Abstract
Artemisinin-based combination therapies (ACTs) are currently used as the first-line therapy for uncomplicated Plasmodium falciparum malaria. However, the recent emergence and/or spread of artemisinin resistance in parts of Greater Mekong Subregion (GMS) of southeast Asia requires close monitoring of the therapeutic efficacy of ACTs. This study was conducted from March 2012 to December 2013 in four clinics and seven villages along the China–Myanmar border. A total of 109 patients with uncomplicated falciparum malaria were treated with dihydroartemisinin–piperaquine (DP) and followed up on days 1, 2, 3, 7, 14, 21, 28, and 42 after treatment. A total of 71 patients (22 children and 49 adults) completed the 42-day follow-up. DP remained highly efficacious for treatment of uncomplicated falciparum malaria with an overall 42-day cure rate of 100%. The day 3 parasite-positive rate was 7.04% (5/71). Within 14 days of treatment, a total of 13 (18.31%) patients had detectable gametocytes and a large proportion of these were persistent from the first three days of treatment. The presence of gametocytes in patients through 14 days after DP treatment suggests that the incorporation of a single dose of primaquine for clearing gametocytemia should be considered for blocking parasite transmission.
Introduction
Malaria is an enormous health problem in the Greater Mekong Subregion (GMS) of southeast Asia.1,2 In recent years, substantial progress has been made in reducing the regional malaria burden; several countries have entered the malaria pre-elimination and elimination phases.3 This success is attributed, in part, to the deployment of artemisinin-based combination therapies (ACTs) in malaria-endemic countries. However, the recent emergence and/or spread of artemisinin resistance in Plasmodium falciparum in parts of GMS pose a serious threat to regional malaria control and elimination campaign.4–6 Since the time when clinical artemisinin resistance was first documented in western Cambodia,5,7,8 intensive monitoring in the GMS has detected artemisinin resistance in at least six areas because of spread, independent emergence, or both.9–12 In vision of a potential catastrophic spread of artemisinin resistance to Africa, the World Health Organization (WHO) developed a containment plan.13 One critical component to deter the spread of artemisinin resistance is to monitor the efficacy of ACTs.
Artemisinin resistance in the GMS is manifested as delayed parasite clearance after ACT treatment. This is better reflected in in vivo parasite clearance rate: whereas sensitive parasites normally have a clearance half-life of ∼2 hours, artemisinin-resistant parasites tend to have a clearance half-life longer than 5 hours.14 Inference of parasite clearance half-life requires determination of peripheral parasitemia by microscopy at multiple time points a day,15 which is difficult to perform in routine monitoring of drug efficacy among outpatients. Alternatively, the proportion of patients remaining parasite-positive 3 days after treatment with drugs containing artemisinin derivatives is often used as a proxy indicator for delayed parasite clearance.16,17 In this regard, WHO proposed a working definition for suspected artemisinin resistance as “an increase in parasite clearance time, as evidenced by > 10% of cases with parasites detectable on day 3 after treatment with an ACT.”13 Many sentinel sites were set up to monitor suspected artemisinin resistance in the GMS, focusing on regions of P. falciparum endemicity and extensive use of artemisinin. The southwestern China's Yunnan province has the longest history of artemisinin use, mostly as monotherapy before 2005. A clinical efficacy study conducted in 2008 demonstrated 100% clinical efficacy of dihydroartemisinin–piperaquine (DP) for the treatment of uncomplicated P. falciparum malaria at the China–Myanmar border area with a day-3 parasitemia-positive rate of 6.2%.18 A recent clinical efficacy study of 7-day artesunate monotherapy in 65 patients indicated an efficacy of 95.9% with a day-3 positivity rate of 18.5%.19 Although these studies cannot be directly compared with each other to suggest potential emergence of artemisinin resistance in this region, it does warrant further scrutiny.
In this study, we evaluated clinical efficacy of DP for treatment of uncomplicated P. falciparum malaria at the China–Myanmar border area, taking advantage of our current epidemiological study conducted in this area.20 Outpatients of falciparum malaria were treated with DP, and parasite clearance and clinical efficacy were determined. Our data confirmed the excellent clinical efficacy of this drug combination, and also revealed information about delayed parasite clearance. This information is deemed important for the detection of clinical artemisinin resistance along the China–Myanmar border.
Materials and Methods
Study region and patient enrollment.
