DOI] [PubMed] [Google Scholar]
- 8.Singer W, Gray CM. Visual feature integration and the temporal correlation hypothesis. Annual review of neuroscience. 1995;18:555–586. doi: 10.1146/annurev.ne.18.030195.003011. [DOI] [PubMed] [Google Scholar]
- 9.Salinas E, Sejnowski TJ. Correlated neuronal activity and the flow of neural information. Nature Reviews Neuroscience. 2001;2:539–550. doi: 10.1038/35086012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez-Burgos G, Lewis DA. GABA neurons and the mechanisms of network oscillations: implications for understanding cortical dysfunction in schizophrenia. Schizophrenia bulletin. 2008;34:944–961. doi: 10.1093/schbul/sbn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uhlhaas PJ, Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52:155–168. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 12.Cho RY, Konecky RO, Carter CS. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haenschel C, Bittner RA, Waltz J, Haertling F, Wibral M, Singer W, et al. Cortical oscillatory activity is critical for working memory as revealed by deficits in early-onset schizophrenia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:9481–9489. doi: 10.1523/JNEUROSCI.1428-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minzenberg MJ, Firl AJ, Yoon JH, Gomes GC, Reinking C, Carter CS. Gamma oscillatory power is impaired during cognitive control independent of medication status in first-episode schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:2590–2599. doi: 10.1038/npp.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spencer KM, Nestor PG, Perlmutter R, Niznikiewicz MA, Klump MC, Frumin M, et al. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:17288–17293. doi: 10.1073/pnas.0406074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee SH, Wynn JK, Green MF, Kim H, Lee KJ, Nam M, et al. Quantitative EEG and low resolution electromagnetic tomography (LORETA) imaging of patients with persistent auditory hallucinations. Schizophrenia research. 2006;83:111–119. doi: 10.1016/j.schres.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 17.Spencer KM, Niznikiewicz MA, Nestor PG, Shenton ME, McCarley RW. Left auditory cortex gamma synchronization and auditory hallucination symptoms in schizophrenia. BMC neuroscience. 2009;10:85. doi: 10.1186/1471-2202-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spencer KM, Salisbury DF, Shenton ME, McCarley RW. Gamma-band auditory steady-state responses are impaired in first episode psychosis. Biol Psychiatry. 2008;64:369–375. doi: 10.1016/j.biopsych.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winterer G, Ziller M, Dorn H, Frick K, Mulert C, Wuebben Y, et al. Schizophrenia: reduced signal-to-noise ratio and impaired phase-locking during information processing. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2000;111:837–849. doi: 10.1016/s1388-2457(99)00322-3. [DOI] [PubMed] [Google Scholar]
- 20.Spencer KM. Baseline gamma power during auditory steady-state stimulation in schizophrenia. Frontiers in human neuroscience. 2012;5:190. doi: 10.3389/fnhum.2011.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rutter L, Carver FW, Holroyd T, Nadar SR, Mitchell-Francis J, Apud J, et al. Magnetoencephalographic gamma power reduction in patients with schizophrenia during resting condition. Human brain mapping. 2009;30:3254–3264. doi: 10.1002/hbm.20746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uhlhaas PJ, Singer W. Neuronal dynamics and neuropsychiatric disorders: toward a translational paradigm for dysfunctional large-scale networks. Neuron. 2012;75:963–980. doi: 10.1016/j.neuron.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Carlsson A, Lindqvist M. Effect of Chlorpromazine or Haloperidol on Formation of 3methoxytyramine and Normetanephrine in Mouse Brain. Acta pharmacologica et toxicologica. 1963;20:140–144. doi: 10.1111/j.1600-0773.1963.tb01730.x. [DOI] [PubMed] [Google Scholar]
- 24.Seeman P, Lee T, Chau-Wong M, Wong K. Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature. 1976;261:717–719. doi: 10.1038/261717a0. [DOI] [PubMed] [Google Scholar]
