Abstract
Objective
Adolescents and young adults remain at high risk for new HIV infections and for unknowingly transmitting the virus to others. Yet, they have demonstrated low rates of testing due to barriers such as stigma and difficulty accessing testing services. Few existing programs have successfully integrated family planning and HIV care services to improve testing and diagnosis rates among young adults and adolescents, particularly those of minority groups. This study describes the process of implementing HIV services into family planning clinics and how to train staff in routine, opt-out testing.
Methods
This study used HIV screening data from 10 family planning clinics serving adolescents and young adults in Houston, Texas. A total of 34,299 patients were tested for HIV during a 48-month study period, from January 2010 through December 2014.
Results
Patients tested included minors <18 years of age (25.5%), males (22.8%), and individuals who had missed opportunities for HIV testing at other health-care settings. From the opt-in period (2006–2007) to the routine, opt-out period (2008–2010), the yearly average number of tests administered more than doubled; the yearly average increased again by 50% from the routine, opt-out period to the routine, rapid period (2011–2014). Eighty-eight (0.3%) patients were diagnosed with HIV, a higher seropositivity rate than CDC's recommended threshold of 0.1% for settings where routine screening is warranted.
Conclusion
Routine, opt-out HIV testing integrated into family planning clinics increased rates of testing acceptance, receipt of test results, and HIV-positive diagnoses among adolescents and young adults.
The human immunodeficiency virus (HIV) epidemic remains a prominent health concern for adolescents and young adults living in the United States, as individuals aged 13–24 years account for approximately one-quarter of all new HIV infections.1 Of those infected, more than 50% are unaware of their HIV-positive status. As such, those aged 13–24 years are at higher risk than older age groups of transmitting the virus to others. Young members of minority groups, particularly African American men who have sex with men (MSM), represent a disproportionate number of new HIV cases in adolescents and young adults. Studies have found that barriers to HIV prevention among adolescents and young adults include low perceived risk, low rates of testing due to stigma, concerns about confidentiality, and difficulty accessing testing services.2,3 A nationally representative sample of 6,628 adolescents and young adults who were interviewed by telephone indicated that fewer than one-third of participants had been tested for HIV. The majority of those screened received the test in a private physician's office (38.0%) or public clinic (31.7%), whereas only 3.1% had been tested at an HIV counseling and testing site.4
Studies have shown that adolescents and young adults are more likely to receive an HIV test if the test is offered vs. requested; if the test is given in a convenient setting, such as an emergency department or school- or community-based clinic; and if the result is available immediately.5–9 A study on HIV testing among individuals aged 13–22 years found that more than 70% of adolescents and young adults preferred rapid HIV testing, which produces preliminary results in 20 minutes, to traditional testing, which produces results after several days.10
In 2006, the Centers for Disease Control and Prevention (CDC) recommended that health-care settings implement routine, opt-out HIV testing to adolescents and adults aged 13–64 years, regardless of risk.11 In the opt-out model, patients are informed that an HIV test will be administered unless they specifically decline. Integrating routine, opt-out HIV screening in medical settings has been identified as a public health priority to reach the undiagnosed and ensure linkage to care.
Family planning (FP) clinics offer high-quality, low-cost health services, particularly to low-income women and men.12 The integration of routine HIV testing into these clinics might help normalize HIV testing, increase testing acceptability and receipt of results, improve health outcomes, and facilitate linkage to care for HIV-positive individuals.13–15 Several studies have reported encouraging outcomes associated with integrating FP clinics and HIV services, such as increased condom use and HIV testing; enhanced patient access to both FP and HIV care services; and improved quality of care.16–20 However, information on the success of these clinics in implementing routine HIV testing among adolescents, especially adolescent males, is limited. Therefore, we examined the implementation of routine, opt-out HIV testing in FP clinics serving adolescents and young adults, and ascertained the effectiveness of identifying individuals who were unaware of their HIV status. Considering the adverse health outcomes associated with undiagnosed HIV-positive individuals—such as unknowing transmission and late linkage to HIV care—it is important to identify integrated programs that effectively screen adolescents and young adults for HIV and connect them to services.
