Abstract
Tumor necrosis factor–α (TNF-α) is a proalgesic cytokine that is commonly expressed following tissue injury. TNF-α expression not only promotes inflammation but can also lead to pain hypersensitivity in nociceptors. With the established link between TNF-α and inflammatory pain, we identified its increased expression in the teeth of patients affected with caries and pulpitis. We generated a transgenic mouse model (TNF-αglo) that could be used to conditionally overexpress TNF-α. These mice were bred with a dentin matrix protein 1 (DMP1)–Cre line for overexpression of TNF-α in both the tooth pulp and bone to study oral pain that would result from subsequent development of pulpitis and bone loss. The resulting DMP1/TNF-αglo mice show inflammation in the tooth pulp that resembles pulpitis while also displaying periodontal bone loss. Inflammatory infiltrates and enlarged blood vessels were observed in the tooth pulp. Pulpitis and osteitis affected the nociceptive neurons innervating the orofacial region by causing increased expression of inflammatory cytokines within the trigeminal ganglia. With this new mouse model morphologically mimicking pulpitis and osteitis, we tested it for signs of oral pain with an oral function assay (dolognawmeter). This assay/device records the time required by a mouse to complete a discrete gnawing task. The duration of gnawing required by the DMP1/TNF-αglo mice to complete the task was greater than that for the controls; extended gnaw time in a dolognawmeter indicates reduced orofacial function. With the DMP1/TNF-αglo mice, we have shown that TNF-α expression alone can produce inflammation similar to pulpitis and osteitis and that this mouse model can be used to study dental inflammatory pain.
Keywords: inflammation, facial pain, cytokine(s), toothache, animal model, Cdk5
Introduction
Tumor necrosis factor–α (TNF-α) is a pleiotropic cytokine that promotes inflammation by promoting recruitment of leukocytes, inducing vasodilation, and stimulating the production of proinflammatory cytokines (Bradley 2008). Elevated levels of TNF-α have often been detected in the serum and tissues of patients with severe infections or autoimmune disorders. For example, upregulation of TNF-α has been discovered in pulpal tissues from teeth with irreversible pulpitis (Pezelj-Ribaric et al. 2002; Kokkas et al. 2007) and in exudates of teeth with apical periodontitis (Safavi and Rossomando 1991). In addition to being proinflammatory, TNF-α may act directly on nociceptive neurons to increase pain sensitivity. The TNF-α receptors, TNFR1 and TNFR2, have been detected on nociceptive neurons that transmit peripheral pain to the central nervous system (Boettger et al. 2008; Schaible 2010). Subcutaneous injection of recombinant TNF-α promotes mechanical allodynia through sensitization of C-fiber nociceptors (Junger and Sorkin 2000), while application of TNF-α to cultured dorsal root ganglion neurons modulates ion channel activity (Czeschik et al. 2008).
Orofacial pain is a widespread public health problem that affects 20% of adults, with toothaches alone afflicting about 22 million Americans (Lipton et al. 1993). Animal models of dental pain often involve exposure of the tooth pulp and administration of lipopolysaccharide to induce an inflammatory immune response (Khan and Hargreaves 2010; Gibbs et al. 2013). We wanted to examine the effect of TNF-α on immune homeostasis within the tooth pulp and determine if overexpression of this inflammatory mediator alone could promote pulpitis. For this purpose, we decided to use a genetic approach to determine the cause of conditional overexpression of TNF-α in the tooth pulp by developing a novel transgenic mouse designated TNF-αglo (Rozas et al., unpublished data). For this genetically modified mouse, the transgene contains a ubiquitous promoter to potentially express TNF-α in any tissue, but expression of TNF-α is blocked by an intervening floxed enhanced green fluorescent protein (EGFP) that must be excised by Cre for TNF-α expression to occur. Rozas et al. (unpublished data) have shown that, without Cre present, only basal levels of TNF-α are expressed in the TNF-αglo transgenic mice. However, when this mouse was crossed with Nav1.8-Cre, targeted overexpression of TNF-α occurred in the nociceptive neurons of the trigeminal ganglia (TG). For our purposes, the TNF-αglo mice were bred with the dentin matrix protein 1 (DMP1)–Cre transgenic line (Lu et al. 2007) to cause recombination in the odontoblasts in teeth and the osteocytes in bone. The resulting double-positive mice (DMP1/TNF-αglo) had increased levels of TNF-α within the teeth, causing recruitment of inflammatory infiltrates and vasodilation of the blood vessels in the tooth pulp. Alveolar bone loss was also seen around the tooth roots of the DMP1/TNF-αglo mice. Because masticatory dysfunction is one indicator of orofacial pain, we measured gnawing function in the DMP1/TNF-αglo mice using a dolognawmeter (Dolan et al. 2010). The device quantifies the duration of gnawing activity required to complete a discrete gnawing task; longer gnaw time in the device is positively correlated with orofacial dysfunction and has been validated as an index of nociception. The DMP1/TNF-αglo mice required more time to gnaw through an obstacle (resin dowel) compared to controls. With masticatory dysfunction in the presence of pulpal inflammation and concurrent periodontitis, we infer nociception-induced gnawing dysfunction. Accordingly, the DMP1/TNF-αglo mice provide a novel genetic mouse model to investigate mechanisms of pain transmission resulting from pulpitis.