- 25.Kellendonk C, Simpson EH, Polan HJ, Malleret G, Vronskaya S, Winiger V, et al. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron. 2006;49:603–615. doi: 10.1016/j.neuron.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 26.Coyle JT. The glutamatergic dysfunction hypothesis for schizophrenia. Harvard review of psychiatry. 1996;3:241–253. doi: 10.3109/10673229609017192. [DOI] [PubMed] [Google Scholar]
- 27.Deutsch SI, Mastropaolo J, Schwartz BL, Rosse RB, Morihisa JM. A “glutamatergic hypothesis” of schizophrenia. Rationale for pharmacotherapy with glycine. Clinical neuropharmacology. 1989;12:1–13. [PubMed] [Google Scholar]
- 28.Javitt DC. Negative schizophrenic symptomatology and the PCP (phencyclidine) model of schizophrenia. The Hillside journal of clinical psychiatry. 1987;9:12–35. [PubMed] [Google Scholar]
- 29.Lahti AC, Holcomb HH, Medoff DR, Tamminga CA. Ketamine activates psychosis and alters limbic blood flow in schizophrenia. Neuroreport. 1995;6:869–872. doi: 10.1097/00001756-199504190-00011. [DOI] [PubMed] [Google Scholar]
- 30.Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry. 1995;52:998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- 31.Tamminga CA. Schizophrenia and glutamatergic transmission. Critical reviews in neurobiology. 1998;12:21–36. doi: 10.1615/critrevneurobiol.v12.i1-2.20. [DOI] [PubMed] [Google Scholar]
- 32.Lodge D, Anis NA. Effects of phencyclidine on excitatory amino acid activation of spinal interneurones in the cat. Eur J Pharmacol. 1982;77:203–204. doi: 10.1016/0014-2999(82)90022-x. [DOI] [PubMed] [Google Scholar]
- 33.Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 34.Hong LE, Summerfelt A, Buchanan RW, O'Donnell P, Thaker GK, Weiler MA, et al. Gamma and delta neural oscillations and association with clinical symptoms under subanesthetic ketamine. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:632–640. doi: 10.1038/npp.2009.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olney JW, Farber NB. NMDA antagonists as neurotherapeutic drugs, psychotogens, neurotoxins, and research tools for studying schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 1995;13:335–345. doi: 10.1016/0893-133X(95)00079-S. [DOI] [PubMed] [Google Scholar]
- 36.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poels EM, Kegeles LS, Kantrowitz JT, Slifstein M, Javitt DC, Lieberman JA, et al. Imaging glutamate in schizophrenia: review of findings and implications for drug discovery. Molecular psychiatry. 2014;19:20–29. doi: 10.1038/mp.2013.136. [DOI] [PubMed] [Google Scholar]
- 38.Beneyto M, Abbott A, Hashimoto T, Lewis DA. Lamina-specific alterations in cortical GABA(A) receptor subunit expression in schizophrenia. Cereb Cortex. 2011;21:999–1011. doi: 10.1093/cercor/bhq169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hashimoto T, Arion D, Unger T, Maldonado-Aviles JG, Morris HM, Volk DW, et al. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Molecular psychiatry. 2008;13:147–161. doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lewis DA, Hashimoto T, Morris HM. Cell and receptor type-specific alterations in markers of GABA neurotransmission in the prefrontal cortex of subjects with schizophrenia. Neurotoxicity research. 2008;14:237–248. doi: 10.1007/BF03033813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morris HM, Hashimoto T, Lewis DA. Alterations in somatostatin mRNA expression in the dorsolateral prefrontal cortex of subjects with schizophrenia or schizoaffective disorder. Cereb Cortex. 2008;18:1575–1587. doi: 10.1093/cercor/bhm186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Volk DW, Matsubara T, Li S, Sengupta EJ, Georgiev D, Minabe Y, et al. Deficits in transcriptional regulators of cortical parvalbumin neurons in schizophrenia. Am J Psychiatry. 2012;169:1082–1091. doi: 10.1176/appi.ajp.2012.12030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woo TU, Walsh JP, Benes FM. Density of glutamic acid decarboxylase 67 messenger RNA-containing neurons that express the N-methyl-D-aspartate receptor subunit NR2A in the anterior cingulate cortex in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2004;61:649–657. doi: 10.1001/archpsyc.61.7.649. [DOI] [PubMed] [Google Scholar]
- 44.Woo TU, Shrestha K, Lamb D, Minns MM, Benes FM. N-methyl-D-aspartate receptor and calbindin-containing neurons in the anterior cingulate cortex in schizophrenia and bipolar disorder. Biol Psychiatry. 2008;64:803–809. doi: 10.1016/j.biopsych.2008.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pratt J, Winchester C, Dawson N, Morris B. Advancing schizophrenia drug discovery: optimizing rodent models to bridge the translational gap. Nat Rev Drug Discov. 2012;11:560–579. doi: 10.1038/nrd3649. [DOI] [PubMed] [Google Scholar]
