Abstract
Attractive toxic sugar bait (ATSB) is a highly effective method which targets mosquitoes based on their sugar foraging behavior, by presenting baits of attractive compounds in combination with sugar and oral toxin to local mosquito populations. Environmental concerns and insecticide selection-pressure have prompted investigations of novel, ecologically-harmless substances which can be used as insecticides. This study examined the efficacy of microencapsulated garlic-oil as the oral toxin component of ATSB for controlling Anopheles sergentii populations inhabiting desert-surrounded wetlands in Israel. ATSB solution containing 0.4% encapsulated garlic oil was applied to local vegetation around a streamlet located in the lower Jordan Valley. To determine the propensity of bait ingestion, and assess the potential ecological impact of the method, mosquito and non-target specimens were collected and tested for the presence of natural plant- or attractive sugar bait (ASB)-derived sugars. Over the experimental period, biting-pressure values in the ATSB treatment site decreased by 97.5%, while at the control site, treated with non-toxic ASB, no significant changes were observed. Approximately 70% of the mosquitoes collected before both treatments, as well as those captured following the application of ASB at the control site, were found to have ingested sugar prior to capture. Non-target insects were minimally affected by the treatment when ATSB was applied to foliage of non-flowering plants. Of the non-Diptera species, only 0.7% of the sampled non-target insects were found to have ingested ASB-solution which was applied to green vegetation, compared with 8.5% which have foraged on ASB-derived sugars applied to flowering plants. Conversely, a high proportion of the non-target species belonging to the order Diptera, especially non-biting midges, were found to have ingested foliage-applied ASB, with more than 36% of the specimens collected determined to have foraged on bait-derived sugars. These results prove that food-grade, EPA-exempt microencapsulated garlic oil is a highly effective insecticide which can be utilized for mosquito population control. The relatively short half-life of this active ingredient makes it a suitable for use in areas where repeated application is possible, limiting the accumulation of deleterious compounds and ensuring minimal environmental impact when applied in accordance with label recommendations.
Keywords: Culicidae, Anopheles sergentii, Sugar-feeding, ATSB, Vector-control, Non-targets
1. Background
Mosquito-borne diseases are responsible for a significant portion of human morbidity and mortality (Tolle, 2009). Consequently, increased attention has been given for development of vector control strategies which aim to reduce mosquito population numbers or their contact with potential human host, with some methods proving highly effective in lowering the incidence of such ailments in many affected areas (Beier et al., 2008; Enayati and Hemingway, 2010). However, the efficacy of current vector control methods is mostly limited to low-transmission environments, such as arid locations or isolated islands (Bhattarai et al., 2007; Kleinschmidt et al., 2007; Keating et al., 2011), whereas they have negligible or no impact in areas where entomological inoculation rates (EIRs) are more substantial (Beier et al., 1999; McKenzie et al., 2001; Shaukat et al., 2010). These shortcomings can be attributed to several factors. Insecticide-based methods such as long lasting insecticide-treated nets (LLINs) and indoor residual spraying (IRS) have been shown to cause selection-pressure and contribute to the emergence of mosquito populations which are resistant to one or several classes of chemical compounds (Oxborough et al., 2008; Ranson et al., 2009, 2011; Beier et al., 2012). In addition, most strategies target host-seeking and blood feeding mosquitoes, relying on potential human hosts to attract the vectors to the vicinity of the insecticide, which is exclusively used within residences. Consequently, the efficacy of such treatments is behavior-dependent, with endophilic species which tend to rest and feed indoors affected more than exophilic, outdoor-feeding vectors (Fornadel et al., 2010; Russell et al., 2011; Derua et al., 2012). Accordingly, a shift in the disease transmission dynamics may perpetuate the incidence of the disease, as a readily available pool of the pathogen is maintained within the latter, impeding attempts to reduce and sustain EIRs below the desired thresholds for prolonged periods (Beier et al., 1999; McKenzie et al., 2001; Shaukat et al., 2010). Hence, while vector control remains a central aspect in the efforts to eradicate mosquito-borne diseases, such drawbacks have highlighted the need to find additional, complementing methods to be utilized in integrative vector management (IVM) for controlling vector populations.
In view of the above, several studies have explored the efficacy of alternative strategy which targets mosquitoes based on physiological requirements or behavioral responses other than the search for blood meals. Both male and female mosquitoes ingest sugars to meet energetic demands, (Yuval, 1992), and are highly-selective in choosing their source (Müller and Schlein, 2006; Schlein and Müller, 2008), which can include fruit, floral nectar, or honeydew. Increased knowledge of mosquito feeding-habits and preferences has enabled targeting the vectors by using attractive toxic sugar bait (ATSB) – a mixture of sugar and oral insecticide dissolved in water, which was initially sprayed on plants known to be highly-attractive to local mosquito populations (Müller and Schlein, 2006; Schlein and Müller, 2008; Müller et al., 2010; Gu et al., 2011). Following studies have improved the method by adding plant-derived attractants to the bait mixture, allowing its application on non-attracting plants (Müller and Schlein, 2008), and in portable bait-stations (Müller et al., 2008; Naranjo et al., 2013).