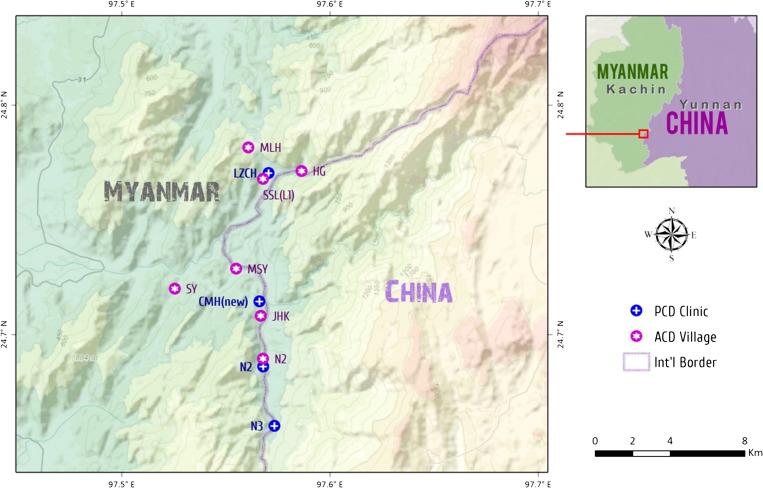
The study was conducted from March 2012 to December 2013 in four clinics and seven villages in and around Laiza township, Kachin State, Myanmar, along the China–Myanmar border (Figure 1 ). The study protocol involved the treatment of patients with uncomplicated falciparum malaria with DP and evaluation of its clinical efficacy for 42 days after treatment. The majority of the patients were recruited from the clinics with our passive case detection efforts, while additional febrile patients were identified from our weekly household visit activity in the seven villages. Thick and thin smears were prepared by the finger-prick method from patients with documented fever or history of fever in the previous 24 hours. Malaria infection was diagnosed based on microscopic examination of blood smears. Patients recruited to the study were those without signs of severe malaria, with P. falciparum mono-infection, and with asexual parasite density below 150,000/μL. Informed consent was obtained from all patients or legal guardians of participants before being included in this study. Assents were also obtained from children aged 7–14 years. The study protocol was reviewed and approved by institutional review boards from Kunming Medical University and the local department of health.
Figure 1.
Map of the study sites.
Laboratory methods.
Thin and thick blood smears were stained with 2% Giemsa for 30 minutes and first read by field microscopists. For quality control, the smears were reexamined at our field station by two experienced microscopists. Discrepancies in the two values by > 30% were reevaluated by a third microscopist to obtain a final consensus of the diagnoses (normally < 10% of the slides). Thin smears were used to determine the parasite species. Parasite densities were calculated from thick blood smears by counting the number of asexual parasites and gametocytes per 200 leukocytes. The average number of parasites per 200 white blood cells (WBCs) was used to estimate the parasite density (number of parasites per microliter blood) assuming 8,000 WBCs/μL blood.
Malaria treatment and follow-up.
Per local government policy, DP is the standard ACT for the treatment of uncomplicated falciparum malaria in the study area. All confirmed falciparum malaria patients received treatment with DP tablets, containing 40 mg dihydroartemisinin and 320 mg piperaquine per tablet (Duocotecxin; Holley Pharm, Chongqing, China). Adult patients received two tablets as the first dose, then two tablets each time at 6, 24, and 36 hours later. Child patients used this same treatment regimen but the total dose was calculated using 6.4 mg dihydroartemisinin and 51.2 mg piperaquine/kg body weight. All treatment time points were directly observed by the staff at the clinics. For follow-ups, participants were asked to return for a check on days 1, 2, 3, 7, 14, 21, 28, and 42 after drug treatment as well as any time in between if they felt sick. Thick and thin blood smears were made for microscopic examinations at these time points.
Data analysis.
Statistical analysis was performed using SigmaStat 3.5 software (San Jose, CA) to compare the parasite densities stratified by patient age. Parasite densities between different age groups were compared using Kruskal–Wallis one-way analysis of variance on ranks. Mean asexual clearance rates between age groups were compared using the Mann–Whitney U test. Clearance of asexual parasites and gametocytes between the two age groups were compared using survival analysis with Kaplan–Meier log-rank test. Day-by-day comparison in asexual parasite and gametocyte positivity between the two age groups was compared using Fisher's exact test.
Results
Patient demographic and clinical profiles.
A total of 109 malaria patients with microscopically confirmed P. falciparum single-species infection and fulfilling the inclusion criteria were recruited to this clinical efficacy study. They were treated with DP and efficacy was followed up through day 42. Five patients were excluded from the analysis because their asexual parasite density at the time of admission was below 100 asexual parasites/μL. Among the malaria patients, 66 were male (60.55%) and 43 were female (39.45%). The ages of the patients varied from 1 to 60 years with a median of 20.5, and 32.11% (35/109) were children aged 15 years and below (Table 1). The asexual parasite density ranged from 140 to 147,600/μL of peripheral blood. There was no statistically significant difference in asexual parasite density among the age groups, albeit the geometric mean parasitemia of the 5–15 year age group was much higher than those of other groups (Kruskal–Wallis one-way analysis, P = 0.373). In addition, 17.43% (19/114) of the patients had detectable gametocytemia at the time of enrollment with a geometric mean of gametocyte density at 54/μL blood (Table 1). At enrollment, major clinical symptoms of the falciparum malaria patients included fever (76.32%), shivering/chills (44.74%), headache (35.96%), and joint pain (14.91%) (Table 2).
Table 1.
Demographic and clinical features of the Plasmodium falciparum patients at the time of enrollment
| Total number of patients (% males) | 109 (60.55%) |
| Age (years) (median/range) | 20.5 (1–60) |
| Feverish patients on day 0 (axillary temperature > 37.5°C) | 77.06% |
| Temperature (°C) [mean (range)] | 38.4 (36.0–40.0) |
| Asexual parasite density on day 0: geometric mean (range) | |
| Under 5 years (N = 6) | 3,203/μL (700–38,880) |
| 5–15 years (N = 29) | 8,290/μL (220–147,600) |
| > 15 years (N = 74) | 3,622/μL (140–83,800) |
| Patients present with gametocytes on day 0 [n (%)] | 19 (17.43%) |
| Gametocyte density on day 0: geometric mean (range) | 54/μL (0–2,540) |
Table 2.
