Abstract
Objective
The Centers for Disease Control and Prevention has recommended emergency department (ED) opt-out HIV screening since 2006. Routine screening can prove challenging due to the ED's complexity and competing priorities. This study examined the implementation and evolution of a routine, integrated, opt-out HIV screening program at an urban academic ED in Alabama since August 2011.
Methods
ED routine, opt-out HIV screening was implemented as a standard of care in September 2011. To describe the outcomes and escalation of the screening program, data analyses were performed from three separate data queries: (1) encounter-level HIV screening questionnaire and test results from September 21, 2011, through December 31, 2013; (2) test-level, fourth-generation HIV results from July 9 through December 31, 2013; and (3) daily HIV testing rates and trends from September 9, 2011, through June 30, 2014.
Results
Of the 46,385 HIV screening tests performed, 252 (0.5%) were confirmed to be positive. Acute HIV infection accounted for 11.8% of all HIV patients identified using the fourth-generation HIV screening assay. Seventy-six percent of confirmed HIV-positive patients had successful linkage to care. Implementation of fourth-generation HIV instrument-based testing resulted in a 15.0% decline in weekly HIV testing rates. Displacement of nursing provider HIV test offers from triage to the bedside resulted in a 31.6% decline in weekly HIV testing rates.
Conclusion
This program demonstrated the capacity for high-volume, routine, opt-out HIV screening. Evolving ED challenges require program monitoring and adaptation to sustain scalable HIV screening in EDs.
The Centers for Disease Control and Prevention (CDC) has endorsed emergency department (ED) opt-out human immunodeficiency virus (HIV) screening since 2006.1 Despite this recommendation, ED-based opt-out HIV screening uptake has been slow due to costs and competing challenges within this setting.2–4 Recent routine, opt-out HIV screening models implemented by EDs have reported varying opt-out rates of 11.1%–76.0%.5–11 Despite broad ranges in screening efficacy, ED-based routine, opt-out screening programs consistently identify HIV prevalence rates of 0.1%–1.7%.5 The cumulative impact of ED-based screening programs remains critical to mitigating the nation's HIV epidemic through increased HIV awareness and linkage to care for disproportionately affected populations.
This study examines our experiences during implementation and evolution of an integrated, routine, opt-out HIV screening program embraced by an urban academic ED from August 2011 through June 2014. We studied program planning and infrastructure development, screening protocols, clinician and staff member roles, operational modifications, program outcomes, and the impact of programmatic changes on temporal trends in daily testing rates.
METHODS
Clinical setting
In 2010, the Alabama Department of Public Health contracted with the University of Alabama at Birmingham (UAB) Emergency Department (UAB-ED) to provide routine HIV screening services. As the only Level 1 trauma center in Alabama, the UAB-ED manages approximately 64,000 visits annually and predominately serves diverse and underserved populations, including patients who are African American (53%), nonwhite, from other races/ethnicities (3%), uninsured (33%), or insured under Medicare or Medicaid (33%). A dedicated 24/7 accredited laboratory supports the 56-bed facility.
Program overview and pilot testing
The integrated UAB-ED screening model aimed to provide routine, opt-out HIV screening services compliant with CDC's 2006 recommendations.1 UAB-ED screening protocols were designed to emphasize patient confidentiality, minimize ED workflow disruption, deliver initial HIV results and counseling for preliminary positive cases prior to discharge, and support effective linkage to care.
Most individuals aged 18–64 years who presented to the UAB-ED with non-life-threatening conditions were eligible for HIV screening, including prisoners and non-English-speaking people. Patients excluded from screening included (1) medically/surgically unstable patients, (2) those unable to respond to the HIV pre-screening questionnaire, (3) those reporting an HIV-positive status or HIV-negative test result performed in the past three months, and (4) those who opted out of HIV screening services.
We pilot-tested HIV screening operations from August through September 2011 during daytime hours (7 a.m.–4 p.m.) to identify potential problems with electronic HIV screening questionnaires and automated test orders, as well as barriers to efficient laboratory procedures. A medical laboratory technician (MLT) devoted to HIV testing during the pilot phase managed all HIV testing in a Clinical Laboratory Improvements Amendment-certified12 UAB-ED laboratory. Full-scale screening operations began in October 2011 with support from three full-time, HIV-devoted MLTs. Certain screening operations have since evolved in various ways to further enhance program efficiency. We address operational enhancements in the following sections.
