Abstract
Although evidence shows depressed moods enhance risk for somatic diseases, molecular mechanisms underlying enhanced somatic susceptibility are ill-defined. Knowledge of these molecular mechanisms will inform development of treatment and prevention strategies across comorbid depressive and somatic illnesses. Existing evidence suggests that interleukin-18 (IL-18; an IL-1 family cytokine) is elevated in depression and implicated in pathophysiology underlying comorbid medical illnesses. We previously identified strong associations between baseline IL-18 and μ-opioid receptor availability in major depressive disorder (MDD) volunteers. Combined with the evidence in animal models, we hypothesized that experimental mood induction would change IL-18, the extent proportional to opioid neurotransmitter release. Using the Velten technique in a [11C]carfentanil positron emission tomography neuroimaging study, we examined the impact of experimentally induced mood (sad, neutral) on plasma IL-18 and relationships with concurrent changes in the central opioid neurotransmission in 28 volunteers (healthy, MDD). Results showed mood induction impacted IL-18 (F2,25=12.2, P<0.001), sadness increasing IL-18 (T27=2.6, P=0.01) and neutral mood reducing IL-18 (T27=−4.1, P<0.001). In depressed volunteers, changes in IL-18 were more pronounced (F2,25=3.6, P=0.03) and linearly proportional to sadness-induced μ-opioid activation (left ventral pallidum, bilateral anterior cingulate cortices, right hypothalamus and bilateral amygdala). These data demonstrate that dynamic changes of a pro-inflammatory IL-1 superfamily cytokine, IL-18, and its relationship to μ-opioid neurotransmission in response to experimentally induced sadness. Further testing is warranted to delineate the role of neuroimmune interactions involving IL-18 in enhancing susceptibility to medical illness (that is, diabetes, heart disease and persistent pain states) in depressed individuals.
Introduction
Evidence shows that depression enhances both susceptibility to and mortality from specific somatic illness.1, 2 Increased concentration of plasma interleukin-18 (IL-18), an indicator of peripheral immune activation, has been identified both in major depressive disorder (MDD)3, 4, 5 and in somatic illness highly comorbid with depression (that is, cardiovascular illness).6, 7, 8 More specifically, in coronary artery disease, elevated plasma IL-18 has been associated with plaque progression9 and increased risk of clinical events.10 Despite common associations between MDD, elevated IL-18, and somatic illness associated with both MDD and elevated IL-18, mechanisms underlying potential immune activating effects of depressed mood are ill-defined. Emerging evidence in depressed participants shows significant correlations between plasma IL-18 and central μ-opioid receptor (μ-OR) availability,5 a measure of endogenous opioid neurotransmitter activity. This neurotransmitter system was previously shown dysregulated in MDD.11 However, whether similar neuroimmune interactions mediate the impact of depression on somatic illness remains unclear. Identifying mechanisms mediating the impact of mood on peripheral immune activation is a critical step in understanding the shared pathophysiology in co-occurring somatic diseases. This knowledge is essential to the subsequent development of novel, personalized immune-based treatment strategies in somatic comorbidities.
IL-18, initially named as interferon-γ-inducing factor, is an IL-1 family cytokine structurally homologous to IL-1β and functionally upstream of IL-1β.12 In animal models, IL-18 is produced, activated and secreted by monocyte-derived immune cells (dendritic cells,13, 14 glial cells15, 16), adrenocortical cells,17, 18 cells in the medial habenula with projections to the interpeduncular nucleus19 and potentially via corticotrophs in the anterior pituitary.20 IL-18 is an important regulator of peripheral immune functions, inducing synthesis of a variety of inflammatory factors (that is, tumor necrosis factor-α, IL-1β, IL-6)21, 22 that are elevated in depressive illness.9, 23, 24 Via a process similar to that involved in activation and release of IL-1β, preformed pro-IL-1825 is activated and readily released as active IL-18 from various cells types. In short, binding of pathogen-associated molecular patterns and damage-associated molecular patterns to cell surface receptors activates intracellular inflammasomes, in turn activating cytoplasmic caspase-1, which then cleaves active IL-18 from its precursor, inactive form, pro-IL-18.26
Evidence to date suggests that stress-induced release of endogenous opioid neurotransmitters can induce activation and release of IL-1 family cytokines, including IL-18. Opioid–cytokine connections involving IL-1 cytokines have been well documented.27, 28 IL-1β has been shown to regulate proenkephalin and μ-OR mRNA expression in astrocyte-enriched cultures.29 IL-18, an IL-1 family cytokine, is a potent inducer of IL-1β. It is plausible that IL-18 could exert autocrine and/or paracrine feedback control over opioidergic neurotransmission and/or opioidergic regulation of immune functioning either directly or indirectly via IL-1β. Although evidence of IL-18's response to psychosocial stress in humans is lacking, several factors suggest that hypothalamic pituitary adrenal-axis (HPA-axis) activation will lead to similar stress-induced elevation in IL-18, as occurs with IL-1β. Activation and release of both IL-18 and IL-1β is achieved through similar mechanisms. IL-18 is a potent inducer of IL-1β, and IL-18 may be susceptible to similar acute stress-related induction as IL-1β.16, 30 In animal models, potent stress-induced elevation of IL-18 has been identified in the adrenal cortex.31 In humans, acute stress has also been shown to induce IL-1 family cytokines.32 A body of existing evidence suggests that regulation of stress-related cytokine induction may be mediated by activation of μ-ORs. Opiates immune-modulating effects33 have been shown to occur via the activation of μ-ORs on the membrane of brain microglia in animal models34 and on peripheral immune cells in vitro in humans.35 Preliminary evidence in humans identifies significant opioid IL-1 family cytokine interactions within the amygdala both in depressed individuals5 and in relation to a painful stressor in healthy controls (IL-1β).36 Taken together, these findings suggest that neuroimmune interactions between μ-ORs and peripheral IL-1 family cytokines, including IL-18, may underlie the impact of depressed mood on precipitation or exacerbation of co-occurring somatic disease. However, whether central neurotransmitter mechanisms involving μ-ORs mediate the impact of mood on peripheral IL-18 concentration has not been investigated, particularly in humans.
Here, we examine the impact of experimental sad mood (and neutral mood) induction on plasma IL-18 in association with cortico-limbic endogenous opioid release using a [11C]carfentanil positron emission tomography (PET) neuroimaging paradigm.5, 37 We hypothesized that mood induction will alter plasma IL-18 concentration (that is, induction of sad mood increasing IL-18, induction of neutral mood reducing IL-18). Furthermore, we anticipated the change in plasma IL-18 to be proportional to endogenous opioid release within brain regions component to circuitry underlying regulation of emotion and in specific regions shown to have both prominent HPA stress-regulatory and immune-regulatory properties (that is, amygdala). To our knowledge, this is the first in vivo molecular evidence of human inflammatory self-regulation with concurrent mechanistic evidence of central opioid neurotransmitter—peripheral inflammatory interactions. That the neuroimmune interactions identified are localized within brain regions component to cortico-limbic circuitry further underscores the impact of our findings.
