Abstract
Objective
Despite common risk factors, screening for hepatitis C virus (HCV) and HIV at the same time as part of routine medical care (dual-routine HCV/HIV testing) is not commonly implemented in the United States. This study examined improvements in feasibility of implementation, screening increase, and linkage to care when a dual-routine HCV/HIV testing model was integrated into routine primary care.
Methods
National Nursing Centers Consortium implemented a dual-routine HCV/HIV testing model at four community health centers in Philadelphia, Pennsylvania, on September 1, 2013. Routine HCV and opt-out HIV testing replaced the routine HCV and opt-in HIV testing model through medical assistant-led, laboratory-based testing and electronic medical record modification to prompt, track, report, and facilitate reimbursement for tests performed on uninsured individuals. This study examined testing, seropositivity, and linkage-to-care comparison data for the nine months before (December 1, 2012–August 31, 2013) and after (September 1, 2013–May 31, 2014) implementation of the dual-routine HCV/HIV testing model.
Results
A total of 1,526 HCV and 1,731 HIV tests were performed before, and 1,888 HCV and 3,890 HIV tests were performed after dual-routine testing implementation, resulting in a 23.7% increase in HCV tests and a 124.7% increase in HIV tests. A total of 70 currently HCV-infected and four new HIV-seropositive patients vs. 101 HCV-infected and 13 new HIV-seropositive patients were identified during these two periods, representing increases of 44.3% for HCV antibody-positive and RNA-positive tests and 225.0% for HIV-positive tests. Linkage to care increased from 27 currently infected HCV--positive and one HIV-positive patient pre-dual-routine testing to 39 HCV--positive and nine HIV-positive patients post-dual-routine testing.
Conclusion
The dual-routine HCV/HIV testing model shows that integrating dual-routine testing in a primary care setting is possible and leads to increased HCV and HIV screening, enhanced seropositivity diagnosis, and improved linkage to care.
Approximately 1.1 million people living in the United States have human immunodeficiency virus (HIV) infection.1 About half of the newly sexually acquired HIV infections are transmitted by the 18% of people living in the United States who are unaware of their HIV-positive status.1,2 HIV in the United States disproportionately affects minorities who bear the largest burden of the disease, with approximately 44% of new HIV cases reported among non-Hispanic black U.S. residents.3 Poverty remains a top predictor of HIV risk among urban heterosexuals.4 An HIV diagnosis relies on multiple tests—a reactive HIV antigen or antibody test confirmed by a supplemental antibody or HIV-1 RNA test.1
An estimated 3.2 million U.S. residents live with the hepatitis C virus (HCV), but most individuals are unaware of their infection.5–8 HCV in the United States disproportionately affects minorities, baby boomers (i.e., those born between 1945 and 1965), injection/intranasal drug users, individuals without a high school education, and those living in households with annual incomes <$25,000.6,9,10 Two tests diagnose HCV: an HCV antibody test that identifies exposure to the virus and an HCV ribonucleic acid (RNA) test that identifies current (chronic) or past (acute) infection.11 Typically, 15%–25% of individuals exposed to HCV clear the virus on their own within the first six months of exposure, while 75%–85% develop HCV infection requiring specialist care.10
Approximately 20% of HIV-infected individuals are coinfected with HCV due to shared risk factors.12 Despite this overlap, dual HCV/HIV screening is not routinely performed in the United States. Dual-routine HCV/HIV testing is defined as an offer for an HCV and HIV test at the same time, as part of routine primary care. Today, HCV and HIV testing still mainly depends on risks and does not follow the Centers for Disease Control and Prevention's (CDC's) 2012 recommendation to administer HCV tests for those born between 1945 and 1965 and 2006 recommendation to integrate routine opt-out HIV testing in primary care settings.13,14 Increased efforts to integrate dual-routine HCV/HIV testing in primary care settings can better diagnose infection in settings where patients testing positive can easily link into both HCV and HIV care cascades. Studies consistently suggest that engagement in clinical care contributes to improved health outcomes for people with HCV and/or HIV infection.13,15
Multiple barriers to dual-routine HCV/HIV testing in primary care exist. A 2014 study found that primary care providers did not integrate recommended HCV testing due to time constraints, lack of training about existing guidelines, and misconceptions about CDC recommendations.16 Likewise, a 2011 HIV study in Massachusetts community health centers (CHCs) reported five HIV testing barriers: provider time constraints, time required to administer counseling, time needed to provide informed consent, lack of funding, and lack of provider training.17
CHCs are in a position to gain the most from integrated dual-routine HCV/HIV testing, as both viruses disproportionately affect the poor and minority patients they serve. We aimed to integrate a dual-routine HCV/HIV testing model in a network of CHCs through staff training, modifications to system-wide health-care infrastructure, and enhanced electronic medical record (EMR) usage. Data presented in this article enforce the paradigm that these modifications in CHCs lead to enhanced dual-routine HCV/HIV testing rates, increased seroprevalence detection rates, and improved linkage to care.
