SUMMARY
Angiogenesis is one of the six originally constituted hallmarks of cancer that has been extensively studied in the last two decades. The aim of our study is to assess the microvessel and macrophageal density in laryngeal carcinoma and its clinicopathological correlations. We immunohistochemically assessed microvessel density (CD34) and macrophage count (CD68) using microarray techniques and then looked for clinicopathological correlations. The mean micro-vessel density in the study group was 14.27 ± 12.92 vessels in a ×200 field with a mean macrophageal infiltration density of 5.19 ± 4.32. Median microvessel density was significantly higher in patients with metastasis than in patients without metastasis. Additionally, linear regression established that macrophageal infiltration density could predict microvessel density in laryngeal carcinoma. We found no association between either factor and recurrence rate or other clinical characteristics. Our study adds additional data to a problem that has been widely studied during the last two decades, even if controversies in this area still remain.
KEY WORDS: Laryngeal carcinoma, Macrophage count, Micro-vessel density, CD31, CD68
RIASSUNTO
L'angiogenesi è uno dei sei principali meccanismi alla base del cancro, ed è stato studiato approfonditamente negli ultimi 20 anni. L'obiettivo del presente studio è stato quello di determinare sia la densità capillare sia l'infiltrato macrofagico nei campioni di carcinoma laringeo e di determinarne la correlazione con gli aspetti clinici e patologici. Sia la densità capillare (CD34) sia l'infiltrato macrofagico (CD68) sono stati determinati con metodiche immunoistochimiche mediante microarray. Il nostro campione ha mostrato una densità capillare media di 14,27 ± 12,92 vasi su campo ingrandito a 200×, e l'infiltrato macrofagico medio è stato di 5,19 ± 4,32. La densità capillare si è dimostrata superiore nei pazienti metastatici. Inoltre uno studio di regressione lineare ha mostrato che l'entità dell'infiltrato macrofagico poteva predire la densità capillare del campione di carcinoma laringeo preso in esame. Non abbiamo invece individuato una correlazione fra ambo i fattori studiati e l'incidenza delle recidive o gli altri fattori clinici presi in esame. Il nostro studio aggiunge dati ad un problema che per quanto studiato a fondo negli ultimi 20 anni resta nella sostanza controverso.
Introduction
Angiogenesis is one of the six originally constituted hallmarks of cancer that has been extensively studied in the last two decades 1. It is an intricate multistep process of endothelial migration and proliferation, capillary maturation, anastomosis and lumen development along with extracellular matrix remodelling 2 3. Numerous studies have demonstrated that highly vascularised tumours show a higher potency to produce metastases compared to less angiogenic neoplasms 4-6. Angiogenesis requires the activation of many receptors by their respective cognate ligands. These include vascular endothelial growth factors (VEGF), placental growth factor (PIGF), fibroblast growth factors (FGF-1 and -2), platelet-derived growth factor (PDGF), hepatocyte growth factor (HGF), angiopoietins (Ang-1 and -2), epidermal growth factor/transforming growth factor-α (EGF/TGF-α) and others. VEGF is known to play the most important role in angiogenesis. The most common antibodies used for microvessel staining at present are against Von Willebrand Factor (Factor VIII), CD31 and CD34.
Macrophages with other inflammatory cell types provide growth factors, cytokines, proteolytic enzymes, proteoglycan, lipid mediators and prostaglandins. All of these factors cause marked changes in inflammatory loci by interacting with epithelial, mesenchymal and vascular endothelial cells 7. Almost 150 years ago, Rudolf Virchow first indicated the concept that lymphoreticular infiltration reflects the origin of cancer at sites of chronic inflammation, suggesting a close connection between inflammation and the development of cancer. Nowadays, the features shared by cancer and inflammation have been highlighted by proposing novel therapeutic strategies targeting the inflammatory responses in tumour stroma. A recent study demonstrated that stromal interactions between malignant cells and inflammatory cells may be closely associated with angiogenesis and progression of cancer 8.
The aim of our study was to assess microvessel and macrophageal density in laryngeal carcinoma and its clinicopathological correlations.
Materials and methods
Patient recruitment and assessment
The study was carried out in the ENT department of University Hospital "Queen Jovanna", Sofia, Bulgaria in cooperation with the Molecular Medicine Center at Medical University of Sofia over the period 2010-2013. Fifty-two patients with histopathologically verified carcinoma of the larynx were enrolled in the study. Informed consent was obtained from all patients and the protocol of the study was approved by the Ethics committee of Medical University of Sofia. A standardised clinical history was obtained for each patient. Detailed descriptions of the endoscopic/ microscopic direct laryngoscopy findings were recorded along with the results of CT scans. All patients were followed for at least two years after their operation and any locoregional recurrences and death events were recorded.
