Abstract
Background
Prescription benzodiazepine overdose continues to cause significant morbidity and mortality in the US. Multiple-provider prescribing, due to either fragmented care or “doctor-shopping,” contributes to the problem.
Objective
To elucidate the effect of provider professional relationships on multiple-provider prescribing of benzodiazepines, using social network analytics.
Design
A retrospective analysis of commercial healthcare claims spanning the years 2008 through 2011. Provider patient-sharing networks were modelled using social network analytics. Care team cohesion was measured using care density, defined as the ratio between the total number of patients shared by provider pairs within a patient’s care team and the total number of provider pairs in the care team. Relationships within provider pairs were further quantified using a range of network metrics, including the number and proportion of patients or collaborators shared.
Main Measures
The relationship between patient-sharing network metrics and the likelihood of multiple prescribing of benzodiazepines.
Participants
Patients between the ages of 18 and 64 years who received two or more benzodiazepine prescriptions from multiple providers, with overlapping coverage of more than 14 days.
Results
A total of 5659 patients and 1448 provider pairs were included in our study. Among these, 1028 patients (18.2 %) received multiple prescriptions of benzodiazepines, involving 445 provider pairs (30.7 %). Patients whose providers rarely shared patients had a higher risk of being prescribed overlapping benzodiazepines; the median care density was 8.1 for patients who were prescribed overlapping benzodiazepines and 10.1 for those who were not (p < 0.0001). Provider pairs who shared a greater number of patients and collaborators were less likely to co-prescribe overlapping benzodiazepines.
Conclusions
Our findings demonstrate the importance of care team cohesion in addressing multiple-provider prescribing of controlled substances. Furthermore, we illustrate the potential of the provider network as a surveillance tool to detect and prevent adverse events that could arise due to fragmentation of care.
KEY WORDS: prescribing patterns, drug overdose, social networks
BACKGROUND
One of the contributors to misuse of controlled prescription drugs is multiple-provider prescribing,1,2 whereby individuals receive concurrent prescriptions for controlled substances from multiple providers, either as a result of fragmentation in patient care or “doctor shopping” among medication diverters.3,4 In 2011, the Centers for Disease Control and Prevention (CDC) reported 22,810 deaths in the US from pharmaceutical overdose.5 Prescription benzodiazepines, used primarily for the relief of anxiety and insomnia, are one of the most common drug classes involved, accounting for 30 % of all overdose deaths.5,6 The 2010 National Survey of Drug Use and Health reported an estimated 186,000 new abusers of benzodiazepines.7 In efforts to curtail the problem, in 1993, the US Congress enacted federal legislation to support the establishment of state-based prescription drug monitoring programs (PDMPs) as a public surveillance tool to track all transactions for controlled substances and to inform clinicians and pharmacists of patients' prescription history. To date, however, uptake of these programs has been slow.8–10
Here, we ask the question: does care coordination diminish the chance of multiple-provider prescribing of benzodiazepines? Care coordination involves a coherent approach to patient management amongst providers, through sharing of care plans and patient information. Fragmentation of care underlies many of the problems in the US healthcare system, contributing to unnecessarily high rates of health services use and spending, and exposing patients to lapses in quality and safety.11 Indeed, failure in care coordination is the root cause of many preventable patient harms, estimated to represent between $25 billion and $45 billion in annual spending.12 The accountable care organization (ACO) model is an attempt to address this problem through the alignment of accountability for providers across the continuum of care.13,14 This is to be accomplished by grouping hospitals and physician practices together to facilitate quality improvement and cost containment. The extent to which care team structures can influence quality of care has yet to be ascertained.
Recent studies have begun to use social network analysis tools to characterize the professional relationships among providers, using the number of shared patients as an indicator of the strength of provider collaborative relationships.15–20 The underlying concept is based on the premise that providers exchange information and establish rapport in the process of providing care to shared patients; providers who share a greater number of patients should therefore have stronger collaborative relationships and will be able to provide better coordinated care. Several studies have demonstrated a direct correlation between the cost of care and hospital readmission rates for individual patients and the number of patients shared among the providers caring for them.15,16
In the context of prescription drug misuse, we hypothesized that 1) individuals whose providers rarely share patients have a greater likelihood of being prescribed controlled substances by multiple providers, and 2) provider pairs who rarely share patients are more likely to co-prescribe controlled substances to their shared patients. We tested these hypotheses, focusing on benzodiazepine prescriptions in the outpatient setting.
METHODS
We conducted a retrospective analysis of healthcare claims from the HealthCore Integrated Research Database (HIRDTM),21 which contains longitudinal data from 14 Anthem-affiliated Blue Cross and/or Blue Shield licensed plans across the US. The data spanned the years 2008 through 2011, and included information on beneficiaries’ demographics, outpatient visits, prescription medications, and providers. Charlson comorbidity index scores were calculated and provided by HealthCore based on comorbid conditions identified across all inpatient and outpatient visits during the study period.22 The study was approved by a central institutional review board used by HealthCore, Inc., and it was designated as exempt by the Committee on Clinical Investigation at Boston Children’s Hospital.