- 46.Bubenikova-Valesova V, Horacek J, Vrajova M, Hoschl C. Models of schizophrenia in humans and animals based on inhibition of NMDA receptors. Neuroscience and biobehavioral reviews. 2008;32:1014–1023. doi: 10.1016/j.neubiorev.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 47.Cunningham MO, Hunt J, Middleton S, LeBeau FE, Gillies MJ, Davies CH, et al. Region-specific reduction in entorhinal gamma oscillations and parvalbumin-immunoreactive neurons in animal models of psychiatric illness. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:2767–2776. doi: 10.1523/JNEUROSCI.5054-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roopun AK, Cunningham MO, Racca C, Alter K, Traub RD, Whittington MA. Region-specific changes in gamma and beta2 rhythms in NMDA receptor dysfunction models of schizophrenia. Schizophr Bull. 2008;34:962–973. doi: 10.1093/schbul/sbn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kittelberger K, Hur EE, Sazegar S, Keshavan V, Kocsis B. Comparison of the effects of acute and chronic administration of ketamine on hippocampal oscillations: relevance for the NMDA receptor hypofunction model of schizophrenia. Brain structure & function. 2012;217:395–409. doi: 10.1007/s00429-011-0351-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hakami T, Jones NC, Tolmacheva EA, Gaudias J, Chaumont J, Salzberg M, et al. NMDA receptor hypofunction leads to generalized and persistent aberrant gamma oscillations independent of hyperlocomotion and the state of consciousness. PLoS One. 2009;4:e6755. doi: 10.1371/journal.pone.0006755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pinault D. N-methyl d-aspartate receptor antagonists ketamine and MK-801 induce wake-related aberrant gamma oscillations in the rat neocortex. Biol Psychiatry. 2008;63:730–735. doi: 10.1016/j.biopsych.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 52.Lazarewicz MT, Ehrlichman RS, Maxwell CR, Gandal MJ, Finkel LH, Siegel SJ. Ketamine modulates theta and gamma oscillations. J Cogn Neurosci. 2010;22:1452–1464. doi: 10.1162/jocn.2009.21305. [DOI] [PubMed] [Google Scholar]
- 53.Leung LW. Spectral analysis of hippocampal EEG in the freely moving rat: effects of centrally active drugs and relations to evoked potentials. Electroencephalography and clinical neurophysiology. 1985;60:65–77. doi: 10.1016/0013-4694(85)90952-6. [DOI] [PubMed] [Google Scholar]
- 54.Ma J, Leung LS. The supramammillo-septal-hippocampal pathway mediates sensorimotor gating impairment and hyperlocomotion induced by MK-801 and ketamine in rats. Psychopharmacology (Berl) 2007;191:961–974. doi: 10.1007/s00213-006-0667-x. [DOI] [PubMed] [Google Scholar]
- 55.Ehrlichman RS, Gandal MJ, Maxwell CR, Lazarewicz MT, Finkel LH, Contreras D, et al. N-methyl-d-aspartic acid receptor antagonist-induced frequency oscillations in mice recreate pattern of electrophysiological deficits in schizophrenia. Neuroscience. 2009;158:705–712. doi: 10.1016/j.neuroscience.2008.10.031. [DOI] [PubMed] [Google Scholar]
- 56.Palenicek T, Fujakova M, Brunovsky M, Balikova M, Horacek J, Gorman I, et al. Electroencephalographic spectral and coherence analysis of ketamine in rats: correlation with behavioral effects and pharmacokinetics. Neuropsychobiology. 2011;63:202–218. doi: 10.1159/000321803. [DOI] [PubMed] [Google Scholar]
- 57.Kulikova SP, Tolmacheva EA, Anderson P, Gaudias J, Adams BE, Zheng T, et al. Opposite effects of ketamine and deep brain stimulation on rat thalamocortical information processing. The European journal of neuroscience. 2012;36:3407–3419. doi: 10.1111/j.1460-9568.2012.08263.x. [DOI] [PubMed] [Google Scholar]