Clinical manifestations of the patients involved in this study
| Clinical manifestation | Cases | Rate (%) |
|---|---|---|
| Fever | 87 | 76.32 |
| Shivering/chills | 51 | 44.74 |
| Headache | 41 | 35.96 |
| Joint pain | 17 | 14.91 |
| Loss of appetite | 9 | 7.89 |
| Vomiting | 9 | 7.89 |
| Dizziness | 9 | 7.89 |
| Coughing | 4 | 3.51 |
| Nausea | 3 | 2.63 |
| Convulsions | 2 | 1.75 |
| Diarrhea | 1 | 0.88 |
| Abdominal pains | 1 | 0.88 |
Malaria treatment outcomes.
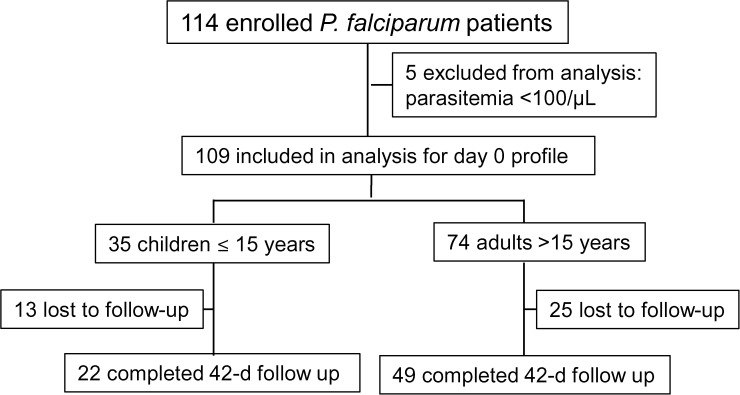
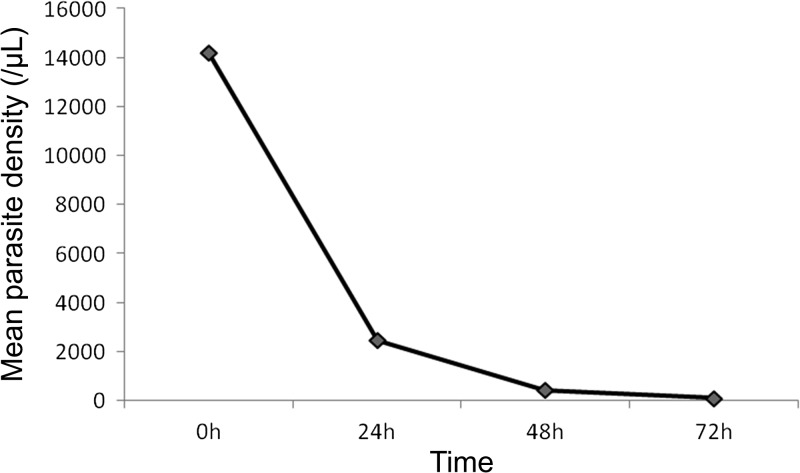
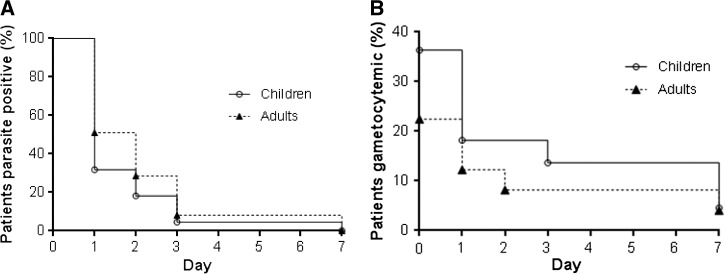
Figure 2 is a schematic illustration of malaria treatment outcomes and results from our follow-up activities. Given that there were only six patients < 5 years, we only compared the results between adults (> 15 years) and children (≤ 15 years). For earlier parasite clearance, parasitemia on day 1, 2, and 3 were closely monitored through microscopic examination of thick and thin blood smears. Since this study was conducted in a military conflict zone, constant human population movement inevitably resulted in high rates of loss to follow-up. For the 109 patients enrolled at the beginning of the study, 71 completed the 42-day follow-up, including 22 of the 35 children and 49 of the 74 adults. All the losses to follow-up were due to relocations of the patients' families to places outside the study area. Clinical efficacy and comparison were made only for the participants who finished the 42-day follow-up. DP remained highly efficacious against P. falciparum and no cases with recurrent parasitemia were detected within the 42-day follow-up. Parasite clearance was followed daily during the first 3 days after initiation of DP treatment (Figure 3 ). Overall, the mean parasite clearance time was estimated to be 36.9 hours (95% confidence interval [CI]: 31.5–42.3). The younger age group cleared asexual parasitemia at 32.6 hours (95% CI: 23.7–41.6), slightly faster than the adult age group (39.0 hours; 95% CI: 32.3–45.6), though the difference was not statistically significant (P = 0.170, Mann–Whitney U test). The asexual clearance profiles of the two age groups did not differ significantly (P > 0.05, Kaplan–Meier log-rank test) (Figure 4A ). On days 1, 2, and 3 post initiation of DP treatment, asexual parasitemia was detected in 45.07% (32/71), 25.35% (18/71), and 7.04% (5/71) patients, respectively (Table 3). Children appeared to have cleared asexual parasitemia faster than adults. Specifically, 7 (31.82%) children, as compared with 25 (51.02%) adults, remained parasitemic on day 1. On day 3, one (4.55%) child patient and four (8.16%) adult patients were parasite positive. However, these differences between the two age groups did not reach statistical significance (P > 0.05, Fisher's exact test). Of the five patients remaining parasitemic on day 3, the geometric mean parasite density on day 0 was 2,167/μL, which was not significantly higher than that of the day 3 negative cases (4,083/μL) (Mann–Whitney rank sum test, P = 0.615 > 0.05). We further examined whether day 3 asexual parasite-positive patients had significantly higher parasitemia at enrollment, and our result showed that there was no significant difference in day 0 parasite density between the day 3 parasite-positive and parasite-negative patients (P > 0.05, t test).
