SUMMARY
Sialendoscopy is a new diagnostic and surgical tool for management of salivary gland diseases that offers the opportunity to treat selected pathologies less invasively and with better results compared to previous techniques. As with any new technique, an adequate training programme involving a gradual learning curve is mandatory to quickly obtain results similar to those reported in the literature. This includes an appropriate diagnostic programme, correct patient selection and knowledge of possible pitfalls. In this retrospective study, the outcomes of the first 141 procedures (74 on the parotid gland and 67 on the submandibular gland) performed with this technique in our Department from 2009 to 2013 were compared with those reported in the literature. Patients were divided into three groups: Group A (the first 49 procedures performed), Group B (the next 50 procedures), and Group C (the last 42 procedures). There were no statistically significant differences relative to mean procedure times, recurrence of symptomatology after treatment, need for further treatments and rates of minor complications between groups. No major complications were seen. The increase in experience resulted in an increased number of interventional sialendoscopies performed under local anaesthesia instead of general anaesthesia (51% vs 18% vs 14%). In only three of 130 glands treated (2.3%) was gland resection required. We also evaluated which technique had been used for stone removal and rate of failure, which was similar in all groups (13.6% vs 15% vs 15%). Our results do not substantially differ from those reported in the literature. Initial difficulties in catheterising the papilla could be overcome with practise on fresh human specimens or fresh pig heads. Lack of precision regarding diagnostic imaging techniques was remedied by improving the competence of the surgeon in performing pre- and postoperative ultrasound. The creation of specialised centres capable of treating up to 1 to 2 million people would be desirable in order to better stratify pathologies, validate the investment in equipment and gain the necessary experience in the various surgical techniques.
KEY WORDS: Sialendoscopy, Learning curve, Training programme
RIASSUNTO
La scialoendoscopia è un nuovo strumento diagnostico e chirurgico che offre l'opportunità di trattare alcune patologie delle ghiandole salivari con procedure non invasive e con risultati potenzialmente superiori alle precedenti tecniche. Come per tutte le nuove tecniche, per raggiungere rapidamente risultati paragonabili a quelli riportati in letteratura, è indispensabile un corretto programma di formazione che segua una graduale curva di apprendimento. Questo include un appropriato programma diagnostico, una corretta selezione dei pazienti e la conoscenza delle possibili insidie operatorie. Abbiamo eseguito uno studio retrospettivo confrontando le prime 141 procedure (74 parotidee e 67 sottomandibolari) eseguite con questa tecnica nel nostro Dipartimento dal 2009 al 2013 con analoghe esperienze riportate in letteratura. I pazienti sono stati divisi in 3 gruppi: Gruppo A (le prime 49 procedure effettuate), gruppo B (le successive 50), Gruppo C (le ultime 42 procedure effettuate). Fra i tre gruppi non sono state evidenziate differenze statisticamente significative nei tempi medi di durata delle procedure, nella percentuale di ricorrenza della sintomatologia dopo il trattamento, nel numero di pazienti che hanno necessitato di più trattamenti e nell'incidenza di complicanze minori. Non sono state riportate complicanze maggiori. Con l'acquisizione di una maggiore esperienza da parte dei chirurghi si è evidenziato un progressivo calo del numero di interventi eseguiti in anestesia generale rispetto a quelli in anestesia locale (51% vs 18% vs 14%). Solo in tre casi su 130 ghiandole trattate (2.3%) è stato necessario eseguire un'asportazione ghiandolare. Per i calcoli salivari è stato valutato il tipo di tecnica utilizzato per l'estrazione e la percentuale d'insuccesso che era analoga nei tre gruppi (13.6% vs 15% vs 15%). I nostri risultati non differiscono sostanzialmente da quelli riportati in letteratura. Abbiamo risolto la difficoltà iniziale nella cateterizzazione del dotto con esercizi chirurgici su cadavere o su teste di maiale. La mancanza di precisione degli strumenti diagnostici radiologici può essere migliorata autonomizzando il chirurgo nell'esecuzione delle ecografie pre e post-operatorie. Viene infine sottolineata l'opportunità di creare dei centri di scialoendoscopia con un bacino di utenza di circa 1 o 2 milioni di abitanti in modo da concentrare le patologie, far fronte agli elevati costi della strumentazione necessaria e poter guadagnare la necessaria esperienza nelle gestione delle varie tecniche chirurgiche.