METHODS
Study setting
We gathered data from a network of 10 FP and primary care clinics in Houston, Texas, that are located in public hospitals, schools, and community settings with documented high rates of teen pregnancy and sexually transmitted infections (STIs). The clinics provide low-cost or free comprehensive FP and reproductive health services to adolescents and young adults aged 13–23 years who reside in Houston, which has a high incidence of HIV among adolescents and young adults. In 2006, an estimated 238 individuals aged 13–24 years were newly diagnosed with HIV in Houston/Harris County.21 By 2011, the number of new HIV cases in Houston/Harris County for those aged 13–24 years was an estimated 286 individuals, a 20.2% increase.22 This age group accounted for 22.8% of all new HIV diagnoses, despite comprising only 5% of the total Houston/Harris County population. The majority of newly HIV-positive individuals aged 13–24 years self-identified as either black or African American (64.7%) or Hispanic or Latino (26.2%), and reported acquiring HIV through MSM activity (73.4%).22
Based on internal reports, the majority of patients seen at the clinics in this study were black or African American and Hispanic or Latino, with the remainder self-identifying as white, Asian, or American Indian (Unpublished data. Smith PB, Buzi RS. Baylor Teen Health Clinic statistical reports. 2014). The average patient age was 18.6 years, and most patients were female (85.2%). Partners of patients, regardless of age, could receive STI and HIV services at the clinics. As per state statute, minors visiting these clinics had the right to consent to STI and HIV testing without parental approval, and the clinics were not required to notify a minor's parents about a positive result.23 Although many states have statutes allowing minors to consent to STI and HIV testing and treatment,24 Title X FP clinics, which offer cost-effective health services underwritten by the U.S. Department of Health and Human Services, are trained to not only meet the needs of adolescents,12 but also promote parent-child communication.25
Routine HIV testing implementation
Opt-in testing.
Prior to the implementation of routine, opt-out screening in 2008, the clinics conducted HIV testing using a risk-based, opt-in screening process through venipuncture blood draw testing. This approach resulted in low rates of HIV testing acceptance, HIV diagnoses, and follow-up, with 35%–38% of patients not returning to receive their results (Unpublished data. Smith PB, Buzi RS. Baylor Teen Health Clinic statistical reports. 2014).
Opt-out testing.
The clinics implemented routine, opt-out HIV testing in 2008. Due to lack of funding sources from 2008 to 2009, only 26% of the tests administered used rapid testing technology. In 2010, 42% of the administered tests used rapid testing technology. The transition to an opt-out system required several system-wide modifications, including a protocol change stemming from revised CDC recommendations to offer routine HIV testing to all clients, regardless of risk, and to eliminate written consent.11 The clinics involved the legal department to assure compliance with state regulations.
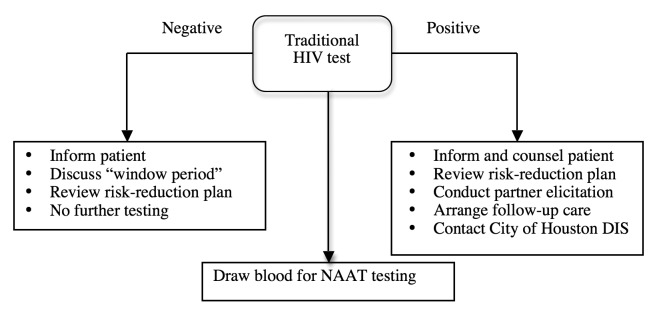
Staff member training was also needed to implement the routine, opt-out system. Obtaining input from staff members and engaging them in the transition process helped secure support for the new provider-initiated HIV testing approach. Staff members were instructed on the new consenting process, including how to use language compatible with the opt-out approach, how to convey information about the test, and how to deliver positive results. With the opt-out system, HIV testing shifted from a patient-initiated to a provider-initiated process. All patients were informed orally that an HIV test would be administered, and they had the opportunity to ask questions or decline testing. Declined HIV tests were documented in patient medical charts. To maintain patient autonomy, staff members were trained to ensure that patients had the capacity to understand the nature, purpose, and risks associated with the HIV test. Staff members also counseled patients on the possible emotional and mental health consequences of receiving an HIV test. The clinics ensured that linkage-to-care services were accessible for HIV follow-up. Step-by-step best practice guides for patient care, rapid HIV testing, and traditional venipuncture HIV testing were developed for staff members to follow when addressing clients (Figure 1; supplemental data available upon request).
Figure 1.
Traditional HIV screening guide used for staff member training at 10 family planning and primary care clinics in Houston, Texas, prior to January 2008
HIV = human immunodeficiency virus
DIS = disease intervention specialist
NAAT = nucleic acid amplification test
Opt-out rapid HIV testing.
In 2011, the clinics fully implemented routine, opt-out HIV screening using rapid HIV testing technology. The type of rapid HIV test used at the clinics was selected based on cost, shelf life, sensitivity, and specificity. The routine, rapid HIV testing approach included a confidential, point-of-service test followed by a 20-minute wait period to retrieve the preliminary results. Blood was drawn for a confirmatory test once the preliminary positive HIV test result was shared with the patient. The clinics developed quality assurance measures for storage and control practices to ensure the validity of the test and its compliance with the opt-out testing model. The clinics' leadership team provided regular, onsite clinical mentorship to staff members, and periodically provided data reports on the prevalence of HIV in the community and the significance of missed testing opportunities. The clinics also designated an HIV champion to motivate staff members and provide supportive supervision. The clinics developed several memorandums of understanding with various HIV service organizations to secure patients' linkage to HIV care.