Workflow
To minimize UAB-ED workflow disruption, we integrated HIV routine, opt-out screening procedures into existing electronic health records (EHRs) by adding the following HIV testing eligibility questions to the triage nursing assessment completed during initial patient evaluations: (1) “Have you been tested for HIV?”, (2) “If yes, when were you last tested?”, and (3) “What was the result of the test?” UAB-ED triage nurses delivered the following statement to patients who had never been tested, were unaware of their prior test results, or had not been tested in the past three months: “We provide a free and confidential, rapid HIV test for all emergency department patients. Please let me know if you have any questions or concerns.” If the patient's responses indicated eligibility, and the patient did not opt out, the EHR generated an automated order for an HIV test to be performed during the ED visit. Nursing providers were trained to deliver HIV screening questionnaires during scheduled sessions before the program launched. Translation services were available to non-English-speaking individuals.
Shortly after full-scale implementation, numerous duplicate tests were identified for individuals previously tested in the UAB-ED within the past three months. To reduce duplicate testing, an EHR rule was developed to cancel automated HIV test orders for people with a documented, nonreactive result in the past 90 days. UAB-ED providers could independently order diagnostic HIV tests if deemed medically necessary.
HIV testing
From initiation through July 2013, MLTs used the OraQuick ADVANCE® Rapid HIV-1/2 Antibody Test (Orasure Technologies, Bethlehem, Pennsylvania) for screening. To offset anticipated high-volume HIV screening and required laboratory labor, we hired three additional HIV-devoted MLTs to individually cover peak volume hours in the UAB-ED. During their absence, preexisting UAB-ED laboratory personnel managed screening tests. Assays were performed using whole-blood specimens obtained during diagnostic work-up or by a separate finger stick executed by the MLTs. MLTs maintained quality control of assay performance. All HIV-antibody-positive individuals had reflexive confirmatory test specimens collected during the UAB-ED encounter. Western blot test results were available within one week of collection.
Counseling and linkage to care
ED result delivery and counseling.
After a preliminary HIV-positive result was documented, patients were counseled on how to reduce the risk of transmission and were linked to HIV care resources. MLTs performing the HIV screening test documented all results in the EHR and personally delivered all reactive results to the UAB-ED physician. The ED provider received a packet of HIV-related materials from the MLT, which outlined the next steps toward linkage to care, provided posttest counseling guidance, and included basic information for the patient about HIV, linkage-to-care steps, and directions to the HIV clinic. Before program initiation, UAB-ED physicians were trained on HIV posttest counseling through didactics and simulated cases. Annual posttest counseling training sessions were performed to educate new faculty and residents. UAB-ED physicians provided posttest counseling to all preliminary HIV-positive individuals. Posttest counseling for these results was tailored to the individual based on his or her HIV risk and clinical presentation.
Linkage-to-care process.
The UAB-ED has worked closely with the UAB 1917 HIV/AIDS Clinic since the beginning of this project. The clinic created a linkage coordinator position whose job was to respond to all individuals who were preliminarily positive in the UAB-ED. UAB-ED MLTs contacted coordinators to deliver preliminary and ribonucleic acid confirmatory tests results. The coordinator also received separate electronic notification of preliminary positive results and contacted each newly diagnosed individual within one to two business days to schedule a linkage visit, with the goal of completing the initial linkage visit within one week of diagnosis. The purpose of the visit was to confirm and explain the HIV results, respond to initial emotional and psychological concerns, review modes of transmission and prevention measures, discuss health-care options for optimal linkage to care, and set the first appointment at the appropriate clinic. Linkage-to-care visits could also take place at the UAB-ED or in the hospital if a patient was admitted or felt to be at risk for loss to follow-up care (e.g., unstable housing/homeless). Through a shared EHR, the UAB-ED and UAB 1917 HIV/AIDS Clinic could access each other's clinical documentation and confirmatory test (e.g., Western blot) results from UAB-ED.
The majority (84.0%) of patients receiving care at the UAB-ED resided within Jefferson County and the four surrounding counties, and were linked to clinics in the Greater Birmingham area. During the first year of expanded testing, the linkage coordinator built relationships with these clinics and became aware of statewide resources allowing for optimal linkage-to-care and active referrals to acquired immunodeficiency syndrome (AIDS) service organizations and other community resources. These relationships were critical in helping patients subsequently link to longitudinal health care and for our program to measure longitudinal outcomes.
Changes made during the program
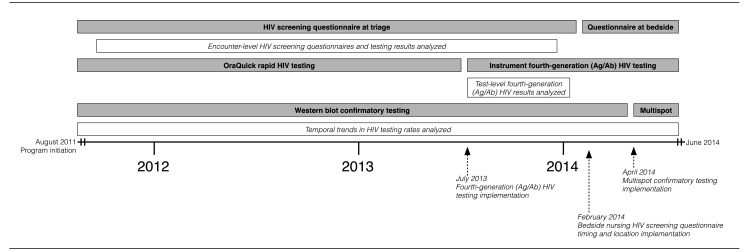
Our program evolved through numerous methodological changes to adapt to new testing technology, improve efficiency, decrease costs, and compensate for the competing challenges in a simultaneously evolving high-volume ED (Figure 1).