Most of the trials which tested the efficacy of ATSB as a vector-control strategy employed one of several chemical compounds (such as spinosad, boric acid, and dinotefuran) as oral insecticides (Müller et al., 2008; Khallaayoune et al., 2013; Naranjo et al., 2013). Possible selection-pressure and development of resistant mosquito populations, as well as ecological and environmental concerns, have prompted studies which tested alternative substances, such as eugenol and garlic oil, which were shown in several studies to possess effective insecticidal properties (Amonkar and Reeves, 1970; Amonkar and Banerji, 1971; Isman, 2000; Cetin et al., 2004; Aboelhadid et al., 2013; Zhao et al., 2013; Khater, 2014; Qualls et al., 2014). This was done in the aim to increase the range of compounds which are used as oral toxins, thereby reducing selection-pressure for specific toxins. In this study we tested the efficacy of the first commercially available ATSB formulation containing a commercial attractant and encapsulated garlic oil as the active ingredient for controlling the population size of Anopheles sergentii, the most common Anopheles species in Israel, and a main vector of malaria in the Afro–Arabian zone (Farid, 1956; Zahar, 1974).
2. Materials and methods
2.1. Study sites
The study was conducted in the Lower Jordan Valley, part of the Dead Sea Rift, which flanks the eastern part of Israel and the Palestinian territories. The climate in the region is arid, with annual precipitation ranging between 50 mm to 100 mm, and an average relative humidity of 20–30%. Local flora and fauna is typical of the Sahara–Arabian phyto-geographical zone, shifting to tropical conditions and associated biota around sporadic natural and anthropogenic water-sources.
Two sections of streamlets (ca. 1.5 km long) with similar vegetation, situated 10 km apart, were chosen as the experimental and control sites. The watercourses of both sites are encompassed by riparian vegetation, which varies between 10–40 m in width (ca. 25 m average), and mainly consists of reeds and Tamarix spp. thickets. A short distance from the water-flow the vegetation abruptly changes to grassland with scattered shrubs and semi-shrubs. The two sites are “island-like” isolated ecological pockets suitable for mosquito breeding, and are predominantly inhabited by the malaria vector A. sergentii, the principle species found in this area.
2.2. Preparation and field application of ATSB solutions
Commercially available industrial-grade bait concentrates were purchased from Terminix®(Memphis, TN, USA). ATSB concentrate containing 0.4% (w/w) Garlic oil encapsulated in beta-cyclodextrin was diluted in tap water (1:3 ratio) containing blue food-dye (0.4% E132, Indigotine “Food Blue No. 1”; Stern, Netanya, Israel). For preparation of ASB (attractive but non-toxic sugar bait), concentrates lacking the active ingredient were similarly diluted in tap water containing green food-dye (0.4% Tartrazine 19140 “Special green”; Stern, Netanya, Israel). ATBS and ABS solutions were applied to a double perimeter of vegetation growing near the banks of either streamlets utilizing a Back-Pack Sprayer (Killaspray, Model 4526, Hozelock, Birmingham, UK) in accordance with label recommendations.
2.3. Experimental design and methods of ATSB field trials
The field trials were conducted over a period of 47 days, starting on September 30th, and ending on November 16th, 2013. Adult mosquitoes were sampled every two days for the first 11 days of the experiments, and every three days following the ATSB/ASB application, which was performed 13 days into the experiment. Female mosquitoes were captured in the evening with a Power Vac Back-Pack unit (John Hock, Gainesville, FL) while attempting to land on the legs of human baits, or in the morning from the surrounding vegetation using entomological sweep-nets. Human bait samples were collected between 20:00 and 22:00, and pooled into 5 min intervals, constituting nine repetitions per day for both the treatment and the control sites. Daily catches collected on human bait and on surrounding vegetation were stored at −70°C until further use.
2.4. Analysis of frequencies of ASB and natural sugar presence in gut
Mosquitoes captured on the surrounding vegetation were pooled according to gender, and random samples of 100 male and female mosquitoes were selected from the total daily catches obtained on four days prior to and six days following the treatment application, totaling 2000 mosquitoes from the control site, and slightly less (n = 1734) from the treatment site due to the gradual reduction in biting-pressure. Random samples of 100 female mosquitoes captured on the human baits were selected from the total daily catches obtained on four days prior to and six days following the bait application, totaling 1000 mosquitoes for the control site and 734 from the treatment site. The presence of natural plant-derived sugars in the gut contents of A. sergentii which were captured before the application of the bait solutions was confirmed by performing cold anthrone assays for fructose (Schlein and Jacobson, 1994). Specimens captured following the treatments were initially inspected visually under a dissecting microscope for the presence of ingested ASB- or ATSB-derived food-dye in the gut tissue, followed by anthrone testing of samples in which food-dye was absent. Each mosquito was placed in the well of a flat-bottomed microtiter plate and soaked with 20 µl of 100% ethanol. Aliquots of 200 µl reaction solution, containing 0.15% anthrone (Sigma, St Louis MO, USA) w/v in 71.7% sulphuric acid were added to the wells and the specimens were homogenized with a glass rod, followed by incubation of the samples for 1 h at 25°C.
