Abstract
Disease-modifying alternatives are sorely needed for the treatment of neurodegenerative disorders, a group of diseases that afflict approximately 50 million Americans annually. Immunotherapy is one of the most developed approaches in this direction. Vaccination against amyloid-β, α-synuclein, or tau has been extensively explored, specially as the discovery that these proteins may propagate cell-to-cell and be accessible to antibodies when embedded into the plasma membrane or in the extracellular space. Likewise, the use of passive immunization approaches with specific antibodies against abnormal conformations of these proteins has also yielded promising results. The clinical development of immunotherapies for Alzheimer’s disease, Parkinson’s disease, frontotemporal dementia, dementia with Lewy bodies, and other neurodegenerative disorders is a field in constant evolution. Results to date suggest that immunotherapy is a promising therapeutic approach for neurodegenerative diseases that progress with the accumulation and prion-like propagation of toxic protein aggregates. Here we provide an overview of the most novel and relevant immunotherapeutic advances targeting amyloid-β in Alzheimer’s disease, α-synuclein in Alzheimer’s disease and Parkinson’s disease, and tau in Alzheimer’s disease and frontotemporal dementia.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-015-0397-z) contains supplementary material, which is available to authorized users.
Key Words: Immunotherapy, Vaccines, Antibodies, Amyloid-β, α-synuclein, Tau
Introduction
Neurodegenerative disorders of the aging population, such as Alzheimer’s disease (AD), Parkinson’s disease (PD) and Frontotemporal dementia (FTD), are characterized by the progressive accumulation of misfolded protein aggregates that initially trigger synaptic damage and network dysfunction, and that eventually lead to loss of selected neuronal populations [1, 2]. In AD, the proteins amyloid-β (Aβ) and tau accumulate in the neocortex, limbic system, and basal forebrain in the form of plaques and neurofibrillary tangles [3]. In PD and related disorders such as PD dementia, dementia with Lewy bodies (DLB), and multiple system atrophy (MSA), the protein α-synuclein (α-syn) accumulates in neuronal and non-neuronal cells in cortical and subcortical nuclei as Lewy bodies, neuronal cytoplasmic inclusions, or glial cytoplasmic inclusions [4, 5]. Furthermore, in FTD (amyotrophic lateral sclerosis spectrum disorder) aggregates of either tau, superoxide dismutase 1, TAR DNA-binding protein 43 (TDP-43), or fused in sarcoma are found [6, 7]. In addition, recent studies have shown that α-syn can accumulate in selected brain regions in AD [8], and that TDP-43 aggregates are found in the limbic system in AD and DLB [9]. These findings reinforce the idea that abnormal protein accumulation is key in most neurodegenerative disorders. Under native conditions, most of these proteins can be found as poorly structured monomers or as dimers or tetramers associated with the plasma membrane [10–12]. However, under pathological conditions such as those associated with AD, PD, and FTD, various molecular weight aggregates of these protein are detected, ranging from small oligomers to protofibrils and fibrils [13–17].
Most recent evidence suggests that oligomers and probably also protofibrils are toxic to neurons by disrupting synaptic function, membrane permeability, calcium homeostasis, gene transcription, mitochondrial activity, autophagy, and/or endosomal transport [18–21]. Moreover, recent studies have shown that propagation and seeding of Aβ, tau, and α-syn in a prion-like manner might also contribute to neurodegeneration [22–28]. Remarkably, there is also evidence that these various protein aggregates can interact with each other [29]. For example, Aβ promotes the aggregation of α-syn and tau in AD and DLB [30, 31], α-syn and tau interact in the brain of patients with PD and DLB [32, 33], α-syn and Aβ can form hetero-oligomers [34, 35], and α-syn can modulate the fibrillization state of Aβ [36].
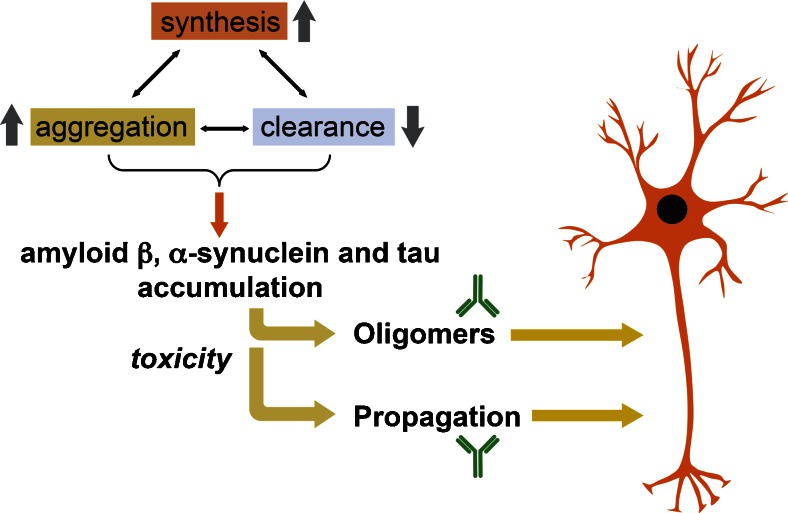
Progressive misfolding and accumulation of neurotoxic Aβ, tau, and α-syn have been associated with an imbalance in the levels of their synthesis, aggregation, and clearance (Fig. 1). Mechanisms of clearance include proteolysis, autophagy, and proteasomal degradation [37, 38]. In this context, it has been suggested that Aβ, tau, and α-syn toxic aggregates might be major therapeutic targets for these neurodegenerative disorders (Fig. 1). Thus, therapeutic strategies for AD, PD, and FTD might require reducing the synthesis, preventing the aggregation and/or enhancing the clearance of Aβ, tau, or α-syn. Numerous strategies directed at reducing the accumulation of these proteins have been developed, including the use of small interfering RNA, antisense RNA [39–43], degrading enzymes (e.g., cathepsin D, neurosin, neprilysin) [44–46], chaperone-like molecules that modulate aggregation state (e.g., Hsp70, β-syn) [47–50], anti-aggregation compounds (e.g., polyphenols) [51–53], and immunotherapy (passive, active, and T-cell-based) [54]. Moreover, the recent discovery that toxic oligomeric forms of α-syn and tau accumulate in the plasma membrane and are secreted to the extracellular environment has provided further rationale for the development of immunotherapeutic approaches for PD, DLB, MSA, FTD, and other neurodegenerative disorders characterized by the abnormal accumulation of these proteins [24, 26, 55–58].
Fig. 1.
Mechanisms of action of immunotherapy for neurodegenerative disorders. The misfolding and accumulation of amyloid-β, α-synuclein, and tau has been associated with an imbalance in the levels of their synthesis, aggregation, and clearance. The toxicity of these proteins is correlated with their ability to adopt specific conformations (oligomers, protofibrils) and to propagate from cell to cell, leading to neurodegeneration. Disease-modifying therapeutic strategies may require reducing the synthesis, preventing the aggregation and/or enhancing the clearance of amyloid-β, α-synuclein, and tau. Specifically, immunotherapeutic approaches are able to target specific conformational species and inhibit cell-to-cell propagation of these proteins
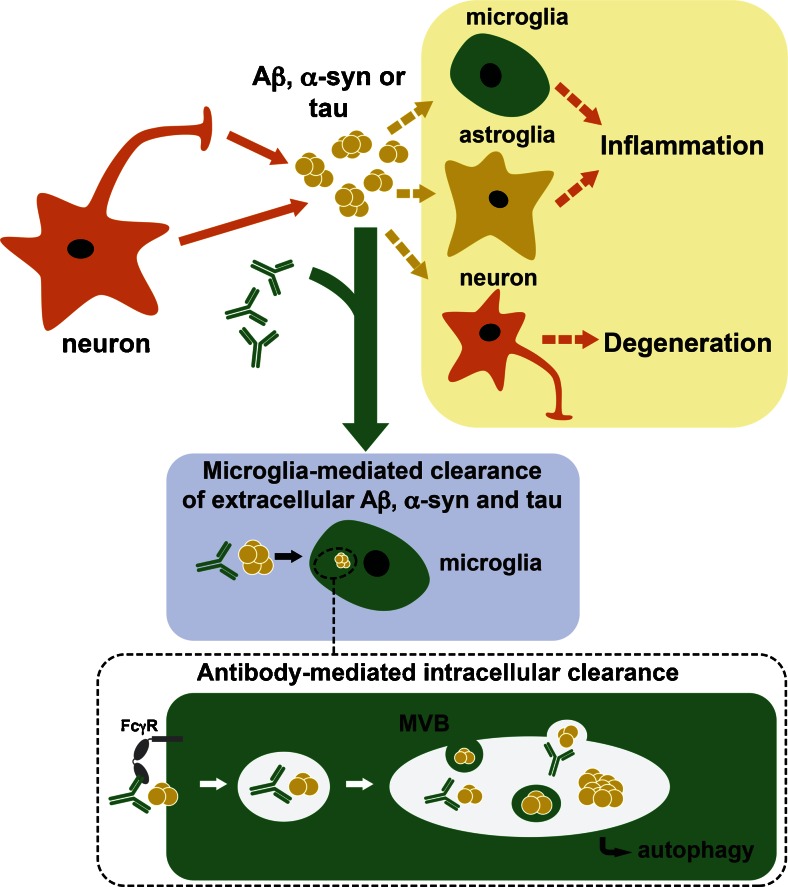
Among these strategies, the development of immunotherapeutic approaches targeting Aβ, tau, and α-syn has received considerable attention in recent years. In this sense, both humoral (active and passive) and T-cell-based approaches have been explored. While active immunization stimulates the immune system to produce antibodies against target proteins, passive immunization consists in directly administering antibodies that confer temporary protection against the disease. A third type of immunotherapy involving the activation of regulatory T-cells has also been explored for the potential treatment of AD and PD [59, 60]. The advantage of humoral immunotherapy over other approaches is that it allows for the generation of antibodies targeting specific conformations of Aβ, tau, or α-syn (monomers, oligomers, and/or fibrils) (Fig. 1). Moreover, antibodies against these proteins can regulate inflammation and facilitate the clearance of target proteins via autophagy or microglia (Fig. 2) [61–66]. Neurodegenerative diseases are associated with signs of chronic neuroinflammation and elevated levels of several proinflammatory cytokines released from microglia, such as interleukin-1β, interleukin-6, and tumor necrosis factor-α [67]. Microgliosis, astrogliosis, and peripheral immune infiltration contribute to the cognitive and motor deficits, and lead to a toxic increase in the levels of reactive oxygen species [68], and to secondary neurodegeneration, characteristic of late disease stages [69]. Immunotherapy has been shown to induce a physiological microglial response (M2 type) and reduce the production of proinflammatory cytokines [65, 66], thus exerting an anti-inflammatory effect in neurodegenerative disorders [70, 71]. However, among the disadvantages of immunotherapy are the potential for autoimmune responses, nonspecific inflammatory reactions such as perivascular edema, need for repetitive administration, lack of response due to senescence of the innate immune system, and limited penetration of antibodies into the central nervous system.