Materials and Methods
Human Samples
Human dental pulp samples from 15 normal teeth, 14 carious teeth without spontaneous pain, and 16 pulpitis teeth were collected from the School and Hospital of Stomatology, Wuhan University. The procedures were performed based on the guidelines of the National Institutes of Health (NIH) regarding the use of human tissues and with permission from the Institutional Ethical Board of Wuhan University, China. Normal tooth samples were collected from teeth extracted as part of orthodontic treatment. Carious teeth without spontaneous pain were diagnosed following an established protocol, including a clinical assessment by endodontic specialists; patients had caries progressing to the dentin and exhibited no history of spontaneous intense pain and teeth with symptoms of pulpitis were excluded. The pulpitis group was composed of patients with a history of spontaneous and intense tooth pain and no history of periodontal disease (Pezelj-Ribaric et al. 2002). Pulpal tissues were extracted from separate groups classified for Western blot analysis. Paraffin-embedded tissue blocks from different groups were used for immunohistochemistry staining.
Transfection of MO6-G3 Cells
To examine recombination of the pCLE–TNF-α transgenic vector and subsequent TNF-α–mediated cell signaling, approximately 2.5 × 105 MO6-G3 cells (MacDougall et al. 1995) were plated onto a 6-cm2 culture dish and incubated overnight before transfection with pCLE–TNF-α and either the Cre expression vector pBS185 (Sauer and Henderson 1990) or an empty vector. Cells were transfected with Lipofectamine 2000 (Thermo Fisher Scientific, MA, USA) according to the manufacturer’s protocol. After 24 h, MO6-G3 supernatants and cell lysates were collected for analysis by enzyme-linked immunosorbent assay (ELISA) and Western blot.
DMP1/TNF-αglo Mice
The TNF-αglo mice were generated as described earlier (Bradley 2008; Rozas et al., unpublished data). Expression of the mouse TNF-α complementary DNA (cDNA) by the CAG promoter is prevented unless Cre is present to excise the intervening floxed EGFP (Bradley et al. 2007; Hall et al. 2010). The resulting pCLE–TNF-α expression vector was then microinjected into the pronucleus of fertilized FVB/N mouse oocyte. The TNF-αglo mice were genotyped by visualization of green fluorescent protein (GFP) by using a Macro Imaging System (Lightools Research, Encinitas, CA, USA). The DMP1-Cre line (Lu et al. 2007) was bred with these TNF-αglo mice for conditional overexpression of TNF-α in the tooth pulp and bone. All the mice tested in our experiments were around 3 to 4 mo of age.
All animal experimental studies and procedures conformed to ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines for preclinical studies and were approved by the Animal Care and Use Committee of the National Institute of Dental and Craniofacial Research, NIH.
Immunohistochemistry and Microradiography
Radiographic, histological, and immunohistochemical analysis of the tissues was performed as previously described (Hall et al. 2013). Sections were stained with the following antibodies: TNF-α (IW-PA1079; IHC World, Woodstock, MD, USA), Phospho–NF-κB p65 (Ser536) (3033; Cell Signaling Technology, Danvers, MA, USA), Mac-2 (CL8942AP; Cedarlane Laboratories, Ontario, Canada), and CD3 (A0452; Dako, Carpinteria, CA, USA). Sections from the TG were stained with antibodies for GFAP (12389; Cell Signaling Technology) and Iba1 (019-19741; Wako Chemicals, Richmond, VA, USA).
Western Blot, ELISA, and Cdk5 Assay
Tissues were collected from 3-mo-old mice and Western blot was performed as previously described (Hall et al. 2013). The following antibodies were used: Cdk5 (sc-173; Santa Cruz Biotechnology, Santa Cruz, CA, USA), p35 (2680; Cell Signaling Technology), phospho–NF-κB p65 (Ser536) (3033; Cell Signaling Technology), NF-κB p65 (Ser536) (4764; Cell Signaling Technology), GFP (11814460001; Roche, Indianapolis, IN, USA), and actin (MAB1501; Millipore, Billerica, MA, USA) as loading control. Quantification of TNF-α levels from either the cultured cells or the mice were performed using an ELISA kit from Thermo Fisher Scientific (KMC3012). Approximately 10 µg of mandibular protein (homogenized in RIPA lysis buffer) was used for the ELISA, while serum samples were diluted 1:10, and supernatants from transfected MO6-G3 cells were diluted 1:2. Cdk5 activity was measured as previously described (Prochazkova et al. 2013). In total, 100 µg of protein from the TG was used to measure Cdk5 activity and the incorporation of P32 onto histone H1 was quantified using ImageJ software to measure band intensity (NIH, Bethesda, MD, USA).
Quantitative Real-Time Polymerase Chain Reaction
Quantitative real-time polymerase chain reaction (q-PCR) to measure the expression of inflammatory markers was performed as previously described (Prochazkova et al. 2013). The TG from 4 female DMP1/TNF-αglo mice were examined along with the corresponding controls.