Materials and methods
This study was approved by the Integrated Scientific and Ethical Review Board at the University of Michigan. Written informed consent was provided by all the study volunteers. Using a modified Velten technique5, 37, 38, 39 in a manner similar to that used in experimental mood inductions previously described,40, 41 both sad and neutral mood states were experimentally induced, 45 min each, in 28 female, right-handed volunteers (13 unmedicated MDD and 15 healthy control volunteers) in the follicular phase of menses during a [11C]carfentanil PET neuroimaging paradigm. All scans began at 1330 hours. Volunteers' age, educational level and ethnicity were similarly distributed across diagnoses. Diagnoses were confirmed using the Structured Clinical Interview for DSM-IV Axis I Disorders.42 Only healthy control volunteers and volunteers determined to have a diagnosis of MDD were included in the study. We were unable to obtain adequate volume of blood samples from one MDD subject and one healthy control subject in order to complete IL-18 ELISA assays, so these subjects were not included in the described analyses. Sad moods were induced by having study volunteers focus on the emotions they experienced during a prior event that was associated with profound sadness (that is, death of a loved one and so on). Neutral moods were induced by having study volunteers who avoid engaging in active thought processes other than acknowledging sensations experienced in the PET scanner. During the diagnostic interview, the interviewer and study volunteer decided upon a specific sad event. Mood inductions were rehearsed upon entry into the study. Volunteers were randomized and counterbalanced with respect to order of mood induction, resulting in half of volunteers inducing neutral mood first, sad mood second and the other half inducing sad mood first, neutral mood second. Positive and negative affect schedule (PANAS-X),43 a measure of internal affective state, was completed at baseline and following both sad and neutral mood induction, and scores were log10 normalized for statistical comparisons. Whole blood was sampled concurrently and centrifuged at 4750 r.p.m. for 15 min with plasma extracted into aliquots and stored at −80 °C. Following completion of PET scanning, volunteers were observed in the PET suite for ~30 min to 1 h for the presence of adverse events and/or worsening of depression. MDD volunteers received a prescription for fluoxetine 20 mg daily and were followed bi-weekly by the study psychiatrist in the local clinic over the course of 10 weeks. On each follow-up visit, volunteers completed objective mood measures and psychiatric screening for worsening depression.
Using plasma samples from three time points: 0 min (study baseline), 45 min and 90 min, we obtained concentrations of IL-18 (standard ELISA, R&D Systems, Minneapolis, MN, USA), adrenocorticotropic hormone (ACTH) and cortisol (Coat a Count kits, Diagnostic Products, Los Angeles, CA, USA). Only subject IDs and time of blood sampling (0 min, 45 min and 90 min) were included on individual sample tubes. Given that subjects were randomized and counterbalanced in the order of mood induction, our investigators were blinded with regards to particular mood state (that is, sad vs neutral) at the time of completion of ACTH, cortisol and IL-18 assays. Assay procedures for quantifying ACTH and cortisol can be found in previously published work.5, 11 In contrast to the various logistic difficulties in reliably quantifying plasma IL-1β, IL-18 is reliably quantified using standard ELISA techniques. Using manufacturer provided IL-18 ELISA kits (R&D Systems) IL-18 concentration was determined from plasma samples run in duplicate. The range of detectable IL-18 concentrations was 12.5 pg ml−1 (minimal detectable concentration) to 1000 pg ml−1 (highest standard concentration). Measures of IL-18, ACTH and cortisol were log10 normalized for statistical comparisons of data within SPSS (IBM, Chicago, IL, USA). Statistical testing for the impact of mood induction on these biological variables was completed using repeated-measures analysis of variance (ANOVA), with the biological variables (that is, IL-18, ACTH and cortisol) representing dependent variables of interest, time (or mood induced) as the repeated factor and diagnosis (that is, MDD, healthy control) as an independent factor of interest. Statistical significance for non-neuroimaging analyses was calculated using a statistical threshold that controls a type-I error rate at P=0.05.
Using PET and the μ-OR selective radiotracer, [11C]carfentanil, we obtained a measure of μ-OR availability (also referred to as non-displaceable binding potential, BPND) during a neutral mood. A measure of sadness-induced central endogenous opioid neurotransmitter release (also referred to as μ-OR activation) was derived from the extent of sadness-induced reduction in μ-OR BPND during the sad mood. Details of neuroimaging processing procedures are discussed in previously published work.5, 11, 37 Planned analyses on [11C]carfentanil PET neuroimaging data included two separate neuroimaging, covariate analyses. Analysis of covariance testing was within SPM8 (Statistical Parametric Mapping, Wellcome Trust, London, UK), examining the effect of affective state on the relationship between IL-18 (covariate) and both central μ-OR availability and central endogenous opioid neurotransmitter release on a voxel-by-voxel basis. Brain images were normalized to the MNI (Montreal Neurological Institute, Montreal, QC, Canada) template. Significant findings within neuroimaging analyses were detected using an uncorrected statistical threshold of P<0.001 for regions hypothesized a priori (rostral anterior cingulate, nucleus accumbens, ventral pallidum, amygdala, medial and posterior thalamus, hypothalamus, periaqueductal gray and raphe). Threshold for significance of findings within non-hypothesized regions was set at P<0.05 after correcting for focal extent to control for a type-1 error.44
Previous evidence identifies strong correlations between baseline plasma IL-18 concentration and central μ-OR BPND bilaterally within the amygdala of MDD volunteers. Using the mediation model within the PROCESS function in SPSS, we tested whether central opioid functioning (bilaterally within the amygdala) during sad-mood induction mediated and moderated the relationship between plasma IL-18 and the resulting affective state using the PANAS-X negative affect subscale.
Several sample size/power calculations were performed using preliminary data. Alternatives to the null hypotheses of (1) no main effects of diagnosis and (2) no effects of mood induction or interactions on the measurements were tested. In all cases studied, sample sizes of n=28 were sufficient for power >85% for main/group effects and within group sample size of n=13 was sufficient for power >80% for correlation effects. Effect sizes were estimated, using G*Power (version 3.1),45 from preliminary data showing mood-induction effect on neurotransmitter release should be an average of at least 15%, effect of diagnosis should range from 10 to 30%, and correlation effects >0.60 based on prior data in healthy controls undergoing a pain challenge. The within-subject variability of PET BPND data is conservatively estimated at CV=0.05–0.10, and between-subject variability at CV=0.07–0.15 based on preliminary data. These conclusions and estimates are supported experimentally by prior experience with these techniques (and radiotracer) in both depressed and healthy control volunteers.