2.5. Ingestion of ASB by non-target insects
Experiments investigating the impact of ATSB on non-target insects were performed at a third site, similar to the two described above. ASB solutions containing no oral-toxins were pre-mixed with either green or yellow food-dyes E102, Tartrazine 19140 (Special green) and E110, Sunset yellow FCF 15985, (Stern, Natanya, Israel) which were then applied to flowerless or blossoming plants, respectively. Target and non-target insects were collected by a variety of methods, including two 6 m Malaise traps (BioQuip, CA), 40 pitfall traps (500 ml plastic cups), and 16 yellow plates (yellow disposable plastic plates, 20 cm diameter) which were used on 15 days throughout the experiments. In addition, collection with entomological nets was performed by two technicians everyday for one hour per day during the entire 15 day period. Six small UV tray traps (Müller et al., 2010) were deployed on eight nights, and a single large light-trap (white cloth with generator-powered 250 Watt mercury Vapor bulb) was used on six nights of the experimental period. All specimens collected were stored at −70°C before being subjected to taxonomic classification and visual inspections of gut-contents with the aid of a dissecting microscope. Presence of green or yellow food–dye in the digestive tissue indicated ingestion of bait from green or blossoming plant-material, respectively.
2.6. Statistical analysis
Statistical analysis was conducted with SPSS software version 20.0 (SPSS inc., Chicago, IL). Biting pressure count data recorded at the control and experimental sites before and after the ATSB treatments was analyzed by performing a general linear model for negative binomial distribution with a log link function to adjust for overdispersion of the data. Wald chi square statistics were used to assess significance, which was taken at P < 0.05. Values used throughout the text represent mean ± S.E.
3. Results
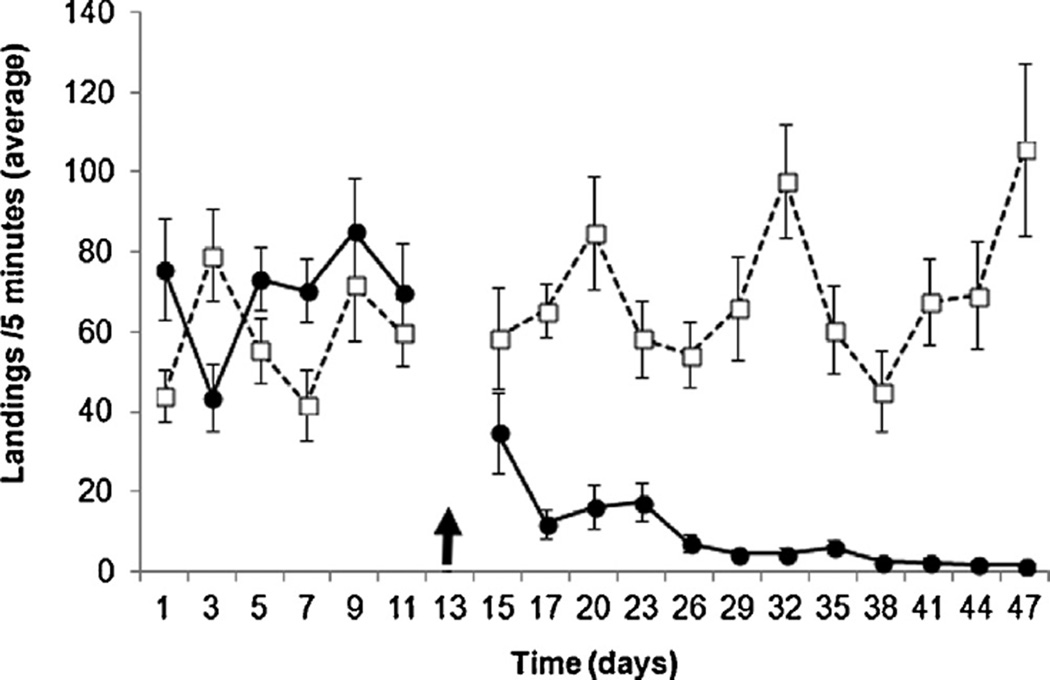
As shown in Fig. 1, application of garlic-oil ATSB greatly diminished the average biting pressure of A. sergentii in the treatment site over the experimental period. A significant reduction (P < 0.01) of more than 81% was seen four days after introduction of the toxic bait, at which the average biting pressure decreased from ca. 14 landings/minute before the treatment (mean ± S.E of all pre-treatment data = 69.7 ± 5.7 landings/5 min) to a transiently-stable 3 landings/minute (15.3 ± 2.1 landings/5 min) four to ten days following the application of the ATSB. Population densities continued to decline throughout the remainder of the experiment, to an average biting pressure of 0.35 landings/minute at the end of the field trials (1.5 ± 0.7 landings/5 min), constituting a decrease of 97.5% in the population density of A. sergentii. In contrast with the reduction seen at the treatment site, no significant changes in biting pressure were observed following application of non-toxic ASB at the control site.