Dolognawmeter
The dolognawmeter is an operant assay validated to measure orofacial dysfunction secondary to inflammation-induced nociception (Dolan et al. 2010). Once loaded into the device, a mouse voluntarily traverses through a confinement tube and gnaws through 2 dowel obstacles (a soft dowel of cross-linked polyethylene foam and then a hard dowel of ethylene vinyl acetate [EVA resin]). Behavioral testing was performed as described previously (Dolan et al. 2010). Gnawing activity was measured with the dolognawmeter before the onset of severe inflammation and concomitant weight loss.
Statistical Analysis
Statistical evaluation of the results was accomplished with GraphPad Prism software, version 6 (GraphPad, San Diego, CA, USA). Statistical differences were assessed using an unpaired t test. All data are expressed as mean ± SEM, and the significance level was set at P < 0.05.
Results
Development of a Mouse Model for Conditional Overexpression of TNF-α by Odontoblasts
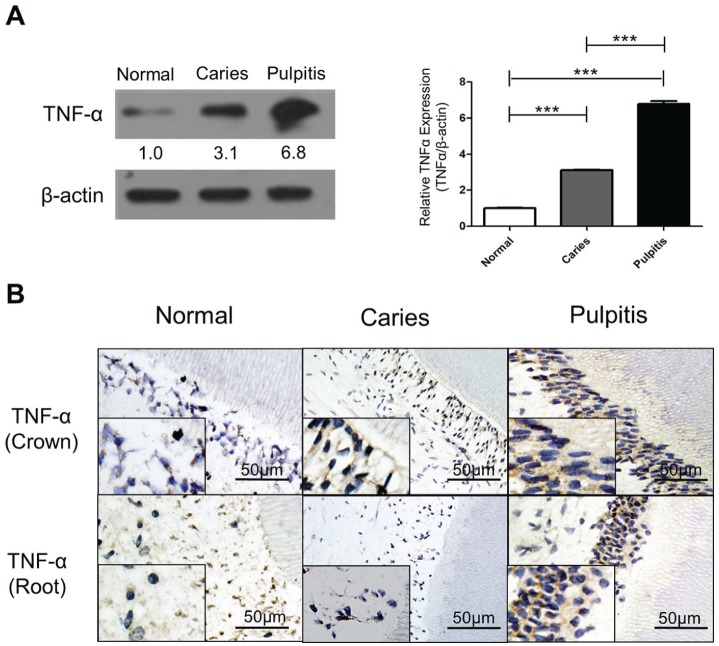
Increased levels of TNF-α have been detected in patients with pulpitis (Pezelj-Ribaric et al. 2002; Kokkas et al. 2007). We first sought to reaffirm whether patients with tooth decay exhibit increased expression of TNF-α. Both Western blot and immunohistochemistry showed that TNF-α levels are increased in patients with dental caries and pulpitis (Fig. 1A, B).
Figure 1.
Tumor necrosis factor–α (TNF-α) expression is elevated by bacterial infection of the human tooth. (A) TNF-α (25 kD) is significantly upregulated during carious progression. A bar graph shows the quantification of the relative TNF-α expression (TNF-α/β-actin) between control, caries, and pulpitis groups (***P < 0.001). (B) Immunohistochemistry staining shows a similar result as the Western blot. Expression of TNF-α is increased in the tooth crown of the caries group and the pulpitis group. However, in the region of the tooth root, the pulpitis group displays a much stronger positive signal of TNF-α than the caries group. Lower inserts show higher magnification in each panel. Bar represents 50 µm.
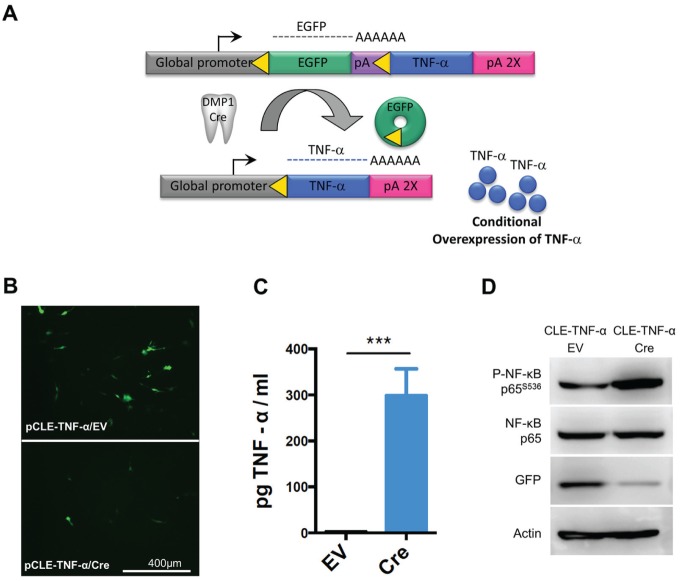
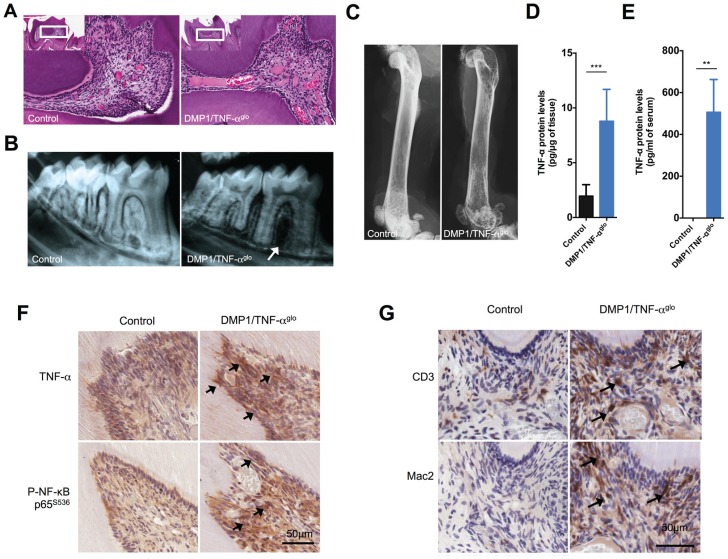
After establishing a link between TNF-α expression and pulpitis, we wanted to create a mouse model in which TNF-α could be conditionally overexpressed in the tooth pulp. A transgenic vector, designated pCLE–TNF-α (Rozas et al., unpublished data), was constructed that requires Cre-mediated transgene recombination in order for TNF-α to be expressed (Fig. 2A). The pCLE–TNF-α vector has a global promoter for potential ubiquitous expression of TNF-α, but transcription is blocked by an intervening floxed EGFP. Therefore, expression of TNF-α only occurs wherever Cre is expressed to excise the floxed EGFP and juxtapose the promoter to the TNF-α cDNA. We first tested pCLE–TNF-α in the immortalized odontoblast cell line MO6-G3 (MacDougall et al. 1995) to ensure that the transgenic construct could promote conditional TNF-α overexpression in the tooth