Figure 1.
Timeline of programmatic changes in HIV test offering, conversion to fourth-generation HIV instrument-based testing, adoption of Multispot for confirmatory testing, and data acquisition, University of Alabama at Birmingham Emergency Department, August 2011 through June 2014
HIV = human immunodeficiency virus
Ag/Ab = antigen/antibody
Fourth-generation HIV testing implementation.
In July 2013, fourth-generation HIV testing through the ARCHITECT i1000SR Immunoassay Analyzer (Abbott Diagnostics, Lake Forest, Illinois) was implemented. This instrument allows on-demand HIV assay performance without the need for sample batching and has a rapid, 28-minute wait for the first result. This fourth-generation HIV assay identifies acute HIV infection by measuring the p24 antigen, which shortens the window period from HIV exposure to detection.
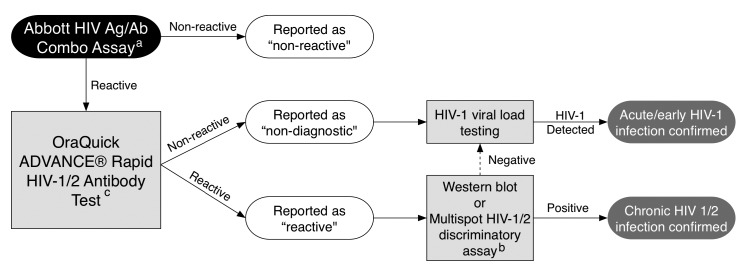
Pre-implementation of the fourth-generation assay required development of a new confirmatory testing algorithm (Figure 2) and system-wide reeducation on result interpretation.13 Additionally, Abbott Diagnostics trained MLTs on instrument performance and maintenance. Although the Multispot HIV-1/HIV-2 Rapid Test (Bio-Rad, Hercules, California) is recommended as the confirmatory test for fourth-generation testing algorithms, this assay was not available in the UAB-ED laboratory, and it was not possible to get the Multispot result during the ED encounter. All repeatedly -reactive fourth-generation assay specimens had a reflexive OraQuick ADVANCE Rapid HIV-1/2 Antibody Test performed on the same blood to reconfirm reactivity and identify potential acute infections.
Figure 2.
Fourth-generation HIV screening algorithm using supplemental OraQuick HIV testing to identify potential acute HIV infection at the point of care, University of Alabama at Birmingham Emergency Department laboratory, July 2013 to June 2014
aAll reactive specimens (signal/cutoff >0.99) would be micro-centrifuged and reanalyzed on the instrument up to two additional times to confirm reactivity per the manufacturer's recommendations.
bWestern blot was the confirmatory test for all reactive specimens until April 2014. Multispot was in the confirmatory testing pathway on all reactive specimens starting in April 2014.
cOraQuick was used before the recommended multispot test was available but is not currently recommended as a confirmatory test.
HIV = human immunodeficiency virus
Ag/Ab = antigen/antibody
If the results of both the fourth-generation and the rapid test were reactive, the sample was reported “reactive.” Reactive specimens were confirmed after the ED encounter in the UAB central laboratory by either Western blot (prior to May 2014) or Multispot (after April 2014). If the fourth-generation assay was reactive, and the rapid test was nonreactive, the result would be reported in the EHR as “non-diagnostic.” Non-diagnostic specimens would be sent for HIV-1 viral load confirmation to identify acute HIV infection, which required a separate specimen collection in a 4-milliliter ethylenediaminetetraacetic acid tube. All final confirmatory results (i.e., Western blot or HIV viral load) were not available during the UAB-ED visit and were reported to patients by our linkage team on subsequent contact. The UAB-ED provider and the UAB-ED program director discussed all non-diagnostic fourth-generation results, which indicate the potential for acute HIV infection, by telephone. Although transition to this new instrument and testing algorithm was time-intensive, implementation of the fourth-generation testing algorithm achieved more efficient confirmation of HIV diagnosis and identification of acute HIV infection.
Nursing HIV screening questionnaire.
In January 2014, the nursing HIV screening questionnaire transitioned from a required triage EHR form at initial contact to an EHR-prompted ad hoc questionnaire form to be filled out once the patient arrived in an ED treatment room. This change reduced the burden of screening questions performed by triage nurses and improved triage efficiency.
Data collection and analysis
Data were collected through a standard query of the UAB-ED EHR. Due to programmatic changes in data collection, the following analyses were performed from separate data queries: (1) encounter-level HIV screening questionnaire and test results from September 21, 2011, to December 31, 2013; (2) test-level, fourth-generation HIV results from July 9 through December 31, 2013; and (3) daily HIV testing rates and trends from September 9, 2011, to June 30, 2014 (Figure 1).