METHODS
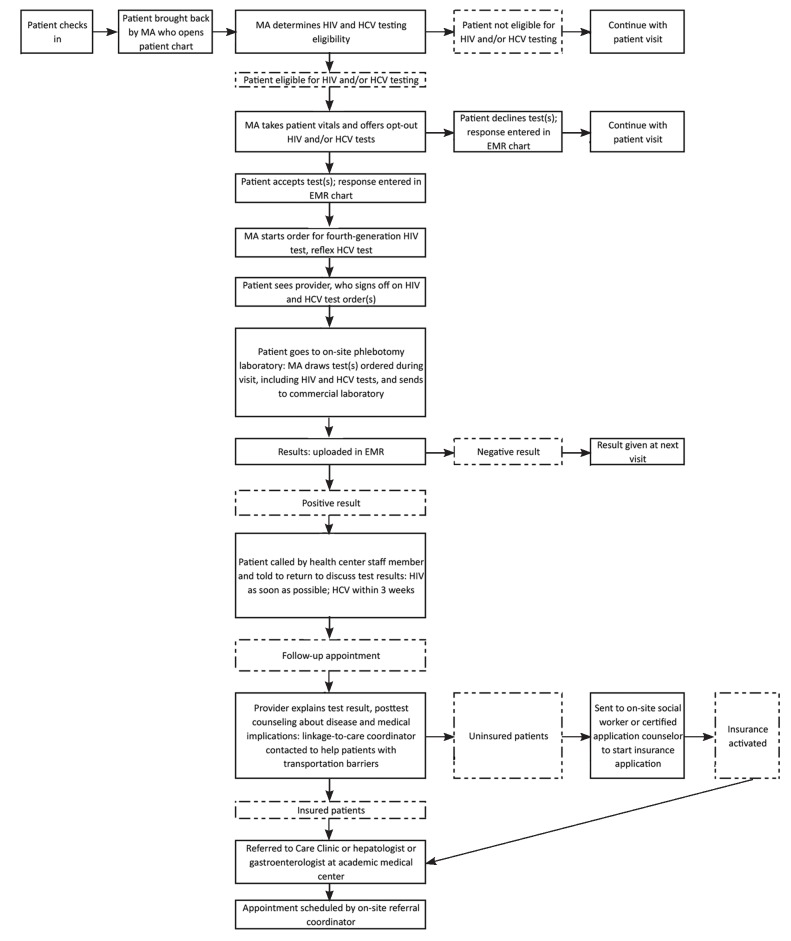
The Philadelphia, Pennsylvania-based National Nursing Centers Consortium (NNCC) and its parent corporation, Public Health Management Corporation (PHMC), partnered on September 1, 2013, to implement a dual-routine HCV/HIV testing model by adding routine, opt-out annual HIV testing to a routine, opt-out HCV testing and linkage-to-care model at four of PHMC's five federally qualified health centers (Figure).18 The four PHMC centers included Mary Howard Health Center, Health Connection, Rising Sun Health Center, and Care Clinic. Mary Howard Health Center cares for an entirely homeless adult patient population, Health Connection and Rising Sun Health Centers provide primary care to public housing residents in adjacent complexes, and Care Clinic provides primary care to all patients and specialty care for HCV and HIV patients.
Figure.
Dual-routine HCV/HIV testing model flowchart showing how HCV and HIV testing and linkage-to-care protocol was integrated during patient visits at four community health centers in Philadelphia, Pennsylvania, September 1, 2013, through May 31, 2014
HCV = hepatitis C virus
HIV = human immunodeficiency virus
MA = medical assistant
EMR = electronic medical record
The existing HCV testing model included medical assistant-initiated, opt-out HCV testing; the use of laboratory-based reflexive testing technology; and EMR modifications that identified baby boomers and patients with current HCV infections, as well as a new account (labeled “Hep C Project”) added for HCV tests performed on uninsured patients.18 HIV testing was opt-in and performed primarily at family planning visits. We classified dual-routine testing as an opt-out HCV test for high-risk patients coupled with an opt-out, non-risk-based HIV test for all patients seen at the four clinics.
Prior to dual-routine HCV/HIV testing implementation, medical staff members attended trainings given by project managers and local experts. Topics included project goals and protocol, defined opt-out testing, Philadelphia's prevalence, novel treatments, and case studies of routine HIV screening success. Medical assistants practiced opt-out vs. opt-in testing and how to educate patients who initially refused testing. A health information team member reinforced how to complete laboratory requisitions.
New EMR modifications made after dual-routine testing implementation included an automatic reminder determined by a daily query that scanned charts of patients (1) with appointments the following day, (2) ≥13 years of age, and (3) without an HIV diagnosis or test result in their medical chart for the past 12 months. HIV data were added to weekly reports, stratified by site, and sent to the project manager who recounted the total number of HCV and HIV tests and names of patients with HCV- and/or HIV-positive tests. These reports were used to track project progress and positive patients through the care cascades. Prior to dual-routine testing, NNCC negotiated competitive pricing with commercial laboratories for HCV tests performed on uninsured patients.18 Similarly, NNCC negotiated pricing for HIV tests. Medical assistants selected the “Hep C Project” account within the EMR for HCV and HIV screening tests performed on uninsured, sliding-scale, and Select Plan (i.e., insurance for family planning visits only) patients, which were paid for with grant funding. Tests performed on insured patients were sent to the capitated commercial laboratory and paid by their insurance company.
Pennsylvania law does not require written consent for HIV testing but does require documentation that a patient accepted or declined an HIV test.19 Patients verbally responded to the medical assistant, who offered the HIV test and documented their responses in fields added to the most commonly used encounters and a new one specifically for HIV testing. Response options included “yes,” “no,” “not eligible because tested within last 12 months,” or “not eligible as HIV positive.” Pennsylvania does not require documented consent for HCV testing. For quality purposes, medical assistants documented when patients refused an HCV test in their medical chart.
Patients eligible for HCV testing, ≥18 years of age, and unaware of their seropositive status were presented in the dataset. Eligibility was based on the following criteria: people born between 1945 and 1965, those who were homeless, or those who had CDC risk factors (e.g., injection/intranasal drug use, piercings/tattoos from non-licensed locations, blood transfusion before 1994, or dialysis).13 We report HIV testing outcomes from individuals ≥18 years of age eligible for annual HIV testing and unaware of their HIV-positive status in this study. We excluded HIV data for patients aged 13–17 years from the current dataset, as they comprised a small fraction of overall testing.