pulp. The MO6-G3 cells were transfected with pCLE–TNF-α and either an empty vector or the Cre expression vector. MO6-G3 cells transfected with the empty vector showed strong EGFP expression (Fig. 2B, D), which suggests that the promoter should also be active within odontoblasts inside the tooth of a mouse. In contrast, recombination of pCLE–TNF-α through cotransfection of Cre led to both diminished GFP expression and increased expression of TNF-α (299.1 ± 33.3 pg TNF-α/mL supernatant for MO6-G3 cells cotransfected with Cre versus 2.8 ± 0.6 [n = 3] for the empty vector) (Fig. 2B–D). In addition, activation of TNF-α–mediated cell signaling was also observed via increased phosphorylation of NF-κB (Fig. 2D). These in vitro experiments demonstrate that the pCLE–TNF-α transgenic construct will be functional in odontoblasts and that active TNF-α is expressed upon recombination. We next made transgenic mice using the pCLE–TNF-α vector for conditional overexpression of TNF-α in a mouse. To induce strong TNF-α expression in the tooth pulp, we bred the TNF-αglo mouse line with the strongest GFP expression to the DMP1-Cre line (Lu et al. 2007; Rozas et al., unpublished data), which expresses Cre in the odontoblasts as well as osteocytes. In the molars of the DMP1/TNF-αglo mice, we observed histological signs of inflammation within the tooth pulp, including inflammatory infiltrates, disruption of the odontoblast layer, and dilated blood vessels (Fig. 3A). In addition, radiographs of the teeth from these mice show alveolar bone loss around the tooth root compared to the controls (Fig. 3B). DMP1 expression has been detected in ameloblasts, cementoblasts, and osteocytes in addition to odontoblasts; expression of TNF-α by all of these cells may cause inflammation that leads to the alveolar bone loss. Due to the TNF-α overexpression in the osteocytes, we also observed overall bone loss in these mice that likely resulted from TNF-α–mediated induction of osteoclast activity (Fig. 3C).
Figure 2.
Transgenic vector for Cre-mediated overexpression of tumor necrosis factor–α (TNF-α). (A) Schematic for conditional overexpression of TNF-α in the odontoblasts of transgenic mice. A transgenic mouse line designated TNF-αglo was generated, which requires Cre-mediated recombination to overexpress TNF-α. These TNF-αglo mice were bred with a dentin matrix protein 1 (DMP1)–Cre line for overexpression of TNF-α in both the tooth pulp and bone. (B) The transgenic vector was first tested in the MO6-G3 odontoblast cell line. The MO6-G3 cells show decreased green fluorescent protein (GFP) expression when cotransfected with Cre versus an empty vector. (C) Supernatants from MO6-G3 cells cotransfected with Cre show higher levels of TNF-α by enzyme-linked immunosorbent assay (***P < 0.001). (D) When cotransfected with Cre, MO6-G3 cells show increased active TNF-α signaling via nuclear factor (NF)–κB phosphorylation and a concomitant decrease in GFP expression. β-Actin was used as loading control. EV, empty vector.
Figure 3.
