Abstract
Aims
Studies have suggested increased cancer incidence associated with long-term dual antiplatelet therapy (DAPT) for acute coronary syndrome (ACS). We evaluated cancer incidence and treatment-related differences in an analysis of DAPT for ACS.
Methods and results
The Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes trial enrolled 9326 participants with ACS, who received aspirin plus clopidogrel or prasugrel. Median treatment exposure was 15 months. Cancer history and screening procedures were collected. Suspected non-benign neoplasm events were reported and adjudicated. The primary outcome was detection of new, non-benign neoplasm. Factors associated with neoplasm events, the relationship of these events to cardiovascular and bleeding endpoints, and treatment-related differences in neoplasm detection were studied. Among 9240 participants who received ≥1 dose of study drug, 1.8% had a confirmed neoplasm event. The efficacy composite of cardiovascular death, myocardial infarction, or stroke occurred more frequently among those with a neoplasm event vs. those without (18.2 vs. 13.5%) as did Global Use of Strategies to Open Occluded Coronary Arteries severe/moderate bleeding (11.2 vs. 1.5%). Screening rates were substantially higher in North America and Western Europe/Scandinavia vs. other regions. Factors most strongly associated with detection of neoplasm events were older age, region, male sex, and current/recent smoking. Among the pre-specified population without a history of neoplasm or previous curative treatment for neoplasm (n = 9105), the incidence of neoplasm events was similar with prasugrel vs. clopidogrel (1.8 vs. 1.7%; HR = 1.04; 95% CI 0.77–1.42; P = 0.79).
Conclusions
Neoplasm events were infrequent during long-term DAPT after ACS, were associated with differential cancer-screening practices across regions, and the frequency of neoplasm detection was similar with prasugrel vs. clopidogrel.
Trial registration
ClinicalTrials.gov identifier: NCT00699998.
Keywords: Neoplasm, Acute coronary syndrome, Antiplatelet drugs, Clopidogrel, Prasugrel, Surveillance, Adjudication, Clinical trial
See page 386 for the editorial comment on this article (doi:10.1093/eurheartj/ehv610)
Introduction
The concurrence of cancer and cardiovascular disease is a controversial topic, given the competing risks for mortality and the confluence and intersection of risk factors for both disease states.1 Numerous conflicting studies regarding the putative risks of cancer associated with the use of long-term cardiovascular therapies have been published, accompanied by waxing and waning public concern regarding the variable findings from these analyses.2–9 Additionally, because cancers detected following a bleeding event are commonly identified with diagnostic procedures used to localize the source of bleeding (e.g. endoscopy, colonoscopy, thoracic/abdominal imaging procedures), detection biases further complicate the task of assessing the potential cancer risk of long-term antithrombotic therapies used to treat cardiovascular disease.10 Nonetheless, factors associated with the development of cancer among patients with cardiovascular disease and the influence of cancer events on the occurrence of both ischaemic and bleeding events in this population have not been well studied.
When combined with background aspirin therapy, the thienopyridine adenosine diphosphate receptor antagonist prasugrel was shown to significantly reduce rates of ischaemic events compared with clopidogrel among participants with acute coronary syndromes (ACS) undergoing percutaneous coronary intervention (PCI) in the TRITON-TIMI 38 trial.11 In this study, the frequency of adverse events (AEs) related to neoplasms was greater with prasugrel than with clopidogrel. This finding prompted additional, post hoc data collection demonstrating the frequency of new, non-benign neoplasms to be higher with prasugrel vs. clopidogrel (1.6 vs. 1.2%). However, neither a causal relationship nor a plausible biological mechanism could be confirmed.12 Subsequently, a comprehensive neoplasm ascertainment process was implemented in the Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes (TRILOGY ACS) trial to further study this issue in a rigorous, prospective, and pre-specified secondary analysis.
We therefore systematically collected data on cancer history and pre- and post-randomization cancer-screening procedures for medically managed ACS patients randomized to dual antiplatelet therapy (DAPT) comprising aspirin plus either prasugrel or clopidogrel in the TRILOGY ACS trial and prospectively adjudicated suspected neoplasms reported during post-randomization follow-up. The present secondary analysis was performed to (i) determine the frequency of and factors associated with new, non-benign neoplasm events among ACS patients treated with DAPT, (ii) ascertain the effect of these events on the occurrence and timing of cardiovascular and bleeding endpoints, and (iii) investigate treatment-related differences in the detection and subsequent progression of new, non-benign neoplasms.
Methods
The design13 and results14 of the TRILOGY ACS study have been previously published. The trial was approved by national and local regulatory authorities of participating countries and at all research sites. All study participants provided written informed consent.
Study design and participants
Participants with unstable angina (UA) or non-ST-segment elevation myocardial infarction (NSTEMI) were enrolled if they had a final treatment strategy of medical management without revascularization (determined within 10 days of presentation for the index ACS event) and were not considered to have a high risk of major bleeding. Participants with a terminal neoplasm with a limited life expectancy were excluded, but there were no exclusions for prior history of neoplasms.
Participants were randomly allocated to prasugrel (10 or 5 mg/day for those aged <75 years and weighing <60 kg and for all ≥75 years) or clopidogrel (75 mg/day) with concomitant aspirin required (a dose of ≤100 mg/day was strongly recommended). The randomized study treatments were continued for a minimum of 6 months and a maximum of 30 months. Over 3 years, 9326 participants were enrolled from 8 geographic regions in 52 countries. Median treatment exposure was 15 months; median follow-up was 17 months.14
Neoplasm data collection, event reporting, and adjudication
History of prior neoplasm occurrence(s) and cancer-screening tests/procedures performed before and after randomization were collected for all participants. Suspected neoplasm events were classified and adjudicated through a comprehensive series of processes detailed in the Neoplasm Clinical Events Committee (CEC) Charter (see Supplementary material online, Appendix S2) and described herein.
First, at the baseline randomization visit, sites were instructed to report confirmed/suspected neoplasm events that occurred prior to randomization and/or were present at randomization. For reported events where the neoplasm onset/diagnosis date was confirmed to be before the date of randomization, information was collected to describe the anatomic/tissue location and to classify prior neoplasm events as (i) no evidence of disease at the time of randomization due to prior curative treatment (i.e. surgical resection, chemotherapy with no evidence of disease recurrence through imaging surveillance, etc.); (ii) stable, inactive disease at the time of randomization with no ongoing treatment; or (iii) active disease at the time of randomization with ongoing treatment. Second, sites were required to report post-randomization neoplasm events that were detected through a series of targeted questions implemented for each participant at every biannual study visit. Third, programmed triggers were implemented within the trial database to prompt reporting of suspected post-randomization neoplasm events (not previously reported by sites) when key data variables were identified, such as serious AEs related to neoplasm or use of concomitant medications related to cancer treatment. Finally, potential post-randomization neoplasm events identified through adjudication of cardiovascular endpoints were triaged to prompt reporting of neoplasm events by sites if those events had not previously been reported. A series of required source documents (oncology treatment notes, imaging reports, histology/pathology reports, etc.) needed to support adjudication of identified post-randomization neoplasm events were collected for all potential unique neoplasm events detected. Suspected non-benign neoplasm events with an uncertain onset/diagnosis date and those with a confirmed onset/diagnosis after the date of randomization were submitted for formal adjudication. Participants could have more than one post-randomization neoplasm event submitted for adjudication if different anatomic/tissue locations were suspected. Additionally, participants could have a suspected post-randomization neoplasm event submitted for adjudication if they had a pre-randomization neoplasm in a different anatomic/tissue location than that of the neoplasm event that was suspected to have occurred post-randomization. Participants could also have multiple post-randomization neoplasm events submitted for adjudication if those events were each considered to be related to a different anatomic/tissue location.