Study procedures
The study included data from HIV testing of female and male adolescents and young adults who attended the network of 10 Houston FP clinics from January 1, 2011, through December 31, 2014. The clinics served patients aged 13–23 years and offered STI and HIV tests to their partners, regardless of age. Only demographic characteristics, such as race/ethnicity, age, and gender, were collected. HIV test results were abstracted from the clinics' internally generated data and tracking system. The data from 2011–2014 were referenced against data from 2006–2010 to compare testing outcomes.
RESULTS
During the 48-month study period, from 2011–2014, the clinics provided services to 39,698 patients, of whom 34,299 (86.4%) received an HIV test using rapid testing (Table 1). Of those tested, 20,191 (58.9%) self-identified as black/African American, 13,641 (39.8%) self-identified as white, 12,896 (37.6%) self-identified as Hispanic, and 7,820 (22.8%) were male. Ages ranged from <15 to ≥30 years, with 17,585 (51.3%) patients aged 20–24 years (Table 2).
Table 1.
Number of clients and HIV tests administered by 10 family planning and primary care clinics in Houston, Texas, January 2011–December 2014
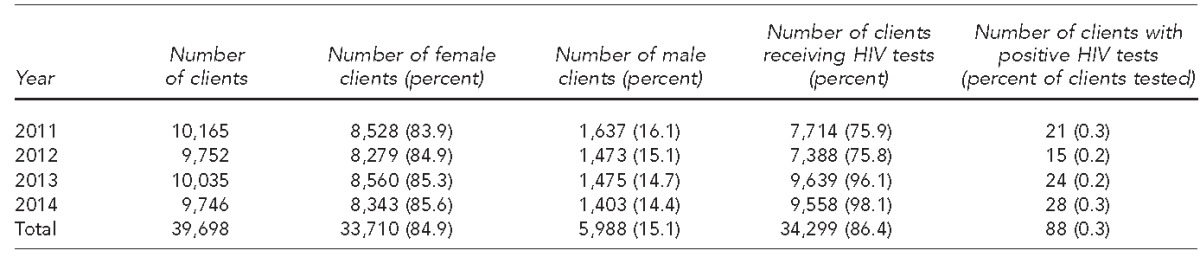
HIV = human immunodeficiency virus
Table 2.
Demographic characteristics and rates of HIV positivity of clients tested for HIV at 10 family planning and primary care clinics in Houston, Texas, January 2011–December 2014
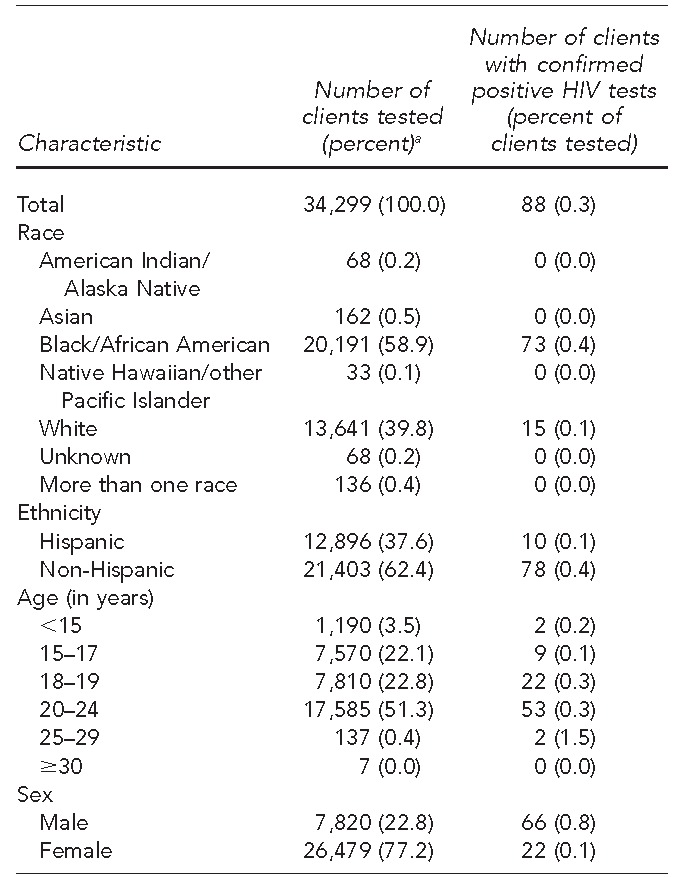
aPercentages may not total to 100 due to rounding.
