Abstract
Importance
Adults with acute myeloid leukemia (AML) typically remain hospitalized after induction or salvage chemotherapy until blood count recovery, with resulting prolonged inpatient stays being a primary driver of healthcare cost. Pilot studies suggest that outpatient management following chemotherapy might be safe and could reduce cost for these patients.
Objective
To compare safety, resource utilization, infections and cost between adults discharged early following AML induction or salvage chemotherapy and inpatient controls.
Design
Non-randomized phase 2 study.
Setting
Single center study conducted at the University of Washington Medical Center in Seattle, WA.
Participants
Over a 43-month period (January 1, 2011 – July 31, 2014), 178 adults receiving intensive AML chemotherapy were enrolled. After completion of chemotherapy, 107 met pre-designated medical and logistical criteria for early discharge (ED), while 29 met medical criteria only and served as inpatient controls.
Interventions for Clinical Trials
ED patients were discharged from the hospital at the completion of chemotherapy, and supportive care was provided in the outpatient setting until count recovery (median 21 days, range 2–45 days). Controls received inpatient supportive care (median 16 days, range 3–42 days).
Main Outcome Measures
1) differences in early mortality 2) differences in resource utilization (ICU days, transfusions/study-day and IV antibiotics/study-day) 3) numbers of infections and 3) total and inpatient charges/study-day between early discharge patients and controls.
Results
Four patients discharged early (4%) but no controls died within 30 days of enrollment (p=0.58). Nine patients discharged early (8%) but no controls required intensive care unit-level care (p=0.20). No differences were noted in the average daily number of red blood cell (p=0.55) or platelet (p=0.31) transfusions. Patients discharged early did have more positive blood cultures (p=0.04) but required fewer days of IV antibiotics (p=0.007). Overall, daily charges among discharged patients were significantly lower (median $3,840 vs. $5,852; p<0.001) despite increased charges per inpatient day when readmitted (median $7,405 vs. $6,267; p<0.001).
Conclusions and Relevance
Early dischargefollowing intensive AML chemotherapy can reduce cost and use of IV antibiotics, but attention should be paid to complications that may occur in the outpatient setting. This study was registered at www.ClinicalTrials.gov (NCT01235572).
INTRODUCTION
Adults with newly diagnosed or relapsed acute myeloid leukemia (AML) or high-risk myelodysplastic syndromes (MDS) commonly require intensive chemotherapy to achieve disease remission.1,2 In many countries, standard practice dictates that these patients remain hospitalized until blood count recovery due to the risk of overwhelming infections and bleeding during pancytopenia. This policy requires hospitalization for an average of 3–4 weeks after completion of chemotherapy.3 Several cost analyses have shown that the resulting prolonged inpatient stays are a driver of exceedingly high costs of leukemia care.4–6 These costs are only expected to rise due to the continued introduction of expensive diagnostic tools and therapeutic modalities. To offset these expenditures, it would be desirable to reduce the time AML patients spend in the hospital during remission induction.
Over the last several years, the use of oral prophylactic antimicrobials has increased, and transfusion support of outpatients has become routine. These advances could support a shift from inpatient to outpatient management for some patients with hematologic malignancies undergoing intensive therapies. Indeed, an increasing number of studies has documented the feasibility, safety, and potential cost savings of treating patients undergoing autologous or allogeneic hematopoietic cell transplantation in the outpatient setting during much of their therapy.7–19 However, only a few retrospective and non-controlled prospective studies, including one conducted at our institution, have investigated whether selected AML patients can be safely discharged after completion of induction chemotherapy.20–25 Our pilot study involving 15 patients discharged early and 5 controls suggested that early hospital discharge is safe and could significantly reduce health care charges.26 This observation motivated our subsequent, larger phase 2 trial to compare the safety, resource utilization, and healthcare charges in adults discharged early following AML induction or salvage chemotherapy with that of inpatient controls.