Once all required source documents and data variables were collected for each potential neoplasm event, the event was adjudicated by an independent Neoplasm CEC whose members were blinded to treatment assignment. The Neoplasm CEC included one gastroenterologist and eight oncologists with expertise in the major subspecialties of oncology. Adjudicated events were confirmed by the Neoplasm CEC as either ‘no event’ or ‘non-benign neoplasm (i.e. malignant).’ Non-benign neoplasms were confirmed as (i) new primary, (ii) recurrence, or (iii) progression, whereas benign neoplasms were confirmed as ‘no event.’
Confirmation of non-benign neoplasm events was primarily based on pathologic data and clinical information. The first priority to confirm the diagnosis was a definitive pathologic diagnosis from a pathology report. If there was no definitive pathologic diagnosis available, then the non-benign neoplasm diagnosis was established by the best pathologic data available, clinical information (such as anatomic distribution of the neoplasm from imaging reports), and the consensus opinion of the adjudication panel. For verified non-benign neoplasm events, the dates of initial detection and histological diagnosis, the anatomic/tissue location of the primary malignant neoplasm and secondary malignant neoplasm (if detected), stage of malignancy (local, regional, or metastatic disease), confirmation of malignant neoplasm recurrence (for malignant neoplasms present prior to randomization), and methods of detection were determined. Neoplasm staging was guided by recommendations from the American Joint Committee on Cancer (AJCC) Cancer Staging Manual (Seventh Edition).15
Confirmed new, recurrent, or progressive non-benign neoplasm events were subsequently reviewed in a blinded fashion at the time of last study contact (final study visit or death) to ascertain disease status and progression for each event. This process was completed before the database was locked and unblinded. For cases in which death occurred after initial positive non-benign neoplasm adjudication, the death was reviewed by the Neoplasm CEC to determine whether the death was malignancy related or non-malignancy related.
Study populations
Among the overall population of 9326 participants randomized into the TRILOGY ACS trial, two subpopulations were pre-specified (in consultation with the U.S. Food and Drug Administration) before study completion and database lock for the analysis of the neoplasm adjudication results. First, the detection of new, non-benign neoplasm events was analysed among all participants treated with ≥1 dose of study drug (n = 9240). Adjudicated recurrent or progressive non-benign neoplasm events were not included in this evaluation. Second, the treatment-related differences (prasugrel vs. clopidogrel) in the detection of non-benign neoplasm events was first analysed among participants who did not have a prior history of a malignant neoplasm or who had curative treatment for a prior malignant neoplasm before randomization (n = 9105) in any anatomic/tissue location, and then also analysed in the overall population (n = 9240). All analyses were performed on a ‘per-participant’ level such that participants with >1 confirmed new, non-benign neoplasm event (in >1 anatomic/tissue location) were counted only once for all analyses described herein.
Cardiovascular and bleeding endpoints
The frequencies of the primary ischaemic efficacy endpoint of the TRILOGY ACS trial [the composite of cardiovascular death, non-fatal myocardial infarction (MI), or non-fatal stroke] and all-cause death were evaluated among participants based upon the detection of a new, non-benign neoplasm event. The frequencies of bleeding endpoints, including Global Use of Strategies to Open Occluded Coronary Arteries (GUSTO) severe, life-threatening, or moderate bleeding not related to coronary artery bypass grafting (CABG), and thrombolysis in myocardial infarction (TIMI) criteria for major or minor bleeding not related to CABG, were also evaluated by neoplasm detection status. Additionally, the occurrence of both ischaemic and bleeding endpoints was evaluated before or after the time/date of new, non-benign neoplasm detection.
Statistical analysis
Baseline characteristics were compared for treated study participants (n = 9240) with vs. without ≥1 new, non-benign neoplasm event confirmed during study follow-up. Continuous variables were presented as medians (25th, 75th percentiles); categorical variables were presented as counts (percentages). The total number and raw percentages of both ischaemic and bleeding events were determined for all treated participants with vs. without a new, non-benign neoplasm event. The breakdown of events before and after detection of a new, non-benign neoplasm event was also determined.
Multivariable Cox proportional hazards regression modelling was used to determine baseline factors associated with risk of a new, non-benign neoplasm event among treated participants (n = 9240). Based upon the number of such events confirmed (n = 170), clinical judgment, and observed regional differences in cancer-screening tests/procedures, the following candidate variables were included: age (continuous), male sex, weight (continuous), NSTEMI vs. UA status for index ACS event, history of hyperlipidaemia, history of diabetes mellitus, current/recent smoking, prior MI, prior PCI, prior CABG, prior peripheral arterial disease, prior heart failure, prior atrial fibrillation, baseline haemoglobin value (continuous), baseline calculated creatinine clearance (continuous), proton-pump inhibitor use at randomization, and geographic region (three-level comparison based upon observed cancer-screening practices and number of patients enrolled within each region: North America vs. Western Europe/Scandinavia vs. Other). Despite similar rates of cancer screening in the Western Europe/Scandinavia and Rest of World (Australia, New Zealand, and South Africa) regions, we chose not to model geographic region with a four-level comparison, given the relatively small number of patients included from the Rest of World region (n = 144).
When fitting the full model, the proportional hazards assumption was checked for each variable and the linearity assumption was checked for each continuous variable. If the proportional hazard assumption was violated, an interaction of the variable with log-transformed time was included in the model. If the linearity assumption was violated, a linear spline was fit to approximate the non-linear relationship of the variable with the outcome. A knot point of 70 years was introduced to assess the non-linear association of increasing age above and below this knot point. To determine which baseline characteristics had the strongest association with development of a new, non-benign neoplasm event, the forward addition sequence for Cox proportional hazards models (reduced model) was constructed via the fast false selection rate variable selection technique.16
Among treated participants without a prior history of malignant neoplasm or with curative treatment for a malignant neoplasm (in any anatomic/tissue location) prior to randomization (n = 9105), differences between treatment groups with regards to detection and timing of new, non-benign neoplasms were compared with the log-rank test stratified by clopidogrel stratum at time of randomization and age group (<75 years vs. ≥75). The same analysis was repeated on the overall population (n = 9240). Kaplan–Meier curves were generated to provide a visual representation of the detection of new, non-benign neoplasm events by treatment assignment during study follow-up among the primary analysis population for this objective (n = 9105). Types of neoplasm, location, stage of malignancy, method of detection, and development of metastatic disease after initial detection were compared between treatment groups using the Fisher exact test.
