Abstract
Background and Objectives
It has been demonstrated that the neutrophil/lymphocyte ratio (NLR) might be a useful marker to predict cardiovascular risk and events. We aimed to investigate the role of the NLR to predict ventricular remodeling (VR) in patients with anterior ST-elevation myocardial infarction (STEMI) who were treated with primary percutaneous coronary intervention.
Subjects and Methods
We prospectively included 274 consecutive anterior STEMI patients. Echocardiography was performed during admission and at six months after myocardial infarction. VR was defined as at least 20% increase from baseline in left ventricular end-diastolic volume. Patients were divided into two groups according to their VR status: VR (n=67) and non-VR (n=207). Total and differential leukocyte count, N-terminal pro-brain natriuretic peptide (NT-proBNP) and other biochemical markers were measured at admission and 24 hours later.
Results
Compared with the non-VR group, peak creatine kinase MB (CK-MB), NT-proBNP (24 h), neutrophil/lymphocyte ratio, presence of diabetes, no-reflow frequency and wall motion score index were significantly higher in patients with VR (p<0.05 for all). On multivariate logistic regression analysis, NLR (β=2.000, 95% confidence interval=1.577-2.537, p<0.001) as well as peak CK-MB, NT-proBNP (24 h), WMSI and diabetes incidence were associated with VR. The cutoff value of the neutrophil/lymphocyte ratio obtained by receiver operator characteristic curve analysis was 4.25 for the prediction of VR (sensitivity: 79 %, specificity: 74%).
Conclusion
In patients with anterior STEMI, initial NLR and NT-proBNP measured 24 hours after admission may be useful for predicting adverse cardiovascular events including left VR.
Keywords: Myocardial infarction; Remodeling; Neutrophils, lymphocytes; BNP; No-reflow
Introduction
Acute myocardial infarction (AMI) is one of the most important causes of morbidity, mortality and heart failure worldwide. Most of these undesirable consequences in patients with AMI are related to the ventricular remodeling (VR) that occurs post-infarction.1) VR, primarily a response of the myocardium to potentially noxious hemodynamic, metabolic, and inflammatory stimuli, is associated with mortality in patients with acute coronary syndromes.2) Furthermore, myocyte hypertrophy, myocyte loss from necrosis or apoptosis, interstitial cell growth and especially fibroblast proliferation, which leads to myocardial fibrosis, are related to VR.3) VR is also affected by preload and afterload activation of the neurohumoral system and other factors that further adversely influence the remodeling process.4)
Inflammation plays a critical role in the development of VR.5) Myocardial ischemia triggers an inflammatory response, mainly characterized by infiltration with neutrophils, followed by monocytes/macrophages and lymphocytes.5) Small numbers of infiltrating lymphocytes also play a key role in remodeling, whereas monocytes cause pro-inflammatory, angiogenic and fibrotic response.6) In the inflammatory phase of the healing of post-myocardial infarction (MI) ventricular remodeling, cytokines lead to the activation of leukocytes.7) Subsequently, leukocytes, neutrophils and fibroblasts remove necrotic cells while releasing cytokines, growth factors and collagen that further form scar tissue.8) As a consequence of all of these processes, tissue injury and local and systemic inflammatory responses occur. The neutrophil/lymphocyte ratio (NLR), an indicator of systemic inflammation, has been proposed as a useful biomarker to predict cardiovascular risk and events.9) According to previous reports, NLR might be used as a prognostic marker in acute coronary syndrome, and it is the most powerful predictor among all white blood cell (WBC) subtypes.9)
Although in previous animal studies, it has been shown that multiple local inflammation biomarkers are activated with post-MI VR,10) the role of a systemic inflammatory marker such as NLR to predict the VR has not been investigated so far. We aimed to assess whether NLR is associated with VR in patients with anterior ST-elevation myocardial infarction (STEMI) who underwent primary percutaneous coronary intervention (PCI).
Subjects and Methods
Study population
We prospectively included 274 consecutive patients with anterior STEMI who underwent primary PCI (223 males, 51 females; mean age 55.3±12.5 years). STEMI was defined as resting chest pain lasting ≥30 min, together with new or presumed new ST-segment elevation in ≥2 contiguous leads with the cutoff point of ≥0.2 mV in anterior leads. The diagnosis was confirmed by coronary angiography in all patients. All patients were started on and continued taking their medications including dual antiplatelet therapy, angiotensin converting enzyme inhibitors (ACE-I) or angiotensin receptor blockers, beta-receptor blockers and statins throughout the study.