HIV = human immunodeficiency virus
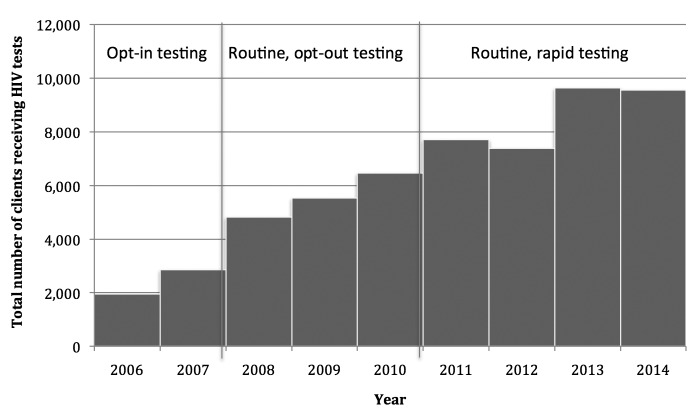
A comparison of HIV testing during the two reference periods, 2006–2007 and 2008–2010, showed that HIV testing more than doubled, from an average of 2,406 tests to an average of 5,611 tests, when the clinics implemented routine, opt-out HIV screening. The number of HIV tests administered increased by more than 50% when the clinics implemented routine, opt-out, rapid HIV testing during the 2011–2014 study period, from a mean of 5,611 tests to a mean of 8,575 tests. The yearly mean number of HIV tests administered from 2011 through 2014 was 3,392 tests more than the yearly mean from 2008 through 2009, an increase of 64.4% (Figure 2).
Figure 2.
Trends in HIV testing among clients served by 10 family planning and primary care clinics in Houston, Texas, January 2006–December 2014
HIV = human immunodeficiency virus
HIV-positive tests
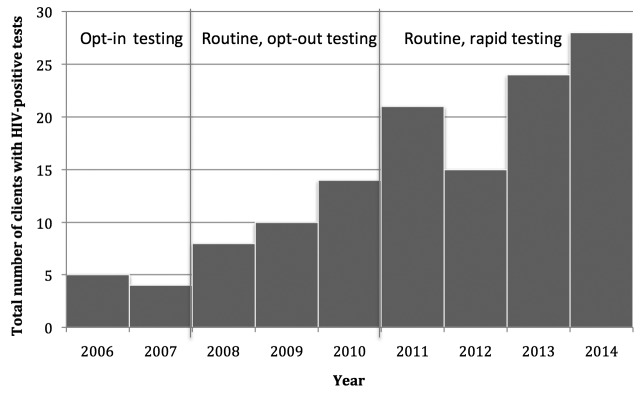
A total of 88 (0.3%) patients tested positive for HIV from 2011 through 2014, of whom 66 (75.0%) were male and 22 (25.0%) were female (Table 2). The mean age of HIV-positive patients was 20.1 years (range 15–25 years). Seventy-three patients self-identified as black/African American and 78 patients self-identified as non-Hispanic (Table 2). The HIV positivity rate for females was 0.1% and for males was 0.8%. The HIV positivity rates did not change from the opt-in to the opt-out period. However, the total number of HIV tests administered nearly doubled, and the percentage of HIV-positive diagnoses increased by 0.5% (Figure 3).
Figure 3.
Trends in HIV-positive cases among clients served by 10 family planning and primary care clinics in Houston, Texas, January 2006–December 2014
HIV = human immunodeficiency virus
Several individuals diagnosed with HIV reported visiting other health-care settings for various conditions, which could have been associated with HIV infection, but were not offered an HIV test. Several patients had been sexually abused and exposed to HIV for a while, but were not aware of their HIV status before visiting the clinics. One of these patients was a young pregnant female whose HIV infection was missed during an emergency room visit, but was diagnosed during routine HIV screening when she visited the clinics for a pregnancy test.
DISCUSSION
The study results demonstrated that the introduction of routine, opt-out HIV screening, in conjunction with system modifications and staff training, was effective in increasing testing acceptance, receipt of results, and HIV diagnoses. Our study results corroborated other studies, which showed testing rates were higher when HIV tests were included in standard care for adolescents and young adults.10,26
Our findings demonstrated that the study clinics were effective in reaching minors (aged ≤17 years). A total of 8,760 minors were tested for HIV during the study period, comprising 25.5% of all patients tested. Of those minors tested, 11 (0.1%) were diagnosed as HIV positive and were able to receive immediate linkage to care. A state assessment of HIV cases among seven FP clinic networks in Texas, including the system of 10 Houston clinics involved in this study, indicated that the clinics in this study were responsible for 57% of the confirmed HIV-positive cases reported.27 Studies have shown that minors are concerned about the need for parental consent in accessing services28 and may be deterred from receiving HIV testing because of parental consent.29 Although the minors in this study were able to access testing without parental consent based on state statutes, the majority of minors agreed to involve their parents in a conversation about their HIV test results following diagnosis. Thus, the clinic had the opportunity to provide HIV education and discuss options and resources for follow-up care to both minors and their parents. The disproportionately high rates of HIV among adolescents and young adults highlight the importance of early diagnosis, which can lessen the time in which an HIV-positive individual may unknowingly transmit the infection to unsuspecting partners.