To adequately capture relationships among providers, we limited our study to four regions where commercial health plans contributing to the HIRD cover a large fraction of the market. These regions were identified by combining ZIP Codes where 1) the HIRD population in the ZIP Code area represented at least 30 % of the local 2010 US census, 2) the ZIP Code was among the top 30 % of the state’s ZIP Codes for HIRD enrollment size, 3) the ZIP Code was surrounded by others meeting criteria 1 and 2, and 4) a 50-mile buffer around the region did not extend into a state where the plan was not a major insurer. Together, 293 ZIP Codes, located primarily in the urban areas of four midwestern and southern states, and 521,145 beneficiaries were included. The median care team size was four providers; on average, each provider had 185 patients. Further details of the regions and network characteristics are described in a previously published study.20
Patient-Sharing Networks
To construct patient-sharing networks, we used outpatient encounter data for all patients in our dataset. We characterized the professional relationships among the full constellation of ambulatory care providers involved in the care of each patient. Providers in specialties not associated with routine ambulatory care (e.g., pathology, anaesthesia, radiology, emergency medicine) were excluded. Furthermore, we excluded providers with fewer than 50 patients during the study period, as they were unlikely to be part of the healthcare network throughout the period, given the small number of patients.
The presence of shared patients was used to infer collaborative relationship between providers. A network tie was formed between two providers if they had an encounter with one or more common patients, including patients not prescribed benzodiazepines during the study period.
Network metrics were derived to infer the degree of collaboration among providers:
Patient-centric measure: To measure care team cohesion for individual patients, care density was calculated, defined as the ratio between the total number of patients shared by provider pairs within a patient’s care team, and the total number of provider pairs within the patient’s care team (Fig. 1).15 A greater care density value indicates a greater number of shared patients among providers within a care team relative to the size of the care team, thus inferring stronger care team cohesiveness.
Provider-centric measures: The strength of the professional relationship between two providers was inferred by the total number of patients they shared as well as the percentage of patients shared (defined as the ratio between the number of patients shared and the total number of patients seen by two providers). To explore additional network-based variables, we computed Jaccard similarity23 to quantify the similarity in the professional network of each provider pair, defined as the ratio between the intersection and union of shared providers (Fig. 1). A greater Jaccard similarity value indicates a greater overlap between the collaborator sets of two providers, thus inferring a stronger tie between the provider pair.
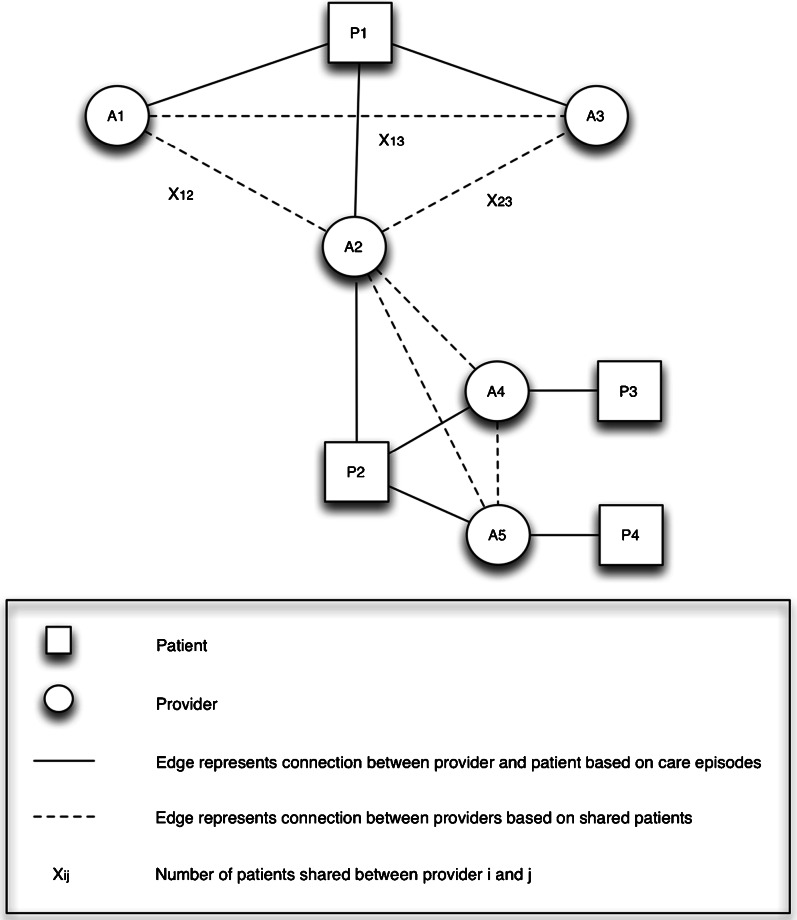
Fig. 1.
Example of patient-sharing network. In this example, the care team for patient P1 consists of three providers (A1, A2, A3), and each provider pair has one shared patient (P1). Care density is defined as the ratio between the total number of patients shared by provider pairs in a care team and the total number of provider pairs in the care team. The care density for patient P1 is calculated as (X12 + X23 + X13)/3, which equates to 1. To measure the relationship between providers A1 and A2, we examine the number of patients they share and the number of providers with whom they collaborate. Provider A1 has one patient (P1), and provider A2 has two patients (P1, P2). There is one shared patient between them (P1), and the proportion of shared patients is 1/2. Provider A1 has two collaborators (A2, A3) and provider A2 has four collaborators (A1, A3, A4, A5). The provider pair A1/A2 has a total of three collaborators, and only one shared collaborator (A3). Jaccard similarity is defined as the ratio between the intersection and union of shared provider. In this example, the Jaccard similarity of provider pair A1/A2 is 1/3.