Results
Impact of mood induction on affective state
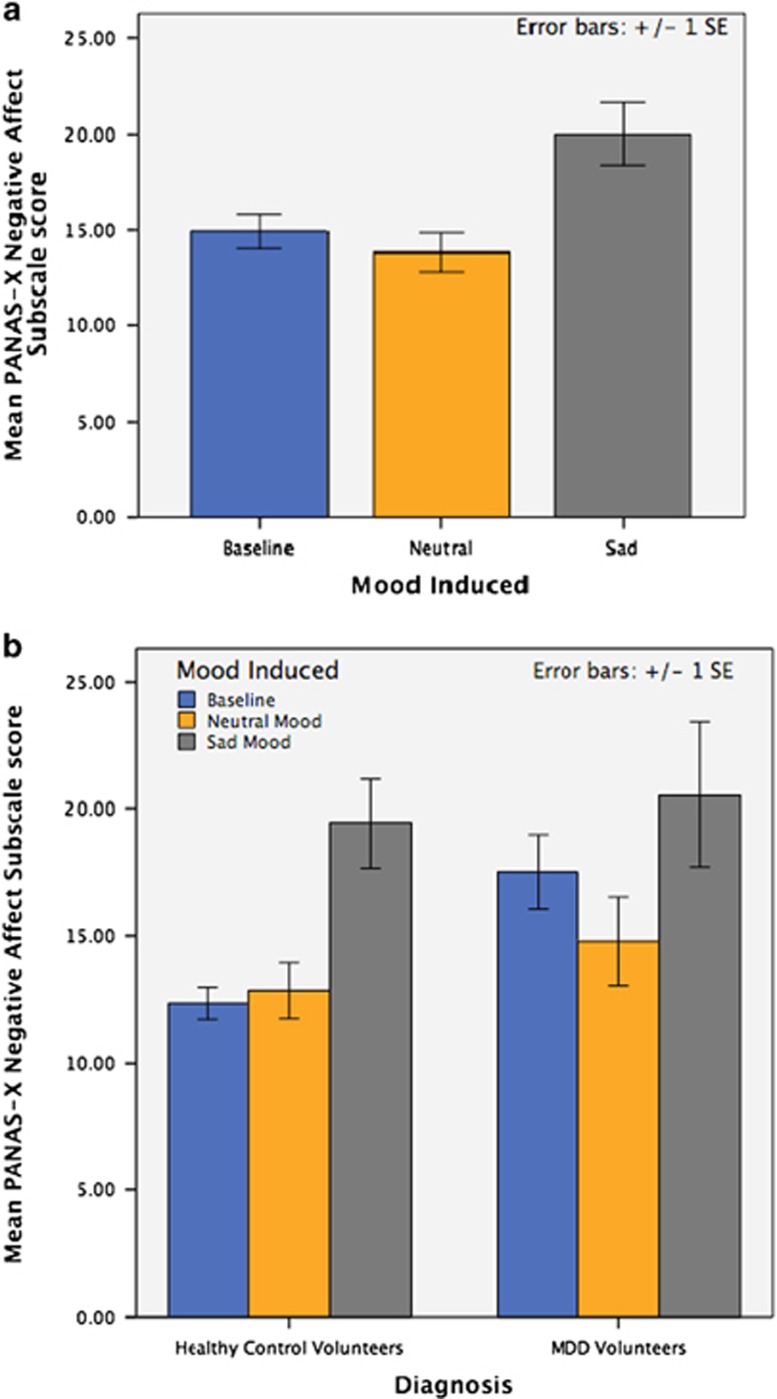
The effect of mood induction on PANAS-X negative affect subscale scores at 45 min intervals over the experimental duration (90 min) is depicted graphically (see Figure 1). Statistical testing with repeated-measures ANOVA identified the impact of mood induction (repeated factor) on negative affect subscale score (dependent variable). As depicted in Figure 1a, experimental mood induction had a significant effect on PANAS negative affect scores (F2,23=11.6, P<0.001), with non-significant reduction following neutral mood induction (mean reduction in negative affect score 0.54, P>0.05) and significant increases following induction of a sustained sadness state (mean increase in negative affect score 7.10, P<0.001). Figure 1b illustrates the impact of mood induction on negative affect subscale scores in two distinct clusters, one for healthy control volunteers and one for MDD volunteers.
Figure 1.
(a) Illustrates volunteers' positive and negative affect schedule (PANAS) negative affect subscale scores at each of three time points (baseline, after neutral induction and after sadness induction) across all study subjects. (b) Illustrates the effect of diagnosis (healthy control vs major depressive disorder (MDD)) on mood-induced changes in PANAS negative affect subscale scores, healthy controls clustered on the left and MDD volunteers clustered on the right. Order of neutral and sustained sadness states were randomized and counterbalanced.
Impact of mood induction on plasma IL-18
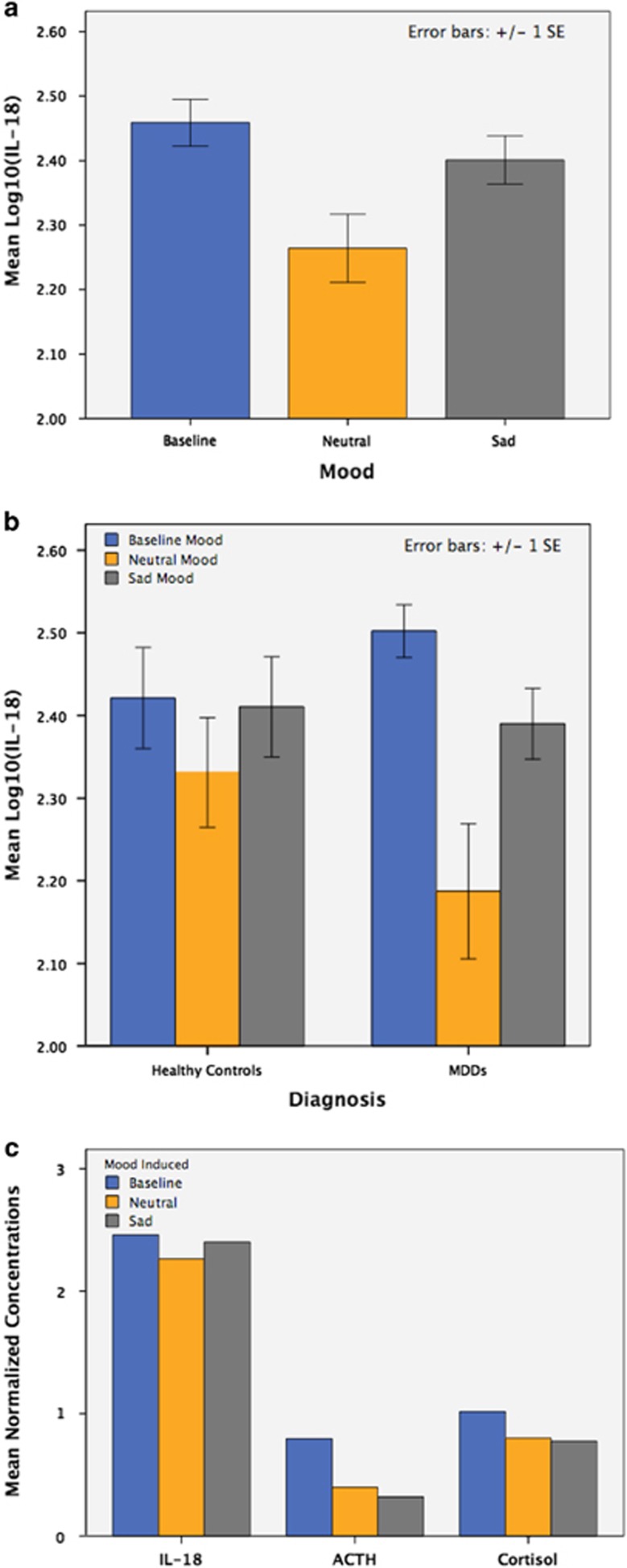
The effect of mood induction and diagnosis on plasma IL-18 was tested using repeated-measures ANOVA with mood induced (challenge condition) as the repeated factor, diagnosis as a factor of interest and IL-18 as the dependent variable. Experimental mood induction significantly regulated IL-18 (F2,52=12.2, P<0.001) with IL-18 reduction following neutral mood induction (T27=−4.1, P<0.001) and IL-18 increase following sad mood induction (T27=2.6, P=0.01; see Figure 2a). In addition, the effect of mood induction on IL-18 was more pronounced in MDD volunteers as compared with healthy control volunteers (F2,52=3.6, P=0.03). MDD volunteers had more substantial IL-18 reduction following neutral mood and greater IL-18 increase following sad mood induction as compared with healthy control volunteers (see Figure 2b).