To determine HCV testing eligibility, medical assistants at Health Connection and Rising Sun, which treat low-risk HCV individuals, asked patients to confirm if they had any risk factors; only those who acknowledged at least one were tested. As Mary Howard and Care Clinic only see patients who are high risk for HCV, all patients were universally HCV tested.18 While taking vital signs, medical assistants informed patients that they would be tested for HCV and HIV unless they declined (Figure). Standing orders allowed the medical assistants to start HCV and HIV laboratory requisitions that the provider subsequently signed during the visit.
Existing U.S. Food and Drug Administration (FDA)-approved HCV and HIV tests were used in this study. For HCV, the test used was the HCV antibody test with reflex to quantitative HCV RNA testing (RealTime HCV/m2000sp/m2000rt, Abbott Laboratories, Abbott Park, Illinois; and COBAS® AMPLICOR HCV Test, Version 2.0, Roche Diagnostics, Indianapolis, Indiana). The HIV test used in our study was the fourth--generation HIV test (ARCHITECT® HIV Ag/Ab Combo, Abbott Laboratories; and GS HIV Combo HIV Ag/Ab EIA, Bio-Rad Laboratories, Hercules, California). As reflexive tests, commercial laboratories used in this project automatically performed confirmatory tests using the same blood specimen if the initial test was positive. We defined a negative HCV antibody test as a lack of existing HCV infection. A positive HCV antibody test and negative HCV RNA test indicated past, acute HCV infection. We defined a positive HCV antibody and positive HCV RNA test as current HCV infection. Patients having chronic or acute HIV detected with the fourth-generation test (as the fourth-generation test can detect acute HIV infection before the body has developed HIV-1 antibodies) were included in the tables.1 Chronic HIV was diagnosed by a positive initial antigen/antibodies combination test and positive second antibody differentiation test (Multispot).1 Acute HIV was diagnosed as positive initial antigen/antibodies combination test, negative second antibody test, and positive HIV-1 RNA test.1 A negative initial antigen/antibodies test using the fourth-generation testing technology indicated a lack of HIV infection.
Test results were automatically uploaded to the patient's chart. Normal results were given at the next visit or by phone. Positive patients were contacted to discuss abnormal test results. At the follow-up visit, providers disclosed the positive test result and counseled patients about medical implications and next steps. Insured patients received referrals to specialists for treatment evaluation. A linkage-to-care coordinator assisted patients who needed help getting to appointments (e.g., lack of transportation). HCV specialists included providers at the Care Clinic or hepatologists or gastroenterologists from the following local academic medical centers: University of Pennsylvania Health System, Einstein Healthcare Network, Philadelphia VA Medical Center, Jefferson University Hospital, Hahnemann University Hospital, Mercy Philadelphia Hospital, and Temple University Hospital. HIV specialists included providers at the Care Clinic or infectious disease specialists at any of the aforementioned local hospitals. Uninsured patients were first referred to the on-site social worker or certified application counselor to complete the insurance process and referred once insurance was activated. Positive HCV or HIV patients at Care Clinic were treated on-site by their primary care provider.