Introduction
Obstructive sialadenitis is the most common non-neoplastic disease of the salivary glands. The most frequent cause of obstructive sialadenitis is sialolithiasis (approximately 66%), which is found more commonly in the submandibular gland (80-90%) than in the parotid gland (5-10%) and sublingual and minor salivary glands (0-5%) 1. Other aetiologies of salivary duct obstruction include stenoses, mucous plugs, anatomic ductal abnormalities, scar tissue and other foreign bodies 2.
Since its introduction into clinical practice more than a decade ago, the modern technique of sialendoscopy has been used in clinical applications and has become the diagnostic tool and treatment of choice for ductal disorders of the salivary glands 3. The natural evolution of proper technical instrumentation and sialoendoscopes has made it a safe, easy and indispensable technique.
Sialendoscopy is a minimally invasive procedure that can be carried out under local anaesthesia. The procedure can be performed in an outpatient setting since complication rates are low and recovery time is short 3 4.
The successful application of sialendoscopy , as with all minimally invasive procedures, requires a well-organised training programme. This includes appropriate diagnostic work-up, operative setting and patient selection. It is also important to understand the possible pitfalls.
Since sialendoscopy requires specific expertise, the surgeon's level of training and experience are key factors in achieving a successful outcome. Learning curves for other endoscopic procedures have already been widely reported; however, the first reports regarding sialendoscopy are very recent 3 5 6. Our objective was to review our experience, compare our outcomes to those of other groups using this technique and present our learning curve in diagnostic and interventional sialendoscopy for obstructive salivary diseases.
Materials and methods
We designed a retrospective study involving 118 patients with symptoms of obstructive sialadenitis who underwent one or more sialendoscopies in our Department from January 2009 to December 2013. We followed the indications that Nahlieli first outlined in detail regarding the diagnosis and treatment of obstructive salivary gland diseases 7. Sialendoscopies were performed by two different surgeons at the Ear, Nose and Throat (ENT) Unit of Sant'Orsola-Malpighi Hospital, Bologna, Italy and the ENT Metropolitan Unit, AUSL Bologna. Both surgeons attended a practical training course on pig heads before starting their surgical sialoendoscopic activity. They also had long-term experience in advanced endoscopic sinus surgery. Assistance by an experienced surgeon was provided at the beginning of their experience for the most difficult cases, especially for large salivary stone removal using combined techniques.
Our equipment included:
0.8, 1.1 and 1.6 Karl Storz semiflexible sialoendoscopes;
Storz Stone Extractor, diameter 0.4 mm, basket with 4 wires;
foreign body forceps, diameter 0.8 mm;
progressive salivary duct probes from size 0000 to size 6;
conic dilatator for salivary ducts;
full HD Image1 Storz camera.
Statistical analysis was carried out using SAS® Version 9.3. (SAS, Inc., Cary, NC). The Kruskal-Wallis, chisquare and Fisher's exact tests were used to compare the groups. Differences were considered significant at a p < 0.05.
All patients were evaluated using preoperative and postoperative ultrasound examination to diagnose sialadenitis and evaluate the outcomes of treatment.
Patients were treated with both local (LA) and general (GA) anaesthesia. Those treated with LA were prepared with lidocaine 10 g/100 ml spray before performing salivary duct dilatation. Once the instrument was introduced, the duct was rinsed with 2% lidocaine and 0.9% sodium chloride at a ratio of 1:1. Local infiltrations with 2% mepivacaine with 1:200,000 epinephrine were performed only when a papillotomy was needed or in the case of a combined technique for salivary stone removal.
At the end of each procedure, a rinsing solution with hydrocortisone 1 g/20 ml in 0.9% sodium chloride in 20 ml syringes was utilised.
Stenoses were dilated with the passage of the sialoendoscope or with forceps as balloon dilatators were not available in our Departments. Mucous plugs were removed by irrigation or by forceps.
Stones were removed using a stone extractor when possible or with a combined technique in cases of larger or impacted stones.
Neither extracorporeal shock-wave lithotripsies (ESWLs) nor laser intra-corporeal lithotripsies were performed due to the absence of these devices in our Departments.
We followed the algorithm proposed by Koch et al. for treatment of obstructive sialadenitis 8. All patients who were candidates for ESWL were sent to another institution with which we collaborate.
The following demographic and clinical data were collected: age, sex, type of treated gland, pre- and post-operative radiological data, pre- and postoperative diagnosis, intraoperative findings and treatment procedure used. Persistence and healing or worsening of the symptomatology for each gland were also analysed.