Fig. 2.
Antibodies promote microglia-mediated clearance of aggregated toxic proteins in neurodegenerative disorders. Toxic oligomeric forms of amyloid-β (Aβ), α-synuclein (α-syn), and tau are released by neurons to the extracellular environment in Alzheimer’s disease, Parkinson’s disease, and frontotemporal dementia brains, where they propagate to neuronal or glial cells leading to neuroinflammation and neurodegeneration. Antibodies bind extracellular or membrane-bound toxic oligomeric conformations of Aβ, α-syn, and tau, and might accelerate their clearance by microglia-mediated mechanisms, probably involving interaction with Fcγ receptors (FcγR) and autophagy degradation (bottom panel). MVB = multivesicular body
Active and passive immunization strategies are being explored in several clinical trials for AD and PD (Table 1). The most advanced studies are those on Aβ immunotherapy, for which 2 active immunization and 5 passive immunization programs are currently underway. For α-syn, there are 2 active and 2 passive immunization programs in phase I. For tauopathies, 2 vaccines and 3 antibodies are currently in phase I studies. Here we will review the most recent contributions and advances on humoral immunotherapy approaches for AD, PD, and FTD targeting Aβ, α-syn, and tau.
Table 1.
Current clinical trials on immunotherapies for neurodegenerative diseases
| Drug | Trial phase | Epitope | Sponsor | Reference(s) | |
|---|---|---|---|---|---|
| Aβ | |||||
| Active immunotherapy: vaccines | |||||
| ACI-24 | I/II | 1–15 aa | AC Immune SA | [72, 73] | |
| Lu AF20513 | I | 1–12 aa, modified | H. Lundbeck A/S | [74] | |
| Passive immunotherapy: antibodies | |||||
| BAN2401 | II | Protofibrils | Eisai Inc. | [75] | |
| Crenezumab | II | 12–23 aa | Genentech | [76] | |
| Flebogamma | III | Immnunoglobulin | Instituto Grifols, S.A. | [77] | |
| Gantenerumab | III | Conformational | Hoffman-La Roche | [78–80] | |
| Solanezumab | III | 13–28 aa | Eli Lilly | [81, 82] | |
| α-Syn | |||||
| Active immunotherapy: vaccines | |||||
| AFFITOPE PD01A | I | NP | Affiris | [66, 83] | |
| AFFITOPE PD03A | I | NP | Affiris | [66, 83] | |
| Passive immunotherapy: antibodies | |||||
| BIIB054 | I | NP | Biogen | ||
| PRX002 | I | C-terminus | Prothena Biosciences | [84] | |
| Tau | |||||
| Active immunotherapy: vaccines | |||||
| AADvac-1 | I | 294–305 aa | Axon Neuroscience SE | [85] | |
| ACI-35 | I | Fragments (pS396, pS404) | AC Immune SA | [86] | |
| Passive immunotherapy: antibodies | |||||
| BMS-986168 | I | eTau | Bristol-Myers Squibb | [87] | |
| C2N-8E12 | I | NP | C2N Diagnostics | ||
| RG7345 | I | pS422 | Hoffmann-La Roche | [88] | |
Information regarding clinical trials was found at clinicaltrials.gov and alzforum.org as of October 2015
Aβ = amyloid-β; α-Syn = α-synuclein; NP = not provided; aa = amino acids
Immunotherapy Targeting Aβ
The Aβ peptide is derived from a larger amyloid precursor protein (APP) by proteolytic cleavage at the β- and γ-secretase sites, resulting in the formation of Aβ1-38, Aβ1-40, Aβ1-42, and Aβ1-43. Patients suffering from AD often produce the longer forms of Aβ (Aβ1-42, Aβ1-43), which are more prone to aggregation and exhibit higher toxicity, while healthy subjects produce more of the shorter Aβ varieties [89, 90]. Therefore, developing antibodies against the longer forms of Aβ allows for the selective targeting of the more fibrillogenic and toxic species in therapy.
Immunotherapy for neurodegenerative disorders was first established for AD by targeting the Aβ peptide. The group of Schenk et al. [91] pioneered active immunization strategies by vaccinating APP transgenic mice with Aβ1-42 (AN-1792) and adjuvant, while Solomon et al. [92] developed a passive immunization approach using monoclonal antibodies against Aβ and showing that they reduce fibrillization in vitro. More recent active immunization strategies have included CAD106 [93], vanutide cridificar [94], and AD02, a synthetic peptide that mimics the N-terminus structure of the Aβ peptide (AFFITOPE; AFFiRiS AG, Vienna, Austria) [95]. Unfortunately, none of these approaches has resulted in significant clinical improvements. However, one of the placebo formulations for AD02 (renamed AD04) had a greater benefit on the primary outcome than the other placebo formulation or any of the 3 AD02 treatment groups in a phase II study, suggesting that it could be further developed. Although the composition of this placebo formulation has not been disclosed, it is possible that includes the adjuvant alum, which has been shown to boost adaptive immunity and induce uric acid production [96], a natural peroxynitrite scavenger [97]. Currently, 2 active immunization trials against Aβ are undergoing (Table 1). Lu AF20513 consists of 3 repetitions of a modified Aβ1-12 sequence, in which the natural T-helper cell epitopes are engineered to reduce the possibility of inducing harmful autoreactive T-cell responses and to improve the ability to mount an effective immune response [74]. ACI-24 (Europe, phase I/II) is a liposome vaccine designed to elicit an antibody response against aggregated Aβ peptides without concomitant proinflammatory T-cell activation. An array of Aβ1-15 sequences are anchored to the surface of liposomes adopting an aggregated β-sheet structure that acts as conformational epitope. In preclinical studies, repeated subcutaneous injection of ACI-24 into AD transgenic mice generated high titers of anti-Aβ antibodies, decreasing the concentration of insoluble Aβ1-40 and Aβ1-42, and of soluble Aβ1-42 [72, 73]. ACI-24 also improved novel object recognition without triggering proinflammatory responses [73]. Finally, DNA vaccines against Aβ1-42 [98, 99], alone or in combination with protein antigens [100, 101], have shown promising results at the preclinical stage. DNA-based vaccination utilizes direct injection of DNA-encoding genes for protein or peptide antigens, and it does not require the use of adjuvants. Co-immunization with a mixture of Aβ1-42 DNA and protein is capable of inducing Th2-type Aβ-specific antibodies while simultaneously suppressing unwanted inflammatory reactions and avoiding T-cell-mediated autoimmune responses [100].
Initial active immunization using AN-1792 (full-length Aβ1-42) highlighted the risk of autoimmune responses when using this type of therapeutic strategy, as 6 % of the patients in that study suffered with meningoencephalitis associated with T-cell infiltration [102]. Since then, numerous efforts have been devoted to reduce antigen-induced inflammatory T-cell activation, including the use of multiple small fragments of Aβ instead of the full sequence (e.g., CAD106, vanutide cridificar), and the use of small synthetic peptides that mimic the original epitope without carrying its sequence (AD02). However, the marginally positive effects observed with active immunization against Aβ suggest that other type of approaches might be more beneficial for patients with AD. In this sense, the use of antibodies directed against specific epitopes or conformations of Aβ has yielded promising results. Passive immunization approaches using monoclonal antibodies against Aβ1-40 [103], Aβ1-42 [104], pyroglutamate Aβ [105], oligomers [106], or protofibrils [107–109] have been developed. Currently, clinical trials with the antibodies BAN2401 (recognizing protofibrils) [75], crenezumab (aggregated species) [76], gantenerumab (fibrils) [78–80], and solanezumab (Aβ mid-domain) [81, 82] are ongoing (Table 1). However, other programs using antibodies such as bapinezumab (N-terminus) [110] and ponezumab (C-terminus) [111] have been discontinued as they did not meet expected goals. Finally, owing to the lack of significant disease modification in phase II and III trials, the use of nonspecific strategies such as intravenous immunoglobulin [Gammagard (Baxter Healthcare Corp., Deerfield, IL, USA), Octagam (Octapharma, Hoboken, NJ, USA), Flebogamma (Instituto Grifois SA, Barcelona, Spain) [77]] is relatively losing momentum for the treatment of AD [112]. These findings suggest that passive immunization against Aβ holds promise for disease modification in AD; however, more research is needed to improve the outcome of the immunotherapeutic treatments. This might include using immunotherapy as a preventive approach prior to the onset of symptoms, co-immunizing with both anti-Aβ and anti-tau antibodies in clinical trials, developing antibodies with better specificity to the toxic forms of Aβ, and boosting antibody penetration into the brain [113].