Multiple-Provider Prescribing of Benzodiazepines
We identified patients who were prescribed overlapping benzodiazepines by multiple providers, with overlapping coverage of 14 or more days, as well as provider pairs who prescribed these medications. All benzodiazepines were considered, including alprazolam, chlordiazepoxide, clonazepam, clorazepate, diazepam, estazolam, flurazepam, halazepam, lorazepam, midazolam, oxazepam, quazepam, temazepam, and triazolam. To ensure that overlapping prescriptions were not a result of a deliberate change in medication regimen, overlapping benzodiazepines dispensed by the same provider were disregarded. Prescription medications were identified by their National Drug Codes.
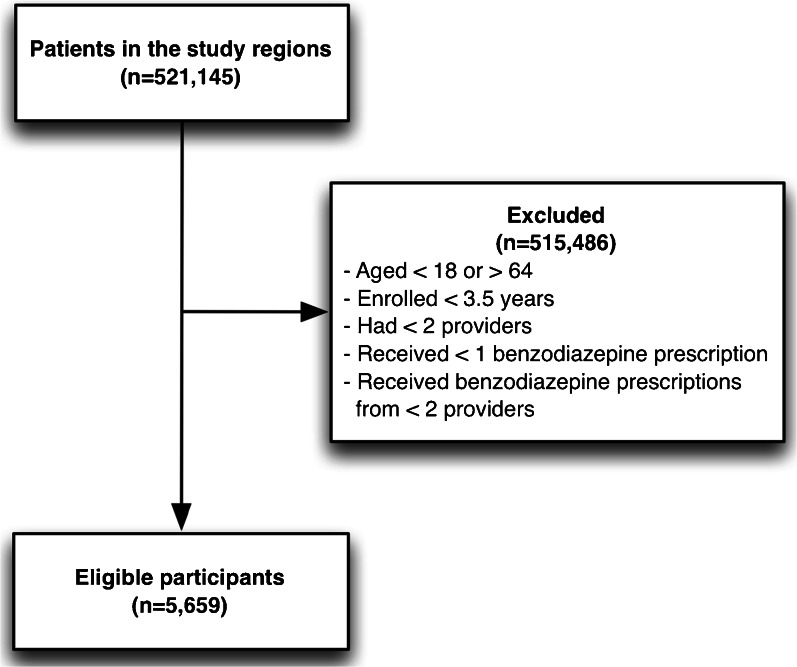
We restricted our patient cohort to all adult beneficiaries (between 18 and 64 years of age) who were continuously enrolled for 3.5 years and who had two or more outpatient prescriptions of benzodiazepines dispensed by multiple providers (Fig. 2). Since we sought to study the impact of provider relationships on prescribing behavior, beneficiaries receiving benzodiazepines from a single provider were excluded.
Fig. 2.
Study participant selection criteria
Study Outcomes
We assessed the impact of provider professional relationships on the occurrence of multiple-provider prescribing of benzodiazepines. Specifically:
Patient-centric measure: We assessed the relationship between the care density of a patient’s care team and the patient’s risk of receiving overlapping benzodiazepine prescriptions;
Provider-centric measure: We examined the relationship between multiple prescribing of benzodiazepines by a provider pair and the number and proportion of patients shared by the two providers and their Jaccard similarity. We further assessed the specialties of each provider pair, and the total number of providers linked to the provider pair.
Statistical Analysis
Descriptive statistics and univariate analysis (t test for continuous variable, chi-square analysis for categorical variables) were performed to characterize and compare the attributes of patients and provider pairs who were involved in one or more multiple provider episodes of benzodiazepine prescriptions with those who were not. Effect size of continuous and categorical variables were calculated using Cohen’s d measure and phi coefficient, respectively.
Backward stepwise logistic regression analyses were conducted to evaluate the independent effect of care density on the likelihood of exposure to overlapping benzodiazepine prescriptions. Care density was dichotomized at the median care density of the study population (defined as greater or less than the median care density). Exposure to overlapping benzodiazepine prescriptions was defined as a dichotomous variable (defined as either no overlapping benzodiazepine prescriptions, or one or more overlapping benzodiazepine prescriptions). Covariates included patient age, gender, Charlson comorbidity index score, number of providers, total number of days benzodiazepines were prescribed, the presence of a primary care provider (PCP) or psychiatrist in a patient’s care team, benzodiazepines prescribed by a PCP or psychiatrist, and the percentage of prescribers who were in a patient’s care team. To detect potential multicollinearity among variables in the multivariate regression analysis, we measured the variance inflation factors (VIF) for each regression variable.24
Analyses were conducted using SAS statistical software, version 9.4 (SAS Institute Inc., Cary, NC, USA). All tests of statistical significance were two-tailed and used an α level of p < 0.05.
RESULTS
There were 521,145 subjects in our dataset; 26,775 (5.1 %) patients received at least one benzodiazepine prescription during the study period. Study participants included 5659 patients who had two or more benzodiazepine prescriptions dispensed by multiple providers (Table 1). Of these patients, 1028 (18.2 %) received multiple prescriptions of benzodiazepines, with overlapping coverage of more than 14 days. A total of 1448 provider pairs were included in our study; 918 (63.4 %) comprised at least one PCP (Table 2). Among provider pairs, 445 (30.7 %) co-prescribed overlapping benzodiazepines to one or more patients. Patients with overlapping benzodiazepine prescriptions had more total benzodiazepine prescriptions over the course of the study period than those without overlapping prescriptions (p < 0.0001).
Table 1.