We enrolled consecutive patients with the first attack of STEMI who had undergone primary PCI within 12 h from the onset of symptoms at our institution. Initially, 363 patients were eligible for this study. Patients with a recent history of myocardial infarction (11 patients), history of previous PCI (8 patients) and previous coronary artery bypass graft (4 patients), late presentation (>12 h) (8 patients), patients with unsuccessful primary PCI (residual stenosis >50% in the culprit lesion after the procedure) and angiographic no-reflow (16 patients), patients with cardiac arrest (4 patients), patients who required an intra-aortic balloon pump (3 patients), patients who died for any reason after primary PCI (4 patients), patients who stopped taking their medications such as ACE-I and beta blockers for any reason during follow-up after primary PCI (5 patients), patients with restenosis and thrombosis after primary PCI (6 patients), and patients who had been pretreated with thrombolytic or glycoprotein IIb/IIIa inhibitor therapy before primary PCI (6 patients), as well as patients with infectious or inflammatory disease (4 patients), severe liver (history of hepatitis or alanin aminotransferase>3 fold the normal value, albumin<2.5 gr/dL) or renal disease (estimeted-glomeruler filtration rate [eGFR] formulated by modification of diet in renal disease (MDRD)<60 mL/min/1.73 m2) (7 patients) and neoplasm, or hematological disorders (3 patients) were excluded from the study. The exclusion criteria were applied to all groups, and as a result, 89 patients were excluded from this study. The Local Ethics Committee approved the study protocol, and each participant provided written informed consent.
After detailed medical history was taken and a complete physical examination was administered, baseline characteristics including age, sex, hypertension, hyperlipidemia, diabetes mellitus, current smoking status, family history of coronary artery disease (CAD), body mass index, and medications were recorded for all patients. Additionally, the time interval from the onset of symptoms to hospital admission was recorded for all patients.
Blood sampling
Venous blood samples were obtained before primary PCI at admission. WBC and differential counts were measured. The total numbers of WBC, neutrophils, monocytes and lymphocytes were measured using a Sysmex K-1000 (Block Scientific, Bohemia, New York, USA) autoanalyzer within 5 min of sampling. Plasma triglyceride, low-density lipoprotein, high-density lipoprotein, glucose, uric acid, and creatinine concentrations were measured with an automated chemistry analyzer (Abbott Aeroset, Minnesota, USA) using commercial kits (Abbott). Creatine kinase MB (CK-MB) activity was measured with an assay that used 2 monoclonal antibodies (CK-MB STAT) on an Elecsys 2010 analyzer (Roche Diagnostics, Basel, Switzerland) by electrochemiluminescence immunoassay. Plasma N-terminal pro-brain natriuretic peptide (NT-proBNP) was measured by electrochemiluminescence (Roche Diagnostics, Basel, Switzerland). eGFR was calculated according to the MDRD formula.
Coronary angiography and percutaneous coronary intervention
Urgent diagnostic coronary angiography was performed according to the standard criteria in all patients. To achieve maximal dilatation, each coronary angiogram was preceded by an intracoronary injection of 100 mg nitroglycerine. Significant CAD was defined as at least 70% luminal diameter stenosis in at least one epicardial coronary artery. Primary PCI procedures were performed using the standard femoral approach with a 7-Fr guiding catheter. All patients were pretreated with loading doses of aspirin (300 mg) and clopidogrel (600 mg); they also received an intravenous bolus of heparin 50 IU/kg. After the guidewire insertion into the infarct related artery (IRA), thromboaspiration (Export™ 6F catheter, Medtronic, Santa Rosa, CA, USA) was performed whenever possible (when the anatomy of the coronary artery e curve and size e allowed it) in all patients with a thrombolysis in myocardial infarction (TIMI) flow grade of 0 and in all patients with a visible thrombus if TIMI flow grade was 1 or more. Then, direct stenting was implanted whenever possible; in the remaining cases, balloon pre-dilatation was carried out. Drug eluting stents were mostly used. In each patient treated with tirofiban, it was administered after the primary PCI procedure in the coronary care unit. Baseline and post-primary PCI thrombolysis in myocardial infarction TIMI flow grade in IRA and post-PPCI TIMI myocardial perfusion grade were assessed by three independent interventional cardiologists. SYNTAX score was calculated as described previously.11)
Echocardiography and left ventricular remodeling
All echocardiograms were performed and interpreted by an experienced blinded echocardiographer. A 16-segment model was used to semi-quantitatively score regional wall motion abnormality. Wall motion was scored as 1=normal, 2=hypokinesia, 3=akinesia, 4=dyskinesia, and a wall motion score index (WMSI) was calculated for each segment using the sum of the score for all segments divided by the total number of segments. The modified Simpson biplane method was used to calculate the left ventricular end-diastolic volumes (LVEDVs) and end-systolic volumes (LVESVs) from 4- and 2-chamber views. LV volumes were corrected for body surface areas. LVR was considered a 20% increase in LVEDV at 6-month follow-up compared with 24-h echocardiogram, based on repeated measurements in individual patients and at the upper 95% confidence limit of intra-observer variability.12) The intra-observer variability values in evaluating the LVEDV and LVESV were 3.7% and 3.5%, respectively.