- 58.Wood J, Kim Y, Moghaddam B. Disruption of prefrontal cortex large scale neuronal activity by different classes of psychotomimetic drugs. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:3022–3031. doi: 10.1523/JNEUROSCI.6377-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Caixeta FV, Cornelio AM, Scheffer-Teixeira R, Ribeiro S, Tort AB. Ketamine alters oscillatory coupling in the hippocampus. Scientific reports. 2013;3:2348. doi: 10.1038/srep02348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Molina LA, Skelin I, Gruber AJ. Acute NMDA receptor antagonism disrupts synchronization of action potential firing in rat prefrontal cortex. PLoS One. 2014;9:e85842. doi: 10.1371/journal.pone.0085842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carlen M, Meletis K, Siegle JH, Cardin JA, Futai K, Vierling-Claassen D, et al. A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Molecular psychiatry. 2012;17:537–548. doi: 10.1038/mp.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Korotkova T, Fuchs EC, Ponomarenko A, von Engelhardt J, Monyer H. NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron. 2010;68:557–569. doi: 10.1016/j.neuron.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 63.Nakao K, Nakazawa K. Brain state-dependent abnormal LFP activity in the auditory cortex of a schizophrenia mouse model. Front Neurosci. 2014;8:168. doi: 10.3389/fnins.2014.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McNally JM, McCarley RW, Brown RE. Chronic Ketamine Reduces the Peak Frequency of Gamma Oscillations in Mouse Prefrontal Cortex Ex vivo. Frontiers in psychiatry. 2013;4:106. doi: 10.3389/fpsyt.2013.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Buzsáki G, Chrobak JJ. Temporal structure in spatially organized neuronal ensembles: a role for interneuronal networks. Current opinion in neurobiology. 1995;5:504–510. doi: 10.1016/0959-4388(95)80012-3. [DOI] [PubMed] [Google Scholar]
- 66.Buzsaki G, Wang XJ. Mechanisms of gamma oscillations. Annual review of neuroscience. 2012;35:203–225. doi: 10.1146/annurev-neuro-062111-150444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cardin JA, Carlén M, Meletis K, Knoblich U, Zhang F, Deisseroth K, et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hansel D, Mato G, Meunier C. Clustering and slow switching in globally coupled phase oscillators. Physical review E, Statistical physics, plasmas, fluids, and related interdisciplinary topics. 1993;48:3470–3477. doi: 10.1103/physreve.48.3470. [DOI] [PubMed] [Google Scholar]
- 70.Borgers C, Kopell N. Synchronization in networks of excitatory and inhibitory neurons with sparse, random connectivity. Neural Comput. 2003;15:509–538. doi: 10.1162/089976603321192059. [DOI] [PubMed] [Google Scholar]
- 71.Börgers C, Epstein S, Kopell NJ. Background gamma rhythmicity and attention in cortical local circuits: a computational study. Proceedings of the National Academy of Sciences. 2005;102:7002–7007. doi: 10.1073/pnas.0502366102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ermentrout GB, Kopell N. Fine structure of neural spiking and synchronization in the presence of conduction delays. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:1259–1264. doi: 10.1073/pnas.95.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vierling-Claassen D, Siekmeier P, Stufflebeam S, Kopell N. Modeling GABA alterations in schizophrenia: a link between impaired inhibition and altered gamma and beta range auditory entrainment. Journal of neurophysiology. 2008;99:2656–2671. doi: 10.1152/jn.00870.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Volman V, Behrens MM, Sejnowski TJ. Downregulation of parvalbumin at cortical GABA synapses reduces network gamma oscillatory activity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:18137–18148. doi: 10.1523/JNEUROSCI.3041-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Spencer KM. The functional consequences of cortical circuit abnormalities on gamma oscillations in schizophrenia: insights from computational modeling. Frontiers in human neuroscience. 2009;3:33. doi: 10.3389/neuro.09.033.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y, et al. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 2010;13:76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Staley KJ. Neurons skip a beat during fast ripples. Neuron. 2007;55:828–830. doi: 10.1016/j.neuron.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 79.Nikolic D, Hausler S, Singer W, Maass W. Distributed fading memory for stimulus properties in the primary visual cortex. PLoS biology. 2009;7:e1000260. doi: 10.1371/journal.pbio.1000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ray S, Maunsell JHR. Differences in gamma frequencies across visual cortex restrict their possible use in computation. Neuron. 