Although women comprise the majority of clients at publicly funded FP clinics, a substantial number of patients who access the study's clinics are male. The proportion of males receiving services in the study's clinics was higher than that of Title X-funded clinics nationwide (15% vs. 8%).30 These data are promising, as studies show that an increase in HIV testing for young males is linked to enhanced opportunities to diagnose HIV-positive patients and link them to care.31 With males accounting for nearly one-quarter of the HIV tests administered and three-quarters of the positive diagnoses, the clinics in this study were effective in reaching out to males by promoting the clinics as safe, supportive environments. In similar HIV and FP integration studies at urban clinics, males comprised <5% of patients accessing HIV services.15,32
The disproportionate ratio of diagnoses reflects the primary modes of transmission among males and females. The main mode of transmission among HIV-positive males at the clinics was MSM intercourse, while the mode for females was heterosexual intercourse. A comparison of risk behaviors among 92 males who visited the clinics in this study, of whom 46 were HIV positive, found that males who tested HIV positive were more likely to identify as MSM than those who were HIV negative.33 These increased rates of diagnosis among young MSM, particularly African American MSM, are consistent with national and local rates.1,22 Studies have found that integrating HIV and FP services can positively facilitate males' involvement in FP, resulting in increased contraceptive use and decreased incidence of pregnancies.34–36 Furthermore, an interview-based study found that males attending HIV clinics were more receptive to receiving FP information and services at integrated clinics compared with FP clinics alone.37
Routine, opt-out HIV testing, in contrast with targeted, risk-based testing, can reduce stigma associated with HIV testing and minimize missed opportunities in diagnosing HIV infection. Studies have shown that multiple missed opportunities for HIV testing are prevalent in health-care settings, particularly in primary care clinics and emergency departments.38,39 These missed opportunities result in late diagnoses and low CD4+ counts. A study conducted in a New York City hospital found that 60% of 253 newly diagnosed HIV patients had made at least one hospital visit within three years prior to diagnosis, with a median of three visits.40 Additionally, these patients had low CD4+ counts, indicating truly missed opportunities. Likewise, in a few cases in our study, patients were unaware of their HIV status before visiting the clinics despite receiving treatment at other health-care settings. Delayed HIV screening also delays linkage to care, which especially concerns pregnant women, whose timely testing and treatment could result in substantial decreases in rates of perinatal transmission.41
With early detection of HIV, infected individuals are able to gain timely access to the HIV treatment cascade, which is critical for viral suppression and transmission prevention.42,43 The early initiation of antiretroviral therapy has been associated with a 96% decrease in linked HIV-1 transmission, and reductions in mortality, progression to acquired immunodeficiency syndrome, and onset of other adverse illnesses.44,45 Although early linkage to care might be an effective form of HIV prevention, studies have shown that adolescents and young adults are more likely than adults to delay linkage to care.46,47 Despite the challenges associated with linking adolescents and young adults to timely HIV care, the clinics in this study were able to link 80% of patients to care. Strategies the clinics used to facilitate immediate linkage to care included developing relationships and memorandums of understanding with various HIV service organizations, accompanying patients to their first appointment, and assisting patients with the eligibility process.
The study's retrospective nature and reliance on internally generated statistical reports limited our knowledge of more specific demographic characteristics, and sexual and risk behavior information. Despite its limitations, our study represented an extensive and diverse sample of young people who lived in a large, urban environment. This study demonstrated not only that routinely screening adolescents and young adults was feasible, but also that the prevalence of new HIV diagnoses (0.3%) exceeded CDC's recommended threshold of 0.1% in settings where routine HIV screening is warranted.11 Therefore, the data from this study can contribute to the limited literature available concerning the integration of routine HIV testing in FP clinics for young people.
CONCLUSION
Our study results suggest that FP clinics, especially those that serve adolescents and young adults, are important settings in which routine, opt-out HIV screening can be integrated, and individuals who are unaware of their HIV status can be identified and linked to care. Several lessons were learned in the process of implementation. Educating staff members about HIV prevalence in the community and the significance of missed testing opportunities, as well as obtaining staff input on routine HIV testing, can secure their support and ensure seamless integration of routine testing into clinic flow. Additionally, a designated HIV champion can motivate staff and provide regular reminders about the importance of offering HIV testing. As large numbers of adolescents and young adults engage in sexually risky behaviors early in life,27 there is an evident need to develop comprehensive FP- and HIV-integrated programs to reduce the impact of HIV infection among adolescents and young adults.
