Abstract
Background
Although AJCC/TNM staging remains the gold standard for prognostic assessment of colon cancer, stage-specific outcomes vary. We therefore prospectively evaluated the prognostic role of immunoprofiling.
Methods
Our cohort included 35 patients from an ongoing prospective trial of ultrastaging for colon cancer. Specimens were analyzed for T cell markers (CD3, CD4, CD8, and FoxP3). The number of tumor-infiltrating lymphocytes was analyzed at the tumor’s margin and center and correlated with AJCC/TNM stage, clinicopathologic variables, and disease-free survival.
Results
There was a significant inverse association between number of CD3+ cells in the tumor center and tumor stage (P=0.05). The tumor center/margin ratio of CD3+ cells also showed an inverse but non-significant relationship with nodal involvement (P=0.07). Body mass index was inversely associated with numbers of CD3+(P=0.04) and CD8+(P=0.02) cells. Longer disease-free survival was correlated with higher CD8+ counts (P=0.07), lower CD4+/CD8+ ratios (P=0.008), and higher CD8+/FoxP3+ ratios (P=0.02).
Conclusions
This is the first prospective validation of immunoprofiling in patients whose colon cancer is staged with strict surgical and pathology quality measures. The apparent correlation between immunophenotypic response and clinical outcome warrants evaluation in a larger prospective trial.
Keywords: Colon cancer, Immunoprofiling, Recurrence, Staging
Introduction
The adaptive immune system plays an integral role in the defense against cancers such as melanoma,1,2 CNS tumors,3 renal carcinomas,4 gynecologic malignancies,5,6 lung cancer,7 breast cancer,8 and gastrointestinal tumors.9–13 However, despite the increasing success of targeted therapies and other immune-based approaches to malignancy, the prognostic potential and assessment of the immune response are not well understood.
Galon et al. proposed that standardized scoring of the immune response to colorectal cancer may be a better prognostic tool than staging based on American Joint Commission on Cancer (AJCC) guidelines. Their immunoscore quantifies two specific lymphocyte populations in the tumor center (CT) and its invasive margin (IM).11 In a series of seminal papers, they demonstrated a strong correlation between immunoscore (high in the setting of a brisk tumor immune response and low in its paucity) and outcome of patients with colorectal cancer. While these data were consistent across a number of recent publications, the findings have not been prospectively validated.11,12,14,15Additionally, while surgical and pathological quality measures have been shown to impact disease outcomes,16,17specifically a minimum of 12 lymph node specimen count, these measures were not considered in earlier immunoscore publications.
In this study, we analyzed prospectively collected data to determine whether the immunoprofile of a primary colon cancer was prognostically significant for patients treated in a clinical trial that used rigorously controlled surgical and pathological quality measures.
Materials and Methods
Our cohort was selected from patients enrolled in an ongoing prospective trial of ultrastaging for colon cancer (NCI trial #00949312). The statistician randomly identified 35 patients with non-metastatic disease (AJCC/TNM Stage I–III), adequate tissue, and complete follow-up.32 Immunohistochemistry was performed on 4-μm formalin-fixed, paraffin-embedded colon cancer slides using the following antibodies: rabbit anti-human CD3 clone 2GV6, rabbit anti-human CD4 clone SP35, rabbit anti-human CD8 clone SP57 (Ventana Medical Systems Tucson, AZ USA), and mouse anti-human FoxP3 clone 236A/E7 (eBiosciences San Diego, CA USA). In brief, sections were deparaffinized in xylene (3 changes), rehydrated through graded alcohols (3 changes 100 % ethanol, 3 changes 95 % ethanol), and rinsed in distilled water. Heat-induced epitope retrieval was performed in a 1200-W microwave oven at 100 % power in 10 mM sodium citrate buffer, pH 6.0. CD3 and CD8 were retrieved for 10 min. CD4 and FoxP3 were retrieved for 20 min. Sections were allowed to cool for 30 min and then rinsed in distilled water. Antibody incubation and detection were carried out on a NexES or Discovery Instrument (Ventana Medical Systems Tucson, AZ USA) using Ventana’s reagent buffer and detection kits unless otherwise noted. Endogenous peroxidase activity was blocked with hydrogen peroxide. FoxP3 was diluted 1:75 in Dulbecco’s phosphate buffered saline and incubated for 12 h at room temperature. CD3, CD4, and CD8 were applied without dilution. All antibodies were detected with Ventana’s biotinylated goat anti-mouse/goat anti-rabbit cocktail followed by application of streptavidin-horseradish-peroxidase conjugate. The complex was visualized with 3,3-diaminobenzidine and enhanced with copper sulfate. Slides were washed in distilled water, counterstained with hematoxylin, dehydrated through graded alcohols, cleared in xylene, and mounted with synthetic permanent media. Appropriate positive and negative controls were included with the study sections.