Figure 2.
(a) Illustrates log10-normalized interleukin-18 (IL-18) concentrations at each of three time points (baseline, after neutral induction, and after sadness induction) across all study subjects. (b) Illustrates the effect of diagnosis on mood-induced changes in IL-18 concentration. A significant interaction between diagnosis and mood induction was observed, the effect of mood induction on IL-18 being greater in MDD volunteers as compared to healthy control volunteers. (c) Illustrates the effect of mood induction on IL-18, adrenocorticotropic hormone (ACTH) and cortisol across all volunteers. In contrast to mood-induced changes in IL-18 (reducing with neutral mood induction, increasing with sad mood induction), both ACTH and cortisol continue to decline over the duration of the study, regardless of whether neutral or sad mood was induced.
Impact of mood induction on ACTH and cortisol
Using a similar repeated-measures ANOVA model, but with both cortisol and ACTH as dependent variables, we showed that mood induction significantly reduced both cortisol and ACTH (F4,13=16.4, P<0.001). However, in contrast to the changes in IL-18 outlined in the above paragraph, changes in ACTH and cortisol were not dependent on type of mood induced with ACTH and cortisol reducing following both neutral and sad mood induction (P<0.001). Further, the mood-induced reduction of both ACTH and cortisol did not differ significantly between MDD and healthy control volunteers (P=0.65; see Figure 2c).
Impact of mood induction on plasma IL-18 when accounting for ACTH and cortisol
Using repeated-measures multivariate ANOVA model, we tested whether the mood induced (repeated factor) had a significant effect on IL-18 while accounting for the impact of mood induction on cortisol and ACTH (three dependent variables; IL-18, ACTH and cortisol). The combined effect of mood induction on the dependent variables (IL-18, ACTH and cortisol) was significant (F6,86=4.7, P<0.001). Mood induction had a significant effect on each dependent variable, including IL-18 (F2,36=8.1, P=0.002), ACTH (F1,22=4.1, P=0.05) and cortisol (F2,38=4.6, P=0.02). IL-18 reduced following neutral mood (F1,22=14.9, P=0.001) and increased following sad mood induction (F1,22=4.8, P=0.04). Neutral mood induction reduced both ACTH (F1,22=3.9, P=0.06; marginally significant) and cortisol (F1,22=6.8, P=0.02), but sad mood induction had no significant effect on either ACTH (P>0.1) or cortisol (P>0.1).
Impact of mood induction on the covariance relationships between IL-18 and central opioid neurotransmission
Using analysis of covariance analyses in SPM8, we tested whether mood-induced changes in IL-18 were proportional to concurrent mood-induced changes in limbic μ-OR measures. Results are outlined below. During neutral mood induction, limbic μ-OR availability, a measure of endogenous opioid tone, covaried with neutral mood-induced changes in plasma IL-18. Similarly, the increase in IL-18 during sad mood induction covaried with sadness-induced release of endogenous opioid neurotransmitter (μ-OR activation).
Neutral mood induction
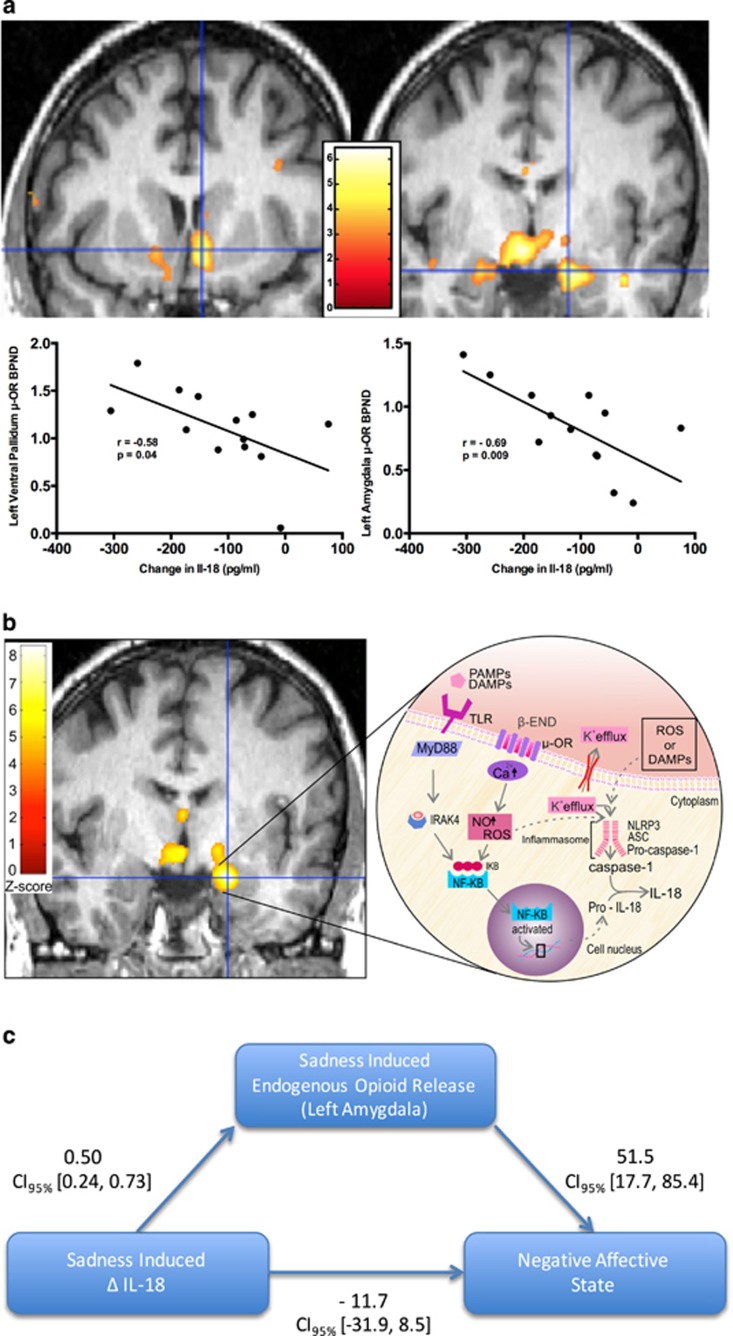
During neutral mood induction, effects were particularly well defined within MDD volunteers. In MDD volunteers, the extent that neutral mood induced a reduction in IL-18 during neutral mood induction was linearly proportional to the extent of μ-OR availability (that is, BPND) within the left ventral pallidum, bilateral anterior cingulate cortices, right hypothalamus, and bilateral amygdala in these depressed volunteers during neutral mood induction. To clarify, amongst the depressed volunteers, greater magnitude of reduction in IL-18 during neutral mood induction was associated with more substantial μ-OR availability (reflecting reduced endogenous opioid tone) within those various brain regions with inherent HPA-modulating properties. In contrast to the widespread effects seen in MDDs, within healthy control volunteers, the linearly proportional relationships between neutral mood-induced reduction in IL-18 and greater μ-OR availability (that is, reduced endogenous opioid tone) were constrained to the right ventral tegmental area (see Table 1, Figure 3a).