Our study presents data for the nine months preceding and following September 1, 2013, when routine HIV testing was added to existing HCV testing across the four PHMC clinics. All four clinics conducted routine HCV/opt-in HIV testing from December 1, 2012, through August 31, 2013. Dual-routine HCV/opt-out HIV testing was performed across all four sites from September 1, 2013, through May 31, 2014. Data were extracted from the centralized EMR. Data analysis ended September 1, 2014. We calculated absolute and percentage differences to show changes in HCV and HIV testing before and after dual-routine testing integration.
RESULTS
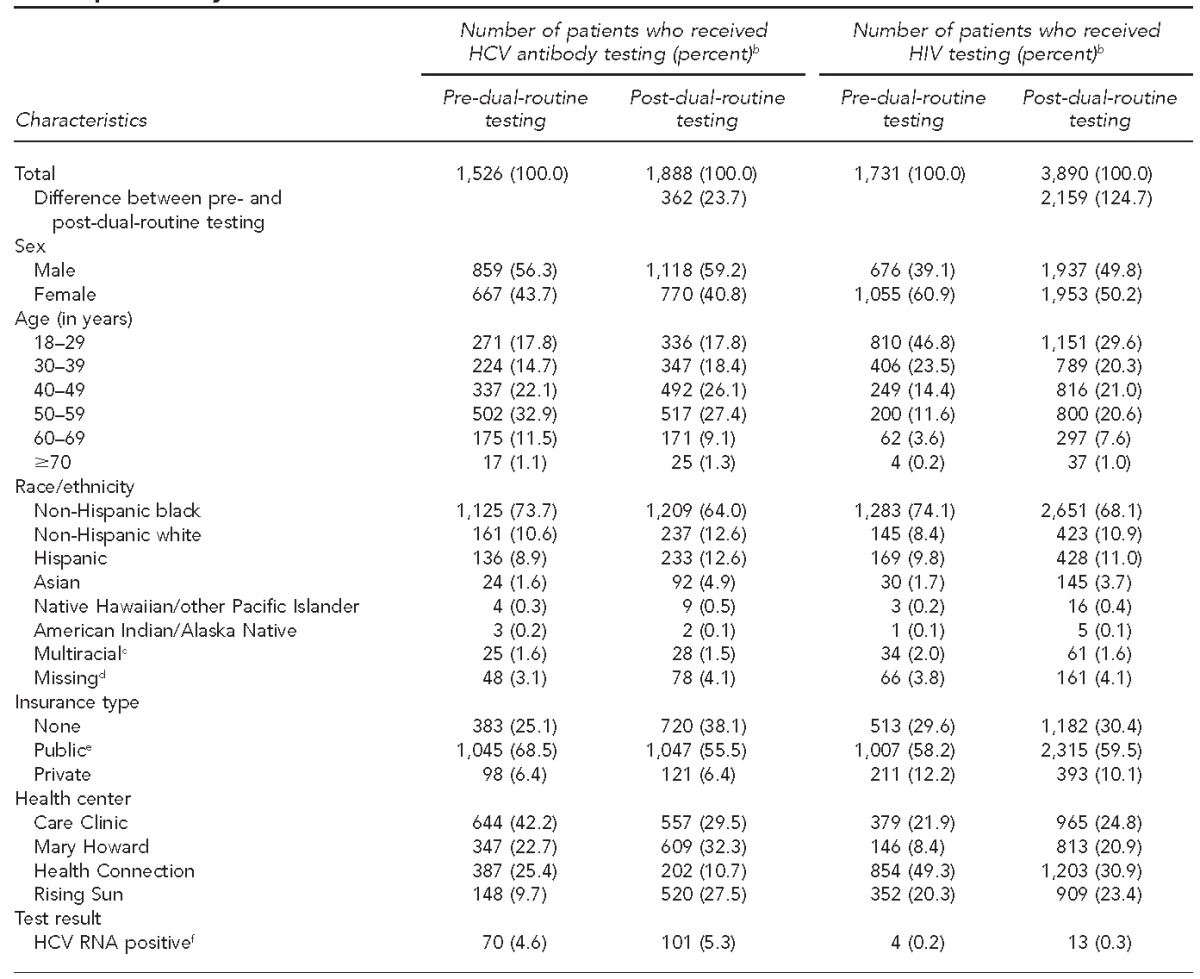
We examined HCV and HIV testing data for 9,035 individuals ≥18 years of age who were eligible for testing and unaware of their HCV or HIV status prior to testing. Of those individuals, 3,414 were tested for HCV and 5,621 were tested for HIV during the 18-month study period (Table 1). A total of 16,364 and 17,364 individuals ≥18 years of age visited the four CHCs during the two study periods, respectively. Mary Howard, Care Clinic, Health Connection, and Rising Sun saw a monthly average of 555, 673, 330, and 286 patients, respectively, in the nine months prior to dual-routine testing implementation, and 597, 686, 345, and 313 patients, respectively, in the nine months following dual-routine testing implementation.
Table 1.
Characteristics of patients who received HCV antibody testing and HIV tests before and after dual-routine HCV/HIV testinga implementation, four urban community health centers, Philadelphia, Pennsylvania, 2012–2014
Dual-routine testing refers to screening for HCV and HIV at the same time as part of routine medical care.
bSome percentages may not total to 100 due to rounding.
cMore than one race/ethnicity
dNo race/ethnicity information listed in medical chart
eMedicaid, Medicare, or Medicaid-managed plan
fHCV antibody and RNA-positive tests
HCV = hepatitis C virus
HIV = human immunodeficiency virus
RNA = ribonucleic acid
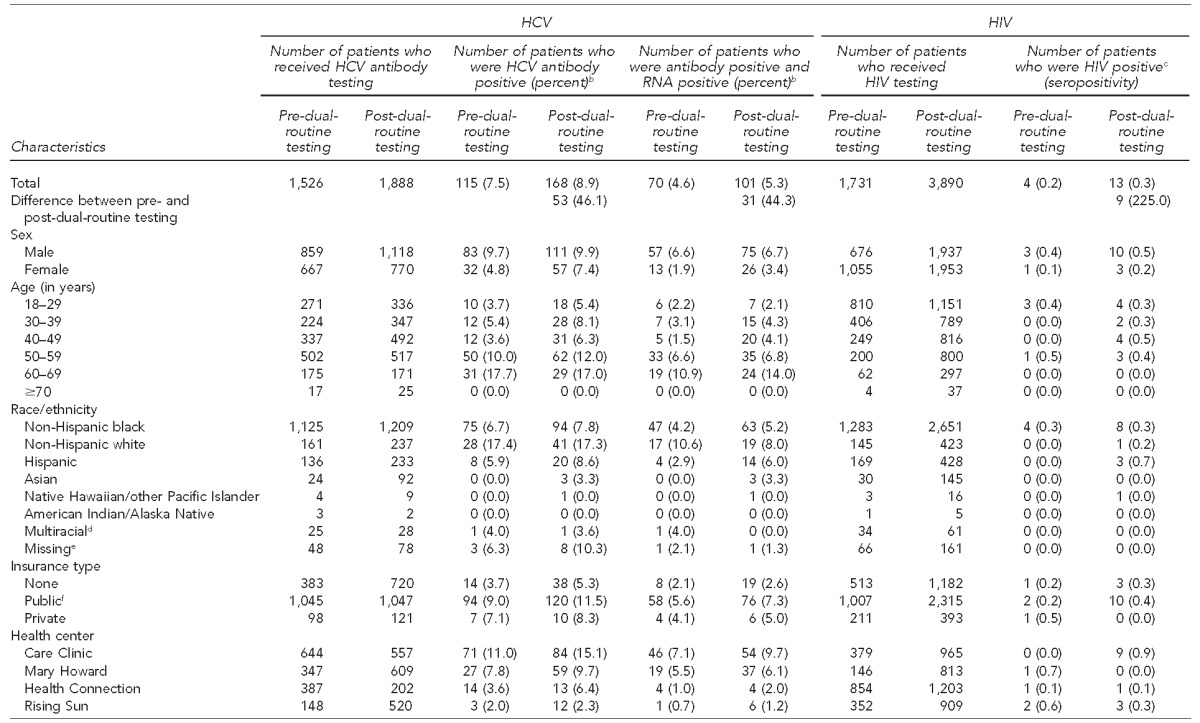
A total of 1,526 individuals were tested for HCV before the dual-routine testing period. This number increased to 1,888 during the dual-routine testing implementation period, which constituted a 23.7% increase between the two nine-month study periods (Table 1). Of those tested, 115 (7.5%) and 168 (8.9%) were HCV antibody positive before and after dual-routine testing implementation, respectively, and 101 (87.8%) and 158 (94.1%) of those testing positive received confirmatory HCV RNA testing during the two study periods. Among individuals tested for HCV, 70 of 1,526 (4.6%) had current HCV infection prior to the dual-routine testing period, and 101 of 1,888 (5.3%) had current HCV infection after dual-routine testing implementation, representing a 44.3% increase in detection. Care Clinic, at 7.1% and 9.7%, followed by Mary Howard, at 5.5% and 6.1%, had the highest rates of current HCV infection during the 18-month study period (Table 2). By race/ethnicity, more non-Hispanic black patients had current HCV infection than any other racial/ethnic group; however, non-Hispanic white patients had higher rates of current HCV infection during the two nine-month periods, at 10.6% and 8.0%, respectively.