All colorectal carcinoma slides were reviewed by a pathologist blinded to all clinical data. Tissue sections were evaluated to ensure the presence of invasive adenocarcinoma, and all immunohistochemical stains were reviewed at scanning magnification (20×) to evaluate the density of staining at both the invasive margin (IM) and tumor center (CT). Areas of greatest density were then evaluated on higher power (100×) to ensure that tumor-infiltrating lymphocytes (TILs) were present, rather than innate lymphoid aggregates and/or germinal centers. If the presence of TILs was confirmed, the area was image captured for digital analysis. In order to standardize this process, image analysis was carried out using ImageJ Java-based image processing program. ImageJ analysis and threshold determination were carried out by a single author for all 35 patients. The median cell count was determined for each marker (CD3=791.5, CD4=792, CD8=569.5, FoxP3=315), and each sample was then classified as “high” if it was above the median and “low” if it was below the median.
Group comparisons were done by Student t test (or Wilcoxon rank sum) or ANOVA (or Kruskal-Wallis test) for continuous variables and chi-square test or Fisher’s exact test for categorical variables. The association of continuous variables was examined by Pearson (or Spearman rank) correlation coefficients. The test of agreement was done using McNemar’s test. Survival estimates were plotted using Kaplan Meier methods and compared using log-rank test. All analyses were done by SAS 9.2 (NC, Cary), and P value <0.05 was considered statistically significant.
Results
The median follow-up time for this study was 46.8 months from time of surgery. Overall and stage-specific patient demographics and tumor characteristics are shown in Table 1. The patient population was 57 % male, with a median age of 74 years. The majority of patients had T3 tumors, and the mean lymph node count was 17.7, with only small variation between stages.
Table 1.
Patient demographics and tumor characteristics
| Parameter | Overall n=35 |
Stage I n=8 |
Stage II n=13 |
Stage III n=14 |
|---|---|---|---|---|
| Male, n | 20 | 3 | 7 | 10 |
| Female, n | 15 | 5 | 6 | 4 |
| Age, median | 74 | 75 | 68 | 71 |
| BMI, mean (±SD) | 25.9 (±3.7) | 25.9 (±3.2) | 25.5 (±3.9) | 26.3 (±4.0) |
| Average tumor size, cm | 4.6 | 2.0 | 5.6 | 5.5 |
| Depth of invasion, (T stage) | T1=5 | T1=5 | – | – |
| T2=4 | T2=3 | – | T2=1 | |
| T3=25 | – | T3=12 | T3=13 | |
| T4=1 | – | T4=1 | – | |
| Node count, mean (±SD) | 17.7 (±11.3) | 11.8 (±4.5) | 21.5 (±14.9) | 17.6 (±9.1) |
| No. of positive LNs, n | – | – | – | 10 |
| 1–3 | 3 | |||
| 4–6 | 1 | |||
| ≥7 | ||||
| Lymphovascular invasion (LVI), n | 4 | 0 | 3 | 1 |
| Adjuvant chemotherapy, n | 19 | 0 | 5 | 14 |
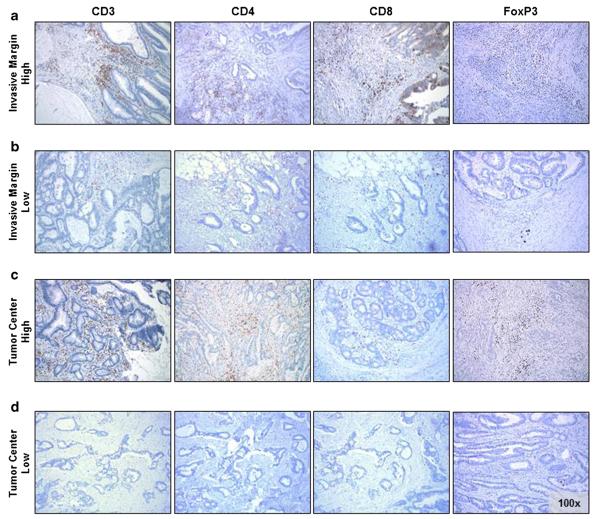
Figure 1 shows a representative immunostain for the pan T cell marker CD3, the helper T cell marker CD4, the cytotoxic T cell marker CD8, and the regulatory T cell marker FoxP3.