Immunotherapy Targeting α-Syn
α-Syn is a synaptic protein involved in synaptic transmission and vesicle release that is specifically upregulated in a discrete population of presynaptic terminals during acquisition-related synaptic rearrangement [114, 115]. α-Syn was initially identified in AD brains associated with plaque formation and neurodegeneration [116, 117]. The abnormal aggregation of α-syn is correlated with the neuropathological changes observed in PD and other synucleinopathies [13, 118], and therefore inhibiting α-syn aggregation would be a key mechanism for preventing its toxicity.
Initial immunotherapeutic studies were performed using vaccination with the full human α-syn protein [119]. Active immunization of α-syn transgenic mouse models of LBD decreased accumulation of aggregated α-syn and reduced neurodegeneration [119]. Furthermore, antibodies produced by immunized mice promoted the degradation of α-syn aggregates, probably via lysosomal pathways [119]. These results suggested that α-syn vaccination is effective in reducing neuronal accumulation of α-syn aggregates and that further development of this approach might have a potential role in the treatment of synucleinopathies.
Other active immunization approaches using AFFITOPEs (AFFiRiS AG) that mimic abnormal conformations of α-syn have been studied in animals model of PD and MSA [65, 66]. AFFITOPEs that mimic the C-terminus region of α-syn are able to elicit an immune response specific to α-syn oligomers [66]. Vaccination with one of these AFFITOPEs (AFF 1) resulted in high antibody titers against α-syn aggregates, decreased accumulation of α-syn oligomers, reduced degeneration of tyrosine hydroxylase fibers in the caudoputamen nucleus, and improved motor and memory deficits in 2 α-syn transgenic models [66]. Moreover, when administered to a transgenic model of MSA, AFF 1 also induced a reduction in neurodegeneration and demyelination in neocortex, striatum, and corpus callosum [65]. The clearance of α-syn induced by AFF 1 involved activation of microglia, increased anti-inflammatory cytokine production, and reduced spreading of α-syn to astroglial cells [65, 66]. These studies suggested that vaccination with AFFITOPEs could help ameliorate the neurodegenerative pathology in synucleinopathies. In this sense, phase I clinical trials with the AFFITOPEs PD01A and PD03A for PD and MSA, respectively, are currently ongoing (Table 1).
Passive immunization approaches using antibodies against α-syn are also being actively pursued. Different groups have investigated which region of the α-syn protein is the best target for the development of disease-modifying monoclonal antibodies. We and others have observed that antibodies that recognize an epitope in the C-terminus of α-syn are more effective at ameliorating the pathology in transgenic mouse models of PD, as they clear intracellular aggregates, inhibit α-syn propagation, and prevent C-terminus cleavage of the protein, which may lead to increased aggregation [54, 84, 120, 121]. However, other groups have reported that antibodies against the N-terminus are also effective at clearing α-syn aggregates, reducing their propagation, and diminishing motor dysfunctions [122, 123]. Together, these reports support the value of immunotherapy with antibodies directed against α-syn for PD, and in this sense the C-terminus antibody PRX002 (AFFiRiS AG) and the antibody BIIB054 (Biogen, Cambridge, MA, USA) are currently being tested in phase I clinical trials (Table 1).
Interestingly, and as mentioned before, antibodies against α-syn may not only reduce α-syn levels, but also reduce its oligomerization and fibrillization in living cells, thus reducing the pathology in mouse models of PD [66, 124, 125]. Furthermore, antibodies may also prevent cell-to-cell propagation of α-syn and facilitate the clearance of extracellular α-syn [84, 121, 122] (Fig. 2) . Importantly, both aggregation and cell-to-cell propagation are intimately related to α-syn toxicity and PD pathology, suggesting that these processes are promising therapeutic targets for immunotherapy.
Immunotherapy Targeting Tau
In AD and other tauopathies such as FTD, hyperphosphorylated tau accumulates within neurons in the form of neurofibrillary tangles [126–131]. Importantly, as cognitive impairments closely correlate with the extension of tau pathology [132, 133], removing neurofibrillary tangles has become one of the main therapeutic goals for the treatment of AD and FTD [134, 135]. In this regard, it has been shown that both active and passive immunization against tau reduce its accumulation and slow or prevent behavioral deficits in transgenic mouse models of tauopathy [135–142].
Active immunotherapy using phosphorylated tau epitopes has shown promising results in animal models [136, 142], and 2 tau vaccines are currently in phase I trial for AD, AADvac-1, and ACI-35 (Table 1). AADvac-1 consists of a synthetic peptide derived from amino acids 294–305 of the tau sequence, although the precise molecular nature of the antigen has not been disclosed. ACI-35 is a liposome-based vaccine that elicits an immune response against pathological conformers of phosphorylated tau without mounting autoimmune B- or T-cell responses against physiological tau conformations. The vaccine contains 16 copies of a synthetic tau fragment phosphorylated at S396 and S404, and anchored into a lipid bilayer. In the tau P301L transgenic mice, ACI-35 injection rapidly generates high titers of polyclonal antibodies specifically directed against phosphorylated tau. The resulting antibodies bind neurofibrillary tangles in mouse brain tissue sections and are able to reduce soluble tau, as well as insoluble, aggregated tau in brain extracts [86].
Antibodies against phospho-tau and tau oligomers have also been developed and tested at preclinical levels. Mechanistically, these antibodies seem to act either by promoting microglial clearance (Fig. 2), or by blocking neuronal uptake of the protein [143]. Passive immunization with anti-phospho-tau antibodies reduce tau pathology and functional deficits [137, 144–146], and antibodies targeting tau oligomers have also shown promise in transgenic models [136, 147], including a concomitant upstream reduction in Aβ pathology [148]. In this sense, there are 3 anti-tau antibodies currently being studied in phase I trials (Table 1). BMS-986168 targets extracellular, N-terminally fragmented forms of tau (eTau) that can induce an increase in Aβ production and contribute to the spreading of the pathology [87]. This antibody reportedly neutralizes eTau toxicity in mouse models of FTD. RG7345 is a humanized monoclonal antibody targeting phospho-tau (pS422). Phosphorylation of tau at S422 has been linked to the relocalization of tau away from microtubules and toward the somatodendritic compartment of the neuron [149]. It has been shown that targeting the pS422 tau epitope with active vaccination decreases levels of insoluble phosphorylated tau and improves behavioral performance in transgenic mouse model of tauopathy [142]. Moreover, in a transgenic mouse model of AD, anti-tau pS422 antibodies are able to reduce accumulation of tau and induce its clearance via lysosomal pathways [88]. Anti-tau antibodies are internalized by neurons with tau aggregates via interaction with Fcγ receptors, and this internalization leads to the clearance of tau pathology in primary neurons [150], a mechanism that is probably shared with antibodies against α-syn (Fig. 2) [121]. Finally, the recombinant humanized anti-tau antibody C2N-8E12 has recently begun a phase I clinical study in patients with progressive supranuclear palsy.
Developing New Technologies for Immunotherapy
Passive immunization using immunoglobulins, voluminous proteins that do not easily cross the blood–brain barrier (BBB) and recognize a limited variety of epitopes, may yield only modest results. Therefore, efforts have recently been focused on the development of therapeutic single chain antibodies [single chain variable fragment (scFv)], fusion proteins of the variable regions of the heavy and light chains of immunoglobulins connected with a short linker peptide of 10–25 amino acids. scFvs retain antigen-binding properties and can be easily screened for desired affinities using phage display methodology. Using this type of approach, scFvs that detect individual conformational species of α-syn have been identified [151–153], and could be potentially used to discriminate among protein conformers for the differential treatment of synucleinopathies or for diagnostic purposes [154]. Moreover, scFvs can be further modified to increase BBB penetrability and facilitate the clearance of α-syn. In this sense, a fusion protein comprising a scFv against α-syn plus the low density lipoprotein domain of apolipoprotein B was recently studied in a transgenic model of DLB. The brain-targeted fusion antibody easily crosses the BBB and gets internalized by neurons using the endosomal sorting complexes required for transport (ESCRT) pathway for enhanced degradation of α-syn aggregates [151], thus attenuating neuronal degeneration in vivo. Similarly, a fusion protein comprising a scFv and a specific protease can further aid in the clearance of aggregation-prone proteins [155]. Finally, the use of gene therapy with intracellular scFv (intrabodies) is also being explored for the detection and clearance of intracellular α-syn aggregates [156–158].
Preclinical and clinical studies suggest that immunotherapy against Aβ, α-syn, and tau is a promising approach for the treatment of AD, PD, and FTD. Furthermore, as these proteins may co-aggregate and/or regulate each other [29, 30, 159–161], immunization against one of them could reduce the aggregation or toxic modification of the others. For example, immunization against Aβ might be helpful at reducing α-syn and tau if administered at early disease stages [62]; likewise, α-syn antibodies might also be helpful in AD [162], and immunization against tau might be useful for PD [161, 163]. In this sense, identifying and targeting polyvalent antigens or using single-chain polyvalent antibodies targeting simultaneously Aβ, tau, and α-syn could have synergistic effects, as it occurs with polyvalent vaccines for certain cancers and infections [164, 165]. In the case of AD, targeting both Aβ and tau at the same time might improve the outcome of immunotherapeutic clinical trials, as it is likely that both proteins synergistically contribute to the progression of the pathology. Unfortunately, owing to the fact that accumulation of toxic proteins is an early event in neurodegenerative diseases, it is possible that immunization would be more successful as an early or preventive strategy rather than therapeutic one. Therefore, clinical trials using active or passive immunization may yield better results if performed in non-diseased or early-stage patients. Immunotherapy also has anti-inflammatory effects, probably by reducing extracellular levels of proinflammatory antigens, stimulating microglial clearance of toxic protein aggregates, and attenuating microglial inflammatory responses, leading to neuroprotective effects that may be also beneficial in late disease stages. As many of the antibodies described here have been developed against specific pathologic conformers of Aβ, α-syn, or tau, these antibodies could also be used as biomarker tools for diagnosis. In this sense, it has been suggested that autoimmune reactions towards specific proteins involved in the disease pathology can be used as biomarkers of neurodegeneration in both AD and PD, especially in early disease stages [166–168]. Antibodies that recognize conformational epitopes specific of amyloid fibrils have been found in sera of healthy and diseased patients [169], suggesting that autoimmune reactivity can play a role as an amyloid clearance mechanism in both health and disease. Many neurodegenerative diseases have similar symptoms as they result from the aggregation of the same protein(s), making diagnosis challenging at times [2]. Using the antibodies developed against specific conformations of these proteins as diagnostic tools would allow the clinician to make accurate decisions about which therapy to prescribe [170–172], greatly benefiting the therapeutic regimen and truly opening the door for personalized medicine. Moreover, it will probably be the case in the future that several immunotherapeutic options will be available for each disease, so therapy customization will become crucial. Finally, proteins other than Aβ, α-syn, and tau do accumulate in neurodegenerative disorders and are potential targets for immunotherapy as well. These include β-secretase [173], presenilin-1, leucine-rich repeat kinase 2 (LRRK2), superoxide dismutase-1 [174–176], TDP-43, and fused in sarcoma, among others. It is possible that simultaneously targeting these proteins would drastically improve the outcome of the immunotherapeutic approach.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(PDF 1224 kb)
Acknowledgments
This work was supported by National Institutes of Health (NIH) grants AG18440, AG022074, and NS044233.