Statistical analysis
Statistical analysis was carried out using SPSS 17.0 for Windows (SPSS Inc., Chicago, Illinois, USA). Data are expressed as mean value±standard deviation. Continuous variables were tested for normality using the Kolmogorov-Smirnov test. An independent-simple t-test was used to analyze the continuous variables. Categorical variables were compared using the Chi-square test. Univariate logistic regression analysis was performed, and the variables that were found to be statistically significant were analyzed with multivariate logistic regression analysis; stepwise backward conditional logistic regression analysis was used to determine the independent predictors of VR. All significant parameters in the univariate analysis were selected in the multivariate model. A receiver operator characteristic (ROC) curve analysis was carried out to identify the optimal NLR cutoff point in patients with STEMI and VR. The value of the area under the curve was calculated as a measure of the test's accuracy. A two-tailed p of less than 0.05 was considered significant.
Results
Patients were divided into two groups according to the presence of ventricular remodeling. The VR and non-VR groups comprised 67 (24.5%) and 207 (75.5%) patients, respectively.
Baseline characteristics, laboratory findings and procedural characteristics
A comparison of the baseline characteristics and laboratory findings is shown in Table 1. Patients with VR had higher rates of diabetes and hypertension and higher BMI, triglyceride, peak CK-MB, NLR, NT-proBNP, and hemoglobin levels compared with the patients in the non-VR group (p<0.05, for all). Additionally, ejection fraction values in the VR group were lower, and WMSI values were higher compared with the non-VR group (p<0.05, for both).
Table 1. Comparison of baseline, clinical, laboratory and echocardiographic characteristics between the groups.
| Variables | Total patient group (n=274) |
Non-remodeling group (n=207) |
Remodeling group (n=67) |
p |
|---|---|---|---|---|
| Baseline and clinical characteristics | ||||
| Age (years) | 55.3±12.5 | 55.3±12.9 | 55.2±11.9 | 0.919 |
| Gender (female) | 51 (18.6) | 42 (20.3) | 9 (13.4) | 0.141 |
| BMI (kg/m2) | 27.2±4.1 | 26.9±3.7 | 28.0±5.1 | 0.050 |
| SBP (mmHg) | 129.2±27.2 | 130.5±27.8 | 125.3±25.0 | 0.180 |
| DBP (mmHg) | 80.4±16.9 | 80.4±17.2 | 80.2±16.2 | 0.938 |
| Heart rate (beat/min) | 86.0±16.1 | 85.7±16.5 | 86.9±14.8 | 0.584 |
| Hypertension | 108 (39.4) | 72 (34.8) | 36 (53.7) | 0.005 |
| Diabetes | 71 (25.9) | 43 (20.8) | 28 (41.8) | 0.001 |
| Hyperlipidemia | 42 (15.3) | 33 (15.9) | 9 (13.4) | 0.390 |
| Smoking | 186 (67.9) | 141 (68.1) | 45 (67.2) | 0.498 |
| Family history | 82 (30) | 57 (27.5) | 25 (37.3) | 0.087 |
| Cerebrovascular accident | 6 (2.3) | 4 (1.9) | 2 (3) | 0.454 |
| Pre-infraction angina | 101 (36.8) | 75 (36.2) | 26 (38.8) | 0.405 |
| Killip class 2-4 | 33 (12) | 24 (11.6) | 9 (13.4) | 0.416 |
| Laboratory findings | ||||
| Glucose (mg/dL) | 148.7±75.3 | 148.6±70.8 | 149.2±88.1 | 0.948 |
| Total cholesterol (mg/dL) | 201.9±45.4 | 199.8±45.4 | 206.2±45.6 | 0.254 |