Fig. 1.
Representative images of immunostaining (CD3 left, CD4 middle left, CD8 middle right, FoxP3 right) at the IM (a and b) and CT (c and d); a and c show images classified as “high”; b and d were classified as “low”
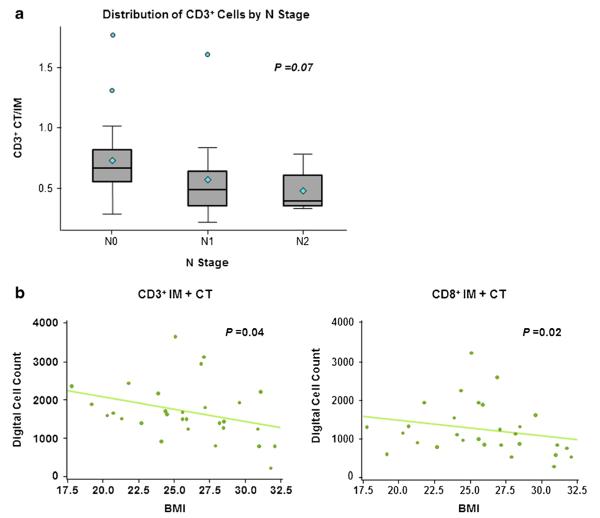
Mean number of CD3+TILs in the CT was inversely correlated with tumor stage (Fig. 2a, P=0.05); this association was not observed for CD3+TILs in the IM (Fig. 2b). The mean CD3+CT/IM cell ratio also was inversely correlated with stage (Fig. 2c, P=0.05); likewise, the mean CD8+ cell count in the CT followed this pattern, although the relationship was not significant (Fig. 2d).
Fig. 2.
Tumor-infiltrating lymphocytes distributed by tumor stage. a CD3+ cells in the CT (left, individual cell counts by patient; right, average cell count); b CD3+ cells in the IM (left, individual cell counts by patient; right, average cell count); c ratio of CD3+ cells in the CT to the IM; d CD8+ cells in the CT (left, individual cell counts by patient; right, average cell count); a, b, d Gray line indicates median cell count per immune marker; above median classified as “high”, below median classified as “low”
The mean CD3+ CT/IM cell ratio was inversely related to the number of tumor-involved nodes (Fig. 3a). Interestingly, there also was a statistically significant inverse association between body mass index (BMI) and number of CD3+TILs (IM+CT; P=0.04) and CD8+TILs (IM+TC; P=0.02) (Fig. 3b), suggesting that obesity attenuates the local tumor immune response.
Fig. 3.