For all analyses, a P-value of <0.05 was considered statistically significant. All data analyses were performed by statisticians at the Duke Clinical Research Institute, Durham, NC, with an independent copy of the database, using SAS version 9.2 and R version 2.14.2.
Results
Neoplasm adjudication results
A total of 706 suspected neoplasm events in 604 participants were identified in the entire study population of 9326 patients, but there were 703 suspected events in 601 participants who received ≥1 dose of study drug (n = 9240; see Supplementary material online, Figure S1). Of these events, 463 were determined not to require adjudication, primarily due to confirmation that the neoplasm onset/diagnosis date occurred before the date of randomization. The remaining 243 events went through the formal adjudication process that confirmed 208 non-benign neoplasm events in 186 participants. After excluding events adjudicated as recurrent or progressive non-benign neoplasms (related to confirmation of pre-randomization neoplasms in the same anatomic/tissue location), 187 distinct new, non-benign neoplasm events were determined to have occurred post-randomization in 170 participants who received ≥1 dose of study drug (representing 1.8% of the population of 9240 treated participants).
Baseline characteristics and prior cancer history
Participants with a new, non-benign neoplasm event were older (71 years old vs. 65), more likely male, more commonly from North America and Western Europe/Scandinavia compared with other regions, more commonly current/recent smokers, more commonly had prior atrial fibrillation and angiography without subsequent revascularization before randomization, and were more likely to be taking a proton-pump inhibitor at baseline (Table 1). Participants with new, non-benign neoplasms were more likely to have a prior history of malignancy in any anatomic/tissue location and to have undergone prior cancer-screening tests/procedures before randomization (see Supplementary material online, Table S1).
Table 1.
Baseline characteristics by neoplasm status
| Characteristic | Detection of new, non-benign neoplasm |
|
|---|---|---|
| Yes (n = 170) | No (n = 9070) | |
| Male sex, n (%) | 126/170 (74.1) | 5497/9070 (60.6) |
| Age, median (IQR) (years) | 71 (64–76) | 65 (58–73) |
| Age ≥75 years, n (%) | 51/170 (30.0) | 2009/9070 (22.1) |
| Weight, median (IQR) (kg) | 80 (69–93) | 75 (65–86) |
| Weight <60 kg, n (%) | 19/170 (11.2) | 1372/9066 (15.1) |
| Region, n (%) | ||
| Central/Eastern Europe | 43/170 (25.3) | 3034/9070 (33.5) |
| East Asia | 3/170 (1.8) | 739/9070 (8.1) |
| Indian Subcontinent | 1/170 (0.6) | 1138/9070 (12.5) |
| Latin America | 17/170 (10.0) | 1253/9070 (13.8) |
| Mediterranean Basin | 10/170 (5.9) | 633/9070 (7.0) |
| North America | 55/170 (32.4) | 1201/9070 (13.2) |
| Western Europe/Scandinavia | 38/170 (22.4) | 931/9070 (10.3) |
| Rest of World | 3/170 (1.8) | 141/9070 (1.6) |
| Presentation characteristics | ||
| Killip Class II–IV on presentation, n (%) | 23/170 (13.5) | 1101/9064 (12.1) |
| Disease classification, n (%) | ||
| UA | 32/170 (18.8) | 2757/9070 (30.4) |
| NSTEMI | 138/170 (81.2) | 6313/9070 (69.6) |
| Cardiovascular risk factors, n (%) | ||
| Family history of CAD | 52/143 (36.4) | 2437/8068 (30.2) |
| Hypertension | 142/170 (83.5) | 7421/9048 (82.0) |
| Hyperlipidaemia | 124/166 (74.7) | 5072/8623 (58.8) |
| Diabetes mellitus | 60/170 (35.3) | 3445/9053 (38.1) |
| Current/recent smoking (within 30 days of randomization) | 40/167 (24.0) | 1788/8983 (19.9) |
| Cardiovascular disease history, n (%) | ||
| Prior MI | 75/166 (45.2) | 3878/8997 (43.1) |
| Prior PCI | 47/169 (27.8) | 2354/9020 (26.1) |
| Prior CABG | 27/168 (16.1) | 1412/9052 (15.6) |
| Prior PAD | 15/165 (9.1) | 663/8912 (7.4) |
| Prior heart failure | 23/168 (13.7) | 1591/9016 (17.6) |
| Prior atrial fibrillation | 20/164 (12.2) | 682/8858 (7.7) |
| Median baseline GRACE risk score (IQR) | 129 (115–145) | 121 (105–139) |
| Baseline laboratory assessments | ||
| Median haemoglobin (IQR) (g/dL) | 13.5 (12.4–14.5) | 13.6 (12.4–14.7) |
| Median creatinine clearance (IQR) (mL/min) | 71.1 (52.1–92.3) | 72.7 (54.1–96.3) |
| Pre-randomization procedures | ||
| Angiography performed, n (%) | 100/170 (58.8) | 3709/9070 (40.9) |
| Concomitant medications at randomization, n (%) | ||
| Aspirin | ||
| Daily dose <100 mg | 63/170 (37.1) | 3028/9070 (33.4) |
| Daily dose 100–250 mg | 78/170 (45.9) | 4843/9070 (53.4) |
| Daily dose >250 mg | 22/170 (12.9) | 638/9070 (7.0) |
| β-Blocker | 138/170 (81.2) | 7060/9070 (77.8) |
| ACE-I/ARB | 135/170 (79.4) | 6834/9070 (75.3) |
| Statin | 143/170 (84.1) | 7568/9070 (83.4) |
| Proton-pump inhibitor | 55/170 (32.4) | 2265/9070 (25.0) |
ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CABG, coronary artery bypass grafting; CAD, coronary artery disease; MI, myocardial infarction; NSTEMI, non-ST-segment elevation myocardial infarction; PAD, peripheral arterial disease; PCI, percutaneous coronary intervention; UA, unstable angina.
Study drug discontinuation
A total of 2153/9240 (23.3%) treated participants permanently discontinued study drug treatment during follow-up, with a significantly higher frequency among those with a new, non-benign neoplasm event (53.5 vs. 22.7%; P < 0.001) (see Supplementary material online, Table S2). Study drug discontinuation for both haemorrhagic and non-haemorrhagic AEs was more common for those with vs. without a new, non-benign neoplasm. Discontinuation for haemorrhagic AEs was more common before neoplasm detection, and discontinuation for non-haemorrhagic AEs was more common after neoplasm detection (see Supplementary material online, Table S3).