Footnotes
As this was a retrospective chart review, the Baylor College of Medicine Institutional Review Board approved the study and waived consent. This project was supported by Gilead Sciences, Inc.
REFERENCES
- 1.Centers for Disease Control and Prevention (US) HIV among youth [cited 2014 Sep 14] Available from: http://www.cdc.gov/hiv/risk/age/youth/index.html?s_cid=tw_drmermin-00186.
- 2.Peralta L, Deeds BG, Hipszer S, Ghalib K. Barriers and facilitators to adolescent HIV testing. AIDS Patient Care STDS. 2007;21:400–8. doi: 10.1089/apc.2006.0112. [DOI] [PubMed] [Google Scholar]
- 3.Hyden C, Allegrante JP, Cohall AT. HIV testing sites' communication about adolescent confidentiality: potential barriers and facilitators to testing. Health Promot Pract. 2014;15:173–80. doi: 10.1177/1524839913499347. [DOI] [PubMed] [Google Scholar]
- 4.Inungu J, Lewis A, Mustafa Y, Wood J, O'Brien S, Verdun D. HIV testing among adolescents and youth in the United States: update from the 2009 Behavior Risk Factor Surveillance System. Open AIDS J. 2011;5:80–5. doi: 10.2174/1874613601105010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schechter-Perkins EM, Koppelman E, Mitchell PM, Morgan JR, Kutzen R, Drainoni ML. Characteristics of patients who accept and decline emergency department rapid HIV testing. Am J Emerg Med. 2014;32:1109–12. doi: 10.1016/j.ajem.2014.05.034. [DOI] [PubMed] [Google Scholar]
- 6.Haines CJ, Uwazuoke K, Zussman B, Parrino T, Laguerre R, Foster J. Pediatric emergency department-based rapid HIV testing: adolescent attitudes and preferences. Pediatr Emerg Care. 2011;27:13–6. doi: 10.1097/PEC.0b013e3182037cde. [DOI] [PubMed] [Google Scholar]
- 7.Jain S, Lowman ES, Kessler A, Harper J, Rumoro DP, Smith KY, et al. Seroprevalence study using oral rapid HIV testing in a large urban emergency department [published erratum appears in J Emerg Med 2013;44:1212] J Emerg Med. 2012;43:269–75. doi: 10.1016/j.jemermed.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 8.Black S, Wallace M, Middelkoop K, Robbertze D, Bennie T, Wood R, et al. Improving HIV testing amongst adolescents through an integrated youth centre rewards program: insights from South Africa. Child Youth Serv Rev. 2014;45:98–105. [Google Scholar]
- 9.Coates TJ, Kulich M, Celentano DD, Zelaya CE, Chariyalertsak S, Chingono A, et al. Effect of community-based voluntary counselling and testing on HIV incidence and social and behavioural outcomes (NIMH Project Accept; HPTN 043): a cluster-randomised trial. Lancet Glob Health. 2014;2:267–77. doi: 10.1016/S2214-109X(14)70032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kowalczyk Mullins TL, Braverman PK, Dorn LD, Kollar LM, Kahn JA. Adolescent preferences for human immunodeficiency virus testing methods and impact of rapid tests on receipt of results. J Adolesc Health. 2010;46:162–8. doi: 10.1016/j.jadohealth.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 11.Branson BM, Handsfield HH, Lampe MA, Janssen RS, Taylor AW, Lyss SB, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1–17. [PubMed] [Google Scholar]
- 12.Department of Health and Human Services (US), Office of Population Affairs. Title X: the national family planning program [cited 2014 Sep 12] Available from: http://www.hhs.gov/opa/pdfs/title-x-national-family-planning-overview.pdf.