Compliance with Ethical Standards
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
References
- 1.Selkoe DJ. Cell biology of protein misfolding: the examples of Alzheimer's and Parkinson's diseases. Nat Cell Biol. 2004;6:1054–1061. doi: 10.1038/ncb1104-1054. [DOI] [PubMed] [Google Scholar]
- 2.Taylor JP, Hardy J, Fischbeck KH. Toxic proteins in neurodegenerative disease. Science. 2002;296:1991–1995. doi: 10.1126/science.1067122. [DOI] [PubMed] [Google Scholar]
- 3.Crews L, Masliah E. Molecular mechanisms of neurodegeneration in Alzheimer's disease. Hum Mol Genet. 2010;19:R12–R20. doi: 10.1093/hmg/ddq160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goedert M, Jakes R, Anthony Crowther R, Grazia Spillantini M. Parkinson's disease, dementia with Lewy bodies, and multiple system atrophy as alpha-synucleinopathies. Methods Mol Med. 2001;62:33–59. doi: 10.1385/1-59259-142-6:33. [DOI] [PubMed] [Google Scholar]
- 5.Spillantini MG. Parkinson's disease, dementia with Lewy bodies and multiple system atrophy are alpha-synucleinopathies. Parkinsonism Relat Disord. 1999;5:157–162. doi: 10.1016/S1353-8020(99)00031-0. [DOI] [PubMed] [Google Scholar]
- 6.Goedert M, Ghetti B, Spillantini MG. Frontotemporal dementia: implications for understanding Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a006254. doi: 10.1101/cshperspect.a006254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrari R, Kapogiannis D, Huey ED, Momeni P. FTD and ALS: a tale of two diseases. Curr Alzheimer Res. 2011;8:273–294. doi: 10.2174/156720511795563700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winslow AR, Moussaud S, Zhu L, et al. Convergence of pathology in dementia with Lewy bodies and Alzheimer's disease: a role for the novel interaction of alpha-synuclein and presenilin 1 in disease. Brain. 2014;137:1958–1970. doi: 10.1093/brain/awu119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arai T, Mackenzie IR, Hasegawa M, et al. Phosphorylated TDP-43 in Alzheimer's disease and dementia with Lewy bodies. Acta Neuropathol. 2009;117:125–136. doi: 10.1007/s00401-008-0480-1. [DOI] [PubMed] [Google Scholar]
- 10.McLean PJ, Kawamata H, Ribich S, Hyman BT. Membrane association and protein conformation of alpha-synuclein in intact neurons. Effect of Parkinson's disease-linked mutations. J Biol Chem. 2000;275:8812–8816. doi: 10.1074/jbc.275.12.8812. [DOI] [PubMed] [Google Scholar]
- 11.Bartels T, Choi JG, Selkoe DJ. alpha-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature. 2011;477:107–110. doi: 10.1038/nature10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pooler AM, Usardi A, Evans CJ, Philpott KL, Noble W, Hanger DP. Dynamic association of tau with neuronal membranes is regulated by phosphorylation. Neurobiol Aging. 2012;33(431):e27–e38. doi: 10.1016/j.neurobiolaging.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Lashuel HA, Petre BM, Wall J, et al. Alpha-synuclein, especially the Parkinson's disease-associated mutants, forms pore-like annular and tubular protofibrils. J Mol Biol. 2002;322:1089–1102. doi: 10.1016/S0022-2836(02)00735-0. [DOI] [PubMed] [Google Scholar]
- 14.Kayed R, Sokolov Y, Edmonds B, et al. Permeabilization of lipid bilayers is a common conformation-dependent activity of soluble amyloid oligomers in protein misfolding diseases. J Biol Chem. 2004;279:46363–46366. doi: 10.1074/jbc.C400260200. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto M, Rockenstein E, Crews L, Masliah E. Role of protein aggregation in mitochondrial dysfunction and neurodegeneration in Alzheimer's and Parkinson's diseases. Neuromolecular Med. 2003;4:21–36. doi: 10.1385/NMM:4:1-2:21. [DOI] [PubMed] [Google Scholar]
- 16.Lasagna-Reeves CA, Castillo-Carranza DL, Sengupta U, et al. Identification of oligomers at early stages of tau aggregation in Alzheimer's disease. FASEB J. 2012;26:1946–1959. doi: 10.1096/fj.11-199851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lasagna-Reeves CA, Sengupta U, Castillo-Carranza D, et al. The formation of tau pore-like structures is prevalent and cell specific: possible implications for the disease phenotypes. Acta Neuropathol Commun. 2014;2:56. doi: 10.1186/2051-5960-2-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lashuel HA, Overk CR, Oueslati A, Masliah E. The many faces of α-synuclein: from structure and toxicity to therapeutic target. Nat Rev Neurosci. 2013;14:38–48. doi: 10.1038/nrn3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winner B, Jappelli R, Maji SK, et al. In vivo demonstration that alpha-synuclein oligomers are toxic. Proc Natl Acad Sci U S A. 2011;108:4194–4199. doi: 10.1073/pnas.1100976108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Umeda T, Tomiyama T, Sakama N, et al. Intraneuronal amyloid β oligomers cause cell death via endoplasmic reticulum stress, endosomal/lysosomal leakage, and mitochondrial dysfunction in vivo. J Neurosci Res. 2011;89:1031–1042. doi: 10.1002/jnr.22640. [DOI] [PubMed] [Google Scholar]
- 21.Meraz-Ríos MA, Lira-De León KI, Campos-Peña V, De Anda-Hernández MA, Mena-López R. Tau oligomers and aggregation in Alzheimer's disease. J Neurochem. 2010;112:1353–1367. doi: 10.1111/j.1471-4159.2009.06511.x. [DOI] [PubMed] [Google Scholar]
- 22.Domert J, Rao SB, Agholme L, et al. Spreading of amyloid-beta peptides via neuritic cell-to-cell transfer is dependent on insufficient cellular clearance. Neurobiol Dis. 2014;65:82–92. doi: 10.1016/j.nbd.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 23.Frost B, Diamond MI. Prion-like mechanisms in neurodegenerative diseases. Nat Rev Neurosci. 2010;11:155–159. doi: 10.1038/nrn2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kfoury N, Holmes BB, Jiang H, Holtzman DM, Diamond MI. Trans-cellular propagation of Tau aggregation by fibrillar species. J Biol Chem. 2012;287:19440–19451. doi: 10.1074/jbc.M112.346072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee SJ, Desplats P, Sigurdson C, Tsigelny I, Masliah E. Cell-to-cell transmission of non-prion protein aggregates. Nat Rev Neurol. 2010;6:702–706. doi: 10.1038/nrneurol.2010.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Desplats P, Lee HJ, Bae EJ, et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci U S A. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stancu IC, Vasconcelos B, Ris L, et al. Templated misfolding of Tau by prion-like seeding along neuronal connections impairs neuronal network function and associated behavioral outcomes in Tau transgenic mice. Acta Neuropathol. 2015;129:875–894. doi: 10.1007/s00401-015-1413-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morales R, Callegari K, Soto C. Prion-like features of misfolded Abeta and tau aggregates. Virus Res. 2015;207:106–112. doi: 10.1016/j.virusres.2014.12.031. [DOI] [PubMed] [Google Scholar]
- 29.Clinton LK, Blurton-Jones M, Myczek K, Trojanowski JQ, LaFerla FM. Synergistic interactions between Abeta, tau, and alpha-synuclein: acceleration of neuropathology and cognitive decline. J Neurosc. 2010;30:7281–7289. doi: 10.1523/JNEUROSCI.0490-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masliah E, Rockenstein E, Veinbergs I, et al. beta-amyloid peptides enhance alpha-synuclein accumulation and neuronal deficits in a transgenic mouse model linking Alzheimer's disease and Parkinson's disease. Proc Natl Acad Sci U S A. 2001;98:12245–12250. doi: 10.1073/pnas.211412398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Do TD, Economou NJ, Chamas A, Buratto SK, Shea JE, Bowers MT. Interactions between amyloid-beta and Tau fragments promote aberrant aggregates: implications for amyloid toxicity. J Phys Chem B. 2014;118:11220–11230. doi: 10.1021/jp506258g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roy B, Jackson GR. Interactions between Tau and alpha-synuclein augment neurotoxicity in a Drosophila model of Parkinson's disease. Hum Mol Genet. 2014;23:3008–3023. doi: 10.1093/hmg/ddu011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moussaud S, Jones DR, Moussaud-Lamodiere EL, Delenclos M, Ross OA, McLean PJ. Alpha-synuclein and tau: teammates in neurodegeneration? Mol Neurodegener. 2014;9:43. doi: 10.1186/1750-1326-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jose JC, Chatterjee P, Sengupta N. Cross dimerization of amyloid-beta and alphasynuclein proteins in aqueous environment: a molecular dynamics simulations study. PloS One. 2014;9:e106883. doi: 10.1371/journal.pone.0106883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsigelny IF, Crews L, Desplats P, et al. Mechanisms of hybrid oligomer formation in the pathogenesis of combined Alzheimer's and Parkinson's diseases. PloS One. 2008;3:e3135. doi: 10.1371/journal.pone.0003135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mandal PK, Pettegrew JW, Masliah E, Hamilton RL, Mandal R. Interaction between Abeta peptide and alpha synuclein: molecular mechanisms in overlapping pathology of Alzheimer's and Parkinson's in dementia with Lewy body disease. Neurochem Res. 2006;31:1153–1162. doi: 10.1007/s11064-006-9140-9. [DOI] [PubMed] [Google Scholar]