Table 1. Shown in the table are brain regions where endogenous opioid neurotransmitter responses to mood induction were associated with concurrent mood-induced changes in plasma IL-18 concentration.
| Brain region | MNI coordinates | Z-score |
|---|---|---|
| Neutral mood induction: MDDs | ||
| Left anterior cingulate cortex | 6, 31, 5 | 3.9 |
| Right anterior cingulate cortex | −11, 36, 11 | 3.9 |
| Left ventral pallidum | 10, 11, −14 | 4.3 |
| Right hypothalamus | −11, 2, −13 | 5.0 |
| Left amygdala | 17, 6, −22 | 5.2 |
| Right amygdala | −27, 1, −22 | 3.6 |
| Neutral mood induction: controls | ||
| Right ventral tegmental area | −4, −7, −8 | 3.8 |
| Sad mood induction: MDDs | ||
| Left medial thalamus | 2, −17, 4 | 5.1 |
| Right medial thalamus | −5, −11, 3 | 5.3 |
| Left ventral pallidum | 7, 14, −6 | 6.0 |
| Left hypothalamus | 17, −1, −10 | 6.7 |
| Right hypothalamus | −10, 0, −12 | 8.3 |
| Left ventral tegmental area | 17, −1, −10 | 6.7 |
| Right ventral tegmental area | −2, −4, −7 | 6.3 |
| Left amygdala | 19, −4, −18 | 8.3 |
| Right amygdala | −24, 0, −21 | 3.8 |
| Sad mood induction: controls | ||
| Right ventral tegmental area | −7, 4, −3 | 5.5 |
| Right hypothalamus | −5, 8, −13 | 4.3 |
| Left amygdala | 22, −3, −24 | 7.8 |
Abbreviations: IL-18, interleukin-18; MDD, major depressive disorder.
These data result from analyses within SPM8 (Wellcome Trust) and Matlab (Mathworks, Natick, MA, USA). Relationships during neutral mood induction are shown in the upper half of the table and relationships following sad mood induction are shown in the lower half of the table. Brain regional peaks of significance are listed with their associated stereotactic coordinates (MNI template) and Z-scores of statistical significance. Significance was set to P<0.001 and at least 10 contiguous voxels for regions with a priori hypotheses and was corrected for multiple comparisons for all other regions.
Figure 3.
Here, we present data depicting relationships between brain endogenous opioid functional measures and plasma IL-18 and the impact of mood induction on these relationships. Within the brain images in a and b, the colors (ranging from red to bright yellow/white) represent regional z-scores of statistical significance superimposed on a standardized brain image. Depicted next to the brain images is the z-scale, a color-coded representation of z-scores of statistical significance. On the scales depicted here, dark red represents z-score of 0 and bright yellow (or white) represents z-scores of 6 and 8 in a and b, respectively. For simplicity of presentation, we have chosen not to present statistical images from every region of significance. All regions are outlined in Table 1. The brain images presented in a illustrate correlations between μ-OR availability and reduction in IL-18 during neutral mood induction in MDDs. The greater the reduction in IL-18, the more substantial the μ-OR availability within the brain regions depicted and following neutral mood induction. In a we present findings within both the amygdala (given its stress and immune regulatory roles) and the ventral pallidum. The brain image presented in b illustrates covariance between sadness-induced endogenous opioid release (also referred to as μ-OR activation) and sadness-induced increase in IL-18 in MDDs. The greater the sadness-induced increase in IL-18, the more substantial the sadness-induced endogenous opioid release within the brain regions depicted (centered on the cross-hair in this image is the left amygdala). Also illustrated in b (in the inset of the figure) are potential biological mechanisms underlying the covariate relationships confirmed via statistical testing in SPM. Release of endogenous opioids can contribute to inflammasome activation and a cascade of events resulting in activation and cellular release of IL-18. (c) Illustrates the mediation model tested using the process function29 within SPSS (IBM, Chicago, IL, USA). IL-18, interleukin-18; MDD, major depressive disorder; μ-OR, μ-opioid receptor.
Sad mood induction
Sad mood induced an increase in plasma IL-18 that covaried with sadness-induced μ-opioid neurotransmitter release in a regionally specific manner in the brain. The regions where these effects occurred differed between MDDs and healthy controls. In MDDs, significant covariance effects were present in a functionally defined pattern bilaterally within the amygdala, ventral tegmental area, hypothalamus, left ventral pallidum and bilateral medial thalamus. More specifically, within these brain regions in MDDs, sadness induced an increase in IL-18 that was linearly proportional to the increase in endogenous opioid neurotransmitter release (that is, μ-OR activation; see Table 1, Figure 3b). In controls, a less functionally defined pattern of covariance effects (both linearly proportional and inversely proportional effects) was identified. In controls, sadness induced an increase in IL-18 that covaried with concurrent reduction in endogenous opioid neurotransmitter release (that is, reduced μ-OR activation) in the left amygdala and right hypothalamus. More specifically, in controls, the sadness-induced increase in IL-18 was inversely proportional to μ-OR activation within the left amygdala and right hypothalamus. However, linearly proportional effects were also present in the control group wherein the sadness-induced increase in IL-18 was linearly proportional to the sadness-induced endogenous opioid neurotransmitter release (that is, μ-OR activation) in the right ventral tegmentum (see Table 1).
Mediation analysis
Given the critical HPA and immune regulatory functions of the amygdala,46 we hypothesized that sadness-induced endogenous opioid neurotransmitter release (that is, μ-OR activation) mediated the relationship between the sadness-induced change in IL-18 and the negative affect score following sad mood induction. Using the process function47 within SPSS we tested this hypothesis. The amygdala endogenous opioid neurotransmitter release (that is, μ-OR activation) data were extracted and entered as the mediator variable in two separate simple mediation analyses. In the first analysis, extracted data from the left amygdala were entered as the mediator (Figure 3c). Results of our mediation analysis showed the model predicted the negative affect score (R2=0.67, F2,9=9.3, P=0.006). Although the indirect effect was significant (indirect effect=25.1, 5% confidence interval=13.5, 95% confidence interval=44.7), the direct effect was not significant (direct effect=−11.7, 5% confidence interval=−31.9, 95% confidence interval=8.5), representing full mediation through the indirect path (see Figure 3c). Similar analysis testing the mediating effects of μ-OR activation data extracted from the right amygdala was not significant (P>0.05).