Figure 2.
Flow chart of the dihydroartemisinin–piperaquine (DP) efficacy study of Plasmodium falciparum patients during 2012–2013.
Figure 3.
Mean Plasmodium falciparum asexual parasite density (per microliter) of P. falciparum patients during the first 3 days after dihydroartemisinin–piperaquine (DP) treatment.
Figure 4.
Kaplan–Meier's survival analysis of the clearance of asexual parasites (A) and gametocytes (B) of the children (≤ 15 years) and adult (> 15 years).
Table 3.
Outcomes of Plasmodium falciparum patients treated with dihydroartemisinin–piperaquine
| Children (≤ 15 years) (N = 22) | Adults (N = 49) | Total (N = 71) | |
|---|---|---|---|
| Patients positive for asexual parasitemia (%)* | |||
| Day 1 | 7 (31.82%) | 25 (51.02%) | 32 (45.07%) |
| Day 2 | 4 (18.18%) | 14 (28.57%) | 18 (25.35%) |
| Day 3 | 1 (4.55%) | 4 (8.16%) | 5 (7.04%) |
| Parasite clearance time (hour) (geometric mean [95% CI]) | 32.6 (23.7–41.6) | 39.0 (32.3–45.6) | 36.9 (31.5–42.3) |
| 42-day cure rate | 100 | 100 | 100 |
| Patients positive for sexual forms (%)† | |||
| Day 0 | 8 (36.36%) | 11 (22.45%) | 19 (26.76%) |
| Day 1 | 4 (18.18%) | 6 (12.24%) | 10 (14.08%) |
| Day 2 | 4 (18.18%) | 4 (18.18%) | 8 (11.27%) |
| Day 3 | 3 (13.64%) | 4 (18.18%) | 7 (9.86%) |
| Day 7 | 1 (4.55%) | 2 (4.08%) | 3 (4.23%) |
| Day 14 | 0 | 1 (2.04%) | 1 (1.41%) |
| Patients gametocyte positive during day 1–14 | 5 (22.73%) | 8 (16.33%) | 13 (18.31%) |
CI = confidence interval.
No significant difference in daily numbers of asexual stage–positive patients between the two age groups (P > 0.05, Fisher's exact test).
No significant difference in daily numbers of gametocyte-positive patients between the two age groups (P > 0.05, Fisher's exact test).
Of the 19 patients that presented with gametocytemia at the time of admission, 14 cleared gametocytes by day 1 (Table 3). However, five patients without gametocytemia on day 0 developed detectable gametocytemia, giving a total of 10 (14.08%) gametocytemic patients on day 1. On day 3, seven were gametocyte positive with five being persistent. On day 7, three patients had detectable gametocytemia, all being persistent. Interestingly, one patient had persistent gametocytemia through day 14. No gametocytes were detected in subsequent time points of the follow-up through day 42. At enrollment, it appeared that more children ≤ 15 years of age carried gametocytes (36.36%). Gametocyte clearance rates were also similar between the two age groups (P > 0.05, Kaplan–Meier log-rank test) (Figure 4B). Daily gametocyte positive rates were not significantly different between the two age groups (P > 0.05, Fisher's exact test).
Discussion
ACTs as the frontline treatment of uncomplicated P. falciparum malaria have been adopted by all falciparum-endemic countries. ACTs involve the combination of an artemisinin family drug with another longer-acting partner drugs: artemisinins can rapidly reduce peripheral parasitemia,21 while the partner drugs clear the remaining parasites.22 The ACTs recommended by the WHO are artemether–lumefantrine (AL), artesunate–amodiaquine, artesunate–mefloquine (AM), DP, and artesunate plus sulfadoxine–pyrimethamine.23 The selection of a particular ACT for a region is affected by several factors and may not always be the most logical with regard to past malaria treatment history. China's Yunnan province and neighboring Myanmar regions adopted the DP combination since 2009. Piperaquine resistance has been detected in China when it was used as a therapeutic replacement of chloroquine.24 This may compromise the role of piperaquine in eliminating parasites that remain after dihydroartemisinin treatment. Nevertheless, clinical trials in Africa and southeast Asia have documented excellent efficacy of DP for treatment of falciparum malaria.25–27 More importantly, several comparative trials of DP versus AL in Africa revealed another advantage of DP in reducing the risk of recurrent parasitemia within 4–9 weeks of treatment.26–29 However, clinical failure with DP was recently reported from Cambodia.23 The clinically resistant parasites from this trial showed increased in vitro IC50 values to piperaquine, suggesting piperaquine resistance may be partially responsible. This study documented clinical failure of another ACT after AM in the GMS, highlighting the necessity of close monitoring of the spread or independent emergence of artemisinin resistance in this region.