2010;67:885–896. doi: 10.1016/j.neuron.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wilson HR, Cowan JD. Excitatory and inhibitory interactions in localized populations of model neurons. Biophysical journal. 1972;12:1–24. doi: 10.1016/S0006-3495(72)86068-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jadi MP, Sejnowski TJ. Cortical oscillations arise from contextual interactions that regulate sparse coding. Proceedings of the National Academy of Sciences of the United States of America. 2014 doi: 10.1073/pnas.1405300111. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jadi MP, Sejnowski TJ. Regulating cortical oscillations in an inhibition-stabilized network. Proc IEEE: Special Issue on Comp Neuro. 2014 doi: 10.1109/JPROC.2014.2313113. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Whittington MA, Traub RD, Jefferys JG. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995;373:612–615. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- 85.Wang XJ, Buzsáki G. Gamma oscillation by synaptic inhibition in a hippocampal interneuronal network model. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16:6402–6413. doi: 10.1523/JNEUROSCI.16-20-06402.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Traub RD, Whittington MA, Colling SB, Buzsaki G, Jefferys JG. Analysis of gamma rhythms in the rat hippocampus in vitro and in vivo. The Journal of physiology. 1996;493(Pt 2):471–484. doi: 10.1113/jphysiol.1996.sp021397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Van Vreeswijk C, Abbott LF, Ermentrout GB. When inhibition not excitation synchronizes neural firing. J Comput Neurosci. 1994;1:313–321. doi: 10.1007/BF00961879. [DOI] [PubMed] [Google Scholar]
- 88.Buia C, Tiesinga P. Attentional modulation of firing rate and synchrony in a model cortical network. J Comput Neurosci. 2006;20:247–264. doi: 10.1007/s10827-006-6358-0. [DOI] [PubMed] [Google Scholar]
- 89.Tiesinga P, Sejnowski TJ. Cortical enlightenment: are attentional gamma oscillations driven by ING or PING? Neuron. 2009;63:727–732. doi: 10.1016/j.neuron.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Traub RD, Kopell N, Bibbig A, Buhl EH, LeBeau FE, Whittington MA. Gap junctions between interneuron dendrites can enhance synchrony of gamma oscillations in distributed networks. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:9478–9486. doi: 10.1523/JNEUROSCI.21-23-09478.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fukuda T, Kosaka T, Singer W, Galuske RA. Gap junctions among dendrites of cortical GABAergic neurons establish a dense and widespread intercolumnar network. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:3434–3443. doi: 10.1523/JNEUROSCI.4076-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Buhl DL, Harris KD, Hormuzdi SG, Monyer H, Buzsaki G. Selective impairment of hippocampal gamma oscillations in connexin-36 knock-out mouse in vivo. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:1013–1018. doi: 10.1523/JNEUROSCI.23-03-01013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brunel N, Wang X-J. What determines the frequency of fast network oscillations with irregular neural discharges? I. Synaptic dynamics and excitation-inhibition balance. Journal of neurophysiology. 2003;90:415–430. doi: 10.1152/jn.01095.2002. [DOI] [PubMed] [Google Scholar]
- 94.Geisler C, Brunel N, Wang XJ. Contributions of intrinsic membrane dynamics to fast network oscillations with irregular neuronal discharges. Journal of neurophysiology. 2005;94:4344–4361. doi: 10.1152/jn.00510.2004. [DOI] [PubMed] [Google Scholar]
- 95.Fuchs EC, Zivkovic AR, Cunningham MO, Middleton S, Lebeau FE, Bannerman DM, et al. Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron. 2007;53:591–604. doi: 10.1016/j.neuron.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 96.Tatard-Leitman VM, Jutzeler CR, Suh J, Saunders JA, Billingslea EN, Morita S, et al. Pyramidal Cell Selective Ablation of N-Methyl-D-Aspartate Receptor 1 Causes Increase in Cellular and Network Excitability. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pfeffer CK, Xue M, He M, Huang ZJ, Scanziani M. Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat Neurosci. 2013;16:1068–1076. doi: 10.1038/nn.