Characteristics of Study Population (n = 5659)
| Characteristics | Patients not prescribed overlapping benzodiazepines (n = 4631) | Patients prescribed overlapping benzodiazepines (n = 1028) | Effect size* | p value |
|---|---|---|---|---|
| Age, mean (SD) | 46.4 (10.6) | 47.3 (10.7) | 0.08 | 0.0203 |
| Female gender, n (%) | 3174 (68.5) | 667 (64.9) | 0.03 | 0.0232 |
| Number of providers, mean (SD) | 7.9 (4.4) | 8.2 (4.9) | 0.05 | 0.1720 |
| Number of benzodiazepines prescribers, mean (SD) | 2.4 (0.7) | 2.8 (1.1) | 0.6 | <0.0001 |
| Total benzodiazepine prescriptions, mean (SD) | 345.4 (318.1) | 688.8 (573.4) | 0.9 | <0.0001 |
| Charlson’s comorbidity index score n (%) | ||||
| 0 | 2178 (47.0) | 453 (44.1) | 0.02 | 0.0847 |
| 1 | 1172 (25.3) | 268 (26.1) | 0.007 | 0.6117 |
| 2 | 580 (12.5) | 122 (11.9) | 0.008 | 0.563 |
| 3 | 281 (6.1) | 75 (7.3) | 0.02 | 0.142 |
| 4 | 138 (3.0) | 33 (3.2) | 0.005 | 0.697 |
| ≥5 | 282 (6.1) | 77 (7.5) | 0.02 | 0.096 |
| ≥1 Psychiatrist in care team, n (%) | 847 (18.3) | 264 (25.7) | 0.07 | <0.0001 |
| ≥1 Benzodiazepine prescription by psychiatrist, n (%) | 987 (21.3) | 340 (33.1) | 0.1 | <0.0001 |
| ≥1 PCP in care team, n (%) | 4529 (97.8) | 1004 (97.7) | 0.003 | 0.7951 |
| ≥1 Benzodiazepine prescription by PCP, n (%) | 3868 (83.5) | 842 (81.9) | 0.02 | 0.2092 |
| Median care density | 10.1 | 8.1 | 0.2 | <0.0001 |
*Effect size was measured using Cohen’s d for continuous variables and phi coefficient for categorical variables
PCP primary care provider
Table 2.
Characteristics of Provider Pairs (n = 1448)
| Characteristics | Provider pairs who did not prescribe overlapping benzodiazepines (n = 1003) | Provider pairs who prescribed overlapping benzodiazepines (n = 445) | Effect size | p value |
|---|---|---|---|---|
| Number of shared patients between 2 providers, mean (SD) | 41.7 (75.7) | 28.7 (66.1) | 0.2 | 0.0010 |
| Percentage of shared patients between 2 providers of their overall panel, mean (SD) | 6.2 (9.6) | 4.5 (9.1) | 0.2 | 0.0013 |
| Total number of providers who had one or more shared patients with either of the provider pair, mean (SD) | 1193.7 (401.4) | 1189.3 (369.7) | 0.01 | 0.8382 |
| Jaccard similarity, mean (SD) | 0.38 (0.15) | 0.35 (0.14) | 0.2 | 0.0003 |
| Most prevalent specialties, n (%) | ||||
| PCP – PCP | 414 (41.3) | 119 (26.7) | 0.1 | <0.0001 |
| PCP – Psychiatrist | 113 (11.3) | 90 (20.2) | 0.1 | <0.0001 |
| PCP – OBGYN | 33 (3.3) | 25 (5.6) | 0.05 | 0.0371 |
| PCP – Neurology | 30 (3.0) | 23 (5.2) | 0.05 | 0.0418 |
| PCP – Nurse practitioner | 28 (2.8) | 8 (1.8) | 0.03 | 0.2624 |
| PCP – Orthopedic surgery | 25 (2.5) | 10 (2.2) | 0.009 | 0.7791 |
| Psychiatrist – Psychiatrist | 22 (2.2) | 18 (4.0) | 0.06 | 0.0473 |
| Other | 338 (33.7) | 152 (34.2) | 0.004 | 0.8649 |
PCP primary care provider
Patient-Centric Measure
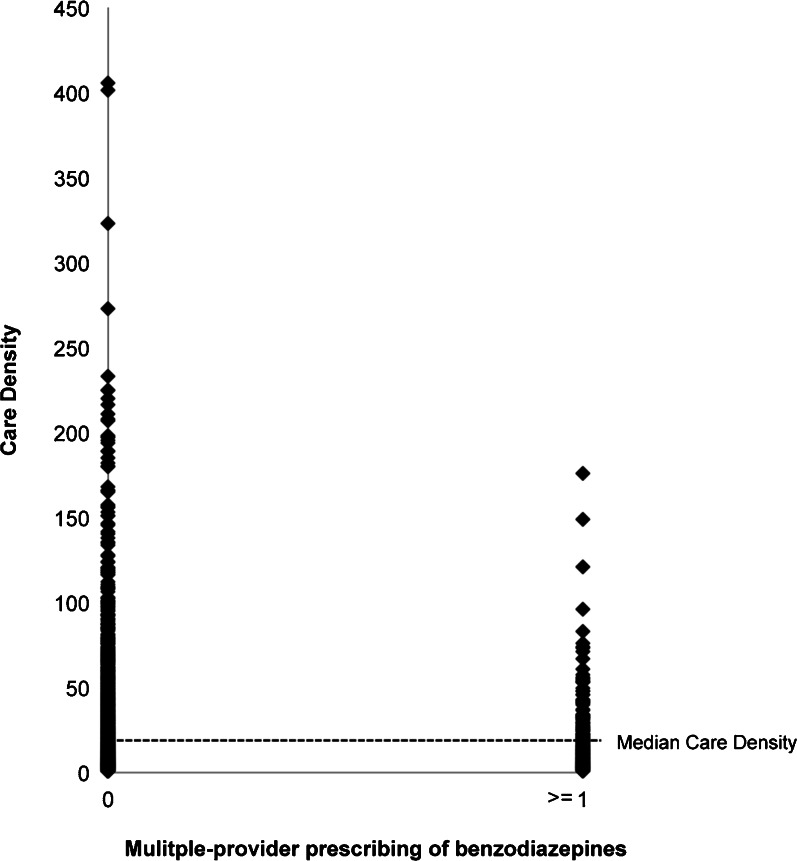
The median care density of the study population was 9.7 (Fig. 3). The median care density of patients who were prescribed overlapping benzodiazepines was lower than that of patients who received no overlapping prescriptions (p < 0.0001) (Table 1).