Dentin matrix protein 1 (DMP1)/tumor necrosis factor–αglo (TNF-αglo) mice show inflammatory infiltrates within the tooth pulp. (A) Hematoxylin and eosin staining of the tooth pulp shows inflammation that is similar to pulpitis with infiltrating cells and enlarged blood vessels. (B) Radiographs also show alveolar bone loss around the teeth of the DMP1/TNF-αglo mice. (C) Femurs from the DMP1/TNF-αglo mice display reduced opacity, suggesting overall systemic bone loss due to overexpression of TNF-α by osteocytes. (D) Increased amounts of TNF-α were detected per µg of mandibular protein within the DMP1/TNF-αglo mice. (E) Serum levels of TNF-α were also increased in the DMP1/TNF-αglo mice as detected by a mouse TNF-α enzyme-linked immunosorbent assay (**P ≤ 0.01). (F) Immunohistochemical staining shows overexpression of TNF-α in the tooth pulp. Increased TNF-α cell signaling was also detected via phospho–NF-κB. Arrows denote sites of increased TNF-α expression and subsequent cell signaling via phosphorylation of NF-κB. (G) CD3 and Mac2 staining shows recruitment of lymphocytes and macrophages, respectively, in the tooth pulp. Arrows identify infiltrating lymphocytes and macrophages.
We next sought to confirm that the observed immunological inflammation in the teeth resulted from overexpression of TNF-α. The mouse mandibles were therefore homogenized and TNF-α levels were measured with an ELISA. In the lower jaw of the DMP1/TNF-αglo mice, there was approximately 8.8 ± 1.2 pg TNF-α per µg of mandibular protein (n = 6), while the control mice had 2.0 ± 0.5 pg TNF-α (n = 5) (Fig. 3D). In the DMP1/TNF-αglo mice, however, we were also able to detect higher circulating levels of TNF-α in the serum (DMP1/TNF-αglo 506.6 ± 78.0 pg TNF-α/mL serum [n = 4] versus 0.0 ± 0.0 [n = 3] for the controls at 100 d of age) (Fig. 3E). Nonetheless, all indications of inflammation were localized to the tooth pulp or the bone without any signs of inflammatory infiltrates in the other major tissues (data not shown). Immunohistochemistry was able to further confirm not only the localized overexpression of TNF-α in the tooth pulp but also the activation of the TNF-α cell-signaling pathway through increased phospho–NF-kB p65 (Fig. 3F, arrows). With validation of localized overexpression of TNF-α by the odontoblasts, we next wanted to characterize the inflammatory infiltrates seen within the tooth pulp. As expected, we observed recruitment of both macrophages (Mac2 staining) and lymphocytes (CD3 staining) into the tooth pulp of the DMP1/TNF-αglo mice as a direct consequence of TNF-α overexpression (Fig. 3G).
Effects of Pulpitis on the Trigeminal Ganglia
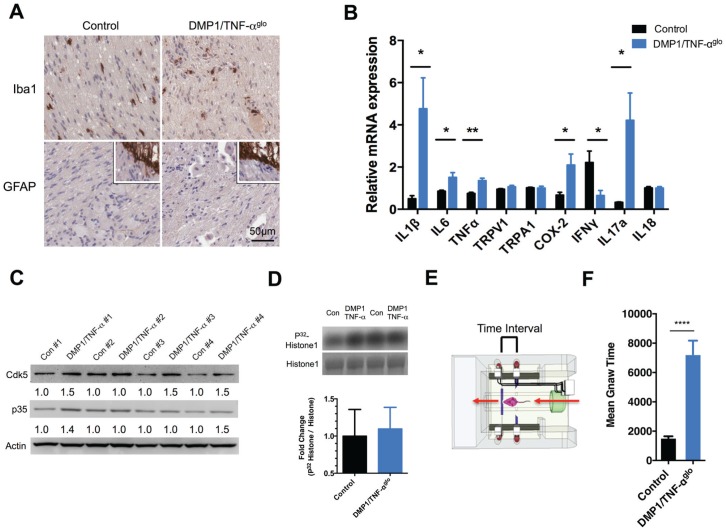
Although signs of inflammation appeared to be primarily localized to the tooth and bone, we wanted to assess the DMP1/TNF-αglo mice for any signs of neuroinflammation. We performed immunostaining for markers of neuroinflammation within the TG but saw no discernable induction of GFAP or increase in activated microglia (Iba1) in the TG of the DMP1/TNF-αglo mice (Fig. 4A). We then examined the TG of the mice for any changes in the expression of inflammatory mediators using q-PCR. The levels of cyclooxygenase 2 (COX-2) and the proinflammatory cytokines interleukin (IL)–1β, IL-6, and TNF-α were increased in the TG of 3 mice compared to the controls (Fig. 4B). Also, the levels of interferon gamma (IFN-γ) were significantly decreased in the DMP1/TNF-αglo mice, which, in turn, possibly allows for increased IL-17 expression—a cytokine that is linked with chronic inflammation and enhanced nociception (Chu et al. 2007; Moynes et al. 2014). Transducers of noxious painful stimuli such as Trpv1 and Trpa1 were not increased in the TG of the DMP1/TNF-αglo mice, but sensitization of these ion channels may be possible as a result of inflammation.
Figure 4.