Tissue microarray construction and immunohistochemistry
In this study, paraffin tissue microarray construction was conducted as routine method. Briefly, a section was cut from each paraffin block and stained with haematoxylin and eosin (H&E). Each donor block was overlaid with the corresponding H&E slide and observed by experienced pathologists. The area in the donor block for tissue microarray sampling was verified according to the H&E slide and marked. An automatic tissue arrayer (Beecher Instruments Inc., USA) was used for array construction. Three representative 1.0-mm cores were removed from each donor block and transferred to a premolded recipient paraffin block with designated orientation. Monoclonal antibody CD31 was used for visualisation of microvessel density and CD68 for macrophageal infiltration density (Dako, Agilent Technologies Inc.). Visualisation was performed with an EnVision FLEX, Mini Kit, High pH (Dako, Agilent Technologies Inc.).
Assessment of tumour microvessel density and macrophageal infiltration
Microvessel density scoring was based on modification of the method described by Weidner et al. 10 in which large microvessels and any single brown-staining endothelial cell clearly separated from adjacent microvessels, tumour cells and other connective tissue elements were considered a single, countable microvessel. Branching structures were counted as 1, unless there was a break in the continuity of the vessel, in which case it was counted as 2 distinct vessels. Microvessel density was scored by counting the number of vessels in three ×200 fields. Positivity for CD68 was scored quantitatively according to the number of positive stromal cells by an experienced pathologist without knowledge of patient characteristics.
Results
The mean age of the study group was 60.15 ± 7.4 years (range 41-76). There were two female patients. All patients had histologically verified squamous cell carcinoma of the larynx. Distribution according to TNM classification was as follows: one patient was staged T1 (1.92%), six T2 (11.54%), 19 T3 (36.5%) and 26 T4 (50%). Thirteen patients (25%) had histologically verified lymph node metastases at the time of surgery.
The mean microvessel density in the entire study group was 14.27 ± 12.92 vessels, ×200 field, and the mean macrophageal infiltration density was 5.19 ± 4.32 (Fig. 1). There was no statistically significant difference for in vessel densities between the different tumour stages (p = 0.268, p = 0.441). A Mann-Whitney U test was run to determine if there were differences in microvessel density between patients with histologically verified metastasis and patients without metastasis at the time of surgery. Median microvessel density was significantly higher in patients with metastasis (33.04 mean rank) than in those without metastasis (24.32 mean rank), U = 338.5, p < 0.05 (Fig. 2).
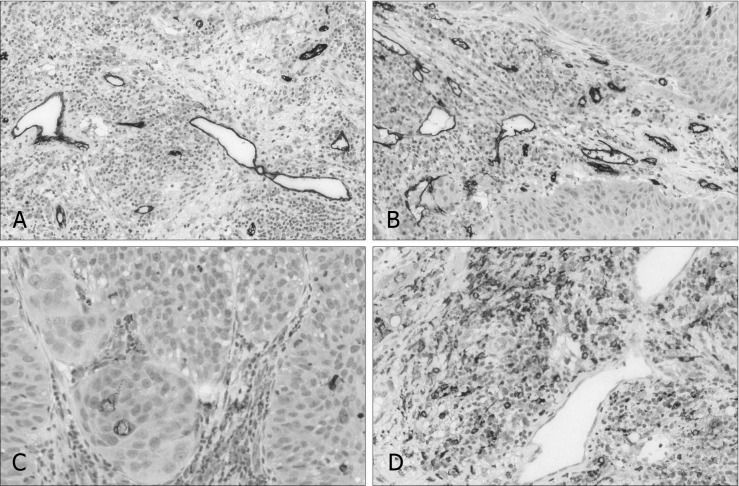
Fig. 1.
CD31+ immunohistochemical visualisation of microvessel density in laryngeal carcinoma (A, B). CD68+ immunohistochemical visualisation of macrophageal infiltration density in laryngeal carcinoma (C, D).
Fig. 2.