Results
During the first 5 years of our experience, 118 patients were treated. In these, 71 parotid glands and 59 submandibular glands were treated involving a total of 141 sialoendoscopic procedures: 74 sialendoscopies on the parotid gland and 67 on the submandibular gland. The mean age of patients was 48.9 years (range 6 to 99). Forty-six patients were male (39%) and 72 were female (61%).
Patients were divided into three groups to compare outcomes. Group A (the first 49 procedures performed from 2009 to 2011), Group B (the second 50 procedures performed in 2012) and Group C (the third 42 procedures performed in 2013).
The mean follow-up was 17 months (range: 1-36 months) for Group A, 11 months (range: 0-22 months) for Group B and 6 months (range: 0-13 months) for Group C. Only two patients were lost to follow-up.
The average time for procedures in each group was compared using the Kruskal-Wallis test.
The comparison was not statistically significant: Group A vs Group B vs Group C (p = 0.3480), Group A vs Group B (p = 0.2347), Group B vs C (p = 0.7896) Group A vs C (p = 0.1979).
No major complications occurred in any group. Only 3 minor complications were reported in Group A (two infections, one wire basket breaking), 4 in Group B (one wire basket breaking, one transient paresis of the VII cranial nerve, one distal stenosis, one syncope) and 2 in Group C (one lingual paraesthesia, one infection) (Table I).
Table I.
Number of glands treated, mean procedure time and standard deviation (SD) for each group, number of complications, number of gland resections, number of cases where failure to catheterise the papilla was reported.
| Group A | Group B | Group C | |
|---|---|---|---|
| Glands treated | 49 | 50 | 42 |
| Mean times (min) | 49.73 (15-110) SD 26.53 | 46.90 (15-189) SD 34.11 | 47.00 (10-180) SD 36.72 |
| Complications | 3 | 4 | 2 |
| Gland resection | 2 | 0 | 1 |
| Failure to catheterise the papilla | 1 | 2 | 2 |
Submandibular gland resection was required in only three of 130 glands treated (2.3%): one for persistent microlithiasis and two for recurrence of an intraparenchymal stone. Failure to catheterise the papilla was reported in only 5 cases (in one case, it was a parotid gland and in 4 cases a submandibular gland).
The number of patients treated with GA (Fig. 1) was higher in Group A (51% vs 18% vs 14%, p < 0.0001) compared to Groups B and C. Using the chi-square test, Group A vs Group B (p = 0.0005) and Group A vs Group C (p = 0.0002) were statistically significant, while Group B vs Group C was not (p = 0.6310).
Fig. 1.
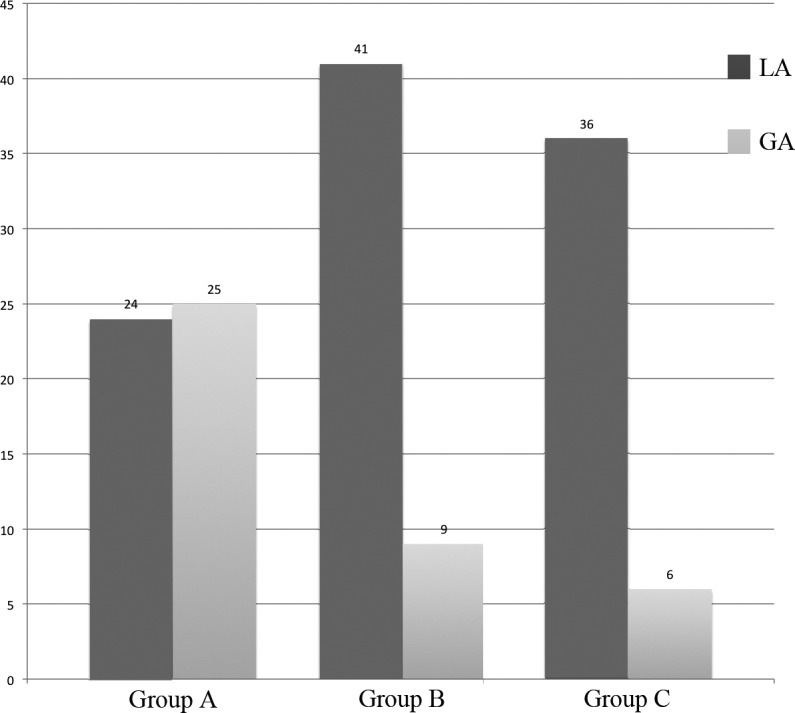
Comparison of patients who underwent LA vs GA (LA: local anaesthesia; GA: general anaesthesia).