Table 2.
Characteristics and seroprevalence of patients with HCV antibody positive tests, current HCV infection, and HIV-positive tests before and after implementation of dual-routine HCV/HIV testing,a four urban community health centers, Philadelphia, Pennsylvania, 2012–2014
Dual-routine testing refers to screening for HCV and HIV at the same time as part of routine medical care.
bSome percentages may not total to 100 due to rounding.
cPositive fourth-generation test detecting either HIV infection (i.e., positive initial antigen/antibodies combination test and positive second antibody differentiation test [Multispot]) or acute HIV infection (i.e., positive initial antigen/antibodies combination test, negative second antibody test, and positive HIV-1 RNA test)
dMore than one race/ethnicity
eNo race/ethnicity information listed in medical chart
fMedicaid, Medicare, or Medicaid-managed plan
HIV = human immunodeficiency virus
HCV = hepatitis C virus
RNA = ribonucleic acid
A total of 1,731 individuals were tested for HIV prior to the dual-routine testing period and 3,890 were tested after dual-routine testing implementation, a 124.7% increase in the number of HIV tests performed (Table 1). Among those tested for HIV, four people tested positive prior to dual-routine testing and 13 people tested positive after dual-routine testing implementation (Table 2). Care Clinic had the highest HIV seroprevalence (nine of 965 tests conducted, 0.9%) after implementation of dual-routine testing (Table 2). Two acute HIV cases were identified during the dual-routine testing implementation period.
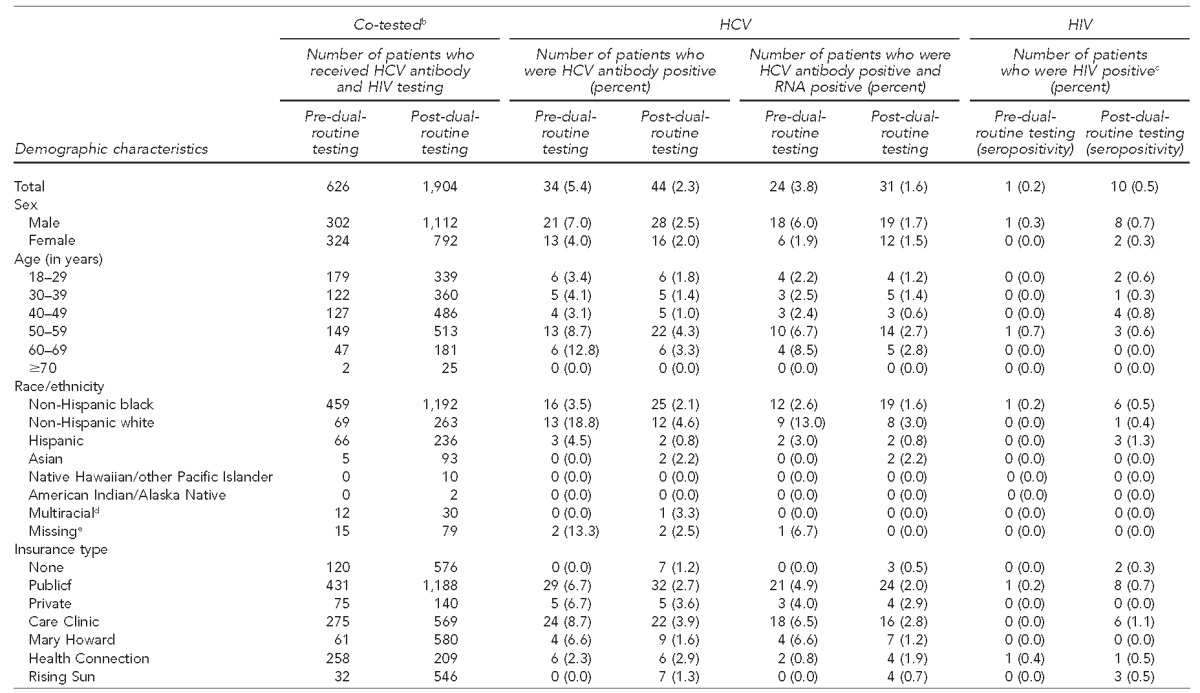
A total of 2,530 individuals were co-tested for HCV and HIV—626 prior to dual-routine testing and 1,904 after dual-routine testing implementation—representing a 204.2% increase. Among the co-tested individuals, 24 and 31 individuals tested positive for current HCV infection during the two study periods (an increase of 29.2%), and one and 10 individuals tested positive for HIV during the two study periods, respectively. No coinfected patients were identified during the study period (Table 3).
Table 3.