PATIENTS AND METHODS
Study cohort
Patients aged 18–75 years were eligible if they had begun (or were to begin within the next week) intensive AML-like chemotherapy (e.g. with “7+3” or a regimen of similar or higher intensity) for untreated or relapsed MDS or AML, excluding acute promyelocytic leukemia. Patients with significant hypersensitivities to prophylactic antimicrobials were excluded. The Institutional Review Board at the Fred Hutchinson Cancer Research Center approved this protocol, and consent was obtained from patients in accordance with the Declaration of Helsinki. This study was registered at www.ClinicalTrials.gov (NCT01235572).
Early discharge eligibility and follow-up
Eligibility for early hospital discharge upon completion of chemotherapy depended on medical and logistics criteria at the time of contemplated discharge. The former were: ECOG performance status of 0–1, bilirubin ≤3× upper limit of normal, glomerular filtration rate ≥25% of the lower limit of normal, and no clinical signs of heart failure or uncontrolled bleeding. Need for intravenous (IV) antimicrobials did not preclude early hospital discharge. The logistical criteria were: residency within 60 minutes of the UW/FHCRC study center, a reliable caregiver, and willingness to frequently follow-up at the primary outpatient care facility (Seattle Cancer Care Alliance, Seattle, WA). Patients meeting both medical and logistics criteria were discharged; if readmitted, subsequent early hospital discharge was possible if all medical/logistics criteria were again met. Patients who met medical but not logistics criteria after completion of chemotherapy remained hospitalized and served as inpatient controls.
Patient management
All patients were prescribed prophylactic antimicrobials (levofloxacin, fluconazole, and acyclovir or medications with similar antimicrobial coverage) until peripheral blood count recovery. Asymptomatic patients received red cell transfusions for a hematocrit <26% and platelet transfusions for a platelet count below <10×109/L; the same transfusion triggers were used for both in- and outpatients. Patients who had fever (as defined by the Infectious Diseases Society of America27) and an absolute neutrophil count (ANC) below 1,000/µL received IV antibiotics in the hospital. Management of neutropenic fever generally consisted of a 3rd generation cephalosporin (unless intolerant) with or without additional gram-positive coverage if indicated. Data regarding vancomycin-resistant enterococcus (VRE) colonization and history of MRSA infections were highlighted in the electronic medical record to allow for tailoring of empiric gram-positive coverage. The inpatient team determined the duration of IV antibiotics and appropriateness to transition back to oral antimicrobials.
Paralleling our institution’s approach to the outpatient care of AML patients following post-remission chemotherapy, the study protocol suggested that outpatients be seen three times weekly by an oncology nurse and weekly by the primary outpatient oncologist or an advanced practice provider if asymptomatic; patients were seen more frequently if mandated by medical problems (e.g. nausea, dehydration, or transfusion needs). Patients and caregivers had 24-hour access to phone consultation with a physician.
Medical complications, resource utilization, and healthcare charges
Information on medical complications (infections, ICU admissions) and use of medical resources (transfusions and IV antibiotics) was collected from electronic medical records. Transfusion requirements were analyzed as number of units of either packed red blood cells (PRBCs) or platelets per study-day. Differences in infectious risk between the two patient cohorts were assessed based on clinically relevant bloodstream and Clostridium difficile (C diff) infections. The former was considered for any positive blood culture except for coagulase-negative Staphylococcus aureus when only 1 of 2 bottles was reported positive. C diff infection was assumed when the toxin B gene was detected via polymerase chain reaction (PCR) test during the study period. Professional and facility gross fees or “charges” associated with inpatient and outpatient management were captured using electronic billing information. Charges (in US$) were measured per study day.
Safety endpoints, definitions, and statistical considerations
For early discharge patients, follow-up time began the day after hospital discharge, which had to occur within 72 hours of chemotherapy completion, and ended when they (a) received additional chemotherapy, (b) attained an ANC ≥0.5×109/L and self-sustained platelet count ≥20×109/L, (c) sought medical attention elsewhere, or (d) 45 days had elapsed from discharge, whichever occurred first. For inpatient controls, follow-up time began the first day they met medical criteria for discharge (but 72 hours of completion of chemotherapy) and ended when they (a) were discharged from the hospital, (b) achieved blood counts as noted above, or (c) received additional chemotherapy. Chemotherapy regimens were categorized as “standard intensity” (“7+3” or “7+3”-like therapy) and “high intensity” (containing cytarabine doses of >1 gram/m2) regimens. Characteristics of discharged patients and inpatient controls were compared with Fisher’s exact (categorical characteristics) and Wilcoxon-Mann-Whitney tests (continuous characteristics). Seven patients were enrolled twice on study; five patients were in the discharge cohort twice, whereas two patients were each in the discharge cohort and the control cohort. Analyses accounted for the potential correlation from patients enrolled twice. Outcome analyses accounted for days on study. Linear (quantitative outcomes) and logistic (binary outcomes) regression analysis was used to evaluate prognostic variables for the proportion of time spent as an inpatient after discharge, the need for hospital readmission, and the risk of bacteremia.