Dentin matrix protein 1 (DMP1)/tumor necrosis factor–αglo (TNF-αglo) mice exhibit orofacial pain due to the TNF-α–induced pulpitis and osteitis. (A) Inflammation initiated by TNF-α is primarily restricted to the tooth pulp and bone and does not cause neuronal injury or glial activation within the trigeminal ganglia (TG) as determined by Iba1 and GFAP staining (lower inserts positive staining for central root astrocytes). (B) The expression of proinflammatory markers within the TG was evaluated by quantitative real-time polymerase chain reaction (*P ≤ 0.05; **P ≤ 0.01). (C) Cdk5 is a protein kinase; its activity can be upregulated by inflammation, so levels of both Cdk5 and its activator p35 were examined within the TG to determine if chronic tooth inflammation increased their expression. In most cases, the levels of both Cdk5 and p35 were higher in the DMP1/TNF-αglo mice versus the littermate control (fold differences were measured as the ratio over β-actin). (D) A Cdk5 kinase assay was performed using protein from the TG. Graph and representative blot showing modest increase in Cdk5 kinase activity. (E) Schematic of the dolognawmeter (Dolan et al. 2010), an assay and device used to quantify nociception by measuring masticatory function. (F) DMP1/TNF-αglo mice require more time to gnaw through a hard dowel compared to Cre− controls, signifying masticatory dysfunction and probable orofacial pain (****P ≤ 0.0001).
We next decided to examine Cdk5 kinase activity within the TG because inflammatory agents such as carrageenan and CFA have been shown to induce Cdk5 activity (Pareek et al. 2006; Yang et al. 2007) in the afferent neurons of the dorsal root ganglia and spinal cord. In addition, TNF-α strongly induced Cdk5 kinase activity in PC12 cells (Utreras et al. 2009) and in the TG from mice with conditional overexpression of TNF-α in nociceptive neurons (Rozas et al., unpublished data). In most cases, we saw an increase in Cdk5 expression in TG of the DMP1/TNF-αglo mice compared to littermate controls along with modest increases in p35 expression (Fig. 4C). With this finding, we also looked for subsequent increases in Cdk5 kinase activity, but we saw only a slight fold increase in Cdk5 activity (1.1 ± 0.1; n = 6) in the DMP1/TNF-αglo mice compared to controls (1.0 ± 0.1; n = 6) (Fig. 4D). Although the change in Cdk5 kinase activity was not statistically significant, the modest trend toward greater activity in the DMP1/TNF-αglo mice may suggest a molecular response to pulpitis because Schwann cells and other sensory neurons within the TG are probably not affected by inflammation to the same degree as the pain-sensing C-fibers.
Masticatory Dysfunction in the DMP1/TNF-αglo Mice
With a mouse model displaying pulpitis and apical periodontitis, we sought to determine whether the DMP1/TNF-αglo mice experience hyperalgesia and allodynia, which are commonly associated with chronic inflammation. We employed an operant assay/device termed a dolognawmeter to measure masticatory dysfunction (a behavioral index of orofacial pain) (Dolan et al. 2010). The dolognawmeter measures the amount of time required for a mouse to gnaw through a series of 2 dowels. The outcome variable is the time required to sever the second, harder, EVA dowel. Four female DMP1/TNF-αglo and control mice were tested using a dolognawmeter starting at age 6 wk. After acclimatizing the mice to the device over the course of 7 preliminary sessions, we recorded the gnaw times of the mice in the last 3 of 10 trials. Control mice required a mean time of 1,538 ± 109.7 s to gnaw through the EVA dowel, while the DMP1/TNF-αglo mice took 7,237 ± 934.2 s (Fig. 4E). We infer that oral inflammation, consisting of both pulpitis and periapical inflammation, led to mechanical allodynia and nociception-induced gnawing dysfunction in the DMP1/TNF-αglo mice.
Discussion
In this study, we confirmed that TNF-α is upregulated in patients with dental caries and with pulpitis. To mimic this inflammatory response, we developed a DMP1/TNF-αglo mouse model in which TNF-α can be genetically upregulated solely in the odontoblasts and osteocytes using the DMP1-Cre mouse line. In the teeth of the DMP1/TNF-αglo mice, the overexpression of TNF-α alone yielded tooth inflammation that mimicked acute pulpitis. Secretion of TNF-α by odontoblasts yielded conditions similar to bacterial infection of the teeth. Cultured human odontoblasts from decayed teeth, for example, show 30-fold increased expression of TNF-α compared to odontoblasts from healthy teeth (Veerayutthwilai et al. 2007). In addition, upregulation of inflammatory genes, including TNF-α, arises primarily in the odontoblast layer during dental caries in humans, while gene expression in the pulp remains unchanged (Horst et al. 2011).
DMP1 is expressed in osteocytes as well as odontoblasts, but overexpression of TNF-α by DMP1-Cre did not appear to cause any developmental defects in these mice. DMP1/TNF-αglo pups were born at the expected genotypic ratio and were phenotypically normal at birth. However, by 9 wk of age, the weight of these mice generally plateaued, and weight differences greater than 5 g appeared after 12 wk of age. Dental injury is known to cause weight loss (Gibbs et al. 2013), but the bone loss and osteitis observed in the DMP1/TNF-αglo mice probably contribute to the differences in body weight. Because of substantial tooth and bone inflammation, the life span of the DMP1/TNF-αglo mice was reduced to a median survival of 21 wk.