Fig. 3.
Distribution of care density, comparing patients who received overlapping benzodiazepines and those who did not. The relationship between care density and multiple prescribing of benzodiazepines is non-linear.
Backward stepwise logistic regression confirmed an independent association between overlapping benzodiazepine prescriptions and median care density (OR 0.84; 95 % CI 0.73–0.98; p = 0.028) (Table 3). The likelihood of being prescribed overlapping benzodiazepines was also associated with seeing a greater number of providers, total benzodiazepine days prescribed, receiving a benzodiazepine prescription from a PCP, and having sought care from a psychiatrist. There was no independent association between overlapping benzodiazepine prescriptions and age, gender, Charlson comorbidity index score, or the presence of a PCP in the patient’s care team, and these variables were thus excluded from the final model (Table 3). Multicollinearity in the multivariate regression analysis was ruled out, since the calculated VIF for all regressor variables was less than 1.01.24
Table 3.
Risk Factors Associated with Overlapping Benzodiazepine Prescriptions Dispensed by Multiple Providers
| Variables | OR (95 % CI) | p value |
|---|---|---|
| Median care density | 0.76 (0.60–0.95) | 0.0178 |
| Number of providers in care team | 1.02 (1.01–1.04) | 0.0138 |
| Total days of benzodiazepine prescriptions | 1.002 (1.002–1.002) | <0.001 |
| Benzodiazepines prescribed by PCP (true/false) | 1.67 (1.34–2.06) | <0.001 |
| Patient has a psychiatrist in care team (true/false) | 1.45 (1.20–1.75) | <0.001 |
| Percentage of prescribers from within care team | 0.98 (0.98–0.98) | <0.001 |
PCP primary care provider
Provider-Centric Measure
Provider pairs who shared a greater number of patients were less likely to co-prescribe overlapping benzodiazepines (p = 0.001) (Table 2). On average, provider pairs who did not co-prescribe overlapping benzodiazepines shared 41.7 patients (6.2 % of all patients under their care), while provider pairs who prescribed overlapping benzodiazepines shared 28.7 patients (4.5 % of all patients under their care). Similarly, a higher percentage of shared patients between two providers was associated with a lower risk of overlapping benzodiazepine prescribing (p = 0.0013). The Jaccard similarity among provider pairs was also significantly associated with the likelihood of overlapping benzodiazepine prescriptions (p = 0.0003). However, the combined total number of collaborators linked to the provider pairs was not associated with the likelihood of overlapping benzodiazepines prescribing. Among provider pairs who co-prescribed overlapping benzodiazepines, 26.7 % involved two PCPs and 20.2 % involved a PCP and a psychiatrist.
DISCUSSION
This is the first study to apply network analysis to examine the impact of provider relationships on multiple-provider prescribing. Using readily available administrative data, we constructed professional networks of providers based on the number of patients shared among providers. Network metrics were then derived to infer care coordination. We quantified the cohesion of a patient’s care team using care density, and demonstrated a significant relationship between care density and the likelihood that a patient was prescribed overlapping benzodiazepines. Furthermore, we extended the concept of shared patients to characterize the professional relationships between individual provider pairs, and used Jaccard similarity to infer the strength of the relationships within provider pairs. We showed that both number and percentage of shared patients between two providers and Jaccard similarity were significant predictors for overlapping benzodiazepine prescriptions by a provider pair. These findings strongly suggest that care team structures and poor care coordination are significant risk factors for unsafe prescribing practices.