Earlier clinical trials in the GMS, which first recorded artemisinin resistance, used artesunate monotherapy for 7 days or a staggered regimen with artesunate for 3 days followed by the ACT partner drugs.5,8,12 These findings prompted strengthened surveillance in the entire GMS to determine whether artemisinin resistance has spread to or emerged in other areas. In 2008–2010, 91 studies were conducted at 32 sentinel sites of the GMS using AM, AL, and DP.11 The most striking finding was that in Palin, Cambodia, where artesunate resistance was first found, the day 3 parasitemia rose sharply from 26% in 2008 to 45% in 2010. In two sites in Vietnam and one site in China, the proportions of day 3 parasite-positive cases also went up to above the 10% cutoff of WHO's working definition of suspected artemisinin resistance,30 whereas in two Myanmar sites bordering with Thailand, day 3 parasitemic cases had increased to almost 20%. An extreme situation was the recent detection of 56% day 2 parasite-positive patients in northwest Cambodia with well-documented clinical failure of DP in 36% of the cases.23 In this study, we monitored clinical efficacy of DP for treatment of uncomplicated falciparum malaria in 109 patients recruited in the northeastern Myanmar. Although our study showed excellent clinical efficacy of DP without the detection of recurrent parasitemia within 42 days of follow-up, we detected ∼7% of patients with peripheral asexual parasitemia on day 3 after initiation of DP treatment, which is slightly higher than the 6.2% day 3 parasite-positive rate observed during an earlier study in the same region.18 It is noteworthy that a study performed in the nearby Yingjiang County, Yunnan Province, showed that 13.6% of falciparum patients were parasite positive on day 3 after DP treatment.11 Another study conducted in 2009 in the same region showed that 7-day artesunate therapy provided excellent efficacy (95.9%), but the day 3 parasite-positive rate was 18.5%,19 much higher than the studies performed with DP.18 The lower levels of day 3 positive rate observed with DP compared with artesunate monotherapy might be due to the effect of the partner drug piperaquine, which should have played an additional role in early parasite clearance. In this regard, piperaquine appeared better than lumefantrine in clearing earlier parasitemia when administered together with an artemisinin drug. For example, a study performed in Uganda showed that earlier treatment failures requiring rescue therapy within 3 days were significantly less frequent with DP than with AL.29 This means that despite we only observed 7.04% day 3 positive cases, artemisinin-resistant parasites were very likely to exist in our study site. It has been recognized that day 3 parasite-positive rate is probably imprecise for predicting treatment outcome.17 Nonetheless, it is easy to perform and would prove useful for identifying potential foci of artemisinin resistance. Combination of treatment efficacy studies with the recently identified molecular marker K1331 would provide an effective way of monitoring artemisinin resistance emergence and spread.12 One major limitation of this study was the large loss-to-follow-up rate, which undermines the generalizability of the finding. In the worst scenario, a modified intent-to-treat analysis by considering the losses to follow-up as treatment failures would drop the DP treatment efficacy to ∼65% with no significant difference found between the children and adult groups (P = 0.83, Fisher's exact test). Yet, the 100% cure rate in our study was consistent with that from an earlier study conducted at the same site with the same ACT,18 suggesting that DP remained highly effective for treating uncomplicated falciparum malaria. Meanwhile, the high mobility of the camp populations also poses an additional difficulty for implementing control measures including effective treatment of the clinical cases. This is even more important for vivax malaria since radical cure of this disease requires a 14-day course of primaquine treatment.
The presence of parasitemia on day 3 after ACT treatment depends on many factors, among which is the baseline parasite density at the time of initial treatment.32 Whereas inclusion of patients with day 0 parasite density above 100,000/μL was allowed in drug efficacy studies in the GMS, only two patients had an initial parasite density between 100,000 and 150,000/μL. However, we did not detect a significant association between day 3 and day 0 parasitemias. At enrollment, the proportion of gametocytemic patients (18.4%) was comparable with those found in the GMS such as in Cambodia (19% in Pailin and 18% in Pursat), much lower than what were typically found in Africa.12,33 Whereas there was a gradual reduction of gametocyte positive cases after DP treatment, gametocytes were detected through day 14 in one patient. Importantly, there were cases where gametocytes were newly detected after DP treatment, suggesting that DP may not be effective in killing gametocytes or preventing transmission. It is noteworthy that microscopy has limited sensitivity in detecting low gametocytemia, and more sensitive molecular methods are needed to validate these findings. Furthermore, the efficacy of the new policy advocated by WHO for including a single dosage of primaquine for eliminating gametocytes and reducing transmission needs to be evaluated.