Fig. 1.
Effect of ATSB and ASB on the biting pressure of female A. sergentii in the treatment site (closed circles) and control site (open squares), respectively. Application of the solutions on the surrounding vegetation was performed on day 13 (black arrow). Values represent mean ± SE of females caught attempting to land on human bait during 5 min intervals over a period of 90 min, constituting 9 repetitions.
The extent of sugar-feeding and ATSB/ASB ingestion by the mosquito population were assessed by anthrone test for sucrose and by visual inspection of guts for the presence of food dye. Field samples were grouped by gender, collection time, location and capturing method (Table 1). Results show the majority of both female and male mosquitoes which were collected from the surrounding vegetation had ingested sugar-meals prior to the application of the treatment in both the experimental (70.7 ± 6.7% of the females and 72.75 ± 2.9% of the males; n = 400 for both groups) and the control sites (females −72 ± 3.7% of the females and 70.5 ± 3.33% of all males; n = 400). Moreover, the intensity of color-reaction indicated meal sizes were relatively large in many of the tested samples. In contrast, only 8.3 + 1.1% and 11.25 ± 2.66% of the female mosquitoes captured on human bait at the treatment and control sites, respectively, were found to have consumed sugars. The consistently low intensity of the anthrone color-reaction may indicate that these specimens had ingested (from natural sources) relatively small sugar-meals prior to capture, in line with reports that extensive ingestion of sugar by female mosquitoes can inhibit their blood-seeking and blood-feeding behaviors (Straif and Beier, 1996; Gary and Foster, 2001). Alternatively, ingestion of the toxic bait may inhibit both blood-feeding and sugar-feeding activities (Junnila et al., submitted), such that previously ingested sugars had been largely digested by the time of capture, considering the relatively long duration required for the insecticide to take effect.
Table 1.
Percent of the samples testing positive for sugar in anthrone assays for sucrose or by visual inspection for the presence of food-dye. Values represent mean ± S.E, sample size is indicated by superscript.
| Pre-treatment |
Post-treatmenta |
||||
|---|---|---|---|---|---|
| Location | Site | Female | Male | Female | Male |
| Vegetation | Control | 72 ± 3.7%b | 70.5 ± 3.3%b | 71.8 ± 3.8%c (27.25 ± 0.9%) | 75.5 ± 3.8%c(37 ± 2.9%) |
| treatment | 70.7 ± 6.7%b | 72.7 ± 2.9%b | 41.5 ± 4.3%d(8.2 ± 1.3%) | 39 ± 3.8%e (11.25 ± 2.8%) | |
| Human | Control | 11.3 ± 2.7%b | 18.7 ± 3.5%c(7 ± 2.5%) | ||
| bait | treatment | 8.3 + 1.1%b | 15.6 ± 3.8%f(2.4 + 1.8%) | ||
Values expressed in prentices are the percentages of the samples found to contain ATSB or ASB-derived food-dye in the gut tissue.
(n = 400).
(n = 600).
(n = 515).
(n = 392).
(n = 324).
Specimens collected after application of the ATSB and ASB were investigated visually for the presence of food-dye, and samples in which coloration was absent were subjected to cold-anthrone assays for sucrose. The fraction of sugar-fed A. sergentii captured on the surrounding vegetation was not affected by the treatment of ASB in the control site, with 71.8 ± 3.8% of the females and 75.5 ± 3.8 of the males testing positive in either assays. ASB-derived sugars accounted for 35% of the sugar-fed females (27.25 ± 0.9% of the total) and 49% of the sugar-fed males (37 ± 2.9% of the total), corresponding to ABS/natural sugar-fed ratios of 0.61 and 0.96 for females and male specimens captured at the control site, respectively. These values show that despite the abundance of natural plant sugar sources, bait-derived sugar was readily ingested by both male and female mosquitoes, indicating the commercial formulation has effectively attracted the target species to the applied bait. As most mosquito species seek and feed on plant sugars at least once a day, exposure to the added insecticide becomes highly likely as time progresses.
The application of ATSB had seemingly lowered the incidence of sugar-feeding in the treatment site by ca. 47% (P < 0.01), with only 41.5 ± 4.3% of the females and 39 ± 3.8% of the males captured on the surrounding vegetation testing positive for traces of food-dye or sucrose, compared with 70.7 ± 6.7% of the females and 72.75 ± 2.9% of the males captured at the same location prior to the application of the treatment. ATSB-derived sugars accounted for 20% of the sugar-fed females (8.2 ± 1.3% of the total) and 28% of the sugar-fed males (11.2 ± 2.8% of the total) captured after the application of ATSB in the treatment site. These correspond to ATBS/natural sugar-fed ratios of 0.25 and 0.39 for females and male specimens captured at the treatment site, respectively. However, consequent mosquito mortality following the ingestion of ATSB likely explains these seemingly low values, since such feeding rates were sufficient for the near annihilation of the mosquito population at the end of the study period. Moreover, these results may be biased due to consequent behavioral changes of the mosquitoes, which are likely less active following ingestion of the garlic oil and are thus less prone to be captured by standard methods. This is further supported by the fact very few blood-seeking females were found to be stained by ASB at the control site, and even fewer were captured following the application of ATSB in the treatment site.