Discussion
These data demonstrate dynamic changes of a pro-inflammatory IL-1 superfamily cytokine, IL-18, and its relationship to μ-opioid neurotransmission in response to experimentally induced sadness, providing preliminary evidence suggesting IL-18 may be mechanistically involved in transferring the somatically deleterious effects of negative affective states. These changes in IL-18 induced by a sustained sadness challenge and their relationship with central μ-opioid neurotransmission were particularly pronounced and present in a functionally defined pattern in MDD volunteers. Taken together, the findings we present provide further evidence in support of IL-18's involvement in MDD. Furthermore, given the abundance of evidence suggesting IL-18's involvement in medical illnesses highly comorbid with MDD (that is, diabetes, heart disease and persistent pain states), the data we present suggest IL-18 may mediate susceptibility to these specific medical comorbidities in depressed individuals.
The moods induced were sufficient to regulate plasma concentration of IL-18, an IL-1 family pro-inflammatory cytokine, in both healthy volunteers and patients diagnosed with MDD, albeit the overall effect of mood induction was more pronounced in MDDs. In addition, changes seen in plasma IL-18 concentration were independent of changes in both ACTH and cortisol, suggesting the presence of distinct mechanisms underlying the impact of mood induction on IL-18 as compared with those underlying the impact of mood induction on both ACTH and cortisol, the latter commonly referred to as products of body's main stress-regulatory system. The directionality of changes in IL-18 observed was dependent on the type of mood induced (that is, sad vs neutral) in direct contrast to the mood-induced change (that is, reduction) in both ACTH and cortisol, independent of type of mood induced. Furthermore, changes in plasma IL-18 concentration from neutral to sadness states were linearly proportional with the activity of endogenous opioid, μ-OR-mediated neurotransmission.
In the setting of lower baseline endogenous opioid tone (for example, higher μ-OR availability), IL-18 is reduced during the neutral mood. In contrast, the extent of sadness-induced endogenous opioid release within the amygdala was linearly proportional to sadness-induced IL-18 elevation. These findings suggest thta dysregulated endogenous opioid processing of emotionally salient events within the amygdala (previously identified in MDD)11 may contribute to disrupted homeostatic regulation of innate immune defenses (and vice versa). Proportional relationships between IL-1 family cytokines and post-synaptic endogenous opioid release have previously been identified in both the central nervous system and peripheral nervous system in animal models. The endogenous opioid neurotransmitter system has long been implicated in the maintenance of homeostasis during stressful challenges,48 and linked, within the amygdala, with pro-inflammatory mechanisms in both animal models49, 50 and humans,5, 36 as well as contributing critical neuroendocrine-immune regulatory roles (amygdala).46 Although the bulk of research evidence to date pertains to IL-1β (or it's antagonist, IL-1ra), given that IL-18 is activated by the same enzyme that activates IL-1β (and in many of the same cells) and that IL-18 is a key (and potent) inducer of IL-1β, it stands to reason that circulating concentrations of circulating IL-18 would also be proportional to post-synaptic endogenous opioid release, specifically within brain regions where IL-18 activity has been identified. It has previously been suggested that in the brain, pre-synaptic endogenous opioid release and binding of endogenous opioids to contiguous, post-synaptic μ-ORs may perform a compensatory role in depressed states in an attempt to dampen existing depressogenic neurotransmitter activity.
Overall, during neutral mood induction, we observed a reduction in IL-18 and the extent of that reduction was found to be linearly proportional to the extent of reduction in opioid release (as determined by increase in μ-OR BPND). Consistent with evidence in animal models, we have identified similar endogenous opioid—IL-1 family cytokine relationships. However, MDD volunteers were depressed at study entry and, compared with healthy control volunteers, had higher pre-mood induction IL-18 concentration. This finding may explain why reduction in IL-18 during neutral mood induction was substantially less in the healthy control group who had lower IL-18 concentration in the presence of a euthymic mood state at baseline. Concurrently, the depressed volunteers evidenced a proportional reduction in endogenous opioid tone, but with more widespread brain localization than occurred in healthy, non-depressed volunteers, particularly pronounced within brain regions previously identified to have substantial immune modulating (amygdala),46 stress regulating (hypothalamus) and pain regulating (thalamus) functions. That these opioid-cytokine interactions were more widespread in depressed volunteers as compared with the healthy control volunteers, underscores the greater need for compensatory opioid modulation of stress (both emotional and physical) during depression in the MDD group.
In contrast, sad mood induction results in a substantial increase in plasma IL-18 concentration, potentially in response to heightened levels of perceived emotional stress consequent to recollection of the prior sad event. The concurrent increase in endogenous opioid release can again be viewed as a compensatory mechanism, in an attempt to reduce the impact of recollecting the sad event. In both groups, the opioid-cytokine proportional relationships were identified within brain regions wherein endogenous opioids have previously been shown enhanced, potentially an attempt to dampen HPA activity that has increased consequent to recollecting the stressful prior sad event. As was the case during neutral mood induction, the significant opioid-cytokine covariance relationships we identified were more widespread in the MDD group, potentially reflecting a greater need for opioid induced modulation of depressogenic neurotransmitter activity during sadness in this group of depressed individuals.
Mediation analyses indicated that the associations between changes in IL-18 plasma levels and changes in affective state during sustained mood induction were mediated by activity of this neurotransmitter system (that is, sadness-induced endogenous opioid release) in the amygdala region. This is a brain region long implicated in vigilance and fear processing as well as in both identification and regulation of emotional salience and response, and where μ-ORs have been shown to have regulatory effects.11, 38, 51, 52 In Figure 3 (see inset in Figure 3b), we illustrate potential mechanisms whereby μ-OR may impact concentration of IL-1 family cytokines. These interactions can occur either acutely (via reactive oxygen species induction of caspase-1 and subsequent activation and release of IL-18), or sub-acutely/chronically via production of pro-IL-18 de novo. The opioid receptor—IL-1 family cytokine interactions outlined in the figure potentially occur in various cells throughout the body, including dendritic cells in the peripheral blood/tissues, or in the central nervous system (that is, microglia, anterior pituitary corticotrophs and so on). Given the known relationships between IL-18 and medical illness6, 7, 8, 10 the basic neuroimmune interactions we present may well represent pathways underlying risk of somatic comorbidity in those somatic illnesses both associated with elevated IL-18 and highly comorbid with MDD, further underscoring the broad ranging clinical implications of these findings. However, what remains unclear is whether mood-induced changes in IL-18 are mediated through neurohormonal stimulation of corticotrophs, adrenal cortical cells, peripheral monocytes or central glial cells remains unclear and of interest for future investigations.