- 13.Abraham AJ, O'Brien LA, Knudsen HK, Bride BE, Smith GR, Roman PM. Patient characteristics and availability of onsite non-rapid and rapid HIV testing in US substance use disorder treatment programs. J Subst Abuse Treat. 2013;44:120–5. doi: 10.1016/j.jsat.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Committee on Pediatric AIDS. Emmanuel PJ, Martinez J. Adolescents and HIV infection: the pediatrician's role in promoting routine testing. Pediatrics. 2011;128:1023–9. doi: 10.1542/peds.2011-1761. [DOI] [PubMed] [Google Scholar]
- 15.Criniti SM, Aaron E, Hilley A, Wolf S. Integration of routine rapid HIV screening in an urban family planning clinic. J Midwifery Womens Health. 2011;56:395–9. doi: 10.1111/j.1542-2011.2011.00031.x. [DOI] [PubMed] [Google Scholar]
- 16.Spaulding AB, Brickley DB, Kennedy C, Almers L, Packel L, Mirjahangir J, et al. Linking family planning with HIV/AIDS interventions: a systematic review of the evidence. AIDS. 2009;23(Suppl 1):79–88. doi: 10.1097/01.aids.0000363780.42956.ff. [DOI] [PubMed] [Google Scholar]
- 17.Church K, Mayhew SH. Integration of STI and HIV prevention, care, and treatment into family planning services: a review of the literature. Stud Fam Plann. 2009;40:171–86. doi: 10.1111/j.1728-4465.2009.00201.x. [DOI] [PubMed] [Google Scholar]
- 18.Lindegren ML, Kennedy CE, Bain-Brickley D, Azman H, Creanga AA, Butler LM, et al. Integration of HIV/AIDS services with maternal, neonatal and child health, nutrition, and family planning services. Cochrane Database Syst Rev. 2012;9:CD010119. doi: 10.1002/14651858.CD010119. [DOI] [PubMed] [Google Scholar]
- 19.Grossman D, Onono M, Newmann SJ, Blat C, Bukusi EA, Shade SB, et al. Integration of family planning services into HIV care and treatment in Kenya: a cluster-randomized trial. AIDS. 2013;27(Suppl 1):77–85. doi: 10.1097/QAD.0000000000000035. [DOI] [PubMed] [Google Scholar]
- 20.Maharaj P, Cleland J. Integration of sexual and reproductive health services in KwaZulu-Natal, South Africa. Health Policy Plan. 2005;20:310–8. doi: 10.1093/heapol/czi038. [DOI] [PubMed] [Google Scholar]
- 21.Houston Department of Health and Human Services. Houston: Bureau of HIV/STD and Viral Hepatitis Prevention; 2011. Enhanced comprehensive HIV prevention planning and implementation for metropolitan statistical areas most affected by HIV/AIDS (ECHPP) for Houston-Baytown-Sugarland, Texas. Also available from: http://www.cdc.gov/hiv/pdf/prevention_demonstrations_echpp_houston_plan.pdf [cited 2014 Aug 16] [Google Scholar]
- 22.Houston Area HIV Services Ryan White Planning Council. The 2013 Houston Area Integrated Epidemiologic Profile for HIV/AIDS Prevention and Care Services Planning [cited 2014 Aug 18] Available from: http://www.rwpchouston.org/publications/2013%20epi%20profile%20--%20approved%20--%2005-09-13.pdf.
- 23.The Center for HIV Law and Policy. State HIV laws: Texas [cited 2014 Aug 18] Available from: http://www.hivlawandpolicy.org/states/texas.
- 24.Culp L, Caucci L. State adolescent consent laws and implications for HIV pre-exposure prophylaxis. Am J Prev Med. 2013;44(Suppl 2):S119–24. doi: 10.1016/j.amepre.2012.09.044. [DOI] [PubMed] [Google Scholar]
- 25.Jones RK. Do U.S. family planning clinics encourage parent-child communication? Findings from an exploratory survey. Perspect Sex Reprod Health. 2006;38:155–61. doi: 10.1363/psrh.38.155.06. [DOI] [PubMed] [Google Scholar]
- 26.Buzi RS, Smith PB, Barrera C. Talk with Tiff: teen's inquiries to a sexual health website. J Sex Marital Ther. 2015;41:126–33. doi: 10.1080/0092623X.2013.857375. [DOI] [PubMed] [Google Scholar]
- 27.Turner SD, Anderson K, Slater M, Quigley L, Dyck M, Guiang CB. Rapid point-of-care HIV testing in youth: a systematic review. J Adolesc Health. 2013;53:683–91. doi: 10.1016/j.jadohealth.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 28.Jackson S, Hafemeister TL. Impact of parental consent and notification policies on the decisions of adolescents to be tested for HIV. J Adolesc Health. 2001;29:81–93. doi: 10.1016/s1054-139x(00)00178-6. [DOI] [PubMed] [Google Scholar]
- 29.Fowler CI, Gable J, Wang J, McClure E. Research Triangle Park (NC): RTI International; 2013. Family planning annual report: 2012 national summary. Also available from: http://www.hhs.gov/opa/pdfs/fpar-national-summary-2012.pdf [cited 2014 Aug 16] [Google Scholar]