- 37.Deleidi M, Maetzler W. Protein clearance mechanisms of alpha-synuclein and amyloid-Beta in lewy body disorders. Int J Alzheimers Dis. 2012;2012:391438. doi: 10.1155/2012/391438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chesser AS, Pritchard SM, Johnson GV. Tau clearance mechanisms and their possible role in the pathogenesis of Alzheimer disease. Front Neurol. 2013;4:122. doi: 10.3389/fneur.2013.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takahashi M, Suzuki M, Fukuoka M, et al. Normalization of overexpressed alpha-synuclein causing Parkinson's disease by a moderate gene silencing with RNA interference. Mol Ther Nucleic Acids. 2015;4:e241. doi: 10.1038/mtna.2015.14. [DOI] [PubMed] [Google Scholar]
- 40.Maraganore DM. Rationale for therapeutic silencing of alpha-synuclein in Parkinson's disease. J Mov Disord. 2011;4:1–7. doi: 10.14802/jmd.11001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nielsen TT, Nielsen JE. Antisense gene silencing: therapy for neurodegenerative disorders? Genes. 2013;4:457–484. doi: 10.3390/genes4030457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu H, Rosler TW, Carlsson T, et al. Tau Silencing by siRNA in the P301S Mouse model of Tauopathy. Curr Gene Ther. 2014;14:343–351. doi: 10.2174/156652321405140926160602. [DOI] [PubMed] [Google Scholar]
- 43.Farr SA, Erickson MA, Niehoff ML, Banks WA, Morley JE. Central and peripheral administration of antisense oligonucleotide targeting amyloid-beta protein precursor improves learning and memory and reduces neuroinflammatory cytokines in Tg2576 (AbetaPPswe) mice. J Alzheimer Dis. 2014;40:1005–1016. doi: 10.3233/JAD-131883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sevlever D, Jiang P, Yen SH. Cathepsin D is the main lysosomal enzyme involved in the degradation of alpha-synuclein and generation of its carboxy-terminally truncated species. Biochemistry. 2008;47:9678–9687. doi: 10.1021/bi800699v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spencer B, Michael S, Shen J, et al. Lentivirus mediated delivery of neurosin promotes clearance of wild-type alpha-synuclein and reduces the pathology in an alpha-synuclein model of LBD. Mol Ther. 2013;21:31–41. doi: 10.1038/mt.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Devi L, Ohno M. A combination Alzheimer's therapy targeting BACE1 and neprilysin in 5XFAD transgenic mice. Mol Brain. 2015;8:19. doi: 10.1186/s13041-015-0110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Danzer KM, Ruf WP, Putcha P, et al. Heat-shock protein 70 modulates toxic extracellular alpha-synuclein oligomers and rescues trans-synaptic toxicity. FASEB J. 2011;25:326–336. doi: 10.1096/fj.10-164624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Evans CG, Wisen S, Gestwicki JE. Heat shock proteins 70 and 90 inhibit early stages of amyloid beta-(1-42) aggregation in vitro. J Biol Chem. 2006;281:33182–33191. doi: 10.1074/jbc.M606192200. [DOI] [PubMed] [Google Scholar]
- 49.Voss K, Combs B, Patterson KR, Binder LI, Gamblin TC. Hsp70 alters tau function and aggregation in an isoform specific manner. Biochemistry. 2012;51:888–898. doi: 10.1021/bi2018078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hashimoto M, Rockenstein E, Mante M, et al. An antiaggregation gene therapy strategy for Lewy body disease utilizing beta-synuclein lentivirus in a transgenic model. Gene Ther. 2004;11:1713–1723. doi: 10.1038/sj.gt.3302349. [DOI] [PubMed] [Google Scholar]
- 51.Caruana M, Högen T, Levin J, Hillmer A, Giese A, Vassallo N. Inhibition and disaggregation of α-synuclein oligomers by natural polyphenolic compounds. FEBS Lett. 2011;585:1113–1120. doi: 10.1016/j.febslet.2011.03.046. [DOI] [PubMed] [Google Scholar]
- 52.Ono K, Hasegawa K, Naiki H, Yamada M. Curcumin has potent anti-amyloidogenic effects for Alzheimer's beta-amyloid fibrils in vitro. J Neurosci Res. 2004;75:742–750. doi: 10.1002/jnr.20025. [DOI] [PubMed] [Google Scholar]
- 53.Wobst HJ, Sharma A, Diamond MI, Wanker EE, Bieschke J. The green tea polyphenol (-)-epigallocatechin gallate prevents the aggregation of tau protein into toxic oligomers at substoichiometric ratios. FEBS Lett. 2015;589:77–83. doi: 10.1016/j.febslet.2014.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Valera E, Masliah E. Immunotherapy for neurodegenerative diseases: focus on α-synucleinopathies. Pharmacol Ther. 2013;138:311–322. doi: 10.1016/j.pharmthera.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Angot E, Brundin P. Dissecting the potential molecular mechanisms underlying alpha-synuclein cell-to-cell transfer in Parkinson's disease. Parkinsonism Relat Disord. 2009;15(Suppl 3):S143–S147. doi: 10.1016/S1353-8020(09)70802-8. [DOI] [PubMed] [Google Scholar]
- 56.Danzer KM, Haasen D, Karow AR, et al. Different species of alpha-synuclein oligomers induce calcium influx and seeding. J Neurosci. 2007;27:9220–9232. doi: 10.1523/JNEUROSCI.2617-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jones EM, Dubey M, Camp PJ, et al. Interaction of tau protein with model lipid membranes induces tau structural compaction and membrane disruption. Biochemistry. 2012;51:2539–2550. doi: 10.1021/bi201857v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee HJ, Patel S, Lee SJ. Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J Neurosci. 2005;25:6016–6024. doi: 10.1523/JNEUROSCI.0692-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reynolds AD, Banerjee R, Liu J, Gendelman HE, Mosley RL. Neuroprotective activities of CD4+CD25+ regulatory T cells in an animal model of Parkinson's disease. J Leukoc Biol. 2007;82:1083–1094. doi: 10.1189/jlb.0507296. [DOI] [PubMed] [Google Scholar]
- 60.Reynolds AD, Stone DK, Hutter JA, Benner EJ, Mosley RL, Gendelman HE. Regulatory T cells attenuate Th17 cell-mediated nigrostriatal dopaminergic neurodegeneration in a model of Parkinson's disease. J Immunol. 2010;184:2261–2271. doi: 10.4049/jimmunol.0901852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang A, Das P, Switzer RC, Golde TE, Jankowsky JL. Robust amyloid clearance in a mouse model of Alzheimer's disease provides novel insights into the mechanism of amyloid-beta immunotherapy. J Neurosci. 2011;31:4124–4136. doi: 10.1523/JNEUROSCI.5077-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oddo S, Billings L, Kesslak JP, Cribbs DH, LaFerla FM. Abeta immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron. 2004;43:321–332. doi: 10.1016/j.neuron.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 63.Gu J, Congdon EE, Sigurdsson EM. Two novel Tau antibodies targeting the 396/404 region are primarily taken up by neurons and reduce Tau protein pathology. J Biol Chem. 2013;288:33081–33095. doi: 10.1074/jbc.M113.494922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morgan D. The role of microglia in antibody-mediated clearance of amyloid-beta from the brain. CNS Neurol Disord Drug Targets. 2009;8:7–15. doi: 10.2174/187152709787601821. [DOI] [PubMed] [Google Scholar]
- 65.Mandler M, Valera E, Rockenstein E, et al. Active immunization against alpha-synuclein ameliorates the degenerative pathology and prevents demyelination in a model of multiple system atrophy. Mol Neurodegener. 2015;10:10. doi: 10.1186/s13024-015-0008-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mandler M, Valera E, Rockenstein E, et al. Next-generation active immunization approach for synucleinopathies: implications for Parkinson's disease clinical trials. Acta Neuropathol. 2014;127:861–879. doi: 10.1007/s00401-014-1256-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smith JA, Das A, Ray SK, Banik NL. Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res Bull. 2012;87:10–20. doi: 10.1016/j.brainresbull.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yuste JE, Tarragon E, Campuzano CM, Ros-Bernal F. Implications of glial nitric oxide in neurodegenerative diseases. Front Cell Neurosci. 2015;9:322. doi: 10.3389/fncel.2015.00322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Frank-Cannon TC, Alto LT, McAlpine FE, Tansey MG. Does neuroinflammation fan the flame in neurodegenerative diseases? Mol Neurodegener. 2009;4:47. doi: 10.1186/1750-1326-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schott JM, Revesz T. Inflammation in Alzheimer's disease: insights from immunotherapy. Brain. 2013;136:2654–2656. doi: 10.1093/brain/awt231. [DOI] [PubMed] [Google Scholar]