Patients were asked at the last follow-up check-up or in a telephone interview if they had any recurrence of preoperative symptoms. Even if a higher recurrence of symptoms after treatment (Fig. 2) was reported in Group A, there was no significant difference between the three groups (34.7% vs 18.4% vs 26.8%: p = 0.1878) [Group A vs Group B (p = 0.0672), Group B vs Group C (p = 0.3362), Group A vs Group C (p = 0.4222)].
Fig. 2.
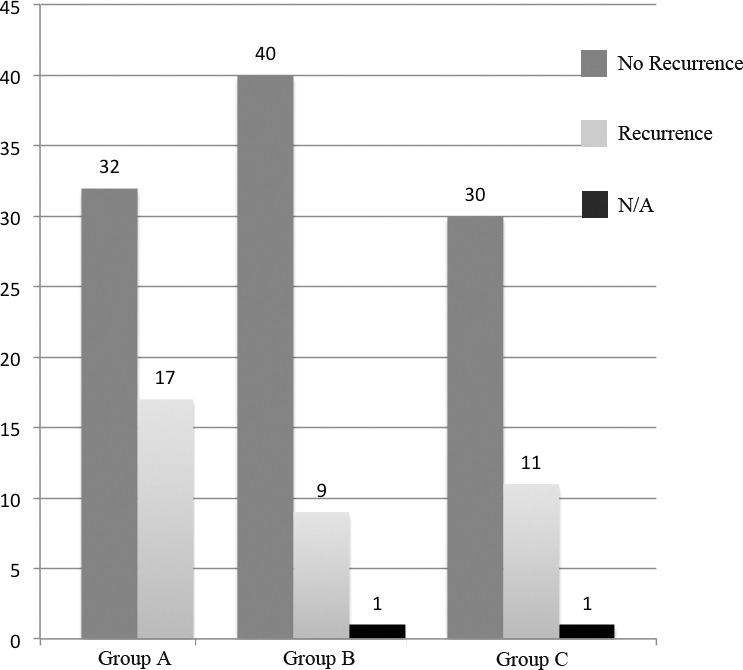
Comparison of cases with recurrence of symptoms (N/A: data not available).
With the chi-square test, there was no significant difference among cases who needed further intervention (medical or surgical) between groups (16.3% vs 12.2% vs 17.1%: p = 0.7814) (Fig. 3) [Group A vs Group B (p = 0.5637) Group B vs Group C (p = 0.5164) Group A vs Group C (p = 0.9246)].
Fig. 3.
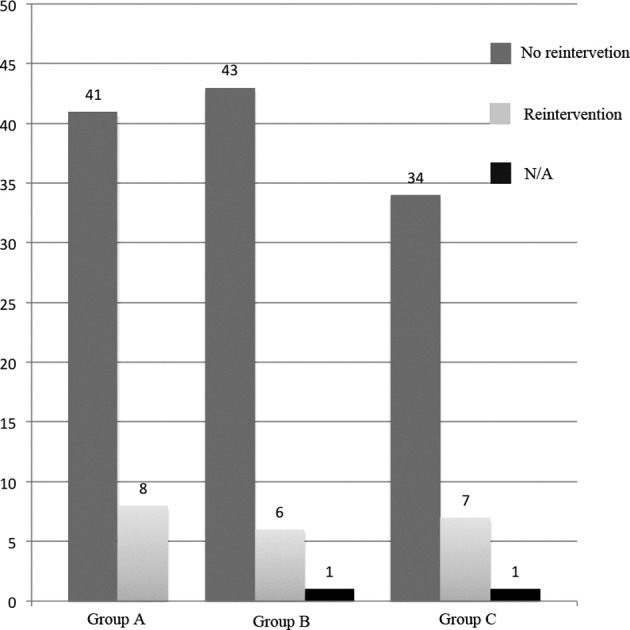
Comparison of cases were further intervention was needed (N/A: data not available).
Sialolithiasis
Sixty-two (44%) glands in 55 patients were treated for a salivary stone: 18 parotid (29%) and 44 submandibular (71%). Twenty-three patients were male (42%) and 32 were female (58%).
In 9 cases, (3 parotid and 6 submandibular), removal of the stones was not possible at the first attempt. In three cases, only a partial removal of the stones was obtained, and in 50 cases (15 parotid, 35 submandibular) complete removal at the first attempt was obtained.