Using this mouse model of pulpitis and osteitis, we sought to identify the molecular changes in the primary afferent nociceptors that innervate teeth and bone that yield inflammatory pain signaling. The tooth pulp is highly innervated with Aδ- and C-fibers (Byers and Närhi 1999); dense innervation can lead to increased pain intensity. The confined space within the hard tissue of the pulp chamber leads to higher pressure in the soft tissue secondary to swelling of the pulp. This anatomic confinement amplifies the intensity of tooth pain. The odontoblasts themselves may also act as pain transducers (Chung et al. 2013). In the DMP1/TNF-αglo mice, there is overexpression of TNF-α in the odontoblasts and the osteocytes, and we needed to establish if the resulting pulpitis and periodontitis caused actual orofacial pain. Numerous behavioral parameters have been employed to evaluate orofacial pain in animal models (Kramer et al. 2012). We used a dolognawmeter because this operant assay directly measures orofacial function (gnawing) in an automated fashion over many weeks while minimizing stress to the mice. With a dolognawmeter, we were able to demonstrate that the DMP1/TNF-αglo mice display masticatory dysfunction indicative of orofacial pain. The shift toward allodynia seen in the DMP1/TNF-αglo mice may be due to inflammatory activation of kinases that, in turn, cause hypersensitivity of the afferent neurons. In the DMP1/TNF-αglo mice, we see a trend toward increased Cdk5 activity, which has been shown to promote hyperalgesia (Pareek et al. 2006; Prochazkova et al. 2013). However, inflammatory mediators such as TNF-α can activate other protein kinases that, in turn, modulate the sensitivity of pain-sensing ion channels in primary afferent neurons and promote peripheral sensitization (Ji et al. 2007).
In conclusion, we have generated a DMP1/TNF-αglo mouse model that mimics the inflammatory conditions resulting from bacterial infection of the tooth pulp. The DMP1/TNF-αglo mice act as a model of pulpal inflammation, periodontitis, and oral pain just by overexpressing the inflammatory cytokine TNF-α.
Author Contributions
B.E. Hall, J.C. Dolan, B.L. Schmidt, A.B. Kulkarni, contributed to conception, design, and data analysis, drafted and critically revised the manuscript; L. Zhang, Z.J. Sun, E. Utreras, M. Prochazkova, A. Cho, A. Terse, contributed to data analysis, drafted the manuscript; P. Arany, contributed to data analysis, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Acknowledgments
We thank Dr. Jerry Feng for providing us DMP1-Cre mice. We also thank Drs. Harish Pant and Niru Amin for their expert advice and help in carrying out Cdk5 kinase assay.
Footnotes
These studies were supported by National Natural Science Foundation of China; the Chilean National Fund for Scientific, Technological Development and Innovation (FONDECYT 1151043), Santiago, Chile; and the Division of Intramural Research, National Institute of Dental and Craniofacial Research, National Institutes of Health. All authors were funded by their own institutions to perform all work relating to the present study.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Boettger MK, Hensellek S, Richter F, Gajda M, Stöckigt R, von Banchet GS, Bräuer R, Schaible HG. 2008. Antinociceptive effects of tumor necrosis factor alpha neutralization in a rat model of antigen-induced arthritis: evidence of a neuronal target. Arthritis Rheum. 58(8):2368–2378. [DOI] [PubMed] [Google Scholar]
- Bradley JR. 2008. TNF-mediated inflammatory disease. J Pathol. 214(2):149–160. [DOI] [PubMed] [Google Scholar]
- Bradley SV, Hyun TS, Oravecz-Wilson KI, Li L, Waldorff EI, Ermilov AN, Goldstein SA, Zhang CX, Drubin DG, Varela K, et al. 2007. Degenerative phenotypes caused by the combined deficiency of murine HIP1 and HIP1r are rescued by human HIP1. Hum Mol Genet. 16(11):1279–1292. [DOI] [PubMed] [Google Scholar]
- Byers MR, Närhi MV. 1999. Dental injury models: experimental tools for understanding neuroinflammatory interactions and polymodal nociceptor functions. Crit Rev Oral Biol Med. 10(1):4–39. [DOI] [PubMed] [Google Scholar]
- Chu CQ, Swart D, Alcorn D, Tocker J, Elkon KB. 2007. Interferon-gamma regulates susceptibility to collagen-induced arthritis through suppression of interleukin-17. Arthritis Rheum. 56(4):1145–1151. [DOI] [PubMed] [Google Scholar]
- Chung G, Jung SJ, Oh SB. 2013. Cellular and molecular mechanisms of dental nociception. J Dent Res. 92(11):948–955. [DOI] [PubMed] [Google Scholar]
- Czeschik JC, Hagenacker T, Schäfers M, Büsselberg D. 2008. TNF-alpha differentially modulates ion channels of nociceptive neurons. Neurosci Lett. 434(3):293–298. [DOI] [PubMed] [Google Scholar]