Number of tumor-infiltrating lymphocytes showed an inverse correlation with nodal stage (a CD3+ CT/IM) and body mass index (b CD3+ IM+CT left, CD8+ IM+CT right)
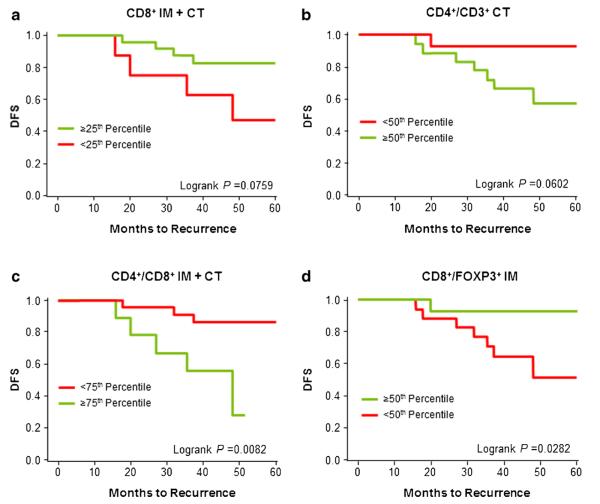
Disease-free survival (DFS) tended to be longer among patients with higher numbers of CD8+ cytotoxic T cells (Fig. 4a) or lower CD4+/CD3+ ratios (Fig. 4b). DFS was significantly longer among patients with lower CD4+/CD8+ ratios (Fig. 4c, P=0.0082). DFS also was significantly longer among patients with higher CD8+/FoxP3+ ratios (Fig. 4d, P=0.0282).
Fig. 4.
Kapan-Meier curves for disease-free survival (DFS) of patients grouped by percentage of tumor-infiltrating lymphocytes. a Patients with CD8+IM+CT counts in the top 25th percentile vs below the 25th percentile; b patients with CD4+/CD3+CT ratios below the 50th percentile vs in 50th percentile or above; c patients with CD4+/CD8+IM+CT ratios below the 75th percentile vs in 75th percentile or above; d patients with CD8+/FoxP3+IM ratios in the top 50th percentile vs below the 50th percentile
Discussion
Although AJCC staging remains standard for prognostic assessment of colon cancer,32 outcomes vary widely among patients with the same stage of disease. For example, up to 25 % of patients with stage I–II tumors will develop recurrence after colectomy. Unfortunately, there is no accurate tool for identifying these high-risk patients. At present, adjuvant chemotherapy for stage II disease is associated with only a 3.6 % absolute improvement in survival18 and is usually limited to those patients with “high-risk features” (poorly differentiated tumors, lymphovascular and/or perineural invasion, perforated tumors, inadequate lymph node sample).19 Conversely, although adjuvant chemotherapy is the standard of care for node-positive (stage III) colon cancer, there may be a low-risk group for whom surgery alone is curative.20
The less than optimal correlation between AJCC stage and clinical outcome mandates a more accurate method of classifying the primary tumor. Proposed techniques include molecular classifications based on tumorigenic pathways, such as analysis for microsatellite instability testing,21 and the analysis of gene expression profiles.22–24 Other classification schemes have included sorting based on morphology (e.g., mucinous histopathology), classifying by mutational analysis, and based on assessment of tumor’s cell of origin. However, these methods fail to account for the body’s immune response to the tumor, and for the tumor’s interaction with its microenvironment.25
The immunoscore quantifies the anti-tumor immune response of two lymphocyte populations in two distinct tumor regions. Its prognostic value has been consistently demonstrated in retrospective cohorts, most of which were in Europe. Furthermore, while the immunoscore is now the subject of a worldwide taskforce dedicated toward its international and prospective validation,26 few studies in colon cancer have analyzed its prognostic utility based on prospectively collected data and those that have utilized older colorectal databases that did not control for surgical and pathologic variables.27
In two recent international multicenter prospective trials, our group has demonstrated that surgical quality and nodal ultrastaging impacts survival in colon cancer. Patients with stage II colon cancer having ≥12 lymph nodes evaluated and found to be negative for micrometastases (IHC negative for pan-cytokeratin) had significantly better DFS than patients with less than the minimum 12 lymph node count evaluated (recurrence rates at 3-year follow-up were 3 vs 18 %, respectively) (P=0.0015).16 Given the prospect of the immunoscore’s prognostic power in colon cancer, the aim of the current study was to address whether the TIL immunophenotype would remain an accurate prognosticator even when evaluated prospectively, in a patient cohort in whom quality of surgery and pathologic evaluation was strictly controlled. Additionally, the bulk of previous immunoscore population studies were conducted in European cohorts, and this is the first study to combine prospectively collected data, with rigorous quality measures, in a North American population of patients.