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pi HJ, Hangya B, Kvitsiani D, Sanders JI, Huang ZJ, Kepecs A. Cortical interneurons that specialize in disinhibitory control. Nature. 2013;503:521–524. doi: 10.1038/nature12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fu Y, Tucciarone JM, Espinosa JS, Sheng N, Darcy DP, Nicoll RA, et al. A cortical circuit for gain control by behavioral state. Cell. 2014;156:1139–1152. doi: 10.1016/j.cell.2014.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Adesnik H, Bruns W, Taniguchi H, Huang ZJ, Scanziani M. A neural circuit for spatial summation in visual cortex. Nature. 2012;490:226–231. doi: 10.1038/nature11526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Grunze HC, Rainnie DG, Hasselmo ME, Barkai E, Hearn EF, McCarley RW, et al. NMDA-dependent modulation of CA1 local circuit inhibition. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16:2034–2043. doi: 10.1523/JNEUROSCI.16-06-02034.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rotaru DC, Lewis DA, Gonzalez-Burgos G. The role of glutamatergic inputs onto parvalbumin-positive interneurons: relevance for schizophrenia. Rev Neurosci. 2012;23:97–109. doi: 10.1515/revneuro-2011-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL, et al. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318:1645–1647. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- 104.Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Konradi C, Yang CK, Zimmerman EI, Lohmann KM, Gresch P, Pantazopoulos H, et al. Hippocampal interneurons are abnormal in schizophrenia. Schizophrenia research. 2011;131:165–173. doi: 10.1016/j.schres.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sakai T, Oshima A, Nozaki Y, Ida I, Haga C, Akiyama H, et al. Changes in density of calcium-binding-protein-immunoreactive GABAergic neurons in prefrontal cortex in schizophrenia and bipolar disorder. Neuropathology : official journal of the Japanese Society of Neuropathology. 2008;28:143–150. doi: 10.1111/j.1440-1789.2007.00867.x. [DOI] [PubMed] [Google Scholar]
- 107.Ali AB, Thomson AM. Synaptic alpha 5 subunit-containing GABAA receptors mediate IPSPs elicited by dendrite-preferring cells in rat neocortex. Cereb Cortex. 2008;18:1260–1271. doi: 10.1093/cercor/bhm160. [DOI] [PubMed] [Google Scholar]
- 108.Hiyoshi T, Kambe D, Karasawa J, Chaki S. Differential effects of NMDA receptor antagonists at lower and higher doses on basal gamma band oscillation power in rat cortical electroencephalograms. Neuropharmacology. 2014;85:384–396. doi: 10.1016/j.neuropharm.2014.05.037. [DOI] [PubMed] [Google Scholar]
- 109.Kirli KK, Ermentrout GB, Cho RY. Computational study of NMDA conductance and cortical oscillations in schizophrenia. Front Comput Neurosci. 2014;8:133. doi: 10.3389/fncom.2014.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Buchanan KA, Blackman AV, Moreau AW, Elgar D, Costa RP, Lalanne T, et al. Target-specific expression of presynaptic NMDA receptors in neocortical microcircuits. Neuron. 2012;75:451–466. doi: 10.1016/j.neuron.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xu H, Jeong HY, Tremblay R, Rudy B. Neocortical somatostatin-expressing GABAergic interneurons disinhibit the thalamorecipient layer 4. Neuron. 2013;77:155–167. doi: 10.1016/j.neuron.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brunel N, Hakim V. Sparsely synchronized neuronal oscillations. Chaos. 2008;18:015113. doi: 10.1063/1.2779858. [DOI] [PubMed] [Google Scholar]
- 113.Brunel N. Dynamics of sparsely connected networks of excitatory and inhibitory spiking neurons. J Comput Neurosci. 2000;8:183–208. doi: 10.1023/a:1008925309027. [DOI] [PubMed] [Google Scholar]
- 114.Kang K, Shelley M, Henrie JA, Shapley R. LFP spectral peaks in V1 cortex: network resonance and cortico-cortical feedback. J Comput Neurosci. 2010;29:495–507. doi: 10.1007/s10827-009-0190-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wallace E, Benayoun M, van Drongelen W, Cowan JD. Emergent oscillations in networks of stochastic spiking neurons. PLoS ONE. 2011;6:e14804. doi: 10.1371/journal.pone.0014804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bressloff PC. Metastable states and quasicycles in a stochastic Wilson-Cowan model of neuronal population dynamics. Phys Rev E Stat Nonlin Soft Matter Phys. 2010;82:051903. doi: 10.1103/PhysRevE.82.051903. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.