Samples of female A. sergentii which were collected on human bait following the applications of ATSB and ASB in the treatment and control sites, respectively, were also subjected to visual inspection and cold anthrone assays for sucrose. In agreement with the results described above, a smaller fraction of the blood-seeking females were found to be stained by ATSB than by ASB following the application of the treatments. Out of 600 specimens sampled at the control site, 11.7 ± 2.7% tested positive for sugar by cold anthrone assay for sucrose, compared to only 7 ± 2.5%, which were found to have ingested ASB by visual inspection. In contrast, while 13.2 ± 3.9% of the females captured at the treatment site (n = 324) tested positive for sucrose by cold anthrone assays, only 2.4 ± 1.8% were stained by ATSB. The ratio of ABS/natural sugar-fed females in the control site (0.59) was significantly higher (P < 0.01) than the ratio of ATBS/natural sugar-fed females which were captured in the treatment site (0.2). These results may be partially due to ATSB-induced mortality of the target insects, but may also reflect changes in feeding behavior of A. sergentii females following ingestion of the microencapsulated garlic oil, which likely inhibits host-seeking and blood-feeding activities.
As many non-target insects depend on plant-derived sugars for their energetic requirements, application of ATSB may have detrimental effects on the ecology of treatment sites. To test the extent of such impacts, additional trials were performed to determine what qualitative and quantitative proportions of the insect populations are prone to ingest ASB/ATSB following its application on different plant-types. Green or yellow food-dyes were added to non-toxic ASB solutions, which were subsequently applied to non-flowering or flowering plants, respectively. Insects were captured using several of techniques on 15 days and 8 nights throughout the experimental period (see methods). Two thousand mosquitoes from nine different morpho-species/ 4 genera and 20,399 non-target insects belonging to nine orders (including non-target Diptera species) and comprising 659 morpho-species were captured, classified, and visually inspected for the presence of green or yellow food dye in the gut tissue with the aid of a dissecting-microscope. Results presented in supplementary Table 1 and described below indicate ASB solutions were mainly ingested by the targeted mosquitoes and non-biting midges, both belonging to the order Diptera.
Out of the 2000 mosquitoes captured at the non-target trials (nine morpho-species 4 genera), 14.75% were found to contain green food-dye, while 18.5% were found to contain yellow food-dye, totaling 33.3% of the population found to ingest ASB from either type of plants.
Of the 4500 specimens of non-target insect belonging to order Diptera, 25% of the higher Diptera species, and 81% of the non-biting midge samples were found to contain food-dye, the former mainly foraging from flowering plants (22.9% containing yellow food-dye compared to only 2.3% which tested positive for green food-dye), while in samples of the latter the proportion of green and yellow-stained specimens were almost equal (36.5% and 44.5%, respectively).
In contrast with target- and non-target insects from the order Diptera, a relatively small fraction of non-target insects belonging to other orders were found to have ingested bait material. Of the 15,899 non-target insects (excluding non-target Diptera), only 1462 (9.2%) were found to have ingested ASB. Out of these, 1356 (93%) ingested bait material which was applied to flowering-plants, while only 106 (7% of the sugar-positive insects, representing 0.65% of the total non-target insects which were sampled) were found to have foraged on ASB applied to the foliage of green plants. These results prove that specific application of the ATSB treatment exclusively to flowerless vegetation, as stated on the product label, can dramatically reduce the impact on non-target insects, while still maintaining a significant effect on target-insects and nuisance pests such as and biting non-biting midges, the latter proliferating into swarms which can impose significant economic burdens to many urban areas around the globe (Richard and Arshad Ali, 2006).
4. Discussion
In agreement with earlier reports (Aboelhadid et al., 2013; Amonkar and Reeves, 1970; Amonkar and Banerji, 1971; Zhao et al., 2013), data obtained in this study confirms the insecticidal properties of microencapsulated garlic-oil. The commercial ATSB formulation with garlic-oil as an active ingredient nearly exterminated the A. sergentii population at the study site, as inferred by the dramatic 97.5% drop from the biting-pressure values recorded prior to the treatment. The impact of garlic-oil ATBS on the mosquito population densities appears to be biphasic: a rapid, significant decrease in biting-pressure over a period of four days, followed by a transient stable period of six days, after which the population gradually declines over 25 days, reaching a mere 2.5% of the biting-pressure recorded prior to the application of the treatment. As larval stages are unlikely to encounter the plant-applied insecticide, this pattern may reflect the duration required for toxin exposure by the entire mosquito population, including all juvenile stages.