Characteristics of individuals co-tested for HCV and HIV and identified as HCV- or HIV-positive, before and after dual-routine testinga implementation, four urban community health centers, Philadelphia, Pennsylvania, 2012–2014
Dual-routine testing refers to screening for HCV and HIV at the same time as part of routine medical care.
bTested for both HIV and HCV within same nine-month timeframe
cPositive fourth-generation test detecting either HIV infection (i.e., positive initial antigen/antibodies combination test and positive second antibody differentiation test [Multispot]) or acute HIV infection (i.e., positive initial antigen/antibodies combination test, negative second antibody test, and positive HIV-1 RNA test)
dMore than one race/ethnicity
eNo race/ethnicity information listed in medical chart
fMedicaid, Medicare, or Medicaid-managed plan
HCV = hepatitis C virus
HIV = human immunodeficiency virus
RNA = ribonucleic acid
The absolute number of currently infected HCV patients seen by specialists increased from 27 to 39 from the study start to the end of data collection in September 2014 (Table 4). The smallest absolute increase was in the number of currently infected HCV patients who were referred to a specialist, from 55 to 59 (7.3%). The biggest improvement in the HIV care continuum between the two study periods was in the number of patients who saw an HIV specialist once referred, improving from one out of three pre-dual-routine testing to nine out of 12 post-dual-routine testing (Table 4).
Table 4.
Linkage-to-care cascade for currently infected HCV- and HIV-positive patients, pre-dual-routine testing (December 2012–August 2013) and post-dual-routine testinga (September 2013–May 2014), four community health centers, Philadelphia, Pennsylvania
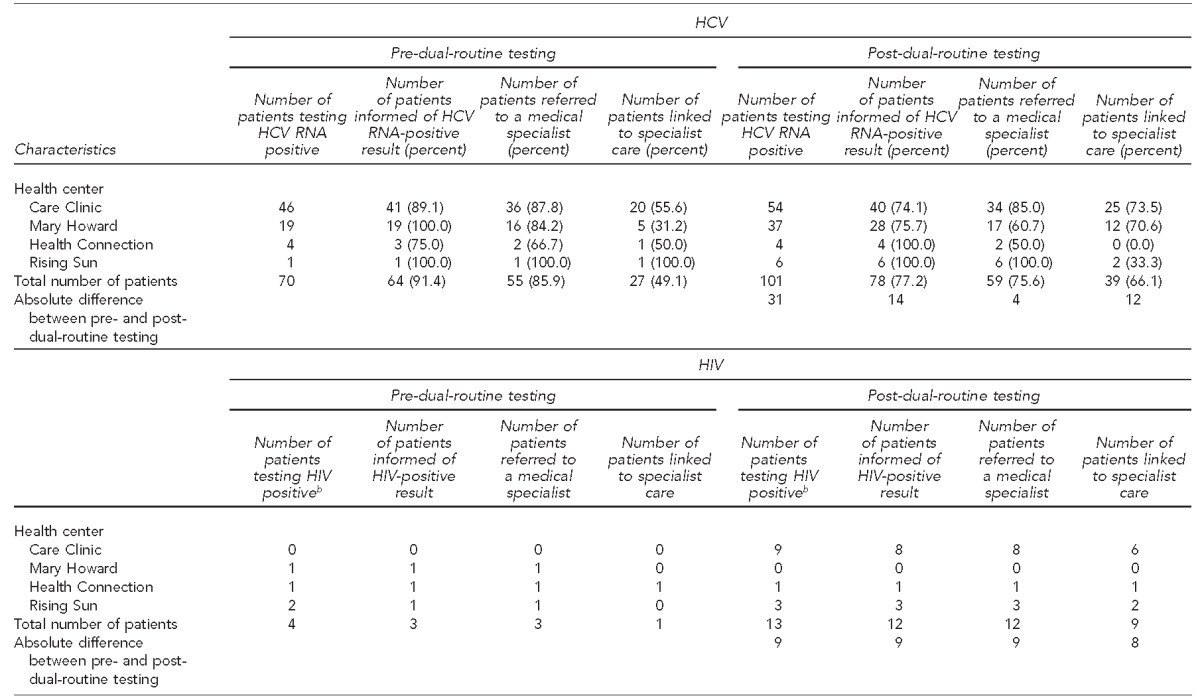
Dual-routine testing refers to screening for HCV and HIV at the same time as part of routine medical care.
bPositive fourth-generation test detecting either HIV infection (i.e., positive initial antigen/antibodies combination test and positive second antibody differentiation test [Multispot]) or acute HIV infection (i.e., positive initial antigen/antibodies combination test, negative second antibody test, and positive HIV-1 RNA test)
HCV = hepatitis C virus
HIV = human immunodeficiency virus
RNA = ribonucleic acid
DISCUSSION
Our dual-routine HCV/HIV testing model had widespread support by clinic staff members and high test acceptance by our patients. Medical assistant-initiated testing ensured both tests were offered. Likewise, non-risk-based HIV testing might have decreased disease stigma, which likely resulted in an increase in the number of both tests performed. Also, as more patients were eligible for HIV testing upon dual-routine testing implementation, medical assistants simultaneously discussed both tests with patients, which led to more HCV tests offered and performed.
Our data show that the percentage increase in HCV/HIV-positive cases detected surpassed the percentage increase in tests performed, indicating that an increase in cases diagnosed was not solely attributable to an increase in testing volume. The reason for this discrepancy might be because increased testing also decreased the missed opportunities for HCV and HIV detection at the two CHCs conducting universal testing (i.e., Mary Howard and Care Clinic). Dual-routine HCV/HIV testing would have allowed more high-risk patients that were missed before dual-routine testing to be tested after dual-routine testing was implemented.
Our percentage of HCV antibody-positive patients who received a confirmatory HCV RNA test was much higher than that seen nationwide and in Philadelphia.20,21 Reflexive testing technology likely resulted in high confirmatory test numbers, as it relies on one blood draw to convey the initial and confirmatory result and eliminates the need for patients to come to the clinic for a second blood draw. This testing technology also led to a more meaningful follow-up clinical visit where confirmed positive patients were educated about their result and were promptly referred to specialists.22
Laboratory price negotiation and designated grant funding for HCV and HIV tests performed on uninsured patients most likely circumvented the test cost as a barrier to routine testing.16,17 As a result, CHCs and patients did not incur any charges. The Hep C Project account also simplified payment for HCV and HIV tests for uninsured individuals because the charges were not on the same invoice as other non-grant-funded tests.