Among the 9 cases in which the procedure failed, 3 underwent a second surgical operation under GA (1 parotid and 2 submandibular) with complete stone removal; in one case, microlithiasis was found (the patient underwent submandibular gland resection one year later), 4 cases are still waiting for removal and one case had spontaneous removal of the stone after a papillotomy of the parotid gland. In the 53 remaining patients, the stones were removed using a basket in 26 cases and with combined techniques in 27 cases (Fig. 4). Endoscopic removal of the stone or a combined approach was carried out in 7 cases vs 12 in Group A (36.8%), 7 vs 10 in Group B (41.2%) and 12 vs 5 in Group C (70.6%), respectively (p = 0.0949) [Group A vs Group B (p = 0.7900), Group B vs Group C (p = 0.0842) and Group A vs Group C (p = 0.0429)]. The techniques adopted for combined removal were: transoral duct slitting (for 21 submandibular glands), opening of the duct over the surface of the stone (for 5 submandibular glands) and endoscopically-assisted transcutaneous stone retrieval (for 1 parotid gland).
Fig. 4.
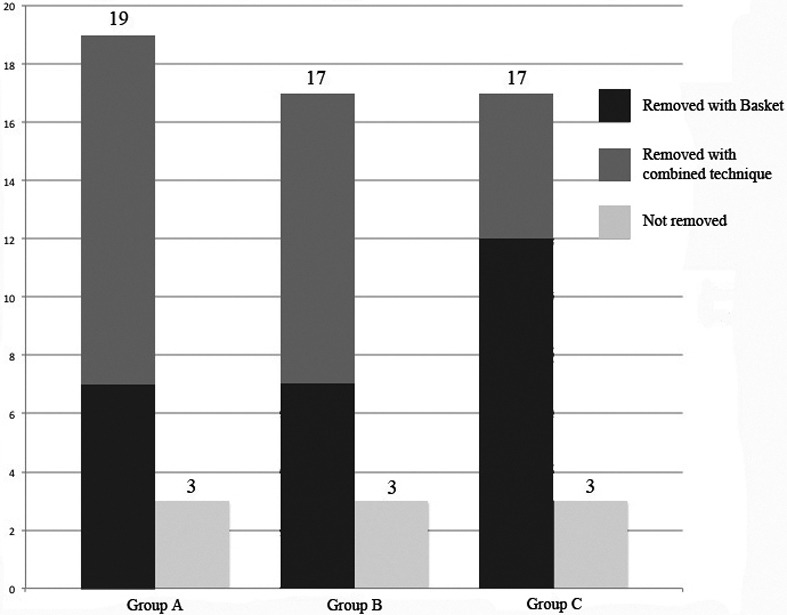
Comparison of salivary stone removal outcomes.
Using Fisher's exact test, there was no significant difference among the outcomes of stone removal between groups (86.4% vs 85% vs 85%: p = 1.0000) [A vs B (p = 1.0000) A vs C (p = 1.0000), B vs C (p = 1.0000)].
Using the Kruskal-Wallis test, there was a decrease in operative time between Group A and Group C even if this was not statistically significant (p = 0.3480) (Table II).
Table II.
Number of glands treated, mean procedure time and standard deviation (SD) for each group.
| Group A | Group B | Group C | |
|---|---|---|---|
| Glands treated | 22 | 20 | 20 |
| Mean times (min) | 69.82 (33-110) SD 24.27 | 66.15 (20-189) SD 41.53 | 61.70 (10-180) SD 42.16 |
Other causes
Twenty-eight glands were treated for ductal stenoses: 17 parotid (12%) and 11 (7.8%) submandibular. In 3 cases, a salivary stone was associated. One stenosis of a parotid gland was due to a previous stone extraction using a basket. Patients had recurrence of symptoms in 4 cases of 8 in Group A, 1 case of 9 in Group B and in 3 cases of 11 in Group C. Using Fisher's exact test, there was no significant difference between the three groups: A vs B (p = 0.1312), A vs C (p = 0.3765), B vs C (p = 0.5913).
In 3 cases, a ductal stent was positioned to avoid restenosis.
In 15 cases, mucous plugs were found (6 were isolated, 5 were associated with a ductal stenosis, 3 with sialolithiasis and 1 with a polyp involving the duct wall).
Four patients were also treated for juvenile recurrent parotitis (2 cases were bilateral). One patient had no additional episodes of sialadenitis after the first treatment, 2 patients had recurrences but less frequently, while the last is scheduled for another treatment due to persistence of the symptomatology.
Forty cases were classified as diagnostic sialendoscopies.
Discussion
The management of salivary obstruction has changed dramatically over the past 20 years 9. The pertinent data suggest that the current standard of practice, which is gland resection, will not be tenable in the future 9. Sialendoscopy is useful since it is less invasive and has a lower morbidity compared to other techniques.