In summary, this study revealed that the current DP regimen as the recommended ACT for treatment of uncomplicated falciparum malaria at the China–Myanmar border area remains highly efficacious. Whereas the day 3 parasitemic cases were still below the 10% cutoff for suspected artemisinin resistance, the increased proportion of patients with day 3 parasitemia to > 10% in a nearby area of China demands further scrutiny of the situation. Besides, our recent analysis of the K13 gene in longitudinally collected samples from this study area showed increasing prevalence of parasites carrying mutations in the kelch domain, suggesting artemisinin resistance may also be evolving in this area.34 Furthermore, given the recently documented failure of DP in Cambodia, continuous monitoring of clinical efficacy of ACT is warranted to deter the emergence and contain the potential spread of artemisinin resistance.
ACKNOWLEDGMENTS
We acknowledge staff from the local hospital and malaria clinics for data collection and slide reading and malaria patients for participation in this study.
Footnotes
Financial support: This work was supported by National Natural Science Foundation of China (no. U1202226, 31260508, and 81271875), a talent introduction project of Yunnan province (no. 2013HA026), and National Institutes of Health, Bethesda, MD (U19AI089672).
Authors' addresses: Ying Wang, Institute of Tropical Medicine, Third Military Medical University, Chongqing, China, E-mail: wangyingtmmu@126.com. Zhaoqing Yang and Lili Yuan, Department of Pathogen Biology and Immunology, Kunming Medical University, Kunming, China, E-mails: zhaoqingy92@hotmail.com and yuanlili201309@gmail.com. Guofa Zhou, Ming-Chieh Lee, and Guiyun Yan, Program in Public Health, University of California at Irvine, Irvine, CA, E-mails: zhoug@uci.edu, mingchil@uci.edu, and guiyuny@uci.edu. Qi Fan, Dalian Institute of Biotechnology, Dalian, Liaoning, China, E-mail: qifan10001@163.com. Yuping Xiao and Yaming Cao, Institute of Pathology and Pathophysiology, China Medical University, Shenyang, China, E-mails: ypxiao@mail.cmu.edu.cn and ymccmu@163.com. Daniel Parker and Liwang Cui, Department of Entomology, Pennsylvania State University, University Park, PA, E-mails: daniel@shoklo-unit.com and luc2@psu.edu.
References
- 1.Cui L, Yan G, Sattabongkot J, Cao Y, Chen B, Chen X, Fan Q, Fang Q, Jongwutiwes S, Parker D, Sirichaisinthop J, Kyaw MP, Su XZ, Yang H, Yang Z, Wang B, Xu J, Zheng B, Zhong D, Zhou G. Malaria in the Greater Mekong Subregion: heterogeneity and complexity. Acta Trop. 2012;121:227–239. doi: 10.1016/j.actatropica.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hewitt S, Delacollette C, Chavez I. Malaria situation in the Greater Mekong Subregion. Southeast Asian J Trop Med Public Health. 2014;44(Suppl 1):46–72. discussion 306–307. [PubMed] [Google Scholar]
- 3.Feachem RG, Phillips AA, Hwang J, Cotter C, Wielgosz B, Greenwood BM, Sabot O, Rodriguez MH, Abeyasinghe RR, Ghebreyesus TA, Snow RW. Shrinking the malaria map: progress and prospects. Lancet. 2010;376:1566–1578. doi: 10.1016/S0140-6736(10)61270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bethell D, Se Y, Lon C, Tyner S, Saunders D, Sriwichai S, Darapiseth S, Teja-Isavadharm P, Khemawoot P, Schaecher K, Ruttvisutinunt W, Lin J, Kuntawungin W, Gosi P, Timmermans A, Smith B, Socheat D, Fukuda MM. Artesunate dose escalation for the treatment of uncomplicated malaria in a region of reported artemisinin resistance: a randomized clinical trial. PLoS One. 2011;6:e19283. doi: 10.1371/journal.pone.0019283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NP, Lindegardh N, Socheat D, White NJ. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noedl H, Se Y, Sriwichai S, Schaecher K, Teja-Isavadharm P, Smith B, Rutvisuttinunt W, Bethell D, Surasri S, Fukuda MM, Socheat D, Chan Thap L. Artemisinin resistance in Cambodia: a clinical trial designed to address an emerging problem in southeast Asia. Clin Infect Dis. 2010;51:e82–e89. doi: 10.1086/657120. [DOI] [PubMed] [Google Scholar]
- 7.Amaratunga C, Sreng S, Suon S, Phelps ES, Stepniewska K, Lim P, Zhou C, Mao S, Anderson JM, Lindegardh N, Jiang H, Song J, Su XZ, White NJ, Dondorp AM, Anderson TJ, Fay MP, Mu J, Duong S, Fairhurst RM. Artemisinin-resistant Plasmodium falciparum in Pursat province, western Cambodia: a parasite clearance rate study. Lancet Infect Dis. 2012;12:851–858. doi: 10.1016/S1473-3099(12)70181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM. Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med. 2008;359:2619–2620. doi: 10.1056/NEJMc0805011. [DOI] [PubMed] [Google Scholar]