To assess the correlation between TIL density and AJCC/TNM stage, the common T cell marker, CD3, was analyzed first. Mean CD3+ values in the CT were significantly smaller in higher tumor stages. While this pattern was not detected in the IM, upon closer examination, the average proportion of the two regions (CD3+ CT/IM) analyzed by stage did indeed reveal a statistically significant decrease with disease progression. These results suggest that not only is the depletion of the lymphocytic response in the CT associated with advancing stage, but that as the tumor progresses, CD3+ lymphocytes may be trapped in the IM, limiting their contribution to the anti-tumor response.
Next, cytotoxic T cells, as represented by CD8+ cells, were examined. We chose to study this marker because the increased presence of CD8+ cells has been implicated with better clinical outcome, and along with CD3 positivity, has been studied in previous immunoscore publications. Consistent with earlier studies, we found that these T cell effectors also followed a down-trending pattern with disease progression, and though not reaching statistical significance, a non-significant association was recognizable in the CT. Additionally, to capture and quantify the remaining T cells populations in the tumor environment, we chose to analyze CD4 staining, which represents the memory T cells, and their subpopulation of FoxP3+ cells, representing the regulatory T cell population.
This pilot study has a number of important limitations. Due to its limited sample size, it was not designed to generate far-reaching conclusions, but rather to be a feasibility platform for the validation of immunoprofiling as an adjunct to AJCC/TNM staging. Additionally, since T3 tumors made up the majority, this may have skewed some of the data presented. Another limitation is that while 19/35 (54 %) of patients received adjuvant treatment (primarily 5FU based), the study due to its limited numbers did not address whether the immunoprofile correlates with response to treatment. Yet, despite the limitations in statistical power, the cornerstone of this study was its prospective adherence to strict quality measures. Since tumors were routinely ultrastaged, undergoing not only standard H&E, but also IHC analysis for pancytokeratin, the finding of increased nodal positivity with decreasing CD3 lymphocyte numbers is novel and gives added credence to immunoprofiling.
Furthermore, a unique and unexpected finding was the significant inverse correlation between BMI and TIL count. Nosho et al. found no association between BMI (dichotomized value of 30) and TILs,27 but our results show that when BMI was analyzed as a continuous variable and as a sum of both tumor regions (IM+TC), increases in BMI were associated with lower CD3+ (P=0.04) and lower CD8+ lymphocytes (P=0.02). These preliminary findings are intriguing, particularly since obesity is increasingly linked with worse outcome in colon cancer. This suggestion that obesity attenuates the immune response, if confirmed in larger patient samples, may explain its putative role in the pathogenesis of colon cancer.
Consistent with previous results,12–15 these findings show that a higher level of infiltrating CD8+ cells correlates with improved DFS. Conversely, a higher ratio of CD4+/CD8+ cells has been shown to correlate with a lesser probability of DFS.28 Since high numbers of tumor-infiltrating FoxP3+ T regulatory cells have been shown to correlate with a poor prognosis in multiple cancer types,8,29,30our finding of better DFS associated with lower CD4+/CD8+ ratio can perhaps be accounted for by the down-regulation of T cell effector cells by the FoxP3+ subpopulation of CD4+TILs. This interpretation is in agreement with the reported inverse correlation between CD8+/FoxP3+ ratio and survival in colon cancer.31 Indeed, we found a significantly improved DFS in patients with a higher CD8+/FoxP3+ ratio.
Conclusion
This is the first evaluation of immunophenotyping of TILs in colon cancer using a prospectively collected patient cohort, in which surgical and pathological quality measures were strictly adhered to. Taken together, the findings in this study are consistent with the mounting evidence in the literature that distinct subpopulations of TILs are associated with disease prognosis. Further investigation will focus on the complex interplay between these distinct T cell populations and their anti-tumor activity. Results can be used to design clinical trials of prognostic tools that quantify TIL populations in patients with colon cancer.