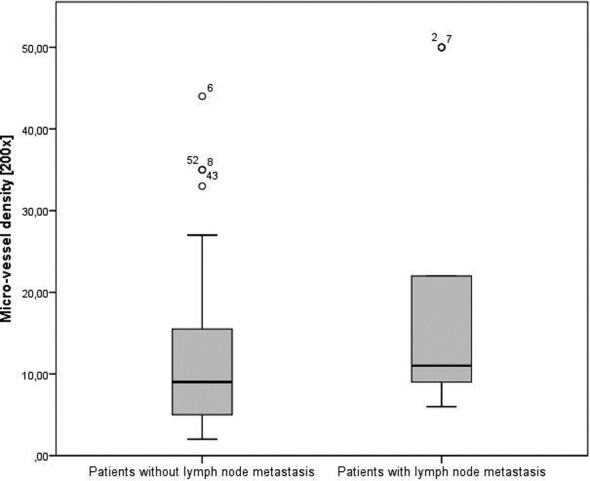
Microvessel density in patients with and without lymph node metastasis at the time of surgery.
Spearman's rank-order correlation was run to assess the relationship between microvessel density and macrophageal infiltration density in the studied group. The relationship was monotonic, as assessed by visual inspection of a scatterplot. There was significant positive correlation between both variables, rs = 0.333, p < 0.05.
Additionally, linear regression established that macrophageal infiltration density could statistically significantly predict microvessel density in laryngeal carcinoma (Fig. 3), F (1, 50) = 43.034, p < 0.0005 and macrophageal infiltration density accounted for 46.3% of the explained variability in micro-vessel density (R2 = 0.463). The regression equation was: microvessel density = -3.704 + 2.035 × (microvessel density).
Fig. 3.
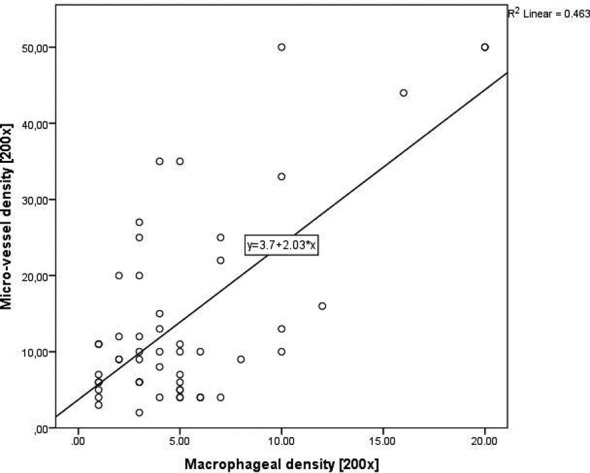
Linear correlation between microvessel and macrophageal infiltration density in laryngeal carcinoma.
We found no association between recurrence rate or other clinical characteristics and microvessel or macrophageal density.
Discussion
The role of the immune system as an important factor that significantly influences the process of tumour neovascularisation, is well-established 2. Despite this fact, we could not find any studies that report data on microvascular density and macrophageal tumour density in laryngeal carcinoma. The results from our study undeniably suggest that the tumour-associated macrophages in laryngeal carcinoma have a significant role in inducing the process of angiogenesis described in other malignancies 10.
When we consider the density of tumour angiogenesis as a prognostic clinical factor, three potential associations should be discussed. (1) Correlation between microvessel density and primary lesion stage (T-stage); (2) Correlation between micro-vessel density and the process of metastasis; (3) Correlation between microvessel density and locoregional recurrences. This is a widely studied area of interest in oncology and after a thorough research of the published literature we have found 15 publications that report results for microvessel density in laryngeal carcinoma. For all three associations, we have found controversial findings. The results from our study suggest that there is no relationship between microvessel density and T-stage, which is supported by the findings of Hagedorn 11 et al. and Tse et al. 12. Kyzas P et al. 13, Lentsch et al. 14 and Zvrko et al. 15 reach the opposite conclusion, showing that higher T-staged tumours also have a higher density of microvessels. Similarly, conflicting evidence have been reported regarding the correlation between microvessel density and dissemination of metastasis. In our study group, patients with higher microvessel density also showed a higher percentage of metastasis at the time of surgery, which was also reported by Murray et al. 16. Later, Tse et al. 12 and Kyzas et al. 13 found no such associations in their studies. The prognostic value of neovascularisation is one of the most studied prognostic factors for locoregional recurrences, which according to our results have no relationship with tumour vessel density. Published studies are divided on this aspect. Hagedorn et al. 11 and Tse et al. 12 confirm our findings, whereas Teknos et al. 17 and Marioni 18 suggest that higher rate of neovascularisation is a poor predictor of locoregional recurrence.