Ischaemic and bleeding endpoints
The frequencies of the primary composite endpoint of cardiovascular death, MI, or stroke (18.2 vs. 13.5%) and all-cause death (28.2 vs. 8.1%) were numerically higher among those treated participants with vs. without a new, non-benign neoplasm (Table 2). Among the 48 deaths that occurred in patients with a new, non-benign neoplasm, 36 (75%) were considered to be malignancy related by the Neoplasm CEC. There were no treatment-related differences in all-cause death as well as malignancy-related deaths (see Supplementary material online, Table S4). The incidence of both GUSTO and TIMI bleeding events was also substantially higher among treated participants with a new, non-benign neoplasm. Non-fatal cardiovascular events (MI and stroke) and bleeding events more commonly occurred before vs. after neoplasm detection (Table 3).
Table 2.
Ischemic and bleeding events by neoplasm status
| Event, n (%) | Detection of new, non-benign neoplasm |
||
|---|---|---|---|
| Total (n = 9240) | Yes (n = 170) | No (n = 9070) | |
| Cardiovascular death, MI, or stroke | 1258 (13.6) | 31 (18.2) | 1227 (13.5) |
| All-cause death | 784 (8.5) | 48 (28.2) | 736 (8.1) |
| GUSTO severe, life-threatening, or moderate bleeding | 158 (1.7) | 19 (11.2) | 139 (1.5) |
| TIMI major or minor bleeding | 174 (1.9) | 20 (11.8) | 154 (1.7) |
Event rates reported as the percentage of treated subjects except for deaths from malignancy as denoted below.
GUSTO, Global Use of Strategies to Open Occluded Arteries; MI, myocardial infarction; TIMI, thrombolysis in myocardial infarction.
Table 3.
Ischaemic and bleeding events before and after detection of new, non-benign neoplasm events
| Event, n (%) | Timing of eventa |
||
|---|---|---|---|
| Total (n = 170) | Before new, non-benign neoplasm | After new, non-benign neoplasm | |
| Cardiovascular death, MI, or stroke | 31 (18.2) | 17 (54.8) | 14 (45.2) |
| All-cause death | 48 (28.2) | 0 | 48 (100.0) |
| GUSTO severe/life-threatening, or moderate bleeding | 19 (11.2) | 13 (68.4) | 6 (31.6) |
| TIMI major or minor bleeding | 20 (11.8) | 16 (80.0) | 4 (20.0) |
Event rates reported as the percentage of treated subjects.
GUSTO, Global Use of Strategies to Open Occluded Arteries; MI, myocardial infarction; TIMI, thrombolysis in myocardial infarction.
aPercentages of total events shown in parentheses for each column.
Cancer screening by geographic region
Among the 9240 treated participants, cancer-screening tests/procedures were performed before randomization most commonly in North America (43%), Rest of World (Australia, New Zealand, and South Africa) (25.2%), and Western Europe/Scandinavia (12.7%) (see Supplementary material online, Table S5). The most common tests/procedures performed for cancer screening were mammography, colonoscopy, and prostate-specific antigen testing. Similar patterns were observed after randomization (during study follow-up) with the highest utilization of cancer-screening tests and procedures in North America (28.5%), Rest of World (Australia, New Zealand, and South Africa) (12.5%), and Western Europe/Scandinavia (12.0%) (see Supplementary material online, Table S6).
Factors associated with neoplasm detection
The association of each of the candidate variables with the detection of new, non-benign neoplasms among the 9240 treated participants is shown in Supplementary material online, Table S7. Seven candidate variables were found to be significantly associated with neoplasm detection in a reduced model, with increasing age up to 70 years, geographic region (North America, Western Europe/Scandinavia, vs. other), male sex, and current/recent smoking identified as the most significant variables (Table 4).
Table 4.
Factors associated with detection of a new, non-benign neoplasm event (reduced model)
| Variables | χ 2 | HR (95% CI) | P-value |
|---|---|---|---|
| Age (years)a | 47.75 | – | <0.001 |
| Per 10 years increase (age ≤70 years) | 38.17 | 3.04 (2.14–4.33) | – |
| Per 10 years increase (age >70 years) | 0.17 | 1.08 (0.76–1.51) | – |
| Region | 36.51 | – | <0.001 |
| North America vs. Other | 30.72 | 3.02 (2.04–4.46) | |
| Western Europe/Scandinavia vs. Other | 18.56 | 2.48 (1.64–3.76) | |
| Male sex | 12.84 | 2.00 (1.37–2.93) | <0.001 |
| Current/recent smoker | 9.07 | 1.80 (1.23–2.63) | 0.003 |
| Prior CABG | 6.64 | 0.56 (0.36–0.87) | 0.010 |
| Hyperlipidaemia | 4.91 | 1.54 (1.05–2.26) | 0.027 |
| Weight per 10 kg decrease | 3.63 | 0.91 (0.83–1.00) | 0.057 |
CABG, coronary artery bypass grafting.
aAge (years) modelled as a linear spline to account for non-linear relationship with detection of new, non-benign neoplasm.
Treatment-related differences in neoplasm detection
Among 9105 treated participants who did not have a history of prior malignant neoplasm in any anatomic/tissue location or had curative treatment for a prior malignancy before randomization, the incidence of new, non-benign neoplasms was statistically similar for prasugrel vs. clopidogrel treatment (1.8 vs. 1.7%; HR = 1.04; 95% CI: 0.77–1.42; P = 0.79). The Kaplan–Meier event curves by randomized treatment assignment had a similar trajectory throughout study follow-up (Figure 1). Among the overall population (n = 9240), regardless of baseline history of malignancy, the number of subjects with new non-benign neoplasms increased by five subjects for both treatment groups [prasugrel, n = 87; (1.9%); clopidogrel, n = 83 (1.8%)] with no change in the observed hazard by treatment assignment (HR = 1.04; 95% CI: 0.77–1.40; P = 0.79).
Figure 1.
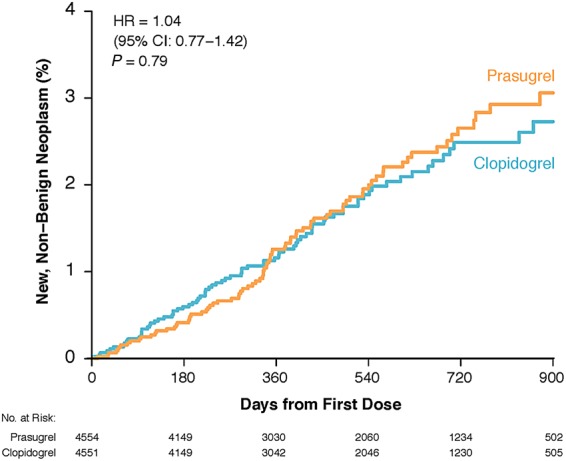
Kaplan–Meier event rates for the detection of new, non-benign neoplasms by treatment assignment (prasugrel vs. clopidogrel) during study follow-up among treated participants who did not have a prior history of malignancy or had curative treatment for a prior malignancy before randomization (n = 9105).