Acknowledgments
The NYULMC Experimental Pathology Immunohistochemistry Core Laboratory is supported in part by the Laura and Isaac Perlmutter Cancer Center Support Grant; NIH /NCI P30CA016087 and the National Institutes of Health Shared Instrumentation (S10) Grant; NIH/ORIP S10OD01058. We would like to thank Selena Rae Granitto for her scientific expertise and selfless contribution of her time. The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Cancer Institute or the National Institutes of Health.
Footnotes
Data from this study were presented orally at the 55th annual meeting of the Society for Surgeons of the Alimentary Tract, May 4, 2014, Chicago, IL.
Contributor Information
Simon Lavotshkin, Department of Surgical Oncology, The John Wayne Cancer Institute at Providence Saint John's Health Center, 2200 Santa Monica Blvd., Santa Monica, CA 90404, USA.
John R. Jalas, Department of Pathology, Providence Saint John’s Health Center, Santa Monica, CA, USA
Hitoe Torisu-Itakura, Department of Surgical Oncology, The John Wayne Cancer Institute at Providence Saint John's Health Center, 2200 Santa Monica Blvd., Santa Monica, CA 90404, USA.
Junko Ozao-Choy, Department of Surgical Oncology, The John Wayne Cancer Institute at Providence Saint John's Health Center, 2200 Santa Monica Blvd., Santa Monica, CA 90404, USA.
Ji Hey Lee, Department of Surgical Oncology, The John Wayne Cancer Institute at Providence Saint John's Health Center, 2200 Santa Monica Blvd., Santa Monica, CA 90404, USA.
Myung Shin Sim, UCLA School of Medicine, Santa Monica, CA, USA.
Alexander Stojadinovic, Uniformed Services University of Health and Sciences, Bethesda, MD, USA.
Zev Wainberg, UCLA School of Medicine, Santa Monica, CA, USA.
Carlo B. Bifulco, Earle A. Chiles Research Institute, Robert W. Franz Cancer Research Center, Providence Portland Medical Center, Portland, OR, USA
Bernard A. Fox, Department of Molecular Microbiology and Immunology, and Knight Cancer Institute, Oregon Health and Science University, Portland, OR, USA
Anton J. Bilchik, Department of Surgical Oncology, The John Wayne Cancer Institute at Providence Saint John's Health Center, 2200 Santa Monica Blvd., Santa Monica, CA 90404, USA; UCLA School of Medicine, Santa Monica, CA, USA; California Oncology Research Institute, Culver City, CA, USA
References
- 1.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–4. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gyorki DE, Yuan J, Mu Z, Zaidi B, Pulitzer M, Busam K, Brady MS, Coit DG, Allison JP, Wolchok JD, Ariyan CE. Immunological insights from patients undergoing surgery on ipilimumab for metastatic melanoma. Annals of surgical oncology. 2013;20:3106–11. doi: 10.1245/s10434-013-2999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang I, Han SJ, Sughrue ME, Tihan T, Parsa AT. Immune cell infiltrate differences in pilocytic astrocytoma and glioblastoma: evidence of distinct immunological microenvironments that reflect tumor biology. Journal of neurosurgery. 2011;115:505–11. doi: 10.3171/2011.4.JNS101172. [DOI] [PubMed] [Google Scholar]
- 4.Nakano O, Sato M, Naito Y, Suzuki K, Orikasa S, Aizawa M, Suzuki Y, Shintaku I, Nagura H, Ohtani H. Proliferative activity of intratumoral CD8(+) T-lymphocytes as a prognostic factor in human renal cell carcinoma: clinicopathologic demonstration of antitumor immunity. Cancer research. 2001;61:5132–6. [PubMed] [Google Scholar]