| Triglyceride (mg/dL) | 138.8±72.8 | 133.7±77.9 | 154.7±41.8 | 0.040 |
| HDL-C (mg/dL) | 39.9±10.7 | 39.3±10.3 | 41.6±12.0 | 0.133 |
| LDL-C (mg/dL) | 133.8±38.1 | 133.8±38.2 | 133.7±38.0 | 0.974 |
| Peak CK-MB (ng/mL) | 161.9±112.7 | 148.1±108.0 | 204.6±116.7 | <0.001 |
| Creatinin (mg/dL) | 0.8±0.2 | 0.87±0.2 | 0.86±0.19 | 0.586 |
| eGFR (mL/min per 1.73 m2) | 110.8±40.7 | 109.9±40.3 | 113.8±42.1 | 0.498 |
| Initial NT-proBNP (pg/mL) | 293.6±293.9 | 291.1±295.6 | 296.0±292.3 | 0.907 |
| 24 hour NT-proBNP (pg/mL) | 783.9±962.7 | 322.7±320.1 | 1245.1±1605.3 | <0.001 |
| Uric acid (mg/dL) | 5.1±1.1 | 5.0±1.1 | 5.6±1.2 | <0.001 |
| Hemoglobin (g/dL) | 14.5±1.6 | 14.4±1.6 | 14.9±1.7 | 0.033 |
| WBC (X1000/µL) | 13.2±4.4 | 13.1±4.3 | 13.6±4.5 | 0.397 |
| Neutrophil (X1000/µL) | 9.1±3.3 | 8.6±3.3 | 10.8±3.2 | <0.001 |
| Lymphocyte (X1000/µL) | 2.5±1.2 | 2.8±1.3 | 1.8±0.7 | <0.001 |
| Monocyte ( X1000/µL) | 0.8±0.4 | 0.9±0.4 | 0.8±0.4 | 0.285 |
| MLR | 0.43±0.25 | 0.37±0.23 | 0.50±0.27 | <0.001 |
| MNR | 14.8±9.2 | 11.3±7.2 | 18.6±11.3 | 0.001 |
| NLR | 5.1±2.4 | 3.6±1.9 | 6.7±3.0 | <0.001 |
| Echocardiography | ||||
| LVESV (mL) | 62.6±19.9 | 62.8±15.8 | 68.5±24.1 | 0.028 |
| Control LVESV (mL) | 72.1±28.4 | 53.9±18.6 | 90.3±38.2 | <0.001 |
| LVEDV (mL) | 113.9±23.4 | 113.9±20.8 | 114.0±26.0 | 0.993 |
| Control LVEDV (mL) | 128.1±30 | 106.8±21.9 | 149.5±38.1 | <0.001 |
| EF (%) | 42.4±6.8 | 44.4±6.5 | 40.4±7.9 | <0.001 |
| Control EF (%) | 44.5±9.6 | 50.2±8.9 | 38.9±10.3 | <0.001 |
| Wall motion score index | 1.4±0.25 | 1.3±0.2 | 1.5±0.3 | <0.001 |
| Previous medications | ||||
| ACEI | 82 (30) | 66 (31.9) | 16 (23.9) | 0.137 |
| ARB use | 11 (0.4) | 6 (2.9) | 5 (7.5) | 0.102 |
| Beta blocker | 20 (7.2) | 13 (6.3) | 7 (10.4) | 0.189 |
| Calcium canal blocker | 10 (3.6) | 7 (3.4) | 3 (4.5) | 0.459 |
| Statin | 34 (12) | 28 (13.5) | 6 (9) | 0.223 |
| OAD use | 63 (23) | 40 (19.3) | 23 (34.3) | 0.010 |
| PPAR gamma agonist | 8 (2.9) | 5 (2.4) | 3 (4.5) | 0.307 |
| Others | 55 (20) | 35 (16.9) | 20 (29.9) | 0.019 |
Data are expressed as mean±standard deviation or n (%). BMI: body mass index, SBP: systolic blood pressure, DBP: diastolic blood pressure, HDL-C: high density lipoprotein cholesterol, LDL-C: low density lipoprotein cholesterol, CK-MB: creatine kinase-MB, eGFR: estimated glemerular filtration rate, NT-proBNP: N-terminal pro-brain natriuretic peptide, WBC: white blood cell, MLR: monocyte to lymphocyte ratio, MNR: monocyte to neutrophil ratio, NLR: neutrophil to lymphocyte ratio, LVESV: left ventricular end systolic volume, LVEDV: left ventricular end diastolic volume, EF: ejection fraction, ACEI: angiotensin converting enzyme inhibitor, ARB: angiotensin receptor blocker, OAD: oral anti diabetic, PPAR: peroxisome proliferator-activated receptor
Procedural characteristics such as door-balloon time, infarction time and SYNTAX score were similar between the groups (p>0.05, for all). A comparison of the procedural characteristics is shown in Table 2.
Table 2. Comparison of procedural characteristics between the groups.