- 71.Hutter-Saunders JA, Mosley RL, Gendelman HE. Pathways towards an effective immunotherapy for Parkinson's disease. Expert Rev Neurother. 2011;11:1703–1715. doi: 10.1586/ern.11.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hickman DT, Lopez-Deber MP, Ndao DM, et al. Sequence-independent control of peptide conformation in liposomal vaccines for targeting protein misfolding diseases. J Biol Chem. 2011;286:13966–13976. doi: 10.1074/jbc.M110.186338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Muhs A, Hickman DT, Pihlgren M, et al. Liposomal vaccines with conformation-specific amyloid peptide antigens define immune response and efficacy in APP transgenic mice. Proc Natl Acad Sci U S A. 2007;104:9810–9815. doi: 10.1073/pnas.0703137104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Davtyan H, Ghochikyan A, Petrushina I, et al. Immunogenicity, efficacy, safety, and mechanism of action of epitope vaccine (Lu AF20513) for Alzheimer's disease: prelude to a clinical trial. J Neurosci. 2013;33:4923–4934. doi: 10.1523/JNEUROSCI.4672-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lannfelt L, Moller C, Basun H, et al. Perspectives on future Alzheimer therapies: amyloid-beta protofibrils—a new target for immunotherapy with BAN2401 in Alzheimer's disease. Alzheimer Res Ther. 2014;6:16. doi: 10.1186/alzrt246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Adolfsson O, Pihlgren M, Toni N, et al. An effector-reduced anti-β-amyloid (Aβ) antibody with unique aβ binding properties promotes neuroprotection and glial engulfment of Aβ. J Neurosci. 2012;32:9677–9689. doi: 10.1523/JNEUROSCI.4742-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boada M, Ramos-Fernandez E, Guivernau B, et al. Treatment of Alzheimer disease using combination therapy with plasma exchange and haemapheresis with albumin and intravenous immunoglobulin: Rationale and treatment approach of the AMBAR (Alzheimer Management By Albumin Replacement) study. Neurologia 2014;pii:S0213-4853(14)00030-9. [DOI] [PubMed]
- 78.Bohrmann B, Baumann K, Benz J, et al. Gantenerumab: a novel human anti-Abeta antibody demonstrates sustained cerebral amyloid-beta binding and elicits cell-mediated removal of human amyloid-beta. J Alzheimer Dis. 2012;28:49–69. doi: 10.3233/JAD-2011-110977. [DOI] [PubMed] [Google Scholar]
- 79.Ostrowitzki S, Deptula D, Thurfjell L, et al. Mechanism of amyloid removal in patients with Alzheimer disease treated with gantenerumab. Arch Neurol. 2012;69:198–207. doi: 10.1001/archneurol.2011.1538. [DOI] [PubMed] [Google Scholar]
- 80.Panza F, Solfrizzi V, Imbimbo BP, et al. Efficacy and safety studies of gantenerumab in patients with Alzheimer's disease. Expert Rev Neurother. 2014;14:973–986. doi: 10.1586/14737175.2014.945522. [DOI] [PubMed] [Google Scholar]
- 81.Farlow M, Arnold SE, van Dyck CH, et al. Safety and biomarker effects of solanezumab in patients with Alzheimer's disease. Alzheimers Dement. 2012;8:261–271. doi: 10.1016/j.jalz.2011.09.224. [DOI] [PubMed] [Google Scholar]
- 82.Siemers ER, Friedrich S, Dean RA, et al. Safety and changes in plasma and cerebrospinal fluid amyloid beta after a single administration of an amyloid beta monoclonal antibody in subjects with Alzheimer disease. Clin Neuropharmacol. 2010;33:67–73. doi: 10.1097/WNF.0b013e3181cb577a. [DOI] [PubMed] [Google Scholar]
- 83.Schneeberger A, Mandler M, Mattner F, Schmidt W. Vaccination for Parkinson's disease. Parkinsonism Relat Disord. 2012;18(Suppl. 1):S11–S13. doi: 10.1016/S1353-8020(11)70006-2. [DOI] [PubMed] [Google Scholar]
- 84.Games D, Valera E, Spencer B, et al. Reducing C-terminal-truncated alpha-synuclein by immunotherapy attenuates neurodegeneration and propagation in Parkinson's disease-like models. J Neurosci. 2014;34:9441–9454. doi: 10.1523/JNEUROSCI.5314-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kontsekova E, Zilka N, Kovacech B, Novak P, Novak M. First-in-man tau vaccine targeting structural determinants essential for pathological tau-tau interaction reduces tau oligomerisation and neurofibrillary degeneration in an Alzheimer's disease model. Alzheimer Res Ther. 2014;6:44. doi: 10.1186/alzrt278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Theunis C, Crespo-Biel N, Gafner V, et al. Efficacy and safety of a liposome-based vaccine against protein Tau, assessed in tau.P301L mice that model tauopathy. PloS One. 2013;8:e72301. doi: 10.1371/journal.pone.0072301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bright J, Hussain S, Dang V, et al. Human secreted tau increases amyloid-beta production. Neurobiol Aging. 2015;36:693–709. doi: 10.1016/j.neurobiolaging.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 88.Collin L, Bohrmann B, Gopfert U, Oroszlan-Szovik K, Ozmen L, Gruninger F. Neuronal uptake of tau/pS422 antibody and reduced progression of tau pathology in a mouse model of Alzheimer's disease. Brain. 2014;137:2834–2846. doi: 10.1093/brain/awu213. [DOI] [PubMed] [Google Scholar]
- 89.Wiltfang J, Esselmann H, Bibl M, et al. Highly conserved and disease-specific patterns of carboxyterminally truncated Abeta peptides 1-37/38/39 in addition to 1-40/42 in Alzheimer's disease and in patients with chronic neuroinflammation. J Neurochem. 2002;81:481–496. doi: 10.1046/j.1471-4159.2002.00818.x. [DOI] [PubMed] [Google Scholar]
- 90.Maddalena AS, Papassotiropoulos A, Gonzalez-Agosti C, et al. Cerebrospinal fluid profile of amyloid beta peptides in patients with Alzheimer's disease determined by protein biochip technology. Neurodegener Dis. 2004;1:231–5. doi: 10.1159/000080991. [DOI] [PubMed] [Google Scholar]
- 91.Schenk D, Barbour R, Dunn W, et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 92.Solomon B, Koppel R, Hanan E, Katzav T. Monoclonal antibodies inhibit in vitro fibrillar aggregation of the Alzheimer beta-amyloid peptide. Proc Natl Acad Sci U S A. 1996;93:452–455. doi: 10.1073/pnas.93.1.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wiessner C, Wiederhold KH, Tissot AC, et al. The second-generation active Abeta immunotherapy CAD106 reduces amyloid accumulation in APP transgenic mice while minimizing potential side effects. J Neurosci. 2011;31:9323–9331. doi: 10.1523/JNEUROSCI.0293-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Arai H, Suzuki H, Yoshiyama T. Vanutide cridificar and the QS-21 adjuvant in Japanese subjects with mild to moderate Alzheimer's disease: results from two phase 2 studies. Curr Alzheimer Res. 2015;12:242–254. doi: 10.2174/1567205012666150302154121. [DOI] [PubMed] [Google Scholar]
- 95.Schneeberger A, Mandler M, Otawa O, Zauner W, Mattner F, Schmidt W. Development of AFFITOPE vaccines for Alzheimer's disease (AD)--from concept to clinical testing. J Nutr Health Aging. 2009;13:264–267. doi: 10.1007/s12603-009-0070-5. [DOI] [PubMed] [Google Scholar]
- 96.Kool M, Soullie T, van Nimwegen M, et al. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med. 2008;205:869–882. doi: 10.1084/jem.20071087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hooper DC, Scott GS, Zborek A, et al. Uric acid, a peroxynitrite scavenger, inhibits CNS inflammation, blood-CNS barrier permeability changes, and tissue damage in a mouse model of multiple sclerosis. FASEB J. 2000;14:691–698. doi: 10.1096/fasebj.14.5.691. [DOI] [PubMed] [Google Scholar]
- 98.Lambracht-Washington D, Qu BX, Fu M, et al. DNA immunization against amyloid beta 42 has high potential as safe therapy for Alzheimer's disease as it diminishes antigen-specific Th1 and Th17 cell proliferation. Cell Mol Neurobiol. 2011;31:867–874. doi: 10.1007/s10571-011-9680-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lambracht-Washington D, Rosenberg RN. Active DNA Abeta42 vaccination as immunotherapy for Alzheimer disease. Transl Neurosci. 2012;3:307–313. doi: 10.2478/s13380-012-0037-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu S, Shi D, Wang HC, Yu YZ, Xu Q, Sun ZW. Co-immunization with DNA and protein mixture: a safe and efficacious immunotherapeutic strategy for Alzheimer's disease in PDAPP mice. Sci Rep. 2015;5:7771. doi: 10.1038/srep07771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lambracht-Washington D, Qu BX, Fu M, et al. A peptide prime-DNA boost immunization protocol provides significant benefits as a new generation Abeta42 DNA vaccine for Alzheimer disease. J Neuroimmunol. 2013;254:63–68. doi: 10.1016/j.jneuroim.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Orgogozo JM, Gilman S, Dartigues JF, et al. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology. 2003;61:46–54. doi: 10.1212/01.WNL.0000073623.84147.A8. [DOI] [PubMed] [Google Scholar]
- 103.McLaurin J, Cecal R, Kierstead ME, et al. Therapeutically effective antibodies against amyloid-beta peptide target amyloid-beta residues 4-10 and inhibit cytotoxicity and fibrillogenesis. Nat Med. 2002;8:1263–1269. doi: 10.1038/nm790. [DOI] [PubMed] [Google Scholar]
- 104.Bard F, Cannon C, Barbour R, et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 105.Frost JL, Liu B, Kleinschmidt M, Schilling S, Demuth HU, Lemere CA. Passive immunization against pyroglutamate-3 amyloid-beta reduces plaque burden in Alzheimer-like transgenic mice: a pilot study. Neurodegener Dis. 2012;10:265–270. doi: 10.1159/000335913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang Y, He JS, Wang X, et al. Administration of amyloid-beta42 oligomer-specific monoclonal antibody improved memory performance in SAMP8 mice. J Alzheimer Dis. 2011;23:551–561. doi: 10.3233/JAD-2010-091195. [DOI] [PubMed] [Google Scholar]