- 5.Nedergaard BS, Ladekarl M, Thomsen HF, Nyengaard JR, Nielsen K. Low density of CD3+, CD4+ and CD8+ cells is associated with increased risk of relapse in squamous cell cervical cancer. British journal of cancer. 2007;97:1135–8. doi: 10.1038/sj.bjc.6604001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah W, Yan X, Jing L, Zhou Y, Chen H, Wang Y. A reversed CD4/CD8 ratio of tumor-infiltrating lymphocytes and a high percentage of CD4(+)FOXP3(+) regulatory Tcells are significantly associated with clinical outcome in squamous cell carcinoma of the cervix. Cellular & molecular immunology. 2011;8:59–66. doi: 10.1038/cmi.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruffini E, Asioli S, Filosso PL, Lyberis P, Bruna MC, Macri L, Daniele L, Oliaro A. Clinical significance of tumor-infiltrating lymphocytes in lung neoplasms. The Annals of thoracic surgery. 2009;87:365–71. doi: 10.1016/j.athoracsur.2008.10.067. discussion 71–2. [DOI] [PubMed] [Google Scholar]
- 8.Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, Banham AH. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:5373–80. doi: 10.1200/JCO.2006.05.9584. [DOI] [PubMed] [Google Scholar]
- 9.Kim ST, Jeong H, Woo OH, Seo JH, Kim A, Lee ES, Shin SW, Kim YH, Kim JS, Park KH. Tumor-infiltrating lymphocytes, tumor characteristics, and recurrence in patients with early breast cancer. American journal of clinical oncology. 2013;36:224–31. doi: 10.1097/COC.0b013e3182467d90. [DOI] [PubMed] [Google Scholar]
- 10.Chen KJ, Zhou L, Xie HY, Ahmed TE, Feng XW, Zheng SS. Intratumoral regulatory T cells alone or in combination with cytotoxic T cells predict prognosis of hepatocellular carcinoma after resection. Medical oncology. 2012;29:1817–26. doi: 10.1007/s12032-011-0006-x. [DOI] [PubMed] [Google Scholar]
- 11.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–4. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 12.Pages F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, Lagorce C, Wind P, Marliot F, Bruneval P, Zatloukal K, Trajanoski Z, et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:5944–51. doi: 10.1200/JCO.2008.19.6147. [DOI] [PubMed] [Google Scholar]
- 13.Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, Ohtani H. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer research. 1998;58:3491–4. [PubMed] [Google Scholar]
- 14.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, Meatchi T, Bruneval P, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. The New England journal of medicine. 2005;353:2654–66. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 15.Mlecnik B, Tosolini M, Kirilovsky A, Berger A, Bindea G, Meatchi T, Bruneval P, Trajanoski Z, Fridman WH, Pages F, Galon J. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:610–8. doi: 10.1200/JCO.2010.30.5425. [DOI] [PubMed] [Google Scholar]
- 16.Bilchik A, Nissan A, Wainberg Z, Shen P, McCarter M, Protic M, Howard R, Elashoff D, Tyler J, Peoples GE, Stojadinovic A. Surgical quality and nodal ultrastaging is associated with long-term disease-free survival in early colorectal cancer: an analysis of 2 international multicenter prospective trials. Annals of surgery. 2010;252:467–74. doi: 10.1097/SLA.0b013e3181f19767. discussion 74–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bilchik AJ, Hoon DS, Saha S, Turner RR, Wiese D, DiNome M, Koyanagi K, McCarter M, Shen P, Iddings D, Chen SL, Gonzalez M, et al. Prognostic impact of micrometastases in colon cancer: interim results of a prospective multicenter trial. Annals of surgery. 2007;246:568–75. doi: 10.1097/SLA.0b013e318155a9c7. discussion 75–7. [DOI] [PubMed] [Google Scholar]
- 18.Quasar Collaborative G. Gray R, Barnwell J, McConkey C, Hills RK, Williams NS, Kerr DJ. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet. 2007;370:2020–9. doi: 10.1016/S0140-6736(07)61866-2. [DOI] [PubMed] [Google Scholar]
- 19.Benson AB, 3rd, Hamilton SR. Path toward prognostication and prediction: an evolving matrix. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:4599–601. doi: 10.1200/JCO.2011.37.8646. [DOI] [PubMed] [Google Scholar]