Conclusions
Our study adds additional data to a problem that has been widely studied during the last two decades. Despite this, the controversies in this area remain and additional studies are necessary.
Acknowledgements
Part of this study was funded by a grant from the Medical University of Sofia 13-D/2013.
References
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. What is the evidence that tumors are angiogenesis dependent? J Nat l Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 3.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 4.Zetter BR. Angiogenesis and tumor metastasis. Annu Rev Med. 1998;49:407–424. doi: 10.1146/annurev.med.49.1.407. [DOI] [PubMed] [Google Scholar]
- 5.Klijanienko J, el-Naggar AK, Braud F, et al. Tumor vascularization, mitotic index, histopathologic grade, and DNA ploidy in the assessment of 114 head and neck squamous cell carcinomas. Cancer. 1995;75:1649–1656. doi: 10.1002/1097-0142(19950401)75:7<1649::aid-cncr2820750715>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 6.Beatrice F, Cammarota R, Giordano C, et al. Angiogenesis: prognostic significance in laryngeal cancer. Anticancer Res. 1998;18:4737–4740. [PubMed] [Google Scholar]
- 7.Ono M. Molecular links between tumor angiogenesis and inflammation: inflammatory stimuli of macrophages and cancer cells as targets for therapeutic strategy. Cancer Sci. 2008;99:1501–1506. doi: 10.1111/j.1349-7006.2008.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wicki A, Christofori G. The Angiogenic Switch in Tumorigenesis. In: Marme D, Fusenig N, editors. Tumor Angiogenesis. 1st ed. Heidelberg: Springer-Verlag; 2008. pp. 79–80. [Google Scholar]
- 10.Leek RD, Lewis CE, Whitehouse R, et al. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res. 1996;56:4625–4629. [PubMed] [Google Scholar]
- 11.Hagedorn HG, Nerlich AG. Microvessel density and endothelial basement membrane composition in laryngeal squamous cell carcinomas. Acta Otolaryngol. 2000;120:891–898. doi: 10.1080/000164800750061796. [DOI] [PubMed] [Google Scholar]
- 12.Tse GM, Chan AW, Yu KH, et al. Strong immunohistochemical expression of vascular endothelial growth factor predicts overall survival in head and neck squamous cell carcinoma. Ann Surg Oncol. 2007;14:3558–3565. doi: 10.1245/s10434-007-9632-0. [DOI] [PubMed] [Google Scholar]
- 13.Kyzas PA, Stefanou D, Batistatou A, et al. Prognostic significance of VEGF immunohistochemical expression and tumor angiogenesis in head and neck squamous cell carcinoma. J Cancer Res Clin Oncol. 2005;131:624–630. doi: 10.1007/s00432-005-0003-6. [DOI] [PubMed] [Google Scholar]
- 14.Lentsch EJ, Goudy S, Sosnowski J, et al. Microvessel density in head and neck squamous cell carcinoma primary tumors and its correlation with clinical staging parameters. Laryngoscope. 2006;116:397–400. doi: 10.1097/01.MLG.0000195286.29613.E1. [DOI] [PubMed] [Google Scholar]
- 15.Zvrko E, Mikic A, Vuckovic L. CD105 expression as a measure of microvessel density in supraglottic laryngeal squamous cell carcinoma. Euro Archiv Otorhinolaryngol. 2009;266:1971–1976. doi: 10.1007/s00405-009-0962-3. [DOI] [PubMed] [Google Scholar]
- 16.Murray JD, Carlson GW, McLaughlin K, et al. Tumor angiogenesis as a prognostic factor in laryngeal cancer. Am J Surg. 1997;174:523–526. doi: 10.1016/s0002-9610(97)00168-2. [DOI] [PubMed] [Google Scholar]
- 17.Teknos TN, Cox C, Barrios MA, et al. Tumor angiogenesis as a predictive marker for organ preservation in patients with advanced laryngeal carcinoma. Laryngoscope. 2002;112:844–851. doi: 10.1097/00005537-200205000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Marioni G, Ottaviano G, Giacomelli L, et al. CD105-assessed micro-vessel density is associated with malignancy recurrence in laryngeal squamous cell carcinoma. Eur J Surg Oncol. 2006;32:1149–1153. doi: 10.1016/j.ejso.2006.08.001. [DOI] [PubMed] [Google Scholar]