- 107.Lord A, Gumucio A, Englund H, et al. An amyloid-beta protofibril-selective antibody prevents amyloid formation in a mouse model of Alzheimer's disease. Neurobiol Dis. 2009;36:425–434. doi: 10.1016/j.nbd.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 108.Englund H, Sehlin D, Johansson AS, et al. Sensitive ELISA detection of amyloid-beta protofibrils in biological samples. J Neurochem. 2007;103:334–345. doi: 10.1111/j.1471-4159.2007.04759.x. [DOI] [PubMed] [Google Scholar]
- 109.Moreth J, Mavoungou C, Schindowski K. Passive anti-amyloid immunotherapy in Alzheimer's disease: What are the most promising targets? Immun Ageing. 2013;10:18. doi: 10.1186/1742-4933-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Blennow K, Zetterberg H, Rinne JO, et al. Effect of immunotherapy with bapineuzumab on cerebrospinal fluid biomarker levels in patients with mild to moderate Alzheimer disease. Arch Neurol. 2012;69:1002–1010. doi: 10.1001/archneurol.2012.90. [DOI] [PubMed] [Google Scholar]
- 111.La Porte SL, Bollini SS, Lanz TA, et al. Structural basis of C-terminal beta-amyloid peptide binding by the antibody ponezumab for the treatment of Alzheimer's disease. J Mol Biol. 2012;421:525–536. doi: 10.1016/j.jmb.2011.11.047. [DOI] [PubMed] [Google Scholar]
- 112.Loeffler DA. Should development of Alzheimer's disease-specific intravenous immunoglobulin be considered? J Neuroinflammation. 2014;11:198. doi: 10.1186/s12974-014-0198-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Spencer B, Masliah E. Immunotherapy for Alzheimer's disease: past, present and future. Front Aging Neurosci. 2014;6:114. doi: 10.3389/fnagi.2014.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fortin DL, Nemani VM, Voglmaier SM, Anthony MD, Ryan TA, Edwards RH. Neural activity controls the synaptic accumulation of alpha-synuclein. J Neurosci. 2005;25:10913–10921. doi: 10.1523/JNEUROSCI.2922-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.George JM, Jin H, Woods WS, Clayton DF. Characterization of a novel protein regulated during the critical period for song learning in the zebra finch. Neuron. 1995;15:361–372. doi: 10.1016/0896-6273(95)90040-3. [DOI] [PubMed] [Google Scholar]
- 116.Uéda K, Fukushima H, Masliah E, et al. Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90:11282–11286. doi: 10.1073/pnas.90.23.11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Masliah E, Iwai A, Mallory M, Ueda K, Saitoh T. Altered presynaptic protein NACP is associated with plaque formation and neurodegeneration in Alzheimer's disease. Am J Pathol. 1996;148:201–210. [PMC free article] [PubMed] [Google Scholar]
- 118.Tsigelny IF, Bar-On P, Sharikov Y, et al. Dynamics of alpha-synuclein aggregation and inhibition of pore-like oligomer development by beta-synuclein. FEBS J. 2007;274:1862–1877. doi: 10.1111/j.1742-4658.2007.05733.x. [DOI] [PubMed] [Google Scholar]
- 119.Masliah E, Rockenstein E, Adame A, et al. Effects of alpha-synuclein immunization in a mouse model of Parkinson's disease. Neuron. 2005;46:857–868. doi: 10.1016/j.neuron.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 120.Masliah E, Rockenstein E, Mante M, et al. Passive immunization reduces behavioral and neuropathological deficits in an alpha-synuclein transgenic model of Lewy body disease. PloS One. 2011;6:e19338. doi: 10.1371/journal.pone.0019338. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 121.Bae EJ, Lee HJ, Rockenstein E, et al. Antibody-aided clearance of extracellular alpha-synuclein prevents cell-to-cell aggregate transmission. J Neurosci. 2012;32:13454–13469. doi: 10.1523/JNEUROSCI.1292-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tran HT, Chung CH, Iba M, et al. Alpha-synuclein immunotherapy blocks uptake and templated propagation of misfolded alpha-synuclein and neurodegeneration. Cell Rep. 2014;7:2054–2065. doi: 10.1016/j.celrep.2014.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shahaduzzaman M, Nash K, Hudson C, et al. Anti-human alpha-synuclein N-terminal peptide antibody protects against dopaminergic cell death and ameliorates behavioral deficits in an AAV-alpha-synuclein rat model of Parkinson's disease. PloS One. 2015;10:e0116841. doi: 10.1371/journal.pone.0116841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Näsström T, Gonçalves S, Sahlin C, et al. Antibodies against alpha-synuclein reduce oligomerization in living cells. PloS One. 2011;6:e27230. doi: 10.1371/journal.pone.0027230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lindstrom V, Fagerqvist T, Nordstrom E, et al. Immunotherapy targeting alpha-synuclein protofibrils reduced pathology in (Thy-1)-h[A30P] alpha-synuclein mice. Neurobiol Dis. 2014;69:134–143. doi: 10.1016/j.nbd.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 126.Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem. 1986;261:6084–6089. [PubMed] [Google Scholar]
- 127.Wolozin BL, Pruchnicki A, Dickson DW, Davies P. A neuronal antigen in the brains of Alzheimer patients. Science. 1986;232:648–650. doi: 10.1126/science.3083509. [DOI] [PubMed] [Google Scholar]
- 128.Lee VM, Otvos L, Schmidt ML, Trojanowski JQ. Alzheimer disease tangles share immunological similarities with multiphosphorylation repeats in the two large neurofilament proteins. Proc Natl Acad Sci U S A. 1988;85:7384–7388. doi: 10.1073/pnas.85.19.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Iwatsubo T, Hasegawa M, Ihara Y. Neuronal and glial tau-positive inclusions in diverse neurologic diseases share common phosphorylation characteristics. Acta Neuropathol. 1994;88:129–136. doi: 10.1007/BF00294505. [DOI] [PubMed] [Google Scholar]
- 130.Hampel H, Teipel SJ. Total and phosphorylated tau proteins: evaluation as core biomarker candidates in frontotemporal dementia. Dement Geriatr Cogn Disord. 2004;17:350–354. doi: 10.1159/000077170. [DOI] [PubMed] [Google Scholar]
- 131.Mandelkow EM, Mandelkow E. Tau as a marker for Alzheimer's disease. Trends Biochem Sci. 1993;18:480–483. doi: 10.1016/0968-0004(93)90011-B. [DOI] [PubMed] [Google Scholar]
- 132.Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992;42:631–639. doi: 10.1212/WNL.42.3.631. [DOI] [PubMed] [Google Scholar]
- 133.Bierer LM, Hof PR, Purohit DP, et al. Neocortical neurofibrillary tangles correlate with dementia severity in Alzheimer's disease. Arch Neurol. 1995;52:81–88. doi: 10.1001/archneur.1995.00540250089017. [DOI] [PubMed] [Google Scholar]
- 134.Götz J, Ittner A, Ittner LM. Tau-targeted treatment strategies in Alzheimer's disease. Br J Pharmacol. 2012;165:1246–1259. doi: 10.1111/j.1476-5381.2011.01713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lasagna-Reeves CA, Castillo-Carranza DL, Jackson GR, Kayed R. Tau oligomers as potential targets for immunotherapy for Alzheimer's disease and tauopathies. Curr Alzheimer Res. 2011;8:659–665. doi: 10.2174/156720511796717177. [DOI] [PubMed] [Google Scholar]
- 136.Asuni AA, Boutajangout A, Quartermain D, Sigurdsson EM. Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements. J Neurosci. 2007;27:9115–9129. doi: 10.1523/JNEUROSCI.2361-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Boutajangout A, Ingadottir J, Davies P, Sigurdsson EM. Passive immunization targeting pathological phospho-tau protein in a mouse model reduces functional decline and clears tau aggregates from the brain. J Neurochem. 2011;118:658–667. doi: 10.1111/j.1471-4159.2011.07337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Boutajangout A, Quartermain D, Sigurdsson EM. Immunotherapy targeting pathological tau prevents cognitive decline in a new tangle mouse model. J Neurosci. 2010;30:16559–16566. doi: 10.1523/JNEUROSCI.4363-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wisniewski T, Boutajangout A. Vaccination as a therapeutic approach to Alzheimer's disease. Mt Sinai J Med. 2010;77:17–31. doi: 10.1002/msj.20156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chai X, Wu S, Murray TK, et al. Passive immunization with anti-Tau antibodies in two transgenic models: reduction of Tau pathology and delay of disease progression. J Biol Chem. 2011;286:34457–34467. doi: 10.1074/jbc.M111.229633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gu J, Sigurdsson EM. Immunotherapy for tauopathies. J Mol Neurosci. 2011;45:690–695. doi: 10.1007/s12031-011-9576-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Troquier L, Caillierez R, Burnouf S, et al. Targeting phospho-Ser422 by active Tau Immunotherapy in the THYTau22 mouse model: a suitable therapeutic approach. Curr Alzheimer Res. 2012;9:397–405. doi: 10.2174/156720512800492503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Funk KE, Mirbaha H, Jiang H, Holtzman DM, Diamond MI. Distinct therapeutic mechanisms of Tau antibodies: promoting microglial clearance vs. blocking neuronal uptake. J Biol Chem. 2015;290:21652–21662. doi: 10.1074/jbc.M115.657924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Umeda T, Eguchi H, Kunori Y, et al. Passive immunotherapy of tauopathy targeting pSer413-tau: a pilot study in mice. Ann Clin Transl Neurol. 