An important point demonstrated in this study is that when utilizing an efficient attractant, ATSB ably attracts sugar-feeding mosquitoes despite the availability of competitive natural-sugar sources, as a considerable fraction of the mosquito population was found to have ingested the bait solution. It should be noted that a larger proportion of both male and females consumed ASB in the control site than ATSB at the treatment site. These differences may be caused by mosquito mortality or behavioral changes, as laboratory trials have demonstrated mosquitoes show no aversion to ATSB when compared with ABS solutions devoid of garlic oil. An added advantage of this method is that older mosquitoes are more greatly impacted, since exposure to the toxin becomes more likely as time progresses. Maturation of infective sporozoite stages of Plasmodium sp. parasites within mosquitoes require several days (Beier, 1998), enhancing the vectorial capacity of older, mature adults. The ability of ATSB treatments to remove the more competent vectors from the environment makes it highly suitable for use in malaria-affected areas.
New vector control strategies must have low ecological-impact, and pose minimal risks to non-target insects. Consequently, the ATSB method may be of some concern, as insecticides are applied to plant species which may be important sugar-sources of a wide variety of species. Indeed, while a relatively low number of insects were found to be stained by either dye (9.2% of all non-target specimens, excluding those belonging to order Diptera), some non-target insects, especially specimens belonging to order Hymenoptera, were similarly prone to ingest ASB-derived sugars (23.6–31.4%, depending on the family). However, all of the non-target insects were found to have foraged almost exclusively from flowering plants (8.5% of the total, constituting 92.3% of the non-target insects which tested positive for food dye). While mosquitoes are also more likely to feed on bait sprayed on flowering plants (18.5%), a significant proportion of the target insects were found to have foraged from solutions applied to flowerless vegetation (14.5% of the total, constituting 44% of the target-insect which tested positive for food dye). These findings that are in agreement with recent studies, which also demonstrated ATSB application has little or no effect on predatory insects (Khallaayoune et al., 2013; Aboelhadid et al., 2013; Revay et al., 2014). Our report further demonstrates that selective targeting of desired vectors with can be greatly enhanced by following simple label guidelines of treatment application. Specifically, application of ATBS exclusively on green, non-flowering plants would cause negligible environmental impact while having minimal effect on the efficacy of vector-control. However, it is noteworthy that collecting methods were focused on adult insects, and foliage-feeding larvae were under sampled in our study. While we expect no major differences in ATSB-sensitivity between phytophagous larvae and foliage-feeding beetles and sap-feeding hemipterans (Table 1), more research is needed to assess the overall impact to the non-target species populations, e.g., through insects surveys 0–2 years following ATSB application.
5. Conclusions
The enduring threats of mosquito-borne diseases and the enormous impact they have on human health in developing countries underline the need for new vector-control strategies which can be easily implemented in affected areas by fairly simple means. A relatively new approach, ATSB is a highly effective method which targets the vectors based on their plant sugar foraging behavior, by combining oral toxins and sugars with potent mosquito attractant of plant origin. The results obtained in this study indicate that the now commercially available ATSB with EPA-exempt, microencapsulated garlic oil can be effectively used for A. sergentii control. The results presented above indicate that when ATSB is applied on flowerless vegetation it has a negligible effect on non target, particularly pollinating insects, making it an ecologically-safe alternative for conventional insecticides. Furthermore, as the efficacy of the treatment is mainly dependent on the attractant rather than the active ingredient which is of lesser importance, this method enables utilization of a wide range of insecticides which to date were deemed ineffective for mosquito control, minimizing resistance development due to intensive application of currently used insecticides. An additional benefit is that ATSB disproportionally impacts older adult mosquitoes which possess the highest vectorial capacity, and should thus greatly decrease the entomological inoculation rates when applied in affected areas.
Supplementary Material
Acknowledgments
Work in this study was supported by the National Institute of Allergy And Infectious Diseases of the National Institutes of Health under Award Number R01AI100968. The content is solely the responsibility of the authors, and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.actatropica.2015.06.018
References