- 20.Andre T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, Bonetti A, Clingan P, Bridgewater J, Rivera F, de Gramont A. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:3109–16. doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 21.Sargent DJ, Marsoni S, Monges G, Thibodeau SN, Labianca R, Hamilton SR, French AJ, Kabat B, Foster NR, Torri V, Ribic C, Grothey A, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:3219–26. doi: 10.1200/JCO.2009.27.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barrier A, Boelle PY, Roser F, Gregg J, Tse C, Brault D, Lacaine F, Houry S, Huguier M, Franc B, Flahault A, Lemoine A, et al. Stage II colon cancer prognosis prediction by tumor gene expression profiling. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:4685–91. doi: 10.1200/JCO.2005.05.0229. [DOI] [PubMed] [Google Scholar]
- 23.Eschrich S, Yang I, Bloom G, Kwong KY, Boulware D, Cantor A, Coppola D, Kruhoffer M, Aaltonen L, Orntoft TF, Quackenbush J, Yeatman TJ. Molecular staging for survival prediction of colorectal cancer patients. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:3526–35. doi: 10.1200/JCO.2005.00.695. [DOI] [PubMed] [Google Scholar]
- 24.Salazar R, Roepman P, Capella G, Moreno V, Simon I, Dreezen C, Lopez-Doriga A, Santos C, Marijnen C, Westerga J, Bruin S, Kerr D, et al. Gene expression signature to improve prognosis prediction of stage II and III colorectal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:17–24. doi: 10.1200/JCO.2010.30.1077. [DOI] [PubMed] [Google Scholar]
- 25.Galon J, Mlecnik B, Bindea G, Angell HK, Berger A, Lagorce C, Lugli A, Zlobec I, Hartmann A, Bifulco C, Nagtegaal ID, Palmqvist R, et al. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. The Journal of pathology. 2014;232:199–209. doi: 10.1002/path.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galon J, Pages F, Marincola FM, Angell HK, Thurin M, Lugli A, Zlobec I, Berger A, Bifulco C, Botti G, Tatangelo F, Britten CM, et al. Cancer classification using the Immunoscore: a worldwide task force. Journal of translational medicine. 2012;10:205. doi: 10.1186/1479-5876-10-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nosho K, Baba Y, Tanaka N, Shima K, Hayashi M, Meyerhardt JA, Giovannucci E, Dranoff G, Fuchs CS, Ogino S. Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer, and prognosis: cohort study and literature review. The Journal of pathology. 2010;222:350–66. doi: 10.1002/path.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diederichsen AC, Hjelmborg J, Christensen PB, Zeuthen J, Fenger C. Prognostic value of the CD4+/CD8+ ratio of tumour infiltrating lymphocytes in colorectal cancer and HLA-DR expression on tumour cells. Cancer immunology, immunotherapy : CII. 2003;52:423–8. doi: 10.1007/s00262-003-0388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamagami W, Susumu N, Tanaka H, Hirasawa A, Banno K, Suzuki N, Tsuda H, Tsukazaki K, Aoki D. Immunofluorescence-detected infiltration of CD4+FOXP3+ regulatory T cells is relevant to the prognosis of patients with endometrial cancer. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2011;21:1628–34. doi: 10.1097/IGC.0b013e31822c271f. [DOI] [PubMed] [Google Scholar]
- 30.Kim HI, Kim H, Cho HW, Kim SY, Song KJ, Hyung WJ, Park CG, Kim CB. The ratio of intra-tumoral regulatory T cells (Foxp3+)/helper T cells (CD4+) is a prognostic factor and associated with recurrence pattern in gastric cardia cancer. Journal of surgical oncology. 2011;104:728–33. doi: 10.1002/jso.22038. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki H, Chikazawa N, Tasaka T, Wada J, Yamasaki A, Kitaura Y, Sozaki M, Tanaka M, Onishi H, Morisaki T, Katano M. Intratumoral CD8(+) T/FOXP3 (+) cell ratio is a predictive marker for survival in patients with colorectal cancer. Cancer immunology, immunotherapy : CII. 2010;59:653–61. doi: 10.1007/s00262-009-0781-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edge SBB DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Manual. 7th Edition Springer; New York: 2010. [Google Scholar]