To assess trends in the number of individuals screened during the study period, we used various time-series functionalities available in Stata® release 12.14 We plotted the total number of patients screened per week, with reference to several important events: (1) full-scale implementation occurring in the 39th week of 2011, (2) requirement of venipuncture-only specimens in the 41st week of 2013 with fourth-generation HIV testing, and (3) movement of the nursing HIV questionnaire from triage to bedside in the fourth week of 2014. To identify a smoothed-curve representation of time trends in screening, we applied the Hodrick-Prescott filter, which is a popular method of decomposition in the macroeconomic literature.15–17 The mean number of patients screened during three time periods related to the aforementioned events were compared, specifying 95% confidence intervals (CIs).
RESULTS
Overall encounter-level HIV screening results
From September 21, 2011, through December 31, 2013, the UAB-ED had 128,748 health-care encounters from 72,462 unique individuals. Among those encounters, 115,225 (89.5%) encounters had the nursing HIV questionnaire performed. We excluded questionnaire data from September 9 through September 21, 2011, and after January 1, 2014, from analysis due to differences in the data collection methods. Among HIV screening questionnaire results, 75,261 (65.3%) encounters were eligible for HIV screening, 39,964 (34.7%) encounters were ineligible for HIV screening, and 189 (0.2%) encounters had incomplete data to determine screening eligibility (Figure 3).
Figure 3.
HIV screening questionnaire and testing results by encounter flowchart, University of Alabama at Birmingham Emergency Department, September 21, 2011, through December 31, 2013
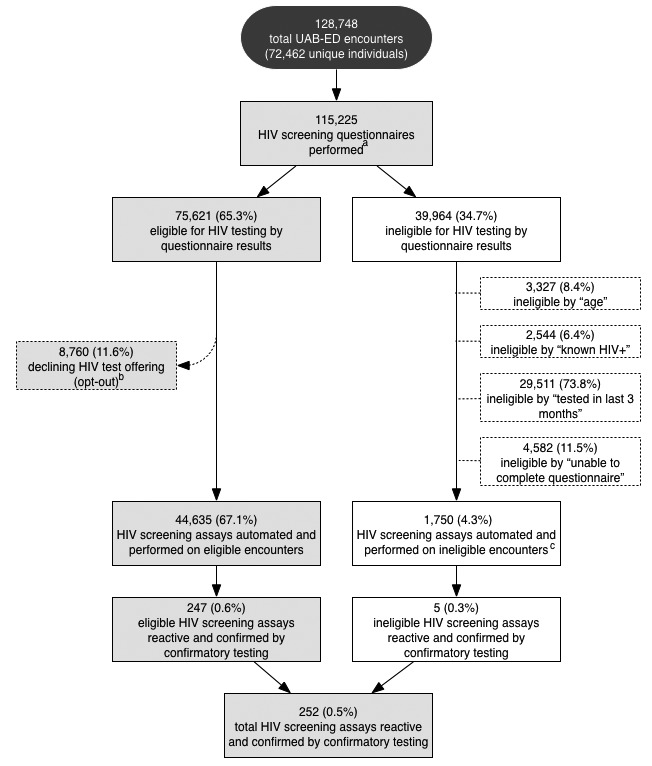
aA total of 13,332 HIV screening questionnaires were not performed due to severity of illness. A total of 189 screening questionnaires were excluded due to incomplete data to determine eligibility.
bA total of 45 diagnostic HIV tests were performed on encounters initially opting out of testing and two were confirmed to have chronic HIV infection.
cThese ineligible encounters incorrectly were offered the opt-out HIV screening test and had the test performed. Those who had an HIV test within the last three months and those aged <18 or >65 years were ineligible for testing.
UAB-ED = University of Alabama at Birmingham emergency department
HIV = human immunodeficiency virus
Ag/Ab = antigen/antibody
Among the 75,261 eligible encounters, 8,760 (11.6%) opted out of screening. The remaining 66,501 encounters had an HIV screening assay order automated by the EHR. A total of 21,866 (32.9%) automated orders did not have an HIV test performed. Reasons for not having a test performed included patient discharge/departure from the ED before specimen collection or a lack of specimen receipt by the laboratory. A total of 44,635 (67.1%) tests were performed among eligible encounters with automated orders. Of the 44,635 HIV screening tests performed on eligible individuals, 257 (0.6%) had a reactive HIV screening assay by rapid testing or fourth-generation instrument testing (Figure 3). Among the 257 reactive HIV screening tests, 243 patients were confirmed to have chronic HIV infection by Western blot, four were confirmed to have acute HIV infection by viral load testing, six had non-diagnostic screening results with negative viral load testing, and four were false positives.