| Procedural characteristics | Total patient group | Non-remodeling group (n=207) |
Remodeling group (n=67) |
p |
|---|---|---|---|---|
| Infarction time (h) | 4.8±3.8 | 4.8±4.0 | 4.9±3.7 | 0.863 |
| Door-balloon time (min) | 26±7.7 | 26.8±7.1 | 25.2±8.4 | 0.121 |
| Initial SYNTAX score | 18.2±6.2 | 17.6±5.9 | 18.8±6.5 | 0.162 |
| Final SYNTAX score | 12.6±5.15 | 12.3±5.2 | 12.9±5.1 | 0.362 |
| Left main disease | 10 (3) | 7 (3.4) | 3 (4.5) | 0.459 |
| Total stent length (mm) | 21.2±5.5 | 21.2±5.4 | 21.3±5.7 | 0.972 |
| Mean stent count (n) | 1.3±0.55 | 1.3±0.6 | 1.4±0.5 | 0.797 |
| Drug eluting stent | 209 (76.2) | 160 (77.3) | 49 (73.1) | 0.294 |
| Bifurcation intervention | 9 (3) | 8 (3.9) | 1 (1.5) | 0.309 |
| Thrombectomy | 32 (11.6) | 23 (11.1) | 9 (13.4) | 0.374 |
| Glycoprotein IIb/IIIa inhibitors | 85 (31) | 60 (29) | 25 (37.3) | 0.130 |
| Initial TIMI flow grade 0 or 1 | 192 (70) | 142 (68.6) | 50 (74.6) | 0.347 |
Data are expressed as mean±standard deviation or n (%). TIMI: thrombolysis in myocardial infarction
Predictors of ventricular remodeling
On multivariate logistic regression analysis, variables were included in the regression model to show the independent predictors of VR. Thereby, it was shown that NLR (β=1.725, 95% confidence interval [CI]=1.431--2.079, p<0.001), peak CK-MB (β=1.004, 95% CI=1.000-1.008, p=0.033) NT-proBNP (β=1.003, 95% CI=1.002-1.004, p<0.001), diabetes (β=2.661, 95% CI=1.114-6.357, p=0.028) and initial WMSI (β=0.965, 95% CI=0.944-0.986, p=0.001) as well as other echocardiographic parameters such as left ventricle end systolic volume (β=0.970, 95% CI=0.945-0.995, p=0.021) were independently associated with VR development (Table 3).
Table 3. Predictors of left ventricular remodeling.
| Variables | p* | Odds ratio | 95% CI (lower-upper) | p |
|---|---|---|---|---|
| Family history | 0.130 | - | - | - |
| Diabetes | 0.001 | 2.661 | 1.114-6.357 | 0.028 |
| Peak CK-MB | <0.001 | 1.004 | 1.000-1.008 | 0.033 |
| 24 h NT-proBNP | <0.001 | 1.003 | 1.002-1.004 | <0.001 |
| NLR | <0.001 | 1.725 | 1.431-2.079 | <0.001 |
| Wall motion score index | <0.001 | 0.965 | 0.944-0.986 | 0.001 |
| EF (%) | <0.001 | 0.978 | 0.864-1.108 | 0.730 |
| LVSV (mL) | <0.001 | 0.970 | 0.945-0.995 | 0.021 |
*Univariate logistic regression analysis. CK-MB: creatine kinase-MB, NT-proBNP: N-terminal pro-brain natriuretic peptide, NLR: neutrophil to lymphocyte ratio, EF: ejection fraction, LVSV: left ventricle systolic volume
Receiver operator characteristic curve analysis
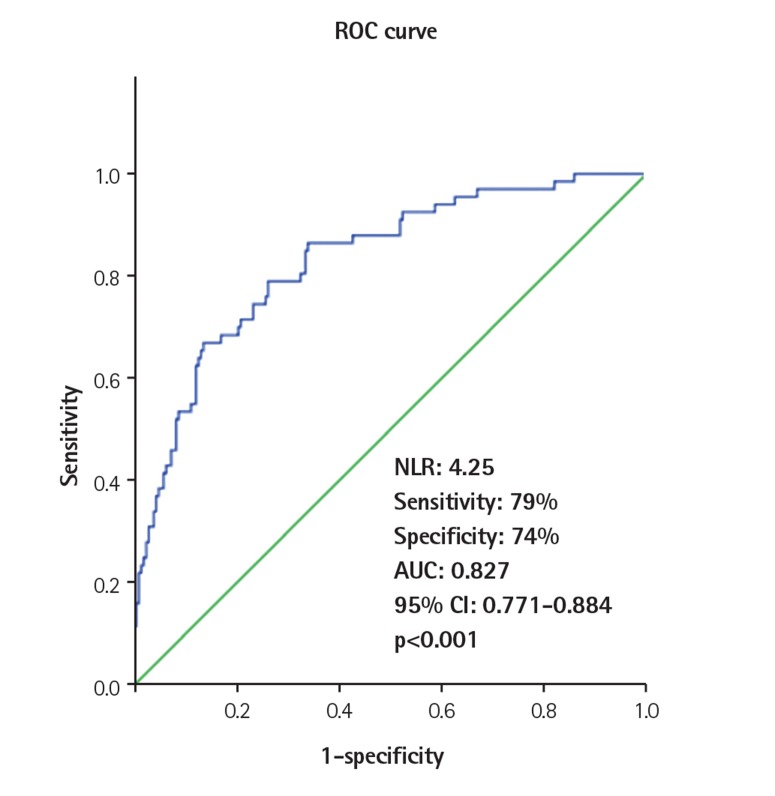
The NLR cutoff value obtained by the ROC curve analysis was 4.25 for predicting VR development (sensitivity: 79%, specificity: 74%). The area under the curve was 0.827 (95% CI: 0.771-0.884, p<0.001). The ROC curve analysis of NLR for predicting the presence of VR is shown in Fig. 1.