- 9.WHO . World Malaria Report 2013. Geneva, Switzerland: World Health Organization; 2013. [Google Scholar]
- 10.Hien TT, Thuy-Nhien NT, Phu NH, Boni MF, Thanh NV, Nha-Ca NT, Thai le H, Thai CQ, Toi PV, Thuan PD, Long le T, Dong le T, Merson L, Dolecek C, Stepniewska K, Ringwald P, White NJ, Farrar J, Wolbers M. In vivo susceptibility of Plasmodium falciparum to artesunate in Binh Phuoc Province, Vietnam. Malar J. 2012;11:355. doi: 10.1186/1475-2875-11-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bustos MD, Wongsrichanalai C, Delacollette C, Burkholder B. Monitoring antimalarial drug efficacy in the Greater Mekong Subregion: an overview of in vivo results from 2008 to 2010. Southeast Asian J Trop Med Public Health. 2013;44(Suppl 1):201–230. discussion 306–307. [PubMed] [Google Scholar]
- 12.Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, Sreng S, Anderson JM, Mao S, Sam B, Sopha C, Chuor CM, Nguon C, Sovannaroth S, Pukrittayakamee S, Jittamala P, Chotivanich K, Chutasmit K, Suchatsoonthorn C, Runcharoen R, Hien TT, Thuy-Nhien NT, Thanh NV, Phu NH, Htut Y, Han KT, Aye KH, Mokuolu OA, Olaosebikan RR, Folaranmi OO, Mayxay M, Khanthavong M, Hongvanthong B, Newton PN, Onyamboko MA, Fanello CI, Tshefu AK, Mishra N, Valecha N, Phyo AP, Nosten F, Yi P, Tripura R, Borrmann S, Bashraheil M, Peshu J, Faiz MA, Ghose A, Hossain MA, Samad R, Rahman MR, Hasan MM, Islam A, Miotto O, Amato R, MacInnis B, Stalker J, Kwiatkowski DP, Bozdech Z, Jeeyapant A, Cheah PY, Sakulthaew T, Chalk J, Intharabut B, Silamut K, Lee SJ, Vihokhern B, Kunasol C, Imwong M, Tarning J, Taylor WJ, Yeung S, Woodrow CJ, Flegg JA, Das D, Smith J, Venkatesan M, Plowe CV, Stepniewska K, Guerin PJ, Dondorp AM, Day NP, White NJ. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2014;371:411–423. doi: 10.1056/NEJMoa1314981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO Global Plan for Artemisinin Resistance Containment (GPARC) 2011. http://apps.who.int/iris/bitstream/10665/44482/1/9789241500838_eng.pdf?ua=1 Available at. Accessed May 1, 2015.
- 14.Phyo AP, Nkhoma S, Stepniewska K, Ashley EA, Nair S, McGready R, ler Moo C, Al-Saai S, Dondorp AM, Lwin KM, Singhasivanon P, Day NP, White NJ, Anderson TJ, Nosten F. Emergence of artemisinin-resistant malaria on the western border of Thailand: a longitudinal study. Lancet. 2012;379:1960–1966. doi: 10.1016/S0140-6736(12)60484-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flegg JA, Guerin PJ, Nosten F, Ashley EA, Phyo AP, Dondorp AM, Fairhurst RM, Socheat D, Borrmann S, Bjorkman A, Martensson A, Mayxay M, Newton PN, Bethell D, Se Y, Noedl H, Diakite M, Djimde AA, Hien TT, White NJ, Stepniewska K. Optimal sampling designs for estimation of Plasmodium falciparum clearance rates in patients treated with artemisinin derivatives. Malar J. 2013;12:411. doi: 10.1186/1475-2875-12-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Das D, Price RN, Bethell D, Guerin PJ, Stepniewska K. Early parasitological response following artemisinin-containing regimens: a critical review of the literature. Malar J. 2013;12:125. doi: 10.1186/1475-2875-12-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stepniewska K, Ashley E, Lee SJ, Anstey N, Barnes KI, Binh TQ, D'Alessandro U, Day NP, de Vries PJ, Dorsey G, Guthmann JP, Mayxay M, Newton PN, Olliaro P, Osorio L, Price RN, Rowland M, Smithuis F, Taylor WR, Nosten F, White NJ. In vivo parasitological measures of artemisinin susceptibility. J Infect Dis. 2010;201:570–579. doi: 10.1086/650301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun X, Zhang Z, Wang J, Deng Y, Yang Y, Lasi J, Sun X, Wang H. Therapeutic efficacy and safety of compound dihydroartemisinin/piperaquine for uncomplicated Plasmodium falciparum infection in Laiza city of Myanmar bordering on China. Chin J Parasitol Parasit Dis. 2011;29:372–375. [PubMed] [Google Scholar]