Among 39,964 individuals determined to be ineligible by the HIV screening questionnaire, 1,750 (4.3%) had automated screening tests performed by nursing miscalculations of eligibility. Reasons for ineligibility among these encounters included either an HIV test within the last three months or age ineligibility. Among the 1,750 tests performed despite ineligibility by the HIV questionnaire, five (0.3%) were reactive and confirmed to be HIV positive. The ED providers overrode the protocol due to clinical suspicion and manually ordered 45 HIV tests for diagnostic purposes on encounters with an HIV screening questionnaire indicating opt-out decline for testing. Two of these diagnostic tests were reactive and confirmed chronic HIV infection. In total, 252 (0.5%) of 46,385 HIV-tested encounters had confirmed identification of HIV infection by Western blot or HIV-1 viral load testing (Figure 3).
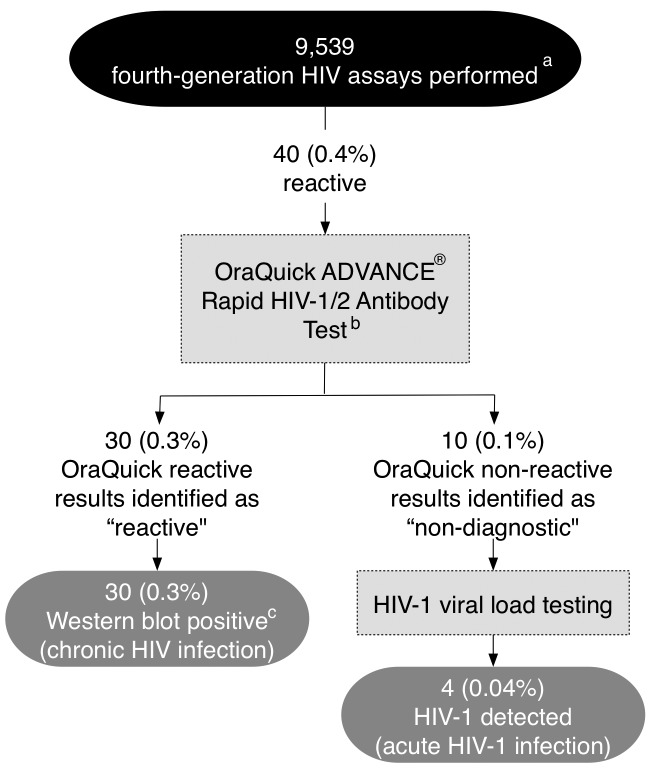
Identification of acute HIV infection
From July 9 through December 31, 2013, a total of 9,539 fourth-generation HIV assays were performed. Thirty (0.3%) tests were reactive and confirmed chronic infection. Ten (0.1%) were non-diagnostic, four of which confirmed acute HIV-1 infection by HIV-1 viral load testing (Figure 4).
Figure 4.
Identification of acute HIV infection using fourth-generation HIV assay, University of Alabama at Birmingham Emergency Department, July 9 through December 31, 2013
aAll initial reactive specimens (signal/cutoff >0.99) would be micro-centrifuged and reanalyzed on the instrument up to two additional times to confirm repeated reactivity per the manufacturer's recommendations prior to identifying the specimen as reactive.
bOraQuick was used before the recommended multispot test was available but is not currently recommended as a confirmatory test.
cWestern blot was used for confirmation of chronic HIV infection due to the lack of availability of an HIV-1/HIV-2 discriminatory assay for confirmation during the project period described. If a specimen with a negative or indeterminate Western blot had occurred, HIV-1 viral load testing would have been initiated.
HIV = human immunodeficiency virus
Methodological changes impacting HIV screening volume, September 9, 2013, through June 30, 2014
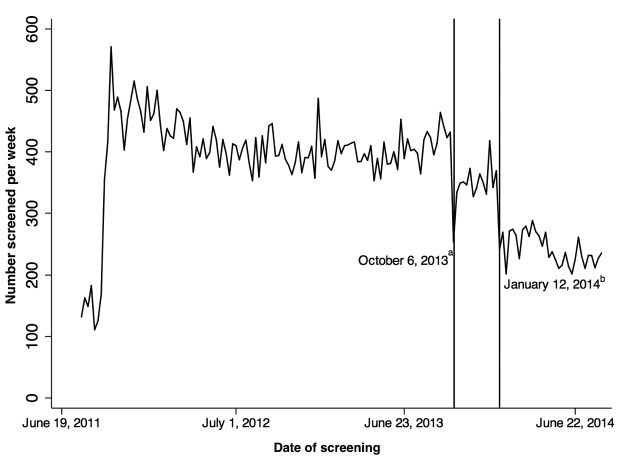
Analysis of weekly HIV screening tests revealed trends in HIV screening rates (Figure 5). During the first 13 weeks of UAB-ED HIV screening—before reaching full capacity—100–175 HIV screening tests were performed per week. We achieved full laboratory staffing and wide-scale implementation in the 13th week after initiation. From initiation through the 40th week of 2013, 415 HIV screening tests were performed weekly.