RESULTS
Study cohort
Between January 1, 2011 and July 31, 2014, 178 patients with MDS/AML were enrolled before or during intensive AML induction/salvage chemotherapy. Forty-two patients (23.6%) were deemed medically ineligible for discharge by the time they finished chemotherapy infusion, primarily due to an ECOG performance status of >1 associated with the development of an acute medical problem (most commonly clinically significant fever/infection and acute toxicity from chemotherapy such as nausea, mucositis, or diarrhea). One hundred seven patients (60.1%) met both medical and logistics criteria and were discharged from the hospital within 72 hours of completing chemotherapy (“early discharge [ED] patients”). The remaining 29 patients (16.3%) met medical but not logistics criteria and therefore remained in hospital, serving as inpatient controls. Demographic and treatment-related characteristics of ED and control patients are summarized in Table 1. More ED patients were female and received therapy for relapsed/refractory AML. From the date of cohort assignment after completion of chemotherapy, ED and control patients spent a median of 21 (range: 2–45) and 16 (range: 3–42) days on study, respectively.
TABLE 1.
Patient characteristics
| Parameter | ED Patients (n=107) |
Control Patients (n=29) |
P-value |
|---|---|---|---|
| Median age, years (range) | 52 (19–73) | 53 (22–70) | 0.82 |
| Male gender, n (%) | 65 (61%) | 23 (79%) | 0.08 |
| Median study days, n (range) | 21 (2–45) | 16 (3–42) | 0.002 |
| Disease, n (%) | |||
| AML | 90 (84%) | 26 (90%) | 0.56 |
| MDS (RAEB2-2) | 17 (16%) | 3 (10%) | |
| Disease status, n (%) | |||
| Newly diagnosed | 43 (40%) | 20 (69%) | 0.007 |
| Relapsed/refractory | 64 (60%) | 9 (31%) | |
| Treatment, n (%) | 0.82 | ||
| Standard intensity | |||
| 7+3 (+/− additional drug)* | 30 (28%) | 9 (31%) | |
| High intensity | |||
| Idarubacin/HiDAC/Pravastatin | 20 (19%) | 5 (17%) | |
| MEC (+/−additional drug)** | 19 (18%) | 2 (7%) | |
| FLAG +/−Idarubicin | 5 (4%) | 2 (7%) | |
| G-CLAC | 16 (15%) | 10 (35%) | |
| G-CLAM | 16 (15%) | 1 (3%) | |
| Other | 1 (1%) | 0 (0%) | |
| Reason for study removal, n(%) | 0.35 | ||
| Blood count recovery | 67 (63%) | 16 (55%) | |
| New treatment | 13 (12%) | 1 (3%) | |
| 45 days without count recovery | 13 (12%) | 0 (0%) | |
| Death | 4 (4%) | 0 (0%) | |
| Clinician decision to discharge off–protocol | 3 (3%) | 11(38%) | |
| Sought care at outside facility | 7 (6%) | --- | |
Additional drugs included azacitidine, decitabine, cladribine, or an oral hedgehog inhibitor.
Additional drugs included decitabine, azacitidine, or MDX 1338.