Taken together, the evidence we present supports the need for further testing to delineate whether neuroimmune interactions involving IL-18 have a mediating role, enhancing susceptibility to certain medical comorbidities (that is, diabetes, heart disease and persistent pain states) in depressed individuals. The topics discussed in this manuscript are of considerable interest across many disciplines and have substantial clinical translational potential. Although the findings we present help to unravel the contribution of neuroimmune interactions potentially underlying the clinically relevant impact of mood states (and changes therein) on peripheral immune functioning, further investigation on a broader sample, potentially including individuals with a comorbid medical illness, will help to validate the relationships presented in this manuscript. In addition, although we noted that various potential confounders (age, ethnicity, body mass index and so on) were similarly distributed across diagnostic groups, our sample size, while more than adequate for a [11C]carfentanil PET neuroimaging study, limited our ability to fully control for all potential confounders. Future investigations on an expanded sample would enable secondary testing to rule out potential contributions of these confounders. In addition, although our sample size was adequate for examining between-group differences, power for examining within group correlation effects would likely improve (that is, above the current 81% power) with a larger sample size. Future studies that include larger sample sizes would help to validate the effects presented here before being generalized to the larger population.
Acknowledgments
Robert Dantzer, Melvin McInnis, Virginia Murphy-Weinberg, Tiffany Love, Heng Wang, Susan Kennedy, Jill Rothley, Edward McKenna, Andrew Weeden, Paul Kison and Shayna Huber provided assistance with a component of this work. This work was funded by K99/R00 DA 033454, Brain and Behavior Research Foundation NARSAD Young Investigators Award, and the University of Michigan Comprehensive Depression Center Rachel Upjohn Clinical Scholars Award (ARP); R01 DA 022520, R01 DA 027492 and the Phil F Jenkins Foundation (JKZ). Presented in part at the annual meeting of the American College of Neuropsychopharmacology (Hawaii, USA; ARP).
Footnotes
AEK has recently been employed by Eli Lilly companies and has consulted for Metastatix, Pennside Partners, Cypress Bioscience, Takeda Pharmaceuticals, NiCox SA, Celtaxsys, Gerson Lehrman Group of Healthcare and Biomedical Advisors, Guidepoint Global, UCB Pharmaceuticals and the Fund for Autoimmune Research and has been an expert for Kirkland and Ellis. AEK has received investigator-initiated research grants for Bristol-Myers Squibb, Roche, and Takeda Pharmaceuticals. The remaining authors declare no conflict of interest.
References
- 1Nicholson A, Kuper H, Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J 2006; 27: 2763–2774. [DOI] [PubMed] [Google Scholar]
- 2Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med 2004; 66: 802–813. [DOI] [PubMed] [Google Scholar]
- 3Bossu P, Salani F, Cacciari C, Picchetto L, Cao M, Bizzoni F et al. Disease outcome, alexithymia and depression are differently associated with serum IL-18 levels in acute stroke. Curr Neurovasc Res 2009; 6: 163–170. [DOI] [PubMed] [Google Scholar]
- 4Merendino RA, Di Rosa AE, Di Pasquale G, Minciullo PL, Mangraviti C, Costantino A et al. Interleukin-18 and CD30 serum levels in patients with moderate-severe depression. Mediators Inflamm 2002; 11: 265–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5Prossin AR, Koch AE, Campbell PL, McInnis MG, Zalcman SS, Zubieta JK. Association of plasma interleukin-18 levels with emotion regulation and mu-opioid neurotransmitter function in major depression and healthy volunteers. Biol Psychiatry 2011; 69: 808–812. [DOI] [PubMed] [Google Scholar]
- 6Blankenberg S, Tiret L, Bickel C, Peetz D, Cambien F, Meyer J et al. Interleukin-18 is a strong predictor of cardiovascular death in stable and unstable angina. Circulation 2002; 106: 24–30. [DOI] [PubMed] [Google Scholar]
- 7Dimsdale JE. Psychological stress and cardiovascular disease. J Am Coll Cardiol 2008; 51: 1237–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8Mallat Z, Corbaz A, Scoazec A, Graber P, Alouani S, Esposito B et al. Interleukin-18/interleukin-18 binding protein signaling modulates atherosclerotic lesion development and stability. Circulation Res 2001; 89: E41–E45. [DOI] [PubMed] [Google Scholar]
- 9Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 2008; 9: 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10Opstad TB, Pettersen AA, Arnesen H, Seljeflot I. The co-existence of the IL-18+183A/G and MMP-9 -1562 C/T polymorphisms is associated with clinical events in coronary artery disease patients. PLoS One 2013; 8: e74498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11Kennedy SE, Koeppe RA, Young EA, Zubieta JK. Dysregulation of endogenous opioid emotion regulation circuitry in major depression in women. Arch Gen Psychiatry 2006; 63: 1199–1208. [DOI] [PubMed] [Google Scholar]
- 12Dinarello CA. Interleukin-18. Methods 1999; 19: 121–132. [DOI] [PubMed] [Google Scholar]
- 13Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat Rev Immunol 2010; 10: 89–102. [DOI] [PubMed] [Google Scholar]
- 14Puren AJ, Fantuzzi G, Dinarello CA. Gene expression, synthesis, and secretion of interleukin 18 and interleukin 1beta are differentially regulated in human blood mononuclear cells and mouse spleen cells. Proc Natl Acad Sci USA 1999; 96: 2256–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15Prinz M, Hanisch UK. Murine microglial cells produce and respond to interleukin-18. J Neurochem 1999; 72: 2215–2218. [DOI] [PubMed] [Google Scholar]
- 16Conti B, Park LC, Calingasan NY, Kim Y, Kim H, Bae Y et al. Cultures of astrocytes and microglia express interleukin 18. Brain Res Mol Brain Res 1999; 67: 46–52. [DOI] [PubMed] [Google Scholar]
- 17Conti B, Sugama S, Kim Y, Tinti C, Kim H, Baker H et al. Modulation of IL-18 production in the adrenal cortex following acute ACTH or chronic corticosterone treatment. Neuroimmunomodulation 2000; 8: 1–7. [DOI] [PubMed] [Google Scholar]
- 18Conti B, Jahng JW, Tinti C, Son JH, Joh TH. Induction of interferon-gamma inducing factor in the adrenal cortex. J Biol Chem 1997; 272: 2035–2037. [DOI] [PubMed] [Google Scholar]
- 19Sugama S, Cho BP, Baker H, Joh TH, Lucero J, Conti B. Neurons of the superior nucleus of the medial habenula and ependymal cells express IL-18 in rat CNS. Brain Res 2002; 958: 1–9. [DOI] [PubMed] [Google Scholar]