The most common locations of new, non-benign neoplasms in the primary analysis population (n = 9105) were lung/bronchus, colorectal, prostate (for male patients), skin (squamous cell and basal cell), and breast (for female patients), with no statistical differences by treatment (Table 5). Numerically, there were more colorectal neoplasms detected in the prasugrel group compared with the clopidogrel group, but there was no statistical difference (14 vs. 6 events, P = 0.10). Patients treated with clopidogrel who had a confirmed neoplasm appeared to be more likely to have advanced-staged neoplasms (Stages III and IV) at the time of neoplasm detection compared with patients treated with prasugrel (Table 5). The circumstances underlying initial neoplasm detection were most commonly symptomatic cancer-screening tests/procedures and evaluation for a bleeding event/anaemia, with no observed statistical differences by study treatment (see Supplementary material online, Table S8). More neoplasm events were detected following gastrointestinal bleeding and anaemia with prasugrel, whereas more neoplasm events were detected following haematuria with clopidogrel.
Table 5.
Neoplasm details by treatment assignment for subjects with new, non-benign neoplasms and without a baseline history of malignancy or curative treatment before randomization, n (%)
| Endpoint | Totala (n = 9105) | Prasugrel (n = 4554) | Clopidogrel (n = 4551) | P-value |
|---|---|---|---|---|
| New, non-benign neoplasm detecteda | 160 (1.8) | 82 (1.8) | 78 (1.7) | 0.79b |
| Locations of new, non-benign neoplasmc | ||||
| Blood | 1 (0.6) | – | 1 (1.3) | 0.49 |
| Bone | 1 (0.6) | 1 (1.2) | – | >0.99 |
| Bone marrow | 8 (5.0) | 4 (4.9) | 4 (5.1) | >0.99 |
| Brain | 1 (0.6) | 1 (1.2) | – | >0.99 |
| Breast | 6 (14.3) | 4 (16.7) | 2 (11.1) | 0.68 |
| Cervix | 1 (2.4) | – | 1 (5.6) | 0.43 |
| Colorectal | 20 (12.5) | 14 (17.1) | 6 (7.7) | 0.10 |
| Esophagus | 4 (2.5) | 1 (1.2) | 3 (3.8) | 0.36 |
| Eye | – | – | – | – |
| Gallbladder | – | – | – | – |
| Kidney | 4 (2.5) | 2 (2.4) | 2 (2.6) | >0.99 |
| Liver | – | – | – | – |
| Lung/bronchus | 23 (14.4) | 11 (13.4) | 12 (15.4) | 0.82 |
| Lymphatics | – | – | – | – |
| Oral cavity | 3 (1.9) | 3 (3.7) | – | 0.25 |
| Ovary | 1 (2.4) | – | 1 (5.6) | 0.43 |
| Pancreas | 3 (1.9) | 2 (2.4) | 1 (1.3) | >0.99 |
| Pharynx | 4 (2.5) | 2 (2.4) | 2 (2.6) | >0.99 |
| Prostate | 15 (12.7) | 7 (12.1) | 8 (13.3) | >0.99 |
| Stomach | 10 (6.2) | 5 (6.1) | 5 (6.4) | >0.99 |
| Urethral | – | – | – | – |
| Urinary bladder | 9 (5.6) | 4 (4.9) | 5 (6.4) | 0.74 |
| Uterus | – | – | – | – |
| Other | 2 (4.8) | 1 (4.2) | 1 (5.6) | >0.99 |
| Unknown primary | 8 (5.0) | 3 (3.7) | 5 (6.4) | 0.49 |
| Skin cancers | ||||
| Basal cell | 19 (11.9) | 8 (9.8) | 11 (14.1) | 0.47 |
| Squamous cell | 20 (12.5) | 11 (13.4) | 9 (11.5) | 0.81 |
| Melanoma | 5 (3.1) | 2 (2.4) | 3 (3.8) | 0.68 |
| Stage of neoplasm at the time of detectionc | ||||
| Stage 0 | 18 (11.2) | 9 (11.0) | 9 (11.5) | >0.99 |
| Stage I | 32 (20.0) | 17 (20.7) | 15 (19.2) | 0.85 |
| Stage II | 13 (8.1) | 10 (12.2) | 3 (3.8) | 0.08 |
| Stage III | 27 (16.9) | 12 (14.6) | 15 (19.2) | 0.53 |
| Stage IV | 41 (25.6) | 15 (18.3) | 26 (33.3) | 0.03 |
| Staging incomplete | 15 (9.4) | 9 (11.0) | 6 (7.7) | 0.59 |
| Unknown | 23 (14.4) | 13 (15.8) | 10 (12.8) | 0.66 |
| Methods used for initial neoplasm detection (not mutually exclusive)d | ||||
| Routine cancer screening | 12 (7.5) | 7 (8.5) | 5 (6.4) | 0.77 |
| Symptomatic cancer screening | 125 (78.1) | 67 (81.7) | 58 (74.4) | 0.34 |
| Evaluation of bleeding event | 40 (25.0) | 20 (24.4) | 20 (25.6) | 0.86 |
| Evaluation of anaemia | 13 (8.1) | 9 (11.0) | 4 (5.1) | 0.25 |
| Diagnostic procedures done for a suspected cancer not associated with a bleeding event or anaemia | 81 (50.6) | 45 (54.9) | 36 (46.2) | 0.34 |
| Diagnostic procedures done for a reason/symptom not related to a suspected cancer bleed event, or anaemia | 26 (16.2) | 9 (11.0) | 17 (21.8) | 0.09 |
| Initial treatment of malignancy (not mutually exclusive)d | ||||
| Radiation | 20 (12.5) | 10 (12.2) | 10 (12.8) | >0.99 |
| Chemotherapy | 27 (16.9) | 7 (8.5) | 20 (25.6) | 0.005 |
| Hormonal therapy | 3 (1.9) | – | 3 (3.8) | 0.11 |
| Surgical resection | 67 (41.9) | 36 (43.9) | 31 (39.7) | 0.63 |
| Immunotherapy | 5 (3.1) | 4 (4.9) | 1 (1.3) | 0.37 |
| Other treatment | 18 (11.2) | 8 (9.8) | 10 (12.8) | 0.62 |
| Metastatic disease detected during studyd | 47 (29.4) | 18 (22.0) | 29 (37.2) | 0.04 |
All values given as number of subjects (%).
aTotal number of subjects treated without baseline history of malignancy or with curative treatment before randomization.
bHazard ratio = 1.04 (95% CI 0.77–1.42)
cFor locations unique to females (breast, cervix, and uterine), the n for the denominator is the n for female patients (42). For the location unique to men (prostate), the n for denominator is the n for male patients (118). Otherwise, the denominator for each cell is the column total. Participants could have had more than one cancer location determined (primary and secondary malignancies) through the adjudication process, so the P-values in this section relate to each specific row separately to account for all malignancies.
dFor the staging data, participants could have had more than one cancer location determined (primary and secondary malignancies) through the adjudication process, so the P-values in this section relate to each specific row separately to account for all malignancies. For the methods of detection, participants could have had more than one test/procedure performed for primary and secondary malignancies. For the initial treatment of malignancy, participants could have had more than one treatment administered. The development of metastatic disease was determined for both primary and secondary malignancies.