Hospital readmissions
While on study, 93 of the 107 ED patients were re-admitted; 19 patients were readmitted twice, five were readmitted three times, and one was readmitted four times. Causes for readmission were neutropenic fever (n=108), bacteremia/sepsis (n=7), localized infection (n=2), and nausea/vomiting (n=2). One patient each was re-admitted for upper GI bleeding, diarrhea, facial swelling, hypotension, mucositis, and monitoring after a motor vehicle accident. ED patients spent a median of 13 (range: 0–42) days as outpatients. The median total number of days spent in the hospital was 8 (range: 0–33) days; thus, early discharge patients spent a median of 61% (range: 0–100%) of the time from discharge until removal from study as outpatients (Table 2). Neither age (>55 vs. ≤55), type of treatment (“7+3-like” vs. higher intensity), nor type of disease (newly diagnosed vs. relapsed/refractory) were associated with re-admissions of ED patients. ED patients age >55 years spent on average 11% less time as inpatients compared to younger ED patients, but this difference was not statistically significant (p=0.64). There was also no statistically significant difference in the proportion of time spent as inpatients among ED patients who received“7+3”-like treatment vs. higher intensity chemotherapy (p=0.97) or among patients with newly diagnosed vs. relapsed/refractory disease (p=0.95).
TABLE 2.
Inpatient/outpatient management and resource utilization of early discharge and inpatient control patients
| ED Patients (n=107) |
Control Patients (n=29) |
p- value |
|
|---|---|---|---|
| Median days on study, n (range) | 21 (2–45) | 16 (3–42) | 0.002 |
| Median days as outpatient, n (range) | 13 (0–42) | --- | |
| Median days as inpatient, n (range) | 8 (0–33) | 16 (3–42) | <0.001 |
| Median number of readmissions, n (range) | 1 (1–4) | --- | |
| Median % days spent as outpatient (range) | 61 (0–100%) | --- | |
| Median number of outpatient MD visits/day, n (range) | 0.09 (0–0.33) | --- | |
| Median number of outpatient Clinic visits/day, n (range) | 0.27 (0–1.12) | --- | |
| Median number of units of RBC transfused/day, n (range) | 0.27 (0–0.94) | 0.29 (0–0.60) | 0.55 |
| Median number of platelet transfusions/day, n (range) | 0.26 (0–1.25) | 0.29 (0.06–0.75) | 0.31 |
| Median days of IV antibiotics/study day, n (range) | 0.48 (0–1) | 0.71 (0–1) | 0.007 |
| Number of patients with bloodstream infections, n (%) | 37 (35%) | 4 (14%) | 0.039 |
| Number of patients with C. diff infections, n (%) | 10 (9%) | 0 | 0.12 |
| Number of patients requiring ICU-level care, n (%) | 9 (8%) | 0 | 0.20 |
| Median days of ICU-level care, n (range) | 2 (1–6) | -- | |
| Early deaths, n (%) | 4 (4%) | 0 (0%) | 0.58 |
ICU-level care needs and deaths
Nine (8%) of the discharged patients required a median of 2 (range: 1–6) days of ICU care (8 for sepsis, 1 for upper GI bleed). There were no patients who required ICU care in the control population (p=0.20). Four ED patients (4%) but no control patient died within 30 days of follow-up on study (p=0.58). These patients were all over age 50 (age 52, 57, 62, and 70, respectively). Two patients were being treated for relapsed/refractory AML, one patient had secondary AML after being previously diagnosed with MDS, and 1 patient developed AML after being previously treated for chronic lymphocytic leukemia with a fludarabine-containing regimen. Causes of death were sepsis in 3 patients and invasive fungal sinusitis (mucormycosis) in the one patient previously treated with fludarabine.
Development of infections and use of IV antibiotics
The number of bloodstream infections was statistically higher among ED patients (p=0.04). On review of the microbiological data, there were 28 gram-positive and 8 gram-negative bacterial infections as well as 5 fungal infections (4 patients had >1 positive culture during follow-up) among ED patients, whereas 4 bacteremias due to gram-positive organisms were noted in inpatient controls. In multivariable analysis, receiving treatment for relapsed/refractory AML/MDS (rather than newly diagnosed disease) was independently associated with the diagnosis of bacteremia (p=0.02), whereas treatment arm (i.e. being discharged early vs. staying inpatient), age, and type of chemotherapy were not. Despite this increase in documented bloodstream infections, ED patients overall received IV antibiotics (given as inpatient and, in many cases, as outpatient after discharge) for less of the study period (median 0.48 vs. 0.71 days of IV antibiotics per study day, p=0.007; Table 2). However, there was no statistically significant difference in the number of IV antibiotics per study day among ED patients vs. controls who had positive cultures (p=0.36). There was a slightly, statistically non-significantly, increased risk of C diff in the ED patients (n=10, 9%) compared with controls (n=0; p=0.12).