- 20Wang N, Sugama S, Conti B, Teramoto A, Shibasaki T. Interleukin-18 mRNA expression in the rat pituitary gland. J Neuroimmunol 2006; 173: 117–125. [DOI] [PubMed] [Google Scholar]
- 21Dinarello CA. IL-18: A TH1-inducing, proinflammatory cytokine and new member of the IL-1 family. J Allergy Clin Immunol 1999; 103: 11–24. [DOI] [PubMed] [Google Scholar]
- 22Reichwald K, Jorgensen TZ, Tougaard P, Skov S. TL1A induces TCR independent IL-6 and TNF-alpha production and growth of PLZF(+) leukocytes. PLoS One 2014; 9: e85793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol 2008; 26: 971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24Maes M, Anderson G, Kubera M, Berk M. Targeting classical IL-6 signalling or IL-6 trans-signalling in depression? Expert Opin Ther Targets 2014; 18: 495–512. [DOI] [PubMed] [Google Scholar]
- 25Kastenmuller W, Torabi-Parizi P, Subramanian N, Lammermann T, Germain RN. A spatially-organized multicellular innate immune response in lymph nodes limits systemic pathogen spread. Cell 2012; 150: 1235–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26van de Veerdonk FL, Netea MG, Dinarello CA, Joosten LA. Inflammasome activation and IL-1beta and IL-18 processing during infection. Trends Immunol 2011; 32: 110–116. [DOI] [PubMed] [Google Scholar]
- 27Peterson PK, Molitor TW, Chao CC. The opioid-cytokine connection. J Neuroimmunol 1998; 83: 63–69. [DOI] [PubMed] [Google Scholar]
- 28Rogers TJ, Peterson PK. Opioid G protein-coupled receptors: signals at the crossroads of inflammation. Trends Immunol 2003; 24: 116–121. [DOI] [PubMed] [Google Scholar]
- 29Ruzicka BB, Akil H. The interleukin-1beta-mediated regulation of proenkephalin and opioid receptor messenger RNA in primary astrocyte-enriched cultures. Neuroscience 1997; 79: 517–524. [DOI] [PubMed] [Google Scholar]
- 30Miyoshi K, Obata K, Kondo T, Okamura H, Noguchi K. Interleukin-18-mediated microglia/astrocyte interaction in the spinal cord enhances neuropathic pain processing after nerve injury. J Neurosci 2008; 28: 12775–12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31Sugama S, Kim Y, Baker H, Tinti C, Kim H, Joh TH et al. Tissue-specific expression of rat IL-18 gene and response to adrenocorticotropic hormone treatment. J Immunol 2000; 165: 6287–6292. [DOI] [PubMed] [Google Scholar]
- 32Yamakawa K, Matsunaga M, Isowa T, Kimura K, Kasugai K, Yoneda M et al. Transient responses of inflammatory cytokines in acute stress. Biol Psychol 2009; 82: 25–32. [DOI] [PubMed] [Google Scholar]
- 33Lawson LJ, Perry VH, Gordon S. Turnover of resident microglia in the normal adult mouse brain. Neuroscience 1992; 48: 405–415. [DOI] [PubMed] [Google Scholar]
- 34Chao CC, Hu S, Shark KB, Sheng WS, Gekker G, Peterson PK. Activation of mu opioid receptors inhibits microglial cell chemotaxis. J Pharmacol Exp Ther 1997; 281: 998–1004. [PubMed] [Google Scholar]
- 35Bonnet MP, Beloeil H, Benhamou D, Mazoit JX, Asehnoune K. The mu opioid receptor mediates morphine-induced tumor necrosis factor and interleukin-6 inhibition in toll-like receptor 2-stimulated monocytes. Anesth Analg 2008; 106: 1142–1149. [DOI] [PubMed] [Google Scholar]
- 36Prossin AR, Zalcman SS, Heitzeg MM, Koch AE, Campbell PL, Phan KL et al. Dynamic Interactions Between Plasma IL-1 Family Cytokines and Central Endogenous Opioid Neurotransmitter Function in Humans. Neuropsychopharmacology 2014; 40: 554–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37Prossin AR, Love TM, Koeppe RA, Zubieta JK, Silk KR. Dysregulation of Regional Endogenous Opioid Function in Borderline Personality Disorder. Am J Psychiatry 2010; 167: 925–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38Zubieta JK, Ketter TA, Bueller JA, Xu Y, Kilbourn MR, Young EA et al. Regulation of human affective responses by anterior cingulate and limbic mu-opioid neurotransmission. Arch Gen Psychiatry 2003; 60: 1145–1153. [DOI] [PubMed] [Google Scholar]
- 39Velten E Jr. A laboratory task for induction of mood states. Behav Res Ther 1968; 6: 473–482. [DOI] [PubMed] [Google Scholar]
- 40George MS, Ketter TA, Parekh PI, Herscovitch P, Post RM. Gender differences in regional cerebral blood flow during transient self-induced sadness or happiness. Biological psychiatry 1996; 40: 859–871. [DOI] [PubMed] [Google Scholar]
- 41Pardo JV, Pardo PJ, Raichle ME. Neural correlates of self-induced dysphoria. Am J Psychiatry 1993; 150: 713–719. [DOI] [PubMed] [Google Scholar]
- 42First MB, Spitzer R. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) 1995.
- 43Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol 1988; 54: 1063–1070. [DOI] [PubMed] [Google Scholar]
- 44Friston KJ WK, Frackowiak RSJ, Mazziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp 1994; 1: 210–220. [DOI] [PubMed] [Google Scholar]
- 45Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007; 39: 175–191. [DOI] [PubMed] [Google Scholar]
- 46Buller KM, Crane JW, Day TA. The central nucleus of the amygdala; a conduit for modulation of HPA axis responses to an immune challenge? Stress 2001; 4: 277–287. [DOI] [PubMed] [Google Scholar]
- 47Hayes AF, Preacher KJ, Myers TA. Mediation and the estimation of indirect effects in political communication research In: Bucy EPH, R L(eds). Sourcebook for Political Communication Research: Methods, Measures, and Analytical Techniques. Routledge: NY, USA, 2011. [Google Scholar]
- 48Ribeiro SC, Kennedy SE, Smith YR, Stohler CS, Zubieta JK. Interface of physical and emotional stress regulation through the endogenous opioid system and mu-opioid receptors. Prog Neuropsychopharmacol Biol Psychiatry 2005; 29: 1264–1280. [DOI] [PubMed] [Google Scholar]
- 49Day HE, Akil H. Differential pattern of c-fos mRNA in rat brain following central and systemic administration of interleukin-1-beta: implications for mechanism of action. Neuroendocrinology 1996; 63: 207–218. [DOI] [PubMed] [Google Scholar]
- 50Day HE, Curran EJ, Watson SJ Jr., Akil H. Distinct neurochemical populations in the rat central nucleus of the amygdala and bed nucleus of the stria terminalis: evidence for their selective activation by interleukin-1beta. J Comp Neurol 1999; 413: 113–128. [PubMed] [Google Scholar]
- 51Liberzon I, Zubieta JK, Fig LM, Phan KL, Koeppe RA, Taylor SF. mu-Opioid receptors and limbic responses to aversive emotional stimuli. Proc Natl Acad Sci USA 2002; 99: 7084–7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry 2001; 6: 13–34. [DOI] [PubMed] [Google Scholar]