Studies in animals and humans have demonstrated how obstruction of the proximal duct does not cause irreversible damage to the salivary glands 10. Recovery of secretory function after stone removal with sialendoscopy is guaranteed in most cases 11 as reported by scintigraphic examination 12 and normalisation of the histological pattern 13.
There are few reports in the literature regarding the learning curve for this new technique.
Conversely, learning curves for other endoscopic procedures have been reported, especially endoscopic sinus surgery 3. Marks has argued that, with rigorous training of the physician, the learning curve can, for the most part, be completed during residency training, allowing the new practitioner to perform endoscopic sinus surgery safely with good results 14. Sialendoscopy , like all endoscopic techniques, requires specific skills. According to Luers, a shorter learning curve can be expected because otolaryngologists are commonly experienced with endoscopic procedures in general, and an experienced supervisor can support the process by direct feedback and practical help 3. However, sialendoscopy differs from other endoscopic procedures in many ways (e.g., smaller endoscopes, newer instruments, endoscopy in a fluid-filled branched system and local anaesthesia) 3.
The actual endpoint of the individual learning curve, with performance results, operating times and rate of complications similar to those reported in the literature, could be identified in approximately 50 cases 3 5.
As with any new technology, there are several barriers for beginning a successful sialendoscopy programme 2.
Kroll, with a statistical survey regarding the prevalence of sialendoscopy in ENT clinics, documented how, in 2009, it was performed in only a minority (24%) of ENT Departments in Germany. Its diffusion was hampered by technical problems, lack of cost benefit, lack of adequate instrumentation and a limited number of patients 15.
To reach levels comparable to those reported in the literature, it is necessary to:
have a good knowledge of the anatomy and physiology of the salivary glands and the floor of the mouth 16;
have adequate instrumentation;
participate in hands on courses, conferences and live surgery;
take advantage of an experienced supervisor using direct feedback and practical help, especially during the initial procedures;
gain experience in surgical techniques for canalising and dilating the duct, and in the use of appropriate endoscopes with fresh human specimens or fresh pig heads;
have competence in managing any potential complications and be comfortable with major salivary gland resection, if required 16.
For the most part, obstructive sialadenitis (66%) is associated with sialolithiasis. However, the frequency of salivary gland stones is higher as documented by postmortem findings (1.2% of the general population) 17. Escudier calculated that 59 patients/million in the general population are hospitalised annually for up to 3 days each year with obstructive salivary gland diseases (stones and chronic sialadenitis) 18. According to Kroll, the range of pathologies that can be treated with sialendoscopy affects 2% of the general population 15. For this reason, it can be assumed that, in the near future, complete removal of a salivary gland that could have been treated with sialendoscopy will be less sustainable 15. Based on these data, it can be calculated that treatment of obstructive salivary stones will probably have to be centralised for populations of about 1 to 2 million 9 10.
The presence of approximately 30 specialised centres in Italy would therefore be desirable, evenly divided in accordance with an appropriate catchment area in order to:
centralise diseases;
validate investment in staff and equipment to provide the service 10;
gain the necessary experience in the various minimally invasive methods.
In fact, there can be many treatment options (sialendoscopy , ESWL, intracorporeal shock wave lithotripsy 19, laser intra-corporeal lithotripsy, interventional radiology, video-assisted conservative surgical removal of parotid and submandibular calculi, and botulinum therapy) 11 20. Each of these techniques may be used as a single therapeutic modality or in combination with one or more of the above-mentioned options 11. Only a centre with an almost complete range of treatment options could therefore have an adequate rate of success in cases of obstructive sialadenitis.
At the outset, the first difficulty encountered with this new technique is represented by the elevated initial cost of the sialoendoscopes and related equipment 2 15.
Sialendoscopy should therefore be initiated after having secured a catchment area which allows amortising the expense.
Technically, the first problem to be encountered is related to difficulties in canalising and dilating the duct to allow for appropriate endoscopic use, bypassing and dilating strictures 2.
In our department, this first obstacle was overcome by organising practical courses on fresh pig heads. The salivary duct anatomy of these animals is similar to that of humans and allows for good preoperative training. The experience of our group, gained with endoscopic dacryocystorhinostomy and tear duct probing, also explains the low rate of failure in our series in locating and dilating the papilla. The five cases reported in Table I (3.6%) include one in Group A, 2 in Group B and 2 in Group C, and cannot be considered as related to lack of experience (in one case, it was due to a distal stenosis). When initial identification and dilatation of the punctum seems challenging, it may be useful to perform it under magnification with loupes or, as reported by other authors, with a microscope 2. In our experience, we preferred to start this part of the procedure using a conic dilatator since, unlike progressive probes, they are less traumatic and lead to a lower risk of creating false paths.