Transfusion needs
As summarized in Table 2, there were no differences between the 2 cohorts in the median number of RBC units transfused per study day (p=0.55) or the number of platelet transfusions given per study day (p=0.31).
Healthcare charges
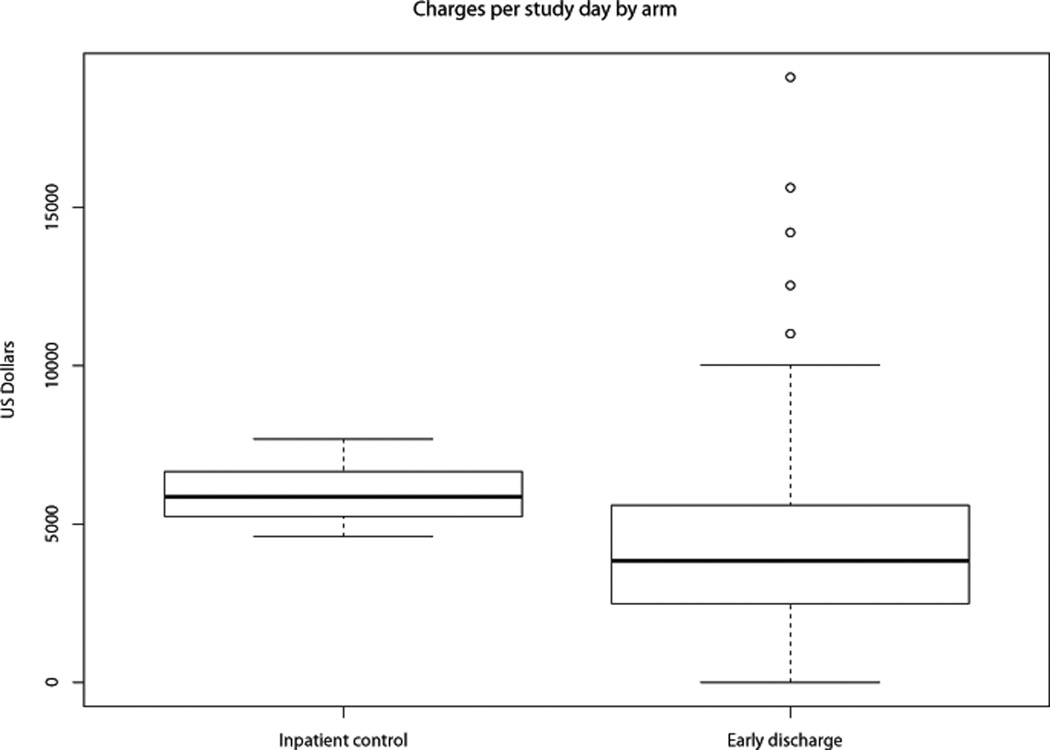
The median charges per study day among ED patients were significantly lower than those of inpatient controls (median $3,840 vs. $5,852, p<0.001; Table 3, Figure 1). This was true although daily inpatient charges were higher for ED patients once they were readmitted compared to the daily inpatient charges incurred by controls (median $7,405 vs. $6,267; p<0.001). Neither age, regimen intensity, nor disease status were associated with daily charges incurred (age ≤ vs. > 55 years: p=0.68; “7+3”-like vs. more intensive chemotherapy: p=0.38; or newly diagnosed vs. relapsed/refractory disease p=0.19).
TABLE 3.
Charges/study day accrued by discharged patients and inpatient controls
| ED Patients (n=105) |
Control Patients (n=29) |
p-value | |
|---|---|---|---|
| Median total charges/study day (range) | $3,840 ($0–$19,105) | 5,852 ($4,599–$7,685) | <0.001 |
| Median total charges/outpatient day (range) | $1,974 ($0–$28,910) | --- | --- |
| Median total charges/inpatient day (range) | $7,405 ($0–$25,917) | 6,282 ($4,599–$8,0278 | <0.001 |
Figure 1.