Fig. 1. The receiver operator characteristic (ROC) curve analysis of the neutrophil to lymphocyte ratio for predicting the presence of ventricular remodeling. NLR: neutrophil to lymphocyte ratio, AUC: area under the curve.
Discussion
To the best of our knowledge, our study is the first in the literature to evaluate the relationship between NLR and VR in patients with STEMI. In the present study, the major finding is that in patients with STEMI who underwent primary PCI, the NLR that was measured on admission was associated with VR. The present study also showed that NT-proBNP, WMSI, presence of no-reflow, peak CK-MB level and diabetes were associated with VR development in our study population.
It is well-known that inflammation plays a critical role in the development of VR following STEMI.5) Myocardial infarction leads to activation of an inflammatory response, which is the main component of healing and scar formation.13),14) As the duration of myocardial ischemia increases, it results in infarction and thus inflammatory response. If the reperfusion of ischemic tissue is warranted, acceleration and augmentation of the inflammatory process are triggered.13) Although the medical treatment has advanced over the past few decades, left VR after AMI remains one of the main clinical issues. In a number of previous studies, it has been demonstrated that long-term VR was related with increased risk of cardiovascular mortality and heart failure.1),15)
In the present study, we demonstrated that NLR, one of the useful inflammatory biomarkers in clinical practice, was independently associated with VR. Numerous previous studies have evaluated the association of total and differential WBC count with the presence and prognosis of CAD.16) WBC, neutrophil count, and NLR were reported as independent prognostic predictors in AMI. It was also shown that NLR was a more important parameter than total WBC with regard to the prognosis of CAD.9),16) Recently, Sahin et al.17) reported that NLR was associated with the extent and complexity of CAD in patients with STEMI. Similarly, Han et al.18) demonstrated that NLR predicts long-term clinical outcomes in patients with acute myocardial infarction, which might be explained by the contribution of VR. Moreover, NLR is associated with impaired myocardial perfusion as well as severity of CAD in patients with stable CAD.19) Therefore, the relationship between NLR and VR is not surprising. The development process of VR following AMI may be divided into 3 partially overlapping phases:7) (1) inflammatory; (2) proliferative; and (3) maturation. The inflammatory phase is triggered by cytokines and leads to the recruitment of leukocytes. The inflammasome, a macromolecular structure that activates caspase-1 and the conversion of pro-interleukin-1b to mature interleukin-1b, is influenced by cell debris.20) As the inflammasome formation and activation occur, tissue injury and local and systemic inflammatory response advance.20) Leukocytes remove necrotic cells as they release cytokines and growth factors. During acute coronary syndromes, neutrophils are the major contributors to adaptive infarct healing, leukocyte platelet aggregate formation and reperfusion injury.21) It has been reported that neutrophil invasion ultimately leads to fibrotic scar formation. Neutrophils play a major role in both promoting fibrosis and inducing myocardial ischemia/infarction by assisting in plaque disruption and by plugging microvessels by coaggregating with platelets.22) It has been shown that neutrophil-mediated microvascular obstruction is not the only potential role of neutrophils; they may also directly injure parenchymal cells by releasing specific toxic products.23) Previous evidence suggests that activated neutrophils in the ischemic and reperfused areas may release proteolytic enzymes or reactive oxygen species to injure surrounding myocytes.23) Adherent neutrophils exclusively secrete nearly all of these toxic products.23) Thus, it was demonstrated that a ligand-specific adhesion of the neutrophils to the cardiac myocytes might play a pivotal role in mediating ischemia-induced myocyte injury.24) During the atherosclerotic process, lymphocytes are one of the key elements in modulating the inflammatory response, and comparative lymphopenia in AMI is accepted as a stress response mediated by augmented endogenous cortisol.25) Compared with the non-VR group, patients in the VR group had increased neutrophil and decreased lymphocyte counts, and therefore, we may speculate that increased NLR suggests that inflammation plays an important role in the VR process.