- 30.Hall HI, Walker F, Shah D, Belle E. Trends in HIV diagnoses and testing among U.S. adolescents and young adults. AIDS Behav. 2012;16:36–43. doi: 10.1007/s10461-011-9944-8. [DOI] [PubMed] [Google Scholar]
- 31.Chabikuli NO, Awi DD, Chukwujekwu O, Abubakar Z, Gwarzo U, Ibrahim M, et al. The use of routine monitoring and evaluation systems to assess a referral model of family planning and HIV service integration in Nigeria. AIDS. 2009;23(Suppl 1):97–103. doi: 10.1097/01.aids.0000363782.50580.d8. [DOI] [PubMed] [Google Scholar]
- 32.Buzi RS, Smith PB, Haas S. Risk-related behaviors associated with HIV among young minority men attending family planning clinics. Int J Mens Health. :1016. In press. [Google Scholar]
- 33.Stephenson R, Vwalika B, Greenberg L, Ahmed Y, Vwalika C, Chomba E, et al. A randomized controlled trial to promote long-term contraceptive use among HIV-serodiscordant and concordant positive couples in Zambia. J Womens Health (Larchmt) 2011;20:567–74. doi: 10.1089/jwh.2010.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wall KM, Vwalika B, Haddad L, Khu NH, Vwalika C, Kilembe W, et al. Impact of long-term contraceptive promotion on incident pregnancy: a randomized controlled trial among HIV-positive couples in Lusaka, Zambia. J Acquir Immune Defic Syndr. 2013;63:86–95. doi: 10.1097/QAI.0b013e31827ee19c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ngure K, Heffron R, Mugo N, Irungu E, Celum C, Baeten JM. Successful increase in contraceptive uptake among Kenyan HIV-1-serodiscordant couples enrolled in an HIV-1 prevention trial. AIDS. 2009;23(Suppl 1):89–95. doi: 10.1097/01.aids.0000363781.50580.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steinfeld RL, Newmann SJ, Onono M, Cohen CR, Bukusi EA, Grossman D. Overcoming barriers to family planning through integration: perspectives of HIV-positive men in Nyanza province, Kenya. AIDS Res Treat. 2013;2013:861983. doi: 10.1155/2013/861983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liddicoat RV, Horton NJ, Urban R, Maier E, Christiansen D, Samet JH. Assessing missed opportunities for HIV testing in medical settings. J Gen Intern Med. 2004;19:349–56. doi: 10.1111/j.1525-1497.2004.21251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sudarshi D, Pao D, Murphy G, Parry J, Dean G, Fisher M. Missed opportunities for diagnosing primary HIV infection. Sex Transm Infect. 2008;84:14–6. doi: 10.1136/sti.2007.026963. [DOI] [PubMed] [Google Scholar]
- 39.Nakao JH, Wiener DE, Newman DH, Sharp VL, Egan DJ. Falling through the cracks? Missed opportunities for earlier HIV diagnosis in a New York City hospital. Int J STD AIDS. 2014;25:887–93. doi: 10.1177/0956462414523944. [DOI] [PubMed] [Google Scholar]
- 40.Davis JA, Yawetz S. Management of HIV in the pregnant woman. Clin Obstet Gynecol. 2012;55:531–40. doi: 10.1097/GRF.0b013e31824f3ae1. [DOI] [PubMed] [Google Scholar]
- 41.Philbin MM, Tanner AE, DuVal A, Ellen JM, Xu J, Kapogiannis B, et al. Factors affecting linkage to care and engagement in care for newly diagnosed HIV-positive adolescents within fifteen adolescent medicine clinics in the United States. AIDS Behav. 2014;18:1501–10. doi: 10.1007/s10461-013-0650-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Das M, Chu PL, Santos GM, Scheer S, Vittinghoff E, MacFarland W, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS One. 2010;5:11068. doi: 10.1371/journal.pone.0011068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anglemyer A, Rutherford GW, Easterbrook PJ, Horvath T, Vitória M, Jan M, et al. Early initiation of antiretroviral therapy in HIV-infected adults and adolescents: a systematic review. AIDS. 2014;28(Suppl 2):105–18. doi: 10.1097/QAD.0000000000000232. [DOI] [PubMed] [Google Scholar]
- 45.Hall HI, Gray KM, Tang T, Li J, Shouse L, Mermin J. Retention in care of adults and adolescents living with HIV in 13 US areas. J Acquir Immune Defic Syndr. 2012;60:77–82. doi: 10.1097/QAI.0b013e318249fe90. [DOI] [PubMed] [Google Scholar]
- 46.Grant AM, Jamieson DJ, Elam-Evans LD, Beck-Sague C, Duerr A, Henderson SL. Reasons for testing and clinical and demographic profile of adolescents with non-perinatally acquired HIV infection. Pediatrics. 2006;117:468–75. doi: 10.1542/peds.2005-0142. [DOI] [PubMed] [Google Scholar]
- 47.Centers for Disease Control and Prevention (US) Youth online: high school YRBS, 1991–2013 results [cited 2014 Sep 14] Available from: http://nccd.cdc.gov/youthonline/app/default.aspx.