Two of our sites—Health Connection and Care Clinic—performed fewer HCV tests after dual-routine testing implementation. This decrease might be explained by the already high testing numbers before the dual-routine testing implementation period, a high number of patients younger than 18 years of age at Health Connection, and patients already diagnosed and in care at Care Clinic, which were exclusion criteria for our study.
Our observed HCV prevalence conflicts with published data, offering important epidemiologic patient insight within a CHC. Our current HCV infection prevalence before and after dual-routine testing implementation (4.6% and 5.3%, respectively) was higher than that reported for the general U.S. population (1.0%–2.0%), baby boomers (3.3%), and a Philadelphia community-based HCV/HIV testing initiative focused in a ZIP Code with high poverty and HIV seropositivity rates (2.8%).7,13,22 Our observed HIV seropositivity of new adult positives pre-dual-routine testing (0.2%) and post-dual-routine testing (0.3%) was higher than the recommended 0.1% positivity threshold for routine HIV testing recommendations from CDC.14 Both of these observed results suggest that dual-routine HCV/HIV screening identified more new positive patients in our study who would otherwise have gone undetected.
Among our patients with positive HCV antibody tests, only 70 of 101 (69.3%) pre-dual-routine testing and 101 of 158 (63.9%) post-dual-routine testing developed current HCV infection. These rates are contrary to the published data in which 75%–85% of individuals with a positive HCV antibody test go on to develop active HCV infection.10 Our reported rates of current infection also varied greatly across the four clinic sites, which warrants further investigation.
Currently infected HCV patients referred to specialists increased 7.3% between the two time periods. After dual-routine testing implementation, Mary Howard showed a drop in the number of patients who were aware of their infection and referred to specialists, from 16 of 19 (84.2%) to 17 of 28 (60.7%). A possible explanation for this drop could be that providers might not have been aware of the new HCV treatment regimens approved during our study. Provider -trainings—an important component of the model—could be an effective way to address future discrepancies. Another explanation for the observed numbers could be that more uninsured patients at the time of diagnosis resulted in a delay in referral. Providers did not refer patients until they obtained insurance to pay for the expensive treatment.
Among the CHCs, Care Clinic consistently showed the highest number of HCV- and HIV-positive patients linked to care before and after dual-routine testing, likely because primary care providers at Care Clinic treat both HCV- and HIV-infected patients. As such, providers did not refer HCV- and HIV-positive patients to an outside practice, except in extenuating circumstances (e.g., advanced liver disease or cirrhosis), when patients can fall out of care. Of the Mary Howard patients referred to an HCV specialist, an increased percentage was seen by medical specialists, from five of 16 (31.2%) to 12 of 17 (70.6%), between the two study periods. Mary Howard's improvement might be attributable to the services provided by our linkage-to-care coordinator, which included escorting currently infected HCV patients to specialist appointments at local academic medical centers. Our study showed that 75% of the HIV patients who were referred to a specialist were actually seen by a specialist between the screening test date and the end of data collection. Although it is possible that more patients saw specialists after the data analysis cutoff point, effective interventions need to be integrated to improve this step in the HIV care cascade. We did not report treatment outcomes, as we are still collecting these data.
Limitations
Our observed HCV and HIV prevalence data were subject to several limitations. Individuals offered dual-routine testing and whose results are provided in this article were engaged in care at one of the CHCs. As such, our data might misrepresent the true community HCV and HIV prevalence and incidence numbers. Another limitation was that linkage-to-care data do not currently include the time it took for specialists to see referred patients with current HCV infection. We are collecting and analyzing data across multiple subspecialty platforms for a follow-up study about linkage to care.
Our study also detected HCV among individuals outside of the 1945–1965 birth-year cohort. As a result, the two clinics that initially only performed high-risk-based HCV testing at the start of our project began universal (non-risk-based) HCV testing for individuals ≥18 years of age as of June 1, 2014. We plan to further analyze the effectiveness of a non-risk-based HCV testing intervention.
Finally, we collected data before Pennsylvania approved Medicaid expansion to increase the number of uninsured patients eligible for insurance. As a result, how insurance type and status will impact HCV care and treatment access remains to be seen.
CONCLUSION
This study provides a replicable template for the integration of dual-routine HCV/HIV testing across CHCs in the United States. Dual-routine HCV/HIV testing implementation in CHCs increases testing rates and seroprevalence detection and improves linkage to care.
Footnotes
The authors thank the medical staff and patients at Mary Howard Health Center, Care Clinic, Health Connection, and Rising Sun for making this study possible. The authors also thank Grace Lee from the National Nursing Centers Consortium for extracting the data from the electronic medical records and Maja Zecevic, PhD, MPH, a Gilead Sciences, Inc. paid consultant, for her editorial assistance.
Catelyn Coyle is a medical advisory member of Gilead Sciences, Inc., for which she received honoraria and travel compensation. This study was supported by grants from the Centers for Disease Control and Prevention (CDC) and HIV on the Frontline of Communities in the United States (FOCUS), a Gilead Sciences, Inc., program. The views presented in this article do not necessarily represent the views of CDC and/or Gilead Sciences, Inc.
The study was considered exempt from institutional review board review because it analyzed program monitoring data that did not include any personally identifiable information from the patients.