NT-pro BNP, a cardiac neurohormone, is released in response to increased left ventricular wall stretch.26) Moreover, myocardial ischemia and infarction stimulate NT-proBNP excretion.27) The present study showed that NT-pro BNP measured 24 hours after the AMI was a strong predictor of VR at 6-month follow-up in patients with STEMI, but no similar relationship was observed between VR and BNP values measured recently after AMI. Very limited data on brain natriuretic peptide (BNP) and NT-pro BNP and their association with the VR are available. Cochet et al.28) reported correlations between NT-pro BNP concentrations on day 3 after AMI and left ventricle ejection fraction as well as infarct size derived from cardiac magnetic resonance (CMR) images. In a previous study by Haeck et al.29) a relationship was found between initial NT-proBNP in presenters less than 6 hours from onset of symptoms and CMR infarct size 4-6 months after AMI. The authors demonstrated that in patients with non-anterior wall STEMI who underwent primary PCI, initial NT-pro BNP level was a strong independent predictor of left ventricular function assessed by CMR imaging at follow-up.29) In contrast to these studies, Kleczynski et al.30) reported that baseline (admission) NT-pro BNP values (<3 hours) did not correlate with infarct size or left ventricular systolic function at 6-month follow-up in patients with STEMI. In fact, the results of that study are consistent with our results. Similar to our study, Kleczynski et al.30) found that VR was not related to BNP measured at patients' first admission after recent AMI. Moreover, the authors of this study speculate that the absence of a relationship between baseline NT-pro BNP and left ventricular function as well as infarct size after 6 months might be the consequence of a contribution of the ischemic stimulus-resolved later after angioplasty-to early NT-pro BNP levels, which might have influenced the correlation between NT-pro BNP and LV hemodynamics.30)
A number of limitations of the present study should be mentioned. We only evaluated NLR as an inflammation marker. The lack of classical inflammatory markers such as high sensitive C-reactive protein, interleukin-6 or eotaxin analysis and/or correlation makes it difficult to draw any consistent conclusion regarding the possibility of NLR to predict cardiovascular events.
In conclusion, measuring initial NLR and 24 hour NT-proBNP levels may be useful for predicting adverse cardiovascular events including left ventricular remodeling in patients with anterior STEMI who were treated with primary PCI. Inflammation has a pivotal role in ventricular remodeling after myocardial infarction, and high NLRs are associated with this undesirable consequence. Predicting adverse cardiovascular event risk such as left ventricular remodeling using NLR after acute MI and applying treatment strategies aimed at modulating inflammation during MI may prevent impaired ventricular tissue and VR and reduce the incidence of heart failure.
Acknowledgment
We thank the fellows of cardiology and the nurses of Adana Numune Training and Research Hospital, Department of Cardiology for their contribution to this study.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Parikh NI, Gona P, Larson MG, et al. Long-term trends in myocardial infarction incidence and case fatality in the National Heart, Lung, and Blood Institute's Framingham Heart study. Circulation. 2009;119:1203–1210. doi: 10.1161/CIRCULATIONAHA.108.825364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoon HJ, Jeong MH, Jeong Y, et al. Progressive dilation of the left atrium and ventricle after acute myocardial infarction is associated with high mortality. Korean Circ J. 2013;43:731–738. doi: 10.4070/kcj.2013.43.11.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olivetti G, Abbi R, Quaini F, et al. Apoptosis in the failing human heart. N Engl J Med. 1997;336:1131–1141. doi: 10.1056/NEJM199704173361603. [DOI] [PubMed] [Google Scholar]
- 4.Heusch G, Libby P, Gersh B, et al. Cardiovascular remodelling in coronary artery disease and heart failure. Lancet. 2014;383:1933–1943. doi: 10.1016/S0140-6736(14)60107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seropian IM, Toldo S, Van Tassell BW, Abbate A. Anti-inflammatory strategies for ventricular remodeling following ST-segment elevation acute myocardial infarction. J Am Coll Cardiol. 2014;63:1593–1603. doi: 10.1016/j.jacc.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 6.Nahrendorf M, Swirski FK. Monocyte and macrophage heterogeneity in the heart. Circ Res. 2013;112:1624–1633. doi: 10.1161/CIRCRESAHA.113.300890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frangogiannis NG. The immune system and the remodeling infarcted heart: cell biological insights and therapeutic opportunities. J Cardiovasc Pharmacol. 2014;63:185–195. doi: 10.1097/FJC.0000000000000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling--concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000;35:569–582. doi: 10.1016/s0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 9.Horne BD, Anderson JL, John JM, et al. Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol. 2005;45:1638–1643. doi: 10.1016/j.jacc.2005.02.054. [DOI] [PubMed] [Google Scholar]