Sialoendoscopic treatment of salivary stones without the need for gland resection may be performed more frequently with increased surgical experience as confirmed by our and other series 6.
There was the same number of cases in each group in which it was not possible to remove the stone (3 cases in each group). In our department, additional improvement and a less frequent need to perform a combined approach can be guaranteed by the introduction of an extracorporeal shock-wave lithotritor and a laser for intra-corporeal lithotripsy.
Another parameter in the advancement of the learning curve is the need to perform the sialoendoscopic procedure under general anaesthesia increasingly less frequently. In Group A, the number of patients operated on under general anaesthesia was almost the same as those operated on under local anaesthesia with a statistically significant improvement in Groups B and C. The first obstacles and difficulties, common to any new procedure, can easily be overcome by performing the first procedures under general anaesthesia, which will help in achieving the learning curve faster. According to Vairel et al., with an increase in experience, an increased number of interventional sialendoscopies could be performed under local anaesthesia, limiting the use of general anaesthesia to more complex cases or to non-compliant patients 5.
Considering our results, there are no statistically significant differences among procedure times, number of complications or rate of success among and after the first 30-50 cases, which were considered the endpoint of the learning curve. According to our experience, we found it more difficult to plan a correct approach for each individual case rather than to perform the surgical procedure itself. This was mainly due to the fact that, in many cases, it was not possible to rely on preoperative radiological findings and, consequently, to accurately predict surgical timing. Nahlieli et al. reported that, of 22 patients treated for submandibular sialolithiasis, 7 (32%) were undetected by imaging techniques (conventional radiography, sialography and ultrasound) and of 10 patients with sialoliths in the parotid gland, 7 (70%) sialoliths were not detected 21.
In our experience, in 20 cases in which a stone was detected with echography, there was no evidence of it with sialendoscopy ; in only 4 of these cases did additional examination demonstrate the presence of the stones in the salivary duct system. In 47 cases, echography was positive for a salivary stone as confirmed by sialendoscopy but, in 9 cases (6.4%), it was undetected and found only with sialendoscopy .
The large variability in the timing of each case due to the lack of pre-operative information can cause problems in planning interventions or in deciding whether to use general or local anaesthesia.
A possible solution would be to increase the competence of the surgeon in carrying out pre- and postoperative ultrasound. This is already the case in German and Swiss centres. This could help in acquiring more experience in preoperative diagnostics and, more precisely, in defining salivary stone position, dimension and relationship with respect to the ductal walls.
Conclusions
In order to achieve good results with sialendoscopy , it is mandatory to carry out complete surgical training with practical courses and/or supervision by an expert surgeon. Previous knowledge of endoscopic sinus surgery can facilitate the first learning phase. Adequate experience with traditional surgery on salivary glands is also required to manage cases of failure involving the endoscopic procedure. Operating on the first cases under general anaesthesia may be helpful in avoiding patient discomfort due to longer procedure times. An aid in reducing false positives and negatives in preoperative imaging is autonomy in preand postoperative ultrasound execution, or direct collaboration with the radiologist.
Acknowledgements
We would like to thank Elisa Carretta for carrying out the statistical analysis.