- Dolan JC, Lam DK, Achdjian SH, Schmidt BL. 2010. The dolognawmeter: a novel instrument and assay to quantify nociception in rodent models of orofacial pain. J Neurosci Methods. 187(2):207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs JL, Urban R, Basbaum AI. 2013. Paradoxical surrogate markers of dental injury–induced pain in the mouse. Pain. 154(8):1358–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BE, Wankhade UD, Konkel JE, Cherukuri K, Nagineni CN, Flanders KC, Arany PR, Chen W, Rane SG, Kulkarni AB. 2013. Transforming growth factor–β3 (TGF-β3) knock-in ameliorates inflammation due to TGF-β1 deficiency while promoting glucose tolerance. J Biol Chem. 288(44):32074–32092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BE, Zheng C, Swaim WD, Cho A, Nagineni CN, Eckhaus MA, Flanders KC, Ambudkar IS, Baum BJ, Kulkarni AB. 2010. Conditional overexpression of TGF-beta1 disrupts mouse salivary gland development and function. Lab Invest. 90(4):543–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst OV, Horst JA, Samudrala R, Dale BA. 2011. Caries induced cytokine network in the odontoblast layer of human teeth. BMC Immunol. 12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Kawasaki Y, Zhuang ZY, Wen YR, Zhang YQ. 2007. Protein kinases as potential targets for the treatment of pathological pain. Handb Exp Pharmacol. 177:359–389. [DOI] [PubMed] [Google Scholar]
- Junger H, Sorkin LS. 2000. Nociceptive and inflammatory effects of subcutaneous TNFalpha. Pain. 85(1–2):145–151. [DOI] [PubMed] [Google Scholar]
- Khan A, Hargreaves KM. 2010. Animal models of orofacial pain. Methods Mol Biol. 617:93–104. [DOI] [PubMed] [Google Scholar]
- Kokkas AB, Goulas A, Varsamidis K, Mirtsou V, Tziafas D. 2007. Irreversible but not reversible pulpitis is associated with up-regulation of tumour necrosis factor–alpha gene expression in human pulp. Int Endod J. 40(3):198–203. [DOI] [PubMed] [Google Scholar]
- Kramer PR, He J, Puri J, Bellinger LL. 2012. A non-invasive model for measuring nociception after tooth pulp exposure. J Dent Res. 91(9):883–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton JA, Ship JA, Larach-Robinson D. 1993. Estimated prevalence and distribution of reported orofacial pain in the United States. J Am Dent Assoc. 124(10):115–121. [DOI] [PubMed] [Google Scholar]
- Lu Y, Xie Y, Zhang S, Dusevich V, Bonewald LF, Feng JQ. 2007. DMP1-targeted Cre expression in odontoblasts and osteocytes. J Dent Res. 86(4):320–325. [DOI] [PubMed] [Google Scholar]
- MacDougall M, Thiemann F, Ta H, Hsu P, Chen LS, Snead ML. 1995. Temperature sensitive simian virus 40 large T antigen immortalization of murine odontoblast cell cultures: establishment of clonal odontoblast cell line. Connect Tissue Res. 33(1–3):97–103. [DOI] [PubMed] [Google Scholar]
- Moynes DM, Vanner SJ, Lomax AE. 2014. Participation of interleukin 17A in neuroimmune interactions. Brain Behav Immun. 41:1–9. [DOI] [PubMed] [Google Scholar]
- Pareek TK, Keller J, Kesavapany S, Pant HC, Iadarola MJ, Brady RO, Kulkarni AB. 2006. Cyclin-dependent kinase 5 activity regulates pain signaling. Proc Natl Acad Sci U S A. 103(3):791–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezelj-Ribaric S, Anic I, Brekalo I, Miletic I, Hasan M, Simunovic-Soskic M. 2002. Detection of tumor necrosis factor alpha in normal and inflamed human dental pulps. Arch Med Res. 33(5):482–484. [DOI] [PubMed] [Google Scholar]
- Prochazkova M, Terse A, Amin ND, Hall B, Utreras E, Pant HC, Kulkarni AB. 2013. Activation of cyclin-dependent kinase 5 mediates orofacial mechanical hyperalgesia. Mol Pain. 9:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safavi KE, Rossomando EF. 1991. Tumor necrosis factor identified in periapical tissue exudates of teeth with apical periodontitis. J Endod. 17(1):12–14. [DOI] [PubMed] [Google Scholar]
- Sauer B, Henderson N. 1990. Targeted insertion of exogenous DNA into the eukaryotic genome by the Cre recombinase. New Biol. 2(6):441–449. [PubMed] [Google Scholar]
- Schaible HG. 2010. The role of TNF-alpha as pain mediator. Z Rheumatol. 69(3):237–239. [DOI] [PubMed] [Google Scholar]
- Utreras E, Futatsugi A, Rudrabhatla P, Keller J, Iadarola MJ, Pant HC, Kulkarni AB. 2009. Tumor necrosis factor–alpha regulates cyclin-dependent kinase 5 activity during pain signaling through transcriptional activation of p35. J Biol Chem. 284(4):2275–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerayutthwilai O, Byers MR, Pham TT, Darveau RP, Dale BA. 2007. Differential regulation of immune responses by odontoblasts. Oral Microbiol Immunol. 22(1):5–13. [DOI] [PubMed] [Google Scholar]
- Yang YR, He Y, Zhang Y, Li Y, Li Y, Han Y, Zhu H, Wang Y. 2007. Activation of cyclin-dependent kinase 5 (Cdk5) in primary sensory and dorsal horn neurons by peripheral inflammation contributes to heat hyperalgesia. Pain. 127(1–2):109–120. [DOI] [PubMed] [Google Scholar]