- 10.Lax A, Sanchez-Mas J, Asensio-Lopez MC, et al. Mineralocorticoid receptor antagonists modulate galectin-3 and interleukin-33/ST2 signaling in left ventricular systolic dysfunction after acute myocardial infarction. JACC Heart Fail. 2015;3:50–58. doi: 10.1016/j.jchf.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 11.Sianos G, Morel MA, Kappetein AP, et al. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. 2005;1:219–227. [PubMed] [Google Scholar]
- 12.Bolognese L, Neskovic AN, Parodi G, et al. Left ventricular remodeling after primary coronary angioplasty: patterns of left ventricular dilation and long-term prognostic implications. Circulation. 2002;106:2351–2357. doi: 10.1161/01.cir.0000036014.90197.fa. [DOI] [PubMed] [Google Scholar]
- 13.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53:31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 14.Frangogiannis NG, Youker KA, Rossen RD, et al. Cytokines and the microcirculation in ischemia and reperfusion. J Mol Cell Cardiol. 1998;30:2567–2576. doi: 10.1006/jmcc.1998.0829. [DOI] [PubMed] [Google Scholar]
- 15.Gajarsa JJ, Kloner RA. Left ventricular remodeling in the post-infarction heart: a review of cellular, molecular mechanisms, and therapeutic modalities. Heart Fail Rev. 2011;16:13–21. doi: 10.1007/s10741-010-9181-7. [DOI] [PubMed] [Google Scholar]
- 16.Tamhane UU, Aneja S, Montgomery D, Rogers EK, Eagle KA, Gurm HS. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am J Cardiol. 2008;102:653–657. doi: 10.1016/j.amjcard.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Sahin DY, Elbasan Z, Gür M, et al. Neutrophil to lymphocyte ratio is associated with the severity of coronary artery disease in patients with ST-segment elevation myocardial infarction. Angiology. 2013;64:423–429. doi: 10.1177/0003319712453305. [DOI] [PubMed] [Google Scholar]
- 18.Han YC, Yang TH, Kim DI, et al. Neutrophil to lymphocyte ratio predicts long-term clinical outcomes in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Korean Circ J. 2013;43:93–99. doi: 10.4070/kcj.2013.43.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanindi A, Erkan AF, Ekici B, Alhan A, Töre HF. Neutrophil to lymphocyte ratio is associated with more extensive, severe and complex coronary artery disease and impaired myocardial perfusion. Turk Kardiyol Dern Ars. 2014;42:125–130. doi: 10.5543/tkda.2014.18949. [DOI] [PubMed] [Google Scholar]
- 20.Mezzaroma E, Toldo S, Farkas D, et al. The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc Natl Acad Sci U S A. 2011;108:19725–19730. doi: 10.1073/pnas.1108586108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maxwell SR, Lip GY. Reperfusion injury: a review of the pathophysiology, clinical manifestations and therapeutic options. Int J Cardiol. 1997;58:95–117. doi: 10.1016/s0167-5273(96)02854-9. [DOI] [PubMed] [Google Scholar]
- 22.Ott I, Neumann FJ, Gawaz M, Schmitt M, Schömig A. Increased neutrophil-platelet adhesion in patients with unstable angina. Circulation. 1996;94:1239–1246. doi: 10.1161/01.cir.94.6.1239. [DOI] [PubMed] [Google Scholar]
- 23.Jaeschke H, Smith CW. Mechanisms of neutrophil-induced parenchymal cell injury. J Leukoc Biol. 1997;61:647–653. doi: 10.1002/jlb.61.6.647. [DOI] [PubMed] [Google Scholar]
- 24.Hermann HP, Zeitz O, Lehnart SE, et al. Potentiation of beta-adrenergic inotropic response by pyruvate in failing human myocardium. Cardiovasc Res. 2002;53:116–123. doi: 10.1016/s0008-6363(01)00437-0. [DOI] [PubMed] [Google Scholar]
- 25.Ducloux D, Challier B, Saas P, Tiberghien P, Chalopin JM. CD4 cell lymphopenia and atherosclerosis in renal transplant recipients. J Am Soc Nephrol. 2003;14:767–772. doi: 10.1097/01.asn.0000048718.43419.44. [DOI] [PubMed] [Google Scholar]
- 26.de Lemos JA, McGuire DK, Drazner MH. B-type natriuretic peptide in cardiovascular disease. Lancet. 2003;362:316–322. doi: 10.1016/S0140-6736(03)13976-1. [DOI] [PubMed] [Google Scholar]
- 27.Staub D, Nusbaumer C, Zellweger MJ, et al. Use of B-type natriuretic peptide in the detection of myocardial ischemia. Am Heart J. 2006;151:1223–1230. doi: 10.1016/j.ahj.2005.06.045. [DOI] [PubMed] [Google Scholar]
- 28.Cochet A, Zeller M, Cottin Y, et al. The extent of myocardial damage assessed by contrast-enhanced MRI is a major determinant of N-BNP concentration after myocardial infarction. Eur J Heart Fail. 2004;6:555–560. doi: 10.1016/j.ejheart.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 29.Haeck JD, Verouden NJ, Kuijt WJ, et al. Comparison of usefulness of N-terminal pro-brain natriuretic peptide as an independent predictor of cardiac function among admission cardiac serum biomarkers in patients with anterior wall versus nonanterior wall ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am J Cardiol. 2010;105:1065–1069. doi: 10.1016/j.amjcard.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 30.Kleczyński P, Legutko J, Rakowski T, et al. Predictive utility of NT-pro BNP for infarct size and left ventricle function after acute myocardial infarction in long-term follow-up. Dis Markers. 2013;34:199–204. doi: 10.3233/DMA-120955. [DOI] [PMC free article] [PubMed] [Google Scholar]