The risk factors for multiple-provider prescribing have been reported in a number of studies.25–28 Consistent with existing literature on the misuse of prescription drugs, we found that patients who had a psychiatrist in the care team were more likely to be exposed to multiple-provider prescriptions of benzodiazepines.25,26 In-depth analysis of care team composition revealed that a significant proportion of patients who received benzodiazepine from a psychiatrist had a concurrent benzodiazepine prescription from a different provider (Table 3), signifying the need for greater care in treating and monitoring these patients. Patients with psychiatric illness often present with other medical conditions; the need for an integrated care approach that promotes collaboration among psychiatrists, PCPs, and other medical professionals caring for these patients is well recognized.29,30 One strategy that has been proven effective in a number of randomized controlled trials is a collaborative care plan that implements active follow-up by a non-physician care manager or nurse, who proactively monitors patients’ progress and facilitates care coordination with their physicians.30–32 While the initial costs of setting up such an intervention may be high, in the long term, they are offset by the reduced need for medical care.33
Our findings differ from existing studies in a number of ways. First, previous studies have found the number of prescribers to be the strongest predictor of multiple-provider prescribing of controlled substances.27,28 Consistent with these studies, in univariate analysis, we showed a significant relationship between the number of prescribers and overlapping benzodiazepine prescriptions; however, the association became insignificant when considered alongside care density in multivariate analysis. This suggests that the root of the problem lies not in the number of prescribers, but rather in the poor coordination of care among the prescribers. Second, we expected patients with higher comorbidity to have a greater risk of overlapping benzodiazepine prescriptions, reflecting the complexity of caring for sicker patients, but this was not observed in the multivariate analysis. Third, contrary to existing literature showing a link between availability of primary care services and improved care quality,27,34 we did not find an association between the presence of a PCP and safer prescribing behavior, and having a PCP in the care team did not prevent multiple-provider prescribing (Table 1). Moreover, our finding that PCPs were involved in a large proportion of multiple benzodiazepine prescriptions suggests a need to re-examine how PCPs can be better equipped to coordinate care effectively. Concerns are increasingly being raised about the declining number of PCPs in the US. An entire generation of PCPs will retire within the next decade, without having been replaced by an equal number of younger PCPs.35 With the growing panels of patients, PCPs can no longer provide a high quality of care.36,37 Because PCPs are the foundation of coordinated care, this need must be urgently addressed.
We chose care density as a measure of collaborative strength based on the premise that providers who shared more patients were more likely to have stronger ties. In the context of multiple prescribing of benzodiazepines, providers who share a greater number of patients are likely to have developed a stronger rapport, and thus are able to detect and prevent overlapping prescriptions. Furthermore, patients involved in “doctor-shopping” are less likely to obtain prescriptions from providers with close ties, and their care-seeking behavior is reflected in an inflated care density value. Care density is especially relevant in identifying patients with substance abuse behavior, as these patients are often “habitual wanderers”38 who are repeatedly seen by different providers. Not only are these patients exposed to the harms associated with fragmented care; they also have a tendency to consume a larger share of healthcare expenditures.38–40 It should be noted, however, that the number of patients shared by providers may also be influenced by other factors, such as cross-coverage within the same practice. Since claims do not capture group practice designations, we were unable to account for intra-organizational patient sharing, and therefore may have overestimated the collaborative ties among cross-covering providers. Providers from the same practice have shared infrastructure and policies, and thus improved care coordination among these providers is expected. Nonetheless, these factors are no less important when designing care teams and in preventing multiple prescribing of benzodiazepines.
Our study lends support to the feasibility of using network metrics derived from provider networks as a surveillance tool, complementing existing PDMPs. Despite implementation of these programs, the incidence of prescription drug overdose in the US continues to rise.41–43 Poor data quality has been implicated as a barrier to the uptake of the programs.44,45 PDMPs are state-based initiatives. Since not all states implement these programs, and because data are generally not shared among states, inter-state prescribing activities cannot be adequately captured.43 The medications being monitored also vary from state to state, with some states monitoring only Schedule II drugs, with others extending surveillance to Schedule II–V drugs. The lack of complete data limits the effectiveness of such programs. In contrast, our approach leveraged existing nationwide administrative data sources to capture prescribing practices. Since all prescriptions are systematically recorded across all states, our data provide a complete medication history of patients. Given the increased use of the electronic health record, network metrics can be easily calculated and integrated into electronically derived measures of coordination, enabling real-time surveillance of prescribing practices without imposing additional work on overburdened providers.
Our study is subject to several limitations. First, we ascertained provider relationships based on the presence of shared patients using claims data from a single commercial health plan. As such, we may have missed shared patients covered by other health plans and out-of-network providers. To mitigate this limitation, we chose regions with substantial market penetration by the health plan that provided the study data. Second, we could not determine whether the overlapping benzodiazepine prescriptions were deliberate. While there is no pharmacological basis for the use of more than one benzodiazepine by the same patient, overlapping benzodiazepine prescriptions may indicate a change in drug regimen. We minimized this limitation by considering only prescriptions that overlapped for more than 14 days. Third, due to data limitations, we could not adequately capture other potential confounders, including geographical variations in care team structure, number of inpatient visits, and benzodiazepine prescriptions that were not reimbursed by the insurer. Future research should examine the temporal patterns of benzodiazepine prescriptions through detailed chart reviews to further inform strategies for reducing multiple-provider prescribing of benzodiazepines. Lastly, we have assumed that providers who share patients have stronger collaborative relationships, and we applied care density to infer the strength of the relationship. We cannot, however, validate this assumption using our current dataset. Nonetheless, care density appears to be a significant predictor of multiple prescribing of benzodiazepines.
CONCLUSIONS
In this study, we applied social network analytics to measure care coordination at a population level, and showed that professional relationships among care providers could have a significant impact on prescribing behavior. Our analysis highlights the importance of care coordination, and demonstrates the feasibility of using patient-sharing network analysis to address multiple-provider prescribing of controlled substances. While our study focuses on multiple-provider prescribing of benzodiazepines, patient-sharing network analysis is a powerful tool that can be applied to measure and predict other patient outcomes.
ACKNOWLEDGMENTS
This work is supported by grant R21GM107645 from the National Institute of General Medical Sciences and G08LM009778 from the National Library of Medicine, NIH. M.S.O. is supported by a fellowship from the National Health and Medical Research Council, Australia (APP1052871). The funding bodies played no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. M.S.O. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
All authors made substantial contribution to the conception, design, acquisition, analysis and interpretation of data and drafting of the work, and gave final approval of the version to be published.