2015;2:241–255. doi: 10.1002/acn3.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sankaranarayanan S, Barten DM, Vana L, et al. Passive immunization with phospho-tau antibodies reduces tau pathology and functional deficits in two distinct mouse tauopathy models. PloS One. 2015;10:e0125614. doi: 10.1371/journal.pone.0125614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.d'Abramo C, Acker CM, Jimenez HT, Davies P. Tau passive immunotherapy in mutant P301L mice: antibody affinity versus specificity. PloS One. 2013;8:e62402. doi: 10.1371/journal.pone.0062402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Castillo-Carranza DL, Sengupta U, Guerrero-Munoz MJ, et al. Passive immunization with Tau oligomer monoclonal antibody reverses tauopathy phenotypes without affecting hyperphosphorylated neurofibrillary tangles. J Neurosci. 2014;34:4260–4272. doi: 10.1523/JNEUROSCI.3192-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Castillo-Carranza DL, Guerrero-Munoz MJ, Sengupta U, et al. Tau immunotherapy modulates both pathological tau and upstream amyloid pathology in an Alzheimer's disease mouse model. J Neurosci. 2015;35:4857–4868. doi: 10.1523/JNEUROSCI.4989-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Buee L, Bussiere T, Buee-Scherrer V, Delacourte A, Hof PR. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev. 2000;33:95–130. doi: 10.1016/S0165-0173(00)00019-9. [DOI] [PubMed] [Google Scholar]
- 150.Congdon EE, Gu J, Sait HB, Sigurdsson EM. Antibody uptake into neurons occurs primarily via clathrin-dependent Fcgamma receptor endocytosis and is a prerequisite for acute tau protein clearance. J Biol Chem. 2013;288:35452–35465. doi: 10.1074/jbc.M113.491001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Spencer B, Emadi S, Desplats P, et al. ESCRT-mediated uptake and degradation of brain-targeted alpha-synuclein single chain antibody attenuates neuronal degeneration in vivo. Mol Ther. 2014;22:1753–1767. doi: 10.1038/mt.2014.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Emadi S, Liu R, Yuan B, et al. Inhibiting aggregation of alpha-synuclein with human single chain antibody fragments. Biochemistry. 2004;43:2871–2878. doi: 10.1021/bi036281f. [DOI] [PubMed] [Google Scholar]
- 153.Emadi S, Barkhordarian H, Wang MS, Schulz P, Sierks MR. Isolation of a human single chain antibody fragment against oligomeric alpha-synuclein that inhibits aggregation and prevents alpha-synuclein-induced toxicity. J Mol Biol. 2007;368:1132–1144. doi: 10.1016/j.jmb.2007.02.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bousset L, Pieri L, Ruiz-Arlandis G, et al. Structural and functional characterization of two alpha-synuclein strains. Nat Commun. 2013;4:2575. doi: 10.1038/ncomms3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Boddapati S, Levites Y, Suryadi V, Kasturirangan S, Sierks MR. Bispecific tandem single chain antibody simultaneously inhibits beta-secretase and promotes alpha-secretase processing of AbetaPP. J Alzheimer Dis. 2012;28:961–969. doi: 10.3233/JAD-2011-111196. [DOI] [PubMed] [Google Scholar]
- 156.Zhou C, Emadi S, Sierks MR, Messer A. A human single-chain Fv intrabody blocks aberrant cellular effects of overexpressed alpha-synuclein. Mol Ther. 2004;10:1023–1031. doi: 10.1016/j.ymthe.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 157.Lynch SM, Zhou C, Messer A. An scFv intrabody against the nonamyloid component of alpha-synuclein reduces intracellular aggregation and toxicity. J Mol Biol. 2008;377:136–147. doi: 10.1016/j.jmb.2007.11.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Joshi SN, Butler DC, Messer A. Fusion to a highly charged proteasomal retargeting sequence increases soluble cytoplasmic expression and efficacy of diverse anti-synuclein intrabodies. MAbs. 2012;4:686–693. doi: 10.4161/mabs.21696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Waxman EA, Giasson BI. Induction of intracellular tau aggregation is promoted by alpha-synuclein seeds and provides novel insights into the hyperphosphorylation of tau. J Neurosci. 2011;31:7604–7618. doi: 10.1523/JNEUROSCI.0297-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Badiola N, de Oliveira RM, Herrera F, et al. Tau enhances alpha-synuclein aggregation and toxicity in cellular models of synucleinopathy. PloS One. 2011;6:e26609. doi: 10.1371/journal.pone.0026609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ishizawa T, Mattila P, Davies P, Wang D, Dickson DW. Colocalization of tau and alpha-synuclein epitopes in Lewy bodies. J Neuropathol Exp Neurol. 2003;62:389–397. doi: 10.1093/jnen/62.4.389. [DOI] [PubMed] [Google Scholar]
- 162.Iwai A. Properties of NACP/alpha-synuclein and its role in Alzheimer's disease. Biochim Biophys Acta. 2000;1502:95–109. doi: 10.1016/S0925-4439(00)00036-3. [DOI] [PubMed] [Google Scholar]
- 163.Arima K, Hirai S, Sunohara N, et al. Cellular co-localization of phosphorylated tau- and NACP/alpha-synuclein-epitopes in lewy bodies in sporadic Parkinson's disease and in dementia with Lewy bodies. Brain Res. 1999;843:53–61. doi: 10.1016/S0006-8993(99)01848-X. [DOI] [PubMed] [Google Scholar]
- 164.Willet M, Kurup D, Papaneri A, et al. Preclinical development of inactivated rabies virus-based polyvalent vaccine against rabies and filoviruses. J Infect Dis. 2015;212(Suppl. 2):S414–S424. doi: 10.1093/infdis/jiv251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ragupathi G, Gathuru J, Livingston P. Antibody inducing polyvalent cancer vaccines. Cancer Treat Res. 2005;123:157–180. doi: 10.1007/0-387-27545-2_7. [DOI] [PubMed] [Google Scholar]
- 166.Yanamandra K, Gruden MA, Casaite V, Meskys R, Forsgren L, Morozova-Roche LA. α-synuclein reactive antibodies as diagnostic biomarkers in blood sera of Parkinson's disease patients. PloS One. 2011;6:e18513. doi: 10.1371/journal.pone.0018513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Maetzler W, Berg D, Synofzik M, et al. Autoantibodies against amyloid and glial-derived antigens are increased in serum and cerebrospinal fluid of Lewy body-associated dementias. J Alzheimer Dis. 2011;26:171–179. doi: 10.3233/JAD-2011-110221. [DOI] [PubMed] [Google Scholar]
- 168.Wilhelm KR, Yanamandra K, Gruden MA, et al. Immune reactivity towards insulin, its amyloid and protein S100B in blood sera of Parkinson's disease patients. Eur J Neurol. 2007;14:327–334. doi: 10.1111/j.1468-1331.2006.01667.x. [DOI] [PubMed] [Google Scholar]
- 169.O'Nuallain B, Hrncic R, Wall JS, Weiss DT, Solomon A. Diagnostic and therapeutic potential of amyloid-reactive IgG antibodies contained in human sera. J Immunol. 2006;176:7071–7078. doi: 10.4049/jimmunol.176.11.7071. [DOI] [PubMed] [Google Scholar]
- 170.Mo JA, Lim JH, Sul AR, Lee M, Youn YC, Kim HJ. Cerebrospinal fluid beta-amyloid1-42 levels in the differential diagnosis of Alzheimer's disease—systematic review and meta-analysis. PloS One. 2015;10:e0116802. doi: 10.1371/journal.pone.0116802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Krishnaswamy S, Lin Y, Rajamohamedsait WJ, Rajamohamedsait HB, Krishnamurthy P, Sigurdsson EM. Antibody-derived in vivo imaging of tau pathology. J Neurosci. 2014;34:16835–16850. doi: 10.1523/JNEUROSCI.2755-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Frisoni GB, Visser PJ. Biomarkers for Alzheimer's disease: a controversial topic. Lancet Neurol. 2015;14:781–783. doi: 10.1016/S1474-4422(15)00150-7. [DOI] [PubMed] [Google Scholar]
- 173.Rakover I, Arbel M, Solomon B. Immunotherapy against APP beta-secretase cleavage site improves cognitive function and reduces neuroinflammation in Tg2576 mice without a significant effect on brain abeta levels. Neurodegener Dis. 2007;4:392–402. doi: 10.1159/000103250. [DOI] [PubMed] [Google Scholar]
- 174.Gros-Louis F, Soucy G, Larivière R, Julien JP. Intracerebroventricular infusion of monoclonal antibody or its derived Fab fragment against misfolded forms of SOD1 mutant delays mortality in a mouse model of ALS. J Neurochem. 2010;113:1188–1199. doi: 10.1111/j.1471-4159.2010.06683.x. [DOI] [PubMed] [Google Scholar]
- 175.Takeuchi S, Fujiwara N, Ido A, et al. Induction of protective immunity by vaccination with wild-type apo superoxide dismutase 1 in mutant SOD1 transgenic mice. J Neuropathol Exp Neurol. 2010;69:1044–1056. doi: 10.1097/NEN.0b013e3181f4a90a. [DOI] [PubMed] [Google Scholar]
- 176.Urushitani M, Ezzi SA, Julien JP. Therapeutic effects of immunization with mutant superoxide dismutase in mice models of amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2007;104:2495–2500. doi: 10.1073/pnas.0606201104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1224 kb)