REFERENCES
- 1.Branson BM. The future of HIV testing. J Acquir Immune Defic Syndr. 2010;55(Suppl 2):102–5. doi: 10.1097/QAI.0b013e3181fbca44. [DOI] [PubMed] [Google Scholar]
- 2.Marks G, Crepaz N, Senterfitt JW, Janssen RS. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: implications for HIV prevention programs. J Acquir Immune Defic Syndr. 2005;39:446–53. doi: 10.1097/01.qai.0000151079.33935.79. [DOI] [PubMed] [Google Scholar]
- 3.Whiteside YO, Cohen SM, Bradley H, Skarbinski J, Hall HI, Lansky A. Progress along the continuum of HIV care among blacks with diagnosed HIV—United States, 2010. MMWR Morb Mortal Wkly Rep. 2014;63(5):85–9. [PMC free article] [PubMed] [Google Scholar]
- 4.Dinenno EA, Oster AM, Sionean C, Denning P, Lansky A. Piloting a system for behavioral surveillance among heterosexuals at increased risk of HIV in the United States. Open AIDS J. 2012;6:169–76. doi: 10.2174/1874613601206010169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denniston MM, Jiles RB, Drobeniuc J, Klevens RM, Ward JW, McQuillan GM, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160:293–300. doi: 10.7326/M13-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ward JW, Valdiserri RO, Koh HK. Hepatitis C virus prevention, care, and treatment: from policy to practice. Clin Infect Dis. 2012;55(Suppl 1):58–63. doi: 10.1093/cid/cis392. [DOI] [PubMed] [Google Scholar]
- 7.Ward JW, Lok AS, Thomas DL, El-Serag HB, Kim WR. Report on a single-topic conference on “chronic viral hepatitis—strategies to improve effectiveness of screening and treatment.”. Hepatology. 2012;55:307–15. doi: 10.1002/hep.24797. [DOI] [PubMed] [Google Scholar]
- 8.Denniston MM, Klevens RM, McQuillan GM, Jiles RB. Awareness of infection, knowledge of hepatitis C, and medical follow-up among individuals testing positive for hepatitis C: National Health and Nutrition Examination Survey 2001–2008. Hepatology. 2012;55:1652–61. doi: 10.1002/hep.25556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tohme RA, Xing J, Liao Y, Holmberg SD. Hepatitis C testing, infection, and linkage to care among racial and ethnic minorities in the United States, 2009–2010. Am J Public Health. 2013;103:112–9. doi: 10.2105/AJPH.2012.300858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen SL, Morgan TR. The natural history of hepatitis C virus (HCV) infection. Int J Med Sci. 2006;3:47–52. doi: 10.7150/ijms.3.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghany MG, Strader DB, Thomas DL, Seeff LB American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335–74. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C virus prevalence among patients infected with human immunodeficiency virus: a cross-sectional analysis of the US adult AIDS clinical trials group. Clin Infect Dis. 2002;34:831–7. doi: 10.1086/339042. [DOI] [PubMed] [Google Scholar]
- 13.Smith BD, Morgan RL, Beckett GA, Falck-Ytter Y, Holtzman D, Teo CG, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965 [published erratum appears in MMWR Recomm Rep 2012;61:43):886] MMWR Recomm Rep. 2012;61(RR-4):1–32. [PubMed] [Google Scholar]
- 14.Branson BM, Handsfield HH, Lampe MA, Janssen RS, Taylor AW, Lyss SB, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1–17. [PubMed] [Google Scholar]
- 15.Morin SF, Kelly JA, Charlebois ED, Remien RH, Rotheram-Borus MJ, Cleary PD. Responding to the National HIV/AIDS Strategy—setting the research agenda. J Acquir Immune Defic Syndr. 2011;57:175–80. doi: 10.1097/QAI.0b013e318222c0f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jewett A, Garg A, Meyer K, Wagner LD, Krauskopf K, Brown KA, et al. Hepatitis C virus testing perspectives among primary care physicians in four large primary care settings. Health Promot Pract. 2015;16:256–63. doi: 10.1177/1524839914532291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mimiaga MJ, Johnson CV, Reisner SL, VanDerwarker R, Mayer KH. Barriers to routine HIV testing among Massachusetts community health center personnel. Public Health Rep. 2011;126:643–52. doi: 10.1177/003335491112600506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coyle C, Viner K, Hughes E, Kwakwa H, Zibbell JE, Vellozzi C, et al. Identification and linkage to care of HCV-infected persons in five health centers—Philadelphia, Pennsylvania, 2012–2014. MMWR Morb Mortal Wkly Rep. 2015;64(17):459–63. [PMC free article] [PubMed] [Google Scholar]
- 19.Momplaisir F, Yehia BR, Harhay MO, Fetzer B, Brady KA, Long JA. HIV testing trends: Southeastern Pennsylvania, 2002–2010. AIDS Patient Care STDS. 2014;28:303–10. doi: 10.1089/apc.2014.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yehia BR, Schranz AJ, Umscheid CA, Lo Re V. The treatment cascade for chronic hepatitis C infection in the United States: a systematic review and meta-analysis. PLoS One. 2014;9:101554. doi: 10.1371/journal.pone.0101554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viner K, Kuncio D, Newbern EC, Johnson CC. The continuum of hepatitis C testing and care. Hepatology. 2015;61:783–9. doi: 10.1002/hep.27584. [DOI] [PubMed] [Google Scholar]
- 22.Spradling PR, Tong X, Rupp LB, Moorman AC, Lu M, Teshale EH, et al. Trends in HCV RNA testing among HCV antibody-positive persons in care 2003–2010. Clin Infect Dis. 2014;59:976–81. doi: 10.1093/cid/ciu509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trooskin SB, Poceta J, Towey CM, Yolken A, Rose JS, Lugman NL, et al. Results from a geographically focused, community-based HCV screening, linkage-to-care, and patient navigation program. J Gen Intern Med. 2015 doi: 10.1007/s11606-015-3209-6. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]