REFERENCES
- 1.McDonald DC, Carlson KE. Estimating the prevalence of opioid diversion by “doctor shoppers” in the United States. PLoS One. 2013;8 doi: 10.1371/journal.pone.0069241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jena AB, Goldman D, Schaeffer LD, Weaver L, Karaca-Mandic P. Opioid prescribing by multiple providers in Medicare: retrospective observational study of insurance claims. BMJ. 2014;348:g1393. doi: 10.1136/bmj.g1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall AJ, Logan JE, Toblin RL, Kaplan JA, Kraner JC, Bixler D, Crosby AE, Paulozzi LJ. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300:2613–20. doi: 10.1001/jama.2008.802. [DOI] [PubMed] [Google Scholar]
- 4.Wilsey BL, Fishman SM, Gilson AM, Casamalhuapa C, Baxi H, Zhang H, Li CS. Profiling multiple provider prescribing of opioids, benzodiazepines, stimulants, and anorectics. Drug Alcohol Depend. 2010;112:99–106. doi: 10.1016/j.drugalcdep.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC) CDC grand rounds: prescription drug overdoses—a U.S. epidemic. MMWR Morb Mortal Wkly Rep. 2012;61:10–3. [PubMed] [Google Scholar]
- 6.Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA. 2013;309:657–9. doi: 10.1001/jama.2013.272. [DOI] [PubMed] [Google Scholar]
- 7.Substance Abuse and Mental Health Services Administration. Highlights of the 2011 drug abuse warning network (DAWN) findings on drug-related emergency department visits. The DAWN Report; 2013. [PubMed]
- 8.Gugelmann HM, Perrone J. Can prescription drug monitoring programs help limit opioid abuse? JAMA. 2011;306:2258–9. doi: 10.1001/jama.2011.1712. [DOI] [PubMed] [Google Scholar]
- 9.Perrone J, Nelson LS. Medication reconciliation for controlled substances—an “ideal” prescription-drug monitoring program. N Engl J Med. 2012;366:2341–3. doi: 10.1056/NEJMp1204493. [DOI] [PubMed] [Google Scholar]
- 10.Feldman L, Williams KS, Coates J, Knox M. Awareness and utilization of a prescription monitoring program among providers. J Pain Palliat Care Pharmacother. 2011;25:313–7. doi: 10.3109/15360288.2011.606292. [DOI] [PubMed] [Google Scholar]
- 11.Naylor MD, Aiken LH, Kurtzman ET, Olds DM, Hirschman KB. The importance of transitional care in achieving health reform. Health Aff. 2011;30:746–54. [DOI] [PubMed]
- 12.Berwick DM, Hackbarth AD. Eliminating waste in the US health care. JAMA. 2012;307(14):1513–6. doi: 10.1001/jama.2012.362. [DOI] [PubMed] [Google Scholar]
- 13.Medicare Payment Advisory Com- mission . Report to the Congress: Improving Incentives in the Medicare Program. Washington (DC): MedPAC; 2009. Accountable care organizations. [Google Scholar]
- 14.Berwick DM. Launching accountable care organizations—the proposed rules for the medicare shared savings program. N Engl J Med. 2011;364(16) doi: 10.1056/NEJMp1103602. [DOI] [PubMed] [Google Scholar]
- 15.Pollack CE, Weissman GE, Lemke KW, Hussey PS, Weiner JP. Patient sharing among providers and costs of care: a network analytic approach to care coordination using claims data. J Gen Intern Med. 2013;28:459–65. doi: 10.1007/s11606-012-2104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uddin S, Hossain L, Kelaher M. Effect of provider collaboration network on hospitalization cost and readmission rate. Eur J Pub Health. 2012;22:629–33. doi: 10.1093/eurpub/ckr153. [DOI] [PubMed] [Google Scholar]
- 17.Barnett ML, Landon BE, O’Malley AJ, Keating NL, Christakis NA. Mapping provider networks with self-reported and administrative data. Health Serv Res. 2011;46:1592–609. doi: 10.1111/j.1475-6773.2011.01262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landon BE, Keating NL, Barnett ML, Onnela JP, Paul S, O’Malley AJ, Keegan T, Christakis NA. Variation in patient-sharing networks of providers across the United States. JAMA. 2012;308:265–73. doi: 10.1001/jama.2012.7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bynum JP, Ross JS. A measure of care coordination? J Gen Intern Med. 2013;28:336–8. doi: 10.1007/s11606-012-2269-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mandl KD, Olson KL, Mines D, Liu C, Tian F. Provider collaboration: cohesion, constellations, and shared patients. J Gen Intern Med. 2014;29:1499–505. doi: 10.1007/s11606-014-2964-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.HealthCore Inc. http://healthcore.com/research-environment. Accessed 1 July 2015.