Figure 5.
Impact of HIV screening programmatic changes implementing fourth-generation instrument-based testing and displacement of HIV test offering from nursing triage on weekly volume of HIV tests performed, University of Alabama at Birmingham Emergency Department, September 9, 2011, through June 30, 2014
aReference line at October 6, 2013, represents venipuncture-only specimen collection for fourth-generation HIV testing. Reference line at January 12, 2014, represents the first week that the nursing HIV screening questionnaire was moved.
bMean number screened per week from September 18, 2011, to October 5, 2013 = 415 (95% confidence interval [CI] 407, 422); October 13, 2013, to January 11, 2014 = 354 (95% CI 340, 367); and January 19, 2014, to August 23, 2014 = 242 (95% CI 233, 251).
HIV = human immunodeficiency virus
In the 41st week of 2013, fourth-generation HIV testing using exclusively venipuncture specimens for the ARCHITECT i1000SR Immunoassay Analyzer completely replaced rapid OraQuick testing using venipuncture or finger stick specimens. This change resulted in a moderate 15% decline from 415 to 354 (95% CI 340, 367) weekly HIV tests performed (Figure 5).
In the fourth week of 2014, the nursing HIV screening questionnaire was moved from the nursing primary assessment performed at triage to a separate, non-required, ad hoc form completed by nursing providers on arrival to an ED room. From the fourth through the 26th week of 2014, weekly HIV screening declined another 31.6%, from 354 to 242 (95% CI 233, 251) weekly HIV tests performed (Figure 5).
Linkage-to-care results
As of December 31, 2013, a total of 252 individuals had received confirmed positive results, and 76% of these individuals had been linked to care (confirmed arrival at both intake/orientation and first provider visit).
DISCUSSION
Routine HIV screening in EDs can be challenging because of the ED's complexity and competing priorities. HIV screening to scale can be limited by multiple challenges, including methods of patient engagement, ED provider adherence to protocols, timing of result delivery, and assay selection. We owe our achievements to the integration of HIV screening into ED clinical care, which required the shared efforts of all ED stakeholders (nurses, laboratory technicians, and physicians). EHR requirements for nursing HIV screening questionnaires and automated test orders for individuals eligible for HIV screening were essential to maintain the highest efficiency.
Some programmatic changes had a negative impact on testing rates over time. We noted a 31.5% decline in weekly HIV tests performed, from 354 to 242, when the required nursing HIV screening questionnaire was moved from its initial location in the ED triage assessment form to a specific ad hoc form the nurses could generate when patients entered an examination room. Although we did not measure them, reasons for decreased adherence to the screening questionnaires included both EHR prompt fatigue and inconvenient placement in the workflow. Additionally, other ED-based programs have identified decreased nursing provider compliance with HIV prescreening questionnaires due to discomfort with the screening offering.18
We also experienced a decrease in our overall HIV testing rate when we moved to a fourth-generation instrument-based HIV testing platform. We owe this decline to the requirement for venipuncture of fourth-generation assays vs. the finger stick sampling for rapid tests. We justified this decline by the increased yield in identification of acute infections that would be missed by current U.S. Food and Drug Administration-approved rapid HIV testing technologies. Additionally, the performance characteristics of our fourth-generation instrument-based assay allowed for a rapid 28-minute wait until first result, lower costs, better quality control, lessened workload for MLTs, and result interface with the EHR. Prior use of instrument-based ED third-generation HIV testing revealed similar benefits; however, the assay used resulted in a two-hour turnaround time, and 25% of newly diagnosed HIV-infected individuals did not obtain their results during the ED visit.11
Overall, a significant proportion (32.8%) of eligible encounters with automated HIV test orders did not have an HIV test performed during the ED visit. Multiple factors contributed to this nonperformance, including patient departure from the ED and patient discharge without a venipuncture or finger stick specimen available to the laboratory. In the future, efforts to reduce testing noncompliance will focus on specimen collection at the time of initial triage.
We also found that a moderate proportion (3.8%) of HIV tests were performed on ineligible encounters early in implementation. For this reason, we created a rule in the EHR to cancel HIV screening orders for people with an HIV nonreactive result documented in the EHR within the last three months. This rule limited ineligible tests performed during the project period. As we transition to billing insurance providers for HIV screening tests, modification within the EHR will be required to further reduce incorrect HIV screening offerings for ineligible patients.