References
- 1.Bodner L. Salivary gland calculi: diagnostic imaging and surgical management. Compendium. 1993;14:572–586. [PubMed] [Google Scholar]
- 2.Maresh A, Kutler DI, Kacker A. Sialoendoscopy in the diagnosis and management of obstructive sialadenitis. Laryngoscope. 2011;121:495–500. doi: 10.1002/lary.21378. [DOI] [PubMed] [Google Scholar]
- 3.Luers JC, Damm M, Klussmann JP, et al. The learning curve of sialoendoscopy with modular sialoendoscopes: a single surgeon's experience. Arch Otolaryngol Head Neck Surg. 2010;136:762–765. doi: 10.1001/archoto.2010.109. [DOI] [PubMed] [Google Scholar]
- 4.Nahlieli O, Nakar LH, Nazarian Y, et al. Sialoendoscopy: a new approach to salivary gland obstructive pathology. J Am Dent Assoc. 2006;137:1394–1400. doi: 10.14219/jada.archive.2006.0051. [DOI] [PubMed] [Google Scholar]
- 5.Vairel B, Bonnecaze G, Shehri S, et al. Courbe d'apprentissage en sialoendoscopie: nos 101 premières procedures. Rev Laryngol Otol Rhinol. 2012;133,4:177–181. [PubMed] [Google Scholar]
- 6.Modest MC, Galinat L, Rabinowitz M, et al. Learning progression in the use of sialoendoscopy for sialolithiasis: effect on gland preservation. Otolaryngol Head Neck Surg. 2014;151:240–245. doi: 10.1177/0194599814533658. [DOI] [PubMed] [Google Scholar]
- 7.Nahlieli O, Baruchin AM. Endoscopic technique for the diagnosis and treatment of obstructive salivary gland diseases. J Oral Maxillofac Surg. 1999;57:1394–1401. doi: 10.1016/s0278-2391(99)90716-4. [DOI] [PubMed] [Google Scholar]
- 8.Koch M, Zenk J, Iro H. Algorithms for treatment of salivary gland obstructions. Otolaryngol Clin North Am. 2009;42:1173–1192. doi: 10.1016/j.otc.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Mc Gurk M, Brown J. Alternatives for the treatment of salivary duct obstruction. Otolaryngol Clin North Am. 2009;42:1073–1085. doi: 10.1016/j.otc.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Iro H, Zenk J, Escudier MP, et al. Outcome of minimally invasive management of salivary calculi in 4,691 patients. Laryngoscope. 2009;119:263–268. doi: 10.1002/lary.20008. [DOI] [PubMed] [Google Scholar]
- 11.Capaccio P, Torretta S, Ottaviani F, et al. Modern management of obstructive salivary diseases. Acta Otorhinolaryngol Ital. 2007;27:161–172. [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshimura Y, Morishita T, Sugihara T. Salivary gland function after sialolithiasis: scintigraphic examination of submandibular glands with 99m Tc-pertechnetate. J Oral Maxillofac Surg. 1989;47:704–710. doi: 10.1016/s0278-2391(89)80009-6. [DOI] [PubMed] [Google Scholar]
- 13.Marchal F, Kurt AM, Dulguerov P, et al. Histopathology of submandibular gland removed for sialolithiasis. Ann Otol Rhinol Laryngol. 2001;110:464–469. doi: 10.1177/000348940111000513. [DOI] [PubMed] [Google Scholar]
- 14.Marks SC. Learning curve in endoscopic sinus surgery. Otolaryngol Head Neck Surg. 1999;120:215–218. doi: 10.1016/S0194-5998(99)70409-2. [DOI] [PubMed] [Google Scholar]
- 15.Kroll T, Finkensieper M, Hauk H, et al. Sialoendoscopylearning curve and nation-wide survey in German ENT-departments. Laryngorhinootologie. 2012;91:561–565. doi: 10.1055/s-0032-1314880. [DOI] [PubMed] [Google Scholar]
- 16.Wilson M, McMullen K, Walvekar R. Sialoendoscopy: endoscopic approach to benign salivary gland diseases. In: Iancu C, editor. Advances in endoscopic surgery. Rijeka, Croatia: InTech; 2011. pp. 101–116. [Google Scholar]
- 17.Ruach S, Gorlin RJ. Diseases of the salivary glands. In: Gorlin RJ, Goldman HM, editors. Oral Pathology, 6th. St Louis, MO: Mosby; 1970. pp. 997–1003. [Google Scholar]
- 18.Escudier MP, McGurk M. Symptomatic sialoadenitis and sialolithiasis in the English population, an estimate of the cost of hospital treatment. Br Den J. 1999;186:463–466. doi: 10.1038/sj.bdj.4800141. [DOI] [PubMed] [Google Scholar]
- 19.Serbetci E, Sengor GA. Sialoendoscopy: experience with the first 60 glands in Turkey and a literature review. Ann Otol Rhinol Laryngol. 2010;119:155–164. doi: 10.1177/000348941011900303. [DOI] [PubMed] [Google Scholar]
- 20.Capaccio P, Torretta S, Pignataro L. The role of adenectomy for salivary gland obstruction in the era of sialoendoscopy and lithotripsy. Otolaryngol Clin North Am. 2009;42:1161–1171. doi: 10.1016/j.otc.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 21.Nahlieli O, Baruchin AM, et al. Sialoendoscopy: three years' experience as a diagnostic and treatment modality. J Oral Maxillofac Surg. 1997;55:912–918. doi: 10.1016/s0278-2391(97)90056-2. [DOI] [PubMed] [Google Scholar]