- 22.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 23.Real R, Vargas JM. The probabilistic basis of Jaccard’s index of similarity. Syst Biol. 1996;45:380–5. doi: 10.1093/sysbio/45.3.380. [DOI] [Google Scholar]
- 24.Mansfield ER, Helms BP. Detecting multicollinearity. Am Stat. 1982;36:158–60. [Google Scholar]
- 25.Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264:2511–8. doi: 10.1001/jama.1990.03450190043026. [DOI] [PubMed] [Google Scholar]
- 26.Dunn KM, Saunders KW, Rutter CM, Banta-Green CJ, Merill JO, Sullivan MD, Weisner CM, Silverberg MJ, Campbell CI, Psaty BM, Von Korff M. Opioid prescriptions for chronic pain and overdose. A cohort study. Ann Intern Med. 2010;152:85–92. doi: 10.7326/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamblyn RM, McLeod PJ, Abrahamowicz M, Laprise R. Do too many cooks spoil the broth? Multiple physician involvement in medical management of elderly patients and potentially inappropriate drug combinations. CMAJ. 1996;154:1177–84. [PMC free article] [PubMed] [Google Scholar]
- 28.Han H, Kass PH, Wilsey BL, Li CS. Individual and county-level factors associated with use of multiple prescribers and multiple pharmacies to obtain opioid prescriptions in California. PLoS One. 2012;7 doi: 10.1371/journal.pone.0046246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katon WJ, Lin EH, Von Korff M, Ciechanowski P, Ludman EJ, Young B, Peterson D, Rutter CM, McGregor M, McCulloch D. Collaborative care for patients with depression and chronic illnesses. JAMA. 1995;273:1026–1031. N Engl J Med. 2010;363:2611–20. doi: 10.1056/NEJMoa1003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coventry P, Lovell K, Dickens C, Bower P, Chew-Graham C, McElvenny D, Hann M, Cherrington A, Garrett C, Gibbons CJ, Baguley C, Roughley K, Adeyemi I, Reeves D, Waheed W, Gask L. Integrated primary care for patients with mental and physical multimorbidity: cluster randomised controlled trial of collaborative care for patients with depression comorbid with diabetes or cardiovascular disease. BMJ. 2015;350:h638. doi: 10.1136/bmj.h638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rollman BL, Belnap BH, LeMenager MS, Mazumdar S, Houck PR, Counihan PJ, Kapoor WN, Schulberg HC, Reynolds CF., 3rd Telephnoe-delivered collaborative care for treating post-CABG depression: a randomized controlled trial. JAMA. 2009;302:2095–103. doi: 10.1001/jama.2009.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Unützer J, Katon W, Callahan CM, Williams JW, Jr, Hunkeler E, Harpole L, Hoffing M, Della Penna RD, Noël PH, Lin EH, Areán PA, Hegel MT, Tang L, Belin TR, Oishi S, Langston C, IMPACT Investigators Improving mood-promoting access to collaborative treatment. Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA. 2002;288:2836–45. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- 33.Unutzer J, Katon WJ, Fan MY, Schoenbaum MC, Lin EH, Della Penna RD, Powers D. Long-term cost effects of collaborative care for late-life depression. Am J Manage Care. 2008;14:95–100. [PMC free article] [PubMed] [Google Scholar]
- 34.Druss BG, Rosenheck RA, Desai MM, Perlin JB. Quality of preventive medical care for patients with mental disorders. Med Care. 2002;40:129–36. doi: 10.1097/00005650-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 35.American College of Physicians. The impending collapse of primary care medicine and its implications for the state of the nation’s health care: a report from the American College of Physicians. Philadelphia (PA): ACP; 2006 Jan 30. Available from: http://www.acponline.org/advocacy/events/state_of_healthcare/ statehc06_1.pdf.
- 36.Bodenheimer T. Primary care—will it survive? N Engl J Med. 2006;355(9):861–4. doi: 10.1056/NEJMp068155. [DOI] [PubMed] [Google Scholar]
- 37.Bodenheimer T. Coordinating care—a perilous journey through the health care system. N Engl J Med. 2008;358(10):1064–71. doi: 10.1056/NEJMhpr0706165. [DOI] [PubMed] [Google Scholar]
- 38.Pankratz L, Jackson J. Habitually wandering patients. N Engl J Med. 1994;331:1752–5. doi: 10.1056/NEJM199412293312606. [DOI] [PubMed] [Google Scholar]
- 39.Schrag D, Xu F, Hanger M, Elkin E, Bickell NA, Bach PB. Fragmentation of care for frequently hospitalized urban residents. Med Care. 2006;44(6):560-7. [DOI] [PubMed]
- 40.Kne T, Young R, Spillane L. Frequent ED users: patterns of use over time. Am J Emerg Med. 1998;16(7):648-52. [DOI] [PubMed]
- 41.Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. 2010;363:1981–5. doi: 10.1056/NEJMp1011512. [DOI] [PubMed] [Google Scholar]
- 42.Paulozzi LJ, Budnitz DS, Xi Y. Increasing deaths from opioid analgesics in the United States. Pharmacoepidemiol Drug Saf. 2006;15:618–27. doi: 10.1002/pds.1276. [DOI] [PubMed] [Google Scholar]
- 43.Paulozzi LJ, Kilbourne EM, Desai HA. Prescription drug monitoring programs and death rates from drug overdose. Pain Med. 2011;12:747–54. doi: 10.1111/j.1526-4637.2011.01062.x. [DOI] [PubMed] [Google Scholar]
- 44.Cepeda MS, Fife D, Yuan Y, Mastrogiovanni G. Distance travelled and frequency of interstate opioid dispensing in opioid shoppers and nonshoppers. J Pain. 2013;14:1158–61. doi: 10.1016/j.jpain.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 45.McLellan T, Turner B. Prescription opioids, overdose deaths, and physician responsibility. JAMA. 2008;300:2672–3. doi: 10.1001/jama.2008.793. [DOI] [PubMed] [Google Scholar]