A high patient acceptance rate of the offered HIV test contributed to our high-volume screening. Our opt-out rate of 11.6% was low compared with widely varying ED opt-out rates of 11.1%–76.0% previously reported. White et al. previously reported a 76.0% opt-out rate when using registration staff members to perform the opt-out HIV test offering.19 In addition, two previous studies reported opt-out rates of 14.6% and 40.3% using a dedicated screening counselor to perform the opt-out HIV test offering.6,9 Similar to our model and result, Sattin et al. described an 11.1% opt-out rate using nursing staff members to offer the opt-out HIV test during the triage process.8 We posit that the reasons for our low opt-out rate included the test offering's routine nature, early integration of nursing stakeholders into testing workflow planning, and inclusion of the test offering in the initial ED nursing assessment.
During the screening period reported, physician providers performed diagnostic HIV tests based on clinical presentation on 45 encounters that had initially opted out. Among these opt-out encounters, 4.4% were confirmed to have chronic HIV infection. This finding highlights that engagement for HIV testing should not be limited to an initial opt-out offering. Patients identified during ED evaluation as at disproportionately high risk for HIV infection (i.e., sexually transmitted infection or high-risk behavior) or presenting with symptoms for acute infection or AIDS-defining illness should be reengaged by providers regarding the importance of being tested.
The recent CDC-endorsed fourth-generation HIV testing algorithm using an HIV-1/HIV-2 discriminatory assay for confirmation now allows for possible confirmation of chronic HIV infection and identification of acute HIV infection during the ED encounter. Because the current U.S. Food and Drug Administration-approved discriminatory assay, Multispot HIV-1/HIV-2 Rapid Test, was not available to our laboratory during the program period, we used OraQuick ADVANCE Rapid HIV-1/2 Antibody Test as a supplemental test in our fourth-generation testing algorithm to preliminarily confirm chronic HIV infection before Western blot. The use of our supplemental HIV testing increased confidence in result accuracy and delivery by ED providers and, more importantly, allowed for identification of possible acute HIV infections during the ED visit. The fourth-generation HIV assay's implementation substantially increased the yield of HIV infections identified, accounting for 11.8% of all confirmed HIV-positive cases during the period. A recent report from an ED performing routine screening using the same fourth-generation assay revealed similar benefits.20
The ED is in a crucial position to initiate interventions to help individuals link to care and establish the foundation for lifelong behaviors ensuring personal and public health. Collaboration with our local HIV clinic and having daily communication of HIV-positive cases occurring overnight and real-time communication during work hours were critical to our linkage success. The benefits of our screening program would be diminished without this effective linkage to care.
The tension between screening for disease and the ED's competing priorities will continue to be an issue as screening for other conditions, such as hepatitis C, are implemented in the ED setting. Maintenance of these programs will require continued engagement of all ED stakeholders to adapt to the common changes and challenges of this unique venue.
Limitations
HIV screening eligibility was determined by the individuals' self-reported HIV status. As a result, screening eligibility might have been affected by recall bias, denial, health literacy, and privacy concerns. We strived to overcome these limitations by conducting the questionnaires in private and training ED nurses to perform the questionnaires. Second, our encounter-level data do not differentiate between newly diagnosed and rediagnosed HIV infection. Third, a small number of patients declined or opted out of HIV screening services; results on these individuals could have amplified the observed seroprevalence rates.
Fourth, filters applied to time-trend analysis may not have detected small fluctuations in the number of patients screened per week, potentially resulting in minimal over- or underrepresentation of trends in the number of patients screened. A realistic application of program implementation planning activities, infrastructure development, and related outcomes may be limited to EDs with similar resource capacity and patient populations.
CONCLUSION
Our screening program demonstrated the capacity for high-volume ED HIV screening and effective linkage to care. Evolving ED challenges require program monitoring and adaptation to sustain ED HIV screening at scale.
Footnotes
The authors thank Susan Butler for her oversight of the laboratory testing process and development, and Kirema Brown and Shyla Campbell for their efforts to provide efficient linkage to care for individuals identified with human immunodeficiency virus infection (HIV) in the emergency department.
This study was approved by the University of Alabama at Birmingham Institutional Review Board (protocol #X131210002). HIV testing is supported through a contract with the Alabama Department of Public Health (CDC-PS10-10138).