- 19.Huang F, Tang L, Yang H, Zhou S, Sun X, Liu H. Therapeutic efficacy of artesunate in the treatment of uncomplicated Plasmodium falciparum malaria and anti-malarial, drug-resistance marker polymorphisms in populations near the China-Myanmar border. Malar J. 2012;11:278. doi: 10.1186/1475-2875-11-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li N, Parker DM, Yang Z, Fan Q, Zhou G, Ai G, Duan J, Lee MC, Yan G, Matthews SA, Cui L, Wang Y. Risk factors associated with slide positivity among febrile patients in a conflict zone of north-eastern Myanmar along the China-Myanmar border. Malar J. 2013;12:361. doi: 10.1186/1475-2875-12-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White NJ. Qinghaosu (artemisinin): the price of success. Science. 2008;320:330–334. doi: 10.1126/science.1155165. [DOI] [PubMed] [Google Scholar]
- 22.Ashley EA, White NJ. Artemisinin-based combinations. Curr Opin Infect Dis. 2005;18:531–536. doi: 10.1097/01.qco.0000186848.46417.6c. [DOI] [PubMed] [Google Scholar]
- 23.WHO . Guidelines for the Treatment of Malaria. 2nd edition. Geneva, Switzerland: World Health Organization; 2010. p. 194 pp. [Google Scholar]
- 24.Davis TM, Hung TY, Sim IK, Karunajeewa HA, Ilett KF. Piperaquine: a resurgent antimalarial drug. Drugs. 2005;65:75–87. doi: 10.2165/00003495-200565010-00004. [DOI] [PubMed] [Google Scholar]
- 25.Ashley EA, McGready R, Hutagalung R, Phaiphun L, Slight T, Proux S, Thwai KL, Barends M, Looareesuwan S, White NJ, Nosten F. A randomized, controlled study of a simple, once-daily regimen of dihydroartemisinin-piperaquine for the treatment of uncomplicated, multidrug-resistant falciparum malaria. Clin Infect Dis. 2005;41:425–432. doi: 10.1086/432011. [DOI] [PubMed] [Google Scholar]
- 26.Yeka A, Dorsey G, Kamya MR, Talisuna A, Lugemwa M, Rwakimari JB, Staedke SG, Rosenthal PJ, Wabwire-Mangen F, Bukirwa H. Artemether-lumefantrine versus dihydroartemisinin-piperaquine for treating uncomplicated malaria: a randomized trial to guide policy in Uganda. PLoS ONE. 2008;3:e2390. doi: 10.1371/journal.pone.0002390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arinaitwe E, Sandison TG, Wanzira H, Kakuru A, Homsy J, Kalamya J, Kamya MR, Vora N, Greenhouse B, Rosenthal PJ, Tappero J, Dorsey G. Artemether-lumefantrine versus dihydroartemisinin-piperaquine for falciparum malaria: a longitudinal, randomized trial in young Ugandan children. Clin Infect Dis. 2009;49:1629–1637. doi: 10.1086/647946. [DOI] [PubMed] [Google Scholar]
- 28.Kamya MR, Yeka A, Bukirwa H, Lugemwa M, Rwakimari JB, Staedke SG, Talisuna AO, Greenhouse B, Nosten F, Rosenthal PJ, Wabwire-Mangen F, Dorsey G. Artemether-lumefantrine versus dihydroartemisinin-piperaquine for treatment of malaria: a randomized trial. PLoS Clin Trials. 2007;2:e20. doi: 10.1371/journal.pctr.0020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wanzira H, Kakuru A, Arinaitwe E, Bigira V, Muhindo MK, Conrad M, Rosenthal PJ, Kamya MR, Tappero JW, Dorsey G. Longitudinal outcomes in a cohort of Ugandan children randomized to artemether-lumefantrine versus dihydroartemisinin-piperaquine for the treatment of malaria. Clin Infect Dis. 2014;59:509–516. doi: 10.1093/cid/ciu353. [DOI] [PubMed] [Google Scholar]
- 30.WHO . Development of a Strategy Towards Elimination of Plasmodium falciparum Parasites with Altered Response to Artemisinins. Geneva, Switzerland: World Health Organization; 2009. p. 52 pp. [Google Scholar]
- 31.Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois AC, Khim N, Kim S, Duru V, Bouchier C, Ma L, Lim P, Leang R, Duong S, Sreng S, Suon S, Chuor CM, Bout DM, Menard S, Rogers WO, Genton B, Fandeur T, Miotto O, Ringwald P, Le Bras J, Berry A, Barale JC, Fairhurst RM, Benoit-Vical F, Mercereau-Puijalon O, Menard D. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature. 2014;505:50–55. doi: 10.1038/nature12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White NJ. Assessment of the pharmacodynamic properties of antimalarial drugs in vivo. Antimicrob Agents Chemother. 1997;41:1413–1422. doi: 10.1128/aac.41.7.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yeka A, Tibenderana J, Achan J, D'Alessandro U, Talisuna AO. Efficacy of quinine, artemether-lumefantrine and dihydroartemisinin-piperaquine as rescue treatment for uncomplicated malaria in Ugandan children. PLoS One. 2013;8:e53772. doi: 10.1371/journal.pone.0053772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Z, Shrestha S, Li X, Miao J, Yuan L, Cabrera M, Grube C, Yang Z, Cui L. Prevalence of K13-propeller polymorphisms in Plasmodium falciparum from China-Myanmar border in 2007–2012. Malar J. 2015;14:168. doi: 10.1186/s12936-015-0672-9. [DOI] [PMC free article] [PubMed] [Google Scholar]