Discussion
In one of the most comprehensive experiences of the collection of cancer data and the adjudication of neoplasm events in a long-term cardiovascular drug therapy trial, we have demonstrated several unique and important findings. First, the detection of new, non-benign neoplasm events (detected through a rigorous, prospectively planned process) occurred infrequently during prolonged treatment with DAPT following an ACS event in a medically managed population, with no statistical differences observed by treatment with prasugrel vs. clopidogrel. Second, the detection of new, non-benign neoplasm events was associated with high rates of permanent study drug discontinuation as well as with ischaemic and bleeding events (before and after neoplasm detection). Finally, substantial differences in the use of pre- and post-randomization cancer-screening tests/procedures were observed across geographic regions.
Based upon the observation of a potential increased frequency of neoplasm with prasugrel vs. clopidogrel for the treatment of ACS patients undergoing PCI in the TRITON-TIMI 38 trial, we implemented a novel process for collecting and adjudicating neoplasm data in TRILOGY ACS that demonstrated a similar risk of developing cancer during treatment with prasugrel vs. clopidogrel (plus aspirin), albeit with limited power given the low event rates observed.12,17 Although this type of process has been implemented in other long-term cardiovascular therapy trials and provides far more robust data and results than prospective or post hoc evaluation of AE data related to neoplasms, its use in evaluating potential cancer risks in future global trials of cardiovascular therapeutics may need to be reconsidered for several reasons.18 First, observed variations in cancer-screening tests/procedures across geographic regions demonstrate practice-related confounding that will always affect neoplasm ascertainment in global trials, especially if the majority of enrolment occurs outside of North America and Western Europe. Second, given the infrequent detection of neoplasm events observed, very large sample sizes would be required to perform adequately powered analyses of treatment-related differences in neoplasm detection. Randomized trials of this size (likely >50 000 patients) are impractical, so less-costly safety surveillance techniques now available in the USA and other countries should be considered for ascertaining cancer risks associated with cardiovascular drugs in the post-approval setting, and streamlined approaches for cancer ascertainment, classification, and adjudication in cardiovascular outcomes trials should be evaluated given the increasing advocacy for large, simple cardiovascular trials.19,20 Finally, given the long-term and complex environmental and genetic influences on cancer development, the effects of a potential neoplasm safety signal cannot be fully ascertained during the typical follow-up period of cardiovascular drug therapy trials (1–3 years).
These issues notwithstanding, more than half of participants with a new, non-benign neoplasm event in TRILOGY ACS permanently discontinued study drug (prasugrel or clopidogrel), and those with neoplasm events had higher frequencies of composite ischaemic events, all-cause mortality, and significant bleeding events. Although the majority of deaths were considered to be malignancy related for participants with a confirmed new, non-benign neoplasm event, more non-fatal cardiovascular events (MI and stroke) and bleeding events occurred before vs. after neoplasm detection, whereas an equal proportion of these participants permanently discontinued study drug before vs. after neoplasm detection.
These exploratory findings highlight the potential pathophysiologic mechanisms related to the use of antiplatelet therapies and the development of ischaemic, bleeding, and neoplasm events in the post-ACS setting, as well as the likely residual confounding that underlies potential explanations. Prior to neoplasm detection, undiagnosed neoplasms may contribute to a pro-thrombotic state that results in ischaemic events despite DAPT treatment, but they may also contribute to the development of significant bleeding (especially for neoplasms in the gastrointestinal tract) leading to premature discontinuation of antiplatelet therapy, which in turn may be associated with subsequent recurrent ischaemic events. While we observed a numerically greater number of colorectal neoplasm events with prasugrel vs. clopidogrel, as was observed in the TRITON-TIMI 38 trial, these observations may be due to detection biases related to the more potent P2Y12 inhibitor, prasugrel, that may have more likely unmasked pre-existing colorectal cancer that was identified with a gastrointestinal bleeding event.11 However, we also observed an apparent greater likelihood for the detection of advanced stages of cancer (Stages III and IV) at the time of neoplasm detection for patients treated with clopidogrel vs. prasugrel. These findings highlight the likely multiple layers of confounding associated with the detection and ascertainment of neoplasm events during DAPT treatment that are difficult to disentangle with respect to the question of whether there are differential pathophysiologic mechanisms for neoplasm detection between prasugrel vs. clopidogrel.
The influence of neoplasm events on mortality rates during extended DAPT in placebo-controlled, randomized clinical trials has been investigated in two recent high-profile studies. In the DAPT trial (a double-blinded, placebo-controlled trial of prolonged DAPT for 30 vs. 12 months following PCI with drug-eluting stent placement), the incidence of all-cause mortality was nominally higher with prolonged DAPT vs. aspirin monotherapy (2.0 vs. 1.5%; P = 0.05), an effect that appeared to be driven by a higher non-cardiovascular mortality rate (1.0 vs. 0.5%; P = 0.002).21 In the prolonged DAPT group, post hoc analyses demonstrated a numeric imbalance of pre-randomization cancers, an increased frequency of malignancy-related deaths (31 vs. 14 deaths; P = 0.02), and no statistical difference in the development of new cancer events. There was no difference in all-cause mortality between treatment groups when patients with a history of cancer prior to randomization were excluded from the analysis.
Subsequently, the DAPT trial results were combined with results from 13 other trials that evaluated extended DAPT vs. aspirin monotherapy or short-duration DAPT (≤6 months) in a comprehensive meta-analysis of almost 70 000 patients. No differences in all-cause, cardiovascular, or non-cardiovascular mortality were observed.22 Collectively, observations from these studies and from TRILOGY ACS indicate that sporadic neoplasms occurring either before or after starting DAPT for a cardiovascular indication appear to significantly influence mortality rates, and thus may confound the interpretation of the results of large, randomized cardiovascular outcomes trials evaluating antiplatelet therapies.
Study limitations
Despite careful planning and implementation of the neoplasm data collection and adjudication process in the TRILOGY ACS trial, certain limitations were present in our study. First, there was no precedent for the classification of new, non-benign neoplasms to guide our decisions for how post-randomization neoplasms were confirmed, located, staged, and described (in terms of primary vs. secondary malignancies, methods of detection, treatments administered, and disease progression by the time of last study contact). The processes we implemented and the results we observed will therefore be useful for informing future neoplasm ascertainment and adjudication efforts in cardiovascular outcomes trials. Second, data on the use of post-randomization cancer-screening tests/procedures were routinely collected and considered in terms of the methods of neoplasm detection, but we could not accurately verify whether these test/procedures were truly done for signs or symptoms caused by a neoplasm or done for routine surveillance without associated signs or symptoms. Finally, this analysis was not powered to definitely evaluate treatment-related differences in neoplasm detection given the low event rates observed, so our findings should be considered to be hypothesis generating.