REFERENCES
- 1.Branson BM, Handsfield HH, Lampe MA, Janssen RS, Taylor AW, Lyss SB, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1–17. [PubMed] [Google Scholar]
- 2.Waxman MJ, Popick RS, Merchant RC, Rothman RE, Shahan JB, Almond G. Ethical, financial, and legal considerations to implementing emergency department HIV screening: a report from the 2007 conference of the National Emergency Department HIV Testing Consortium. Ann Emerg Med. 2011;58(Suppl 1):33–43. doi: 10.1016/j.annemergmed.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 3.Mumma BE, Suffoletto BP. Less encouraging lessons from the front lines: barriers to implementation of an emergency department-based HIV screening program. Ann Emerg Med. 2011;58(Suppl 1):44–8. doi: 10.1016/j.annemergmed.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 4.Haukoos JS, Hopkins E, Hull A, Dean C, Donahoe K, Ruzas CM, et al. HIV testing in emergency departments in the United States: a national survey. Ann Emerg Med. 2011;58(Suppl 1):10–6. doi: 10.1016/j.annemergmed.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 5.Haukoos JS. The impact of nontargeted HIV screening in emergency departments and the ongoing need for targeted strategies. Arch Intern Med. 2012;172:20–2. doi: 10.1001/archinternmed.2011.538. [DOI] [PubMed] [Google Scholar]
- 6.Brown J, Shesser R, Simon G, Bahn M, Czarnogorski M, Kuo I, et al. Routine HIV screening in the emergency department using the new U.S. Centers for Disease Control and Prevention guidelines: results from a high-prevalence area. J Acquir Immune Defic Syndr. 2007;46:395–401. doi: 10.1097/qai.0b013e3181582d82. [DOI] [PubMed] [Google Scholar]
- 7.Haukoos JS, Hopkins E, Conroy AA, Silverman M, Byyny RL, Eisert S, et al. Routine opt-out rapid HIV screening and detection of HIV infection in emergency department patients. JAMA. 2010;304:284–92. doi: 10.1001/jama.2010.953. [DOI] [PubMed] [Google Scholar]
- 8.Sattin RW, Wilde JA, Freeman AE, Miller KM, Dias JK. Rapid HIV testing in a southeastern emergency department serving a semiurban-semirural adolescent and adult population. Ann Emerg Med. 2011;58(Suppl 1):60–4. doi: 10.1016/j.annemergmed.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 9.Wheatley MA, Copeland B, Shah B, Heilpern K, Del Rio C, Houry D. Efficacy of an emergency department-based HIV screening program in the Deep South. J Urban Health. 2011;88:1015–9. doi: 10.1007/s11524-011-9588-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White DA, Sadoun T, Tran T, Alter HJ. Increased acceptance rates of HIV screening using opt-out consent methods in an urban emergency department. J Acquir Immune Defic Syndr. 2011;58:277–82. doi: 10.1097/QAI.0b013e318231916d. [DOI] [PubMed] [Google Scholar]
- 11.Hoxhaj S, Davila JA, Modi P, Kachalia N, Malone K, Ruggerio MC, et al. Using nonrapid HIV technology for routine, opt-out HIV screening in a high-volume urban emergency department. Ann Emerg Med. 2011;58(Suppl 1):79–84. doi: 10.1016/j.annemergmed.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Medicare & Medicaid Services (US) Clinical Laboratory Improvement Amendments (CLIA) [cited 2015 Jun 16] Available from: https://www.cms.gov/regulations-and-guidance/legislation/CLIA.
- 13.Branson BM, Owen SM, Wesolowski LG, Bennett B, Werner BG, Wroblewski KE, et al. Laboratory testing for the diagnosis of HIV infection [cited 2014 Jun 27] Available from: http://stacks.cdc.gov/view/cdc/23447.
- 14.Stata Corp. Stata®: Release 12. College Station (TX): StataCorp; 2013. [Google Scholar]
- 15.Hodrick RJ, Prescott EC. Postwar US business cycles: an empirical investigation. J Money Credit Bank. 1997;29:1–16. [Google Scholar]
- 16.Kowal P. Matlab implementation of commonly used filter [cited 2015 Jul 1] Available from: https://ideas.repec.org/c/wpa/wuwppr/0507001.html.
- 17.Ravn MO, Uhlig H. On adjusting the Hodrick-Prescott filter for the frequency of observations. Rev Econ Stat. 2002;84:371–80. [Google Scholar]
- 18.Minniear TD, Gilmore B, Arnold SR, Flynn PM, Knapp KM, Gaur AH. Implementation of and barriers to routine HIV screening for adolescents. Pediatrics. 2009;124:1076–84. doi: 10.1542/peds.2009-0237. [DOI] [PubMed] [Google Scholar]
- 19.White DA, Scribner AN, Vahidnia F, Dideum PJ, Gordon DM, Frazee BW, et al. HIV screening in an urban emergency department: comparison of screening using an opt-in versus an opt-out approach. Ann Emerg Med. 2011;58(Suppl 1):89–95. doi: 10.1016/j.annemergmed.2011.03.032. [DOI] [PubMed] [Google Scholar]
- 20.Geren K, Moore E, Tomlinson C, Hobohm D, Gardner A, Reardon-Maynard D, et al. Detection of acute HIV infection in two evaluations of a new HIV diagnostic testing algorithm—United States, 2011–2013. MMWR Morb Mortal Wkly Rep. 2013;62(24):489–94. [PMC free article] [PubMed] [Google Scholar]