- Aboelhadid SM, Kamel AA, Arafa WM, Shokier KA. Effect of Allium sativum and Allium cepa oils on different stages of Boophilus annulatus. Parasitol. Res. 2013;112:1883–1890. doi: 10.1007/s00436-013-3344-0. [DOI] [PubMed] [Google Scholar]
- Amonkar SV, Banerji A. Isolation and characterization of larvicidal principle of garlic. Science. 1971;174:1343–1344. doi: 10.1126/science.174.4016.1343. [DOI] [PubMed] [Google Scholar]
- Amonkar SV, Reeves EL. Mosquito control with active principle of garlic, Allium sativum. J. Econ. Entomol. 1970;63:1172–1175. doi: 10.1093/jee/63.4.1172. [DOI] [PubMed] [Google Scholar]
- Beier JC, Killeen GF, Githure JI. Short report: entomologic inoculation rates and Plasmodium falciparum malaria prevalence in Africa. Am. J. Trop. Med. Hyg. 1999;61:109–113. doi: 10.4269/ajtmh.1999.61.109. [DOI] [PubMed] [Google Scholar]
- Beier JC, Keating J, Githure JI, Macdonald MB, Impoinvil DE, Novak RJ. Integrated vector management for malaria control. Malar. J. 2008;7:1–10. doi: 10.1186/1475-2875-7-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier JC, Müller GC, Gu W, Arheart KL, Schlein Y. Attractive toxic sugar bait (ATSB) methods decimate populations of Anopheles malaria vectors in arid environments regardless of the local availability of favored sugar-source blossoms. Malar. J. 2012;11:31. doi: 10.1186/1475-2875-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier JC. Malaria parasite development in mosquitoes. Annu. Rev. Entomol. 1998;43:519–543. doi: 10.1146/annurev.ento.43.1.519. [DOI] [PubMed] [Google Scholar]
- Bhattarai A, Ali AS, Kachur SP, Martensson A, Abbas AK, Khatib R, Al-Mafazy AW, Ramsan M, Rotllant G, Gerstenmaier JF. Impact of artemisinin-based combination therapy and insecticide-treated nets on malaria burden in Zanzibar. PLoS Med. 2007;4:e309. doi: 10.1371/journal.pmed.0040309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetin H, Erler F, Yanikoglu A. Larvicidal activity of a botanical natural product, AkseBio2, against Culex pipiens. Fitoterapia. 2004;75:724–728. doi: 10.1016/j.fitote.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Derua YA, Alifrangis M, Hosea KM, Meyrowitsch DW, Magesa SM, Pedersen EM, Simonsen PE. Change in composition of the Anopheles gambiae complex and its possible implications for the transmission of malaria and lymphatic filariasis in north-eastern Tanzania. Malar. J. 2012;11:188. doi: 10.1186/1475-2875-11-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enayati A, Hemingway J. Malaria management: past, present, and future. Annu. Rev. Entomol. 2010;55:569–591. doi: 10.1146/annurev-ento-112408-085423. [DOI] [PubMed] [Google Scholar]
- Farid MA. The implications of Anopheles sergentii for malaria eradication programmes east of the Mediterranean. Bull. World Health Org. 1956;15:821–828. [PMC free article] [PubMed] [Google Scholar]
- Fornadel CM, Norris LC, Glass GE, Norris DE. Analysis of Anopheles arabiensis blood feeding behavior in southern Zambia during the two years after introduction of insecticide-treated bed nets. Am. J. Trop. Med. Hyg. 2010;83:848–853. doi: 10.4269/ajtmh.2010.10-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gary RE, Foster WA. Effects of available sugar on the reproductive fitness and vectorial capacity of the malaria vector Anopheles gambiae (Diptera: Culicidae) J. Med. Entomol. 2001;38:22–28. doi: 10.1603/0022-2585-38.1.22. [DOI] [PubMed] [Google Scholar]
- Gu W, Müller GC, Schlein Y, Novak RJ, Beier JC. Natural plant sugar sources of anopheles mosquitoes strongly impact malaria transmission potential. PLoS One. 2011;6:e15996. doi: 10.1371/journal.pone.0015996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isman MB. Plant essential oils for pest and disease management. Crop Prot. 2000;19:603–608. [Google Scholar]
- Keating J, Locatelli A, Gebremichael A, Ghebremeskel T, Mufunda J, Mihreteab S, Berhane D, Carneiro P. Evaluating indoor residual spray for reducing malaria infection prevalence in Eritrea: results from a community randomized control trial. Acta Trop. 2011;119:107–113. doi: 10.1016/j.actatropica.2011.04.015. [DOI] [PubMed] [Google Scholar]
- Khallaayoune K, Qualls WA, Revay EE, Allan SA, Arheart KA, Kravchenko VD, Xue RD, Schlein Y, Beier JC, Müller GC. Attractive toxic sugar baits: control of mosquitoes with the low risk active ingredient dinotefuran and potential impacts on non-target organisms in Morocco. Environ. Entomol. 2013;42:1040–1045. doi: 10.1603/EN13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khater HF. Bioactivities of some essential oils against the camel nasal botfly, Cephalopina titillator. Parasitol. Res. 2014;113:593–605. doi: 10.1007/s00436-013-3688-5. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt I, Torrez M, Schwabe C, Benavente L, Seocharan I, Jituboh D, Nseng G, Sharp B. Factors influencing the effectiveness of malaria control in Bioko Island, Equatorial Guinea. Am. J. Trop. Med. Hyg. 2007;76:1027–1032. [PMC free article] [PubMed] [Google Scholar]