Conclusions
We have demonstrated how the detection and classification of neoplasms during the conduct of a global cardiovascular outcomes trial informs the epidemiologic description of the natural histories of concurrent cardiovascular disease and cancer and relates to variability in cancer-screening practices across geographic regions. Nonetheless, these results provide further evidence to inform the debate about cancer risks associated with prolonged DAPT treatment with prasugrel vs. clopidogrel (plus aspirin) after ACS, but also highlight the complexities of implementing a neoplasm adjudication process in a global cardiovascular outcomes trial. Future studies should therefore investigate the relative value of novel data surveillance approaches to ascertain the potential cancer risks of cardiovascular drug therapies when used in large patient populations.
Supplementary material
Supplementary Material is available at European Heart Journal online.
Authors’ contributions
D.D.C. performed statistical analysis. M.T.R. handled funding and supervision. M.T.R. acquired the data. M.T.R. and E.M.O. conceived and designed the research. M.T.R. drafted the manuscript. D.E., P.J.S., M.A.M., K.L.B., D.J.G., N.E.R., S.Y.Z., A.W.B., J.H.S., J.E.O., K.J.W., L.H., D.Z., S.D.W., H. D.W., D.P., K.A.A.F., P.W.A., E.M.O., and D.D.C. made critical revision of the manuscript for key intellectual content.
Funding
This work and the TRILOGY ACS study was supported by Daiichi Sankyo, Incorporated, and Eli Lilly and Company. The study sponsors had no role in the conception and design of this study or in creating the first draft of the manuscript. Employees of Eli Lilly (Dr Winters and Ms. Houterloot) and Daiichi Sankyo Development Ltd. (Dr Zamoryakhin) participated as authors during subsequent drafts of the manuscript.
Conflict of interest: M.T.R. receives research funding from Eli Lilly, Sanofi-Aventis, Daiichi Sankyo, Janssen Pharmaceuticals, Ferring Pharmaceuticals, American College of Cardiology, the American Heart Association, and the Familial Hypercholesterolemia Foundation. He also receives consulting payments or honoraria from AstraZeneca, Boehringer Ingelheim, Merck, Amgen, PriMed, and Elsevier Publishers. All conflicts of interest are listed at https://www.dcri.org/about-us/conflict-of interest. D.C., P.S., S.Y.Z., and D.E. have no conflicts of interest. M.M. reports research grants or consultancies with Advanced Liquid Logic, Inc., American Physician Institute, American Society for Clinical Investigation, Amgen, Inc., Bayer, Best Doctors, Bristol-Myers Squibb, Cape Fear Valley Hospital, Caris, Dava Oncology, Defined Health, Etubics Corporation, Genentech, Inc. (Roche Holding), Genomic Health, Inc., Georgia Society of Clinical Oncology, Gerson Lehrman Group, Inc., Health Advances, MarketLab dba Focus Pointe Global, National Institutes of Health, Novartis, Onyx, PPD, Inc., Pfizer Inc., PhytoChem Pharmaceuticals, Inc., Prometheus Laboratories Inc., Regeneron Pharmaceuticals, Sanofi-Aventis, Schlesinger Associates, Watermark, and Xcenda, LLC (AmerisourceBergen Corp). K.B. reports research grants or consultancies with Amgen, Inc., Cowen and Company, Research To Practice, Statistical Collaborative, Inc., WebMD, Institute for Continuing Healthcare Education, and QED Communications. N.R. reports research grants or consultancies with Bristol-Myers Squibb, Stem CentRX Inc., and Onyx. A.B. reports research grants or consultancies with Celgene Corporation, Seattle Genetics, and GlaxoSmithKline. J.S. reports research grants or consultancies with Amgen, Exelixis Inc., Bayer, Onyx, Celgene, and Marval Biosciences. J.O. reports research grants or consultancies with Abbott Laboratories, Bristol-Myers Squibb, Gilead Sciences, Inc., Luitpold Pharmaceuticals, Inc. (Daiichi Sankyo), and Schering-Plough Corp. (owned by Merck & Co, USA). K.W. and L.H. are employees and minor shareholders of Eli Lilly and Company. D.Z. is an employee of Daiichi Sankyo Development Ltd. S.W. reports grant and research support from Eli Lilly, AstraZeneca, Merck, and Eisai; and consulting fees and honoraria from Eli Lilly, Daiichi Sankyo, AstraZeneca, Bristol-Myers Squibb, Sanofi-Aventis, and Eisai. H.W. reports receiving grant support from Sanofi-Aventis, Eli Lilly, The Medicines Company, NIH, Pfizer, Roche, Johnson & Johnson, Schering-Plough, Merck Sharpe & Dohme, AstraZeneca, GlaxoSmithKline, Daiichi Sankyo Pharma Development, and Bristol-Myers Squibb; he also participates in advisory boards for Merck Sharpe & Dohme, Roche, and Regado Biosciences. D.P. reports receiving research grants from Eli Lilly and the Medtronic Foundation and honoraria from Eli Lilly. K.F. reports receiving research grants from Lilly, Bayer, Johnson & Johnson, and AstraZeneca; speakers bureau payments from Bayer, Johnson & Johnson, AstraZeneca, and Sanofi-Aventis; and consulting/other payments from Lilly, Bayer, Johnson & Johnson, AstraZeneca, Sanofi-Aventis, Boehringer Ingelheim, and Eli Lilly. P.A. reports receiving consulting fees from Eli Lilly, Hoffmann-La Roche, Merck, Axio Research, and Orexigen; grant support from Boehringer Ingelheim, Hoffmann-La Roche, Sanofi-Aventis, Scios, Ortho Biotech, Johnson & Johnson, Janssen Pharmaceuticals, GlaxoSmithKline, Amylin Pharmaceuticals, and Merck; and payment for developing educational presentations from AstraZeneca and Eli Lilly and Company. E.M.O. reports receiving grant support and travel expenses from Daiichi Sankyo and Eli Lilly; consulting fees from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen Pharmaceuticals, Liposcience, Merck, Pozen, Hoffmann-La Roche, Sanofi-Aventis, The Medicines Company, and Web MD; grant support from Gilead Sciences; and lecture fees from Gilead Sciences, Boehringer Ingelheim, and The Medicines Company.
Supplementary Material
Acknowledgements
The authors thank the following: Karen Pieper, MS, for expert coordination and management of the statistical analytic team; Jonathan McCall, MS, for expert editorial assistance, and Kerry Stenke for expert graphics assistance. Ms. Pieper, Mr. McCall, and Ms. Stenke are employees of the Duke Clinical Research Institute, Durham, NC; none received any compensation for their work on this manuscript other than their usual salaries.
References
- 1. Messerli FH, Bangalore S, Torp-Pedersen C, Staessen JA, Kostis JB. Cardiovascular drugs and cancer: of competing risk, smallpox, Bernoulli, and d'Alembert. Eur Heart J 2013;34:1095–1098. [DOI] [PubMed] [Google Scholar]
- 2. Sipahi I, Debanne SM, Rowland DY, Simon DI, Fang JC. Angiotensin-receptor blockade and risk of cancer: meta-analysis of randomised controlled trials. Lancet Oncol 2010;11:627–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bangalore S, Kumar S, Kjeldsen SE, Makani H, Grossman E, Wetterslev J, Gupta AK, Sever PS, Gluud C, Messerli FH. Antihypertensive drugs and risk of cancer: network meta-analyses and trial sequential analyses of 324,168 participants from randomised trials. Lancet Oncol 2011;12:65–82. [DOI] [PubMed] [Google Scholar]
- 4. ARB Trialists Collaboration. Effects of telmisartan, irbesartan, valsartan, candesartan, and losartan on cancers in 15 trials enrolling 138,769 individuals. J Hypertens 2011;29:623–635. [DOI] [PubMed] [Google Scholar]
- 5. Pasternak B, Svanström H, Callréus T, Melbye M, Hviid A. Use of angiotensin receptor blockers and the risk of cancer. Circulation 2011;123:1729–1736. [DOI] [PubMed] [Google Scholar]
- 6. Lindholm LH, Carlberg B. Blood-pressure drugs and cancer: much ado about nothing? Lancet Oncol 2011;12:6–8. [DOI] [PubMed] [Google Scholar]
- 7. Peto R, Emberson J, Landray M, Baigent C, Collins R, Clare R, Califf R. Analyses of cancer data from three ezetimibe trials. N Engl J Med 2008;359:1357–1366. [DOI] [PubMed] [Google Scholar]
- 8. Rossebø AB, Pedersen TR, Boman K, Brudi P, Chambers JB, Egstrup K, Gerdts E, Gohlke-Bärwolf C, Holme I, Kesäniemi YA, Malbecq W, Nienaber CA, Ray S, Skjaerpe T, Wachtell K, Willenheimer R, SEAS Investigators. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med 2008;359:1343–1356. [DOI] [PubMed] [Google Scholar]
- 9. Pfeffer MA. Cancer in cardiovascular drug trials and vice versa: a personal perspective. Eur Heart J 2013;34:1089–1094. [DOI] [PubMed] [Google Scholar]
- 10. Asiimwe A, Li JJ, Weerakkody G, Vangerow H, Delisle F, Benoit K, Heath L, Wernicke J, Motsko S. Diagnoses of gastrointestinal cancers after gastrointestinal bleeding in patients receiving clopidogrel or warfarin. Curr Drug Saf 2013;8:261–269. [DOI] [PubMed] [Google Scholar]
- 11. Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM, TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007;357:2001–2015. [DOI] [PubMed] [Google Scholar]
- 12. Unger EF. Weighing benefits and risks – the FDA's review of prasugrel. N Engl J Med 2009;361:942–945. [DOI] [PubMed] [Google Scholar]
- 13. Chin CT, Roe MT, Fox KA, Prabhakaran D, Marshall DA, Petitjean H, Lokhnygina Y, Brown E, Armstrong PW, White HD, Ohman EM, TRILOGY ACS Steering Committee. Study design and rationale of a comparison of prasugrel and clopidogrel in medically managed patients with unstable angina/non-ST-segment elevation myocardial infarction: the TaRgeted platelet Inhibition to cLarify the Optimal strateGy to medicallY manage Acute Coronary Syndromes (TRILOGY ACS) trial. Am Heart J 2010;160:16–22.e1. [DOI] [PubMed] [Google Scholar]
- 14. Roe MT, Armstrong PW, Fox KA, White HD, Prabhakaran D, Goodman SG, Cornel JH, Bhatt DL, Clemmensen P, Martinez F, Ardissino D, Nicolau JC, Boden WE, Gurbel PA, Ruzyllo W, Dalby AJ, McGuire DK, Leiva-Pons JL, Parkhomenko A, Gottlieb S, Topacio GO, Hamm C, Pavlides G, Goudev AR, Oto A, Tseng CD, Merkely B, Gasparovic V, Corbalan R, Cinteză M, McLendon RC, Winters KJ, Brown EB, Lokhnygina Y, Aylward PE, Huber K, Hochman JS, Ohman EM, TRILOGY ACS Investigators. Prasugrel versus clopidogrel for acute coronary syndromes without revascularization. N Engl J Med 2012;367:1297–1309. [DOI] [PubMed] [Google Scholar]
- 15. American Joint Committee on Cancer (AJCC) – Cancer Staging Manual. 7th ed New York, NY: Springer Publishers; 2010. [Google Scholar]
- 16. Boos DD, Stefanski LA, Wu Y. Fast FSR variable selection with applications to clinical trials. Biometrics 2009;65:692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ohman EM, Roe MT, Armstrong PW, Fox KA, Prabhakaran D, White HD. Public sensationalism and clinical trials: how to address the challenges of science? Am J Med 2010;123:481–483. [DOI] [PubMed] [Google Scholar]
- 18. Blazing MA, Giugliano RP, Cannon CP, Musliner TA, Tershakovec AM, White JA, Reist C, McCagg A, Braunwald E, Califf RM. Evaluating cardiovascular event reduction with ezetimibe as an adjunct to simvastatin in 18,144 patients after acute coronary syndromes: final baseline characteristics of the IMPROVE-IT study population. Am Heart J 2014;168:205–212.e1. [DOI] [PubMed] [Google Scholar]
- 19. Eapen ZJ, Lauer MS, Temple RJ. The imperative of overcoming barriers to the conduct of large, simple trials. JAMA 2014;311:1397–1398. [DOI] [PubMed] [Google Scholar]
- 20. Psaty BM, Breckenridge AM. Mini-Sentinel and regulatory science – big data rendered fit and functional. N Engl J Med 2014;370:2165–2167. [DOI] [PubMed] [Google Scholar]
- 21. Mauri L, Kereiakes DJ, Yeh RW, Driscoll-Shempp P, Cutlip DE, Steg PG, Normand SL, Braunwald E, Wiviott SD, Cohen DJ, Holmes DR Jr, Krucoff MW, Hermiller J, Dauerman HL, Simon DI, Kandzari DE, Garratt KN, Lee DP, Pow TK, Ver Lee P, Rinaldi MJ, Massaro JM, DAPT Study Investigators. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med 2014;371:2155–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Elmariah S, Mauri L, Doros G, Galper BZ, O'Neill KE, Steg PG, Kereiakes DJ, Yeh RW. Extended duration dual antiplatelet therapy and mortality: a systematic review and meta-analysis. Lancet 2015;385:792–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