- Müller GC, Schlein Y. Sugar questing mosquitoes in arid areas gather on scarce blossoms that can be used for control. Int. J. Parasitol. 2006;36:1077–1080. doi: 10.1016/j.ijpara.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Müller GC, Schlein Y. Efficacy of toxic sugar baits against adult cistern-dwelling Anopheles claviger. Trans. R. Soc. Trop. Med. Hyg. 2008;102:480–484. doi: 10.1016/j.trstmh.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Müller GC, Kravchenko VD, Schlein Y. Decline of Anopheles sergentii and Aedes caspius populations following presentation of attractive, toxic (Spinosad), sugar bait stations in an oasis. J. Am. Mosq. Control Assoc. 2008;24:147–149. doi: 10.2987/8756-971X(2008)24[147:DOASAA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Müller GC, Beier JC, Traore SF, Toure MB, Traore MM, Bah S, Doumbia S, Schlein Y. Successful field trial of attractive toxic sugar bait (ATSB) plant-spraying methods against malaria vectors in the Anopheles gambiae complex in Mali, West Africa. Malar. J. 2010;9:210. doi: 10.1186/1475-2875-9-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie FE, Killeen GF, Beier JC, Bossert WH. Seasonality, parasite diversity, and local extinctions in Plasmodium falciparum malaria. Ecology. 2001;82:2673–2681. doi: 10.1890/0012-9658(2001)082[2673:spdale]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naranjo DP, Qualls WA, Alimi TO, Roque DD, Samson DM, Arheart KC, Beier JC, Xue RD. Evaluation of boric acid sugar baits against Aedes albopictus (Diptera:Culicidae) in tropical environments. Parasitol. Res. 2013;112:1583–1587. doi: 10.1007/s00436-013-3312-8. [DOI] [PubMed] [Google Scholar]
- Oxborough RM, Mosha FW, Matowo J, Mndeme R, Feston E, Hemingway J, Roland M. Mosquitoes and bednets: testing the spatial positioning of insecticide on nets and the rationale behind combination insecticide treatments. Ann. Trop. Med. Parasitol. 2008;102:717–727. doi: 10.1179/136485908X337553. [DOI] [PubMed] [Google Scholar]
- Qualls WA, Müller GC, Revay EE, Allan SA, Arheart AL, Beier JC, Smith ML, Scott JM, Kravchenko VD, Hausmann A, Yefremova ZA, Xue RD. Evaluation of attractive toxic sugar bait (ATSB) – barrier for control of vector and nuisance mosquitoes and its effect on non-target organisms in sub-tropical environments in Florida. Acta Trop. 2014;131:104–110. doi: 10.1016/j.actatropica.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranson H, Abdallah H, Badolo A, Guelbeogo WM, Kerah-Hinzoumbe C, Yangalbe-Kalnone E, Sagnon N, Simard F, Coetzee M. Insecticide resistance in Anopheles gambiae: data from the first year of a multi-country study highlight the extent of the problem. Malar. J. 2009;8:299. doi: 10.1186/1475-2875-8-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranson H, N’Guessan R, Lines J, Moiroux N, Nkuni Z, Corbel V. Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends Parasitol. 2011;27:91–98. doi: 10.1016/j.pt.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Revay EE, Müller GC, Qualls WA, Kline DL, Naranjo DP, Arheart KL, Kravchenko VD, Yefremova Z, Hausmann A, Beier JC, Schlein Y, Xue R. Control of Aedes albopictus with attractive toxic sugar baits (ATSB) and potential impact on non-target organisms in St. Augustine, Florida. Parasitol. Res. 2014;113:73–79. doi: 10.1007/s00436-013-3628-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard J, Arshad Ali, Lobinske Population monitoring, ecology and control possibilities for nuisance midges (Diptera: Chironomidae) Tech. Bull. Florida Mosq. Control Assoc. 2006;7:63–66. [Google Scholar]
- Russell TL, Govella NJ, Azizi S, Drakeley CJ, Kachur SP, Killeen GF. Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malar. J. 2011;10:80. doi: 10.1186/1475-2875-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlein Y, Jacobson RL. Mortality of Leishmania major in Phlebotomus papatasi caused by plant feeding of the sandflies. Am. J. Trop. Med. Hyg. 1994;50:20–27. [PubMed] [Google Scholar]
- Schlein Y, Müller GC. An approach to mosquito control: using the dominant attraction of flowering Tamarix jordanis trees against Culex pipiens. J. Med. Entomol. 2008;45:384–390. doi: 10.1603/0022-2585(2008)45[384:aatmcu]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Shaukat AM, Breman JG, McKenzie FE. Using the entomological inoculation rate to assess the impact of vector control on malaria parasite transmission and elimination. Malar. J. 2010;9:122. doi: 10.1186/1475-2875-9-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straif SC, Beier JC. Effects of sugar availability on the blood-feeding behavior of Anopheles gambiae (Diptera: Culicidae) J. Med. Entomol. 1996;33:608–612. doi: 10.1093/jmedent/33.4.608. [DOI] [PubMed] [Google Scholar]
- Tolle MA. Mosquito-borne diseases. Curr. Prob. Pediatr. Adolesc. Health Care. 2009;39:97–140. doi: 10.1016/j.cppeds.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Yuval B. The other habit: sugar feeding by mosquitoes. Bull. Soc. Vector Ecol. 1992;17:150–156. [Google Scholar]
- Zahar AR. Review of the ecology of malaria vectors in the WHO Eastern Mediterranean Region. Bull. World Health Org. 1974;50:427–440. [PMC free article] [PubMed] [Google Scholar]
- Zhao NN, Zhang H, Zhang XC, Luan XB, Zhou C, Liu QZ, Shi WP, Liu ZL. Evaluation of acute toxicity of essential oil of garlic (Allium sativum) and its selected major constituent compounds against overwintering Cacopsylla chinensis (Hemiptera: Psyllidae) J. Econ. Entomol. 2013;106:1349–1354. doi: 10.1603/ec12191. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.