Boxplot depicting charges per study day in inpatient controls vs. early discharge patients.
DISCUSSION
The delivery of effective supportive care for patients with AML has increased substantially in the past 1–2 decades with introduction of oral broad-spectrum antimicrobials and routine use of transfusions. Nonetheless, in many countries including the U.S., patients receiving induction regimens such as 7+3 continue to spend many weeks “pre-emptively” as inpatients for close monitoring at large financial and possibly psychological detriment. We therefore conducted a pilot study (NCT00844441) between April 2009 and April 2010 to explore early discharge of adult AML/MDS patients.26 Among 39 enrollees, 15 were discharged early and 5 patients served as inpatient controls. No patient died within 30 days of enrollment, and patients discharged early incurred lower median daily charges ($3,270 vs. $5,467; p=0.01) than controls.26 The current study extends our experience to a much larger patient population. Consistent with the data from the pilot study, early discharge patients spent a median of >50% of their time as outpatients, demonstrating the feasibility of this care strategy. Moreover, this follow-up study indicates that a policy of early hospital discharge following intensive chemotherapy for AML/MDS decreases healthcare charges (Figure 1) without significantly increasing treatment-related mortality.
Over the last 2 decades, the rates of early death (“treatment-related mortality”) have significantly decreased in patients with AML given intensive induction chemotherapy,28 likely as a result of improved supportive care measures. Indeed, we observed only 4 deaths within the first 30 days after completion of chemotherapy among the 136 patients we followed in this study (2.9%), and only 9 patients required ICU-level care while on study. However, all patients who required ICU-level care or who experienced early death were allocated to the early discharge arm. The rates of ICU-level care or early death were not statistically significantly different between ED and control patients (p=0.58 and p=0.20, respectively), and they appeared well within the expected range, suggesting that early discharge is safe. Nevertheless, the rarity of these outcomes does not exclude the possibility of increased risk of life-threatening complications when discharged early after intensive chemotherapy. Thus, this finding supports a cautious approach to this care strategy and our recommendation that patients be monitored very closely in well-equipped outpatient facilities with immediate access to a hospital.
The rising costs associated with cancer care pose a significant burden to individual patients and to society in general.29–32 It is well established that patients with hematologic malignancies such as AML or MDS incur some of the highest costs.33,34 Thus, care strategies aimed at reducing these costs are of high economic interest. As health care cost for AML patients are largely driven by inpatient care,3 we posited that a policy of early hospital discharge might lead to a reduction in healthcare expenditures. Consistent with the findings from our pilot study,26 the results from this phase 2 study strongly support this idea by demonstrating that discharge after completion of intensive chemotherapy for AML or high risk MDS is associated with reduced daily charges when compared to standard practice. This is true despite the fact that the use of red blood cell or platelet transfusions was similar and, when re-hospitalized, the daily inpatient charges were higher than for a control patient who never left the hospital. Ours is the first large prospective study designed to determine the fiscal impact of these two approaches. The median difference in charges (approximately $2,000/study day) accrued between our inpatient and discharged populations during the supportive phase of care is similar to what was seen in our pilot study,26 arguing for the validity of our findings.
While several methods for studying healthcare cost have been utilized,4–6 we chose to use hospital “charges”, which are appreciably higher than the actual amount reimbursed by payers on behalf of the patient. This allowed us to avoid inconsistencies in reimbursement policies negotiated between the institution and various payers and perform a valid comparison between the study groups; however, we would caution against using our results to estimate the direct cost of AML/MDS care. Moreover, while we avoided this discrepancy by performing all in- and outpatient care within a hospital-based clinic facility, hospital-based healthcare system charges may be greater than non-hospital-based outpatient clinic charges due to the larger amount of overhead required to operate large healthcare facilities. This possibility suggests that even greater savings may be incurred if early discharge policies could be implemented in non-hospital-based outpatient clinics.
One objective of increased outpatient patient management is to reduce the morbidity and/or mortality from nosocomial or healthcare-related infections. Indeed, one retrospective study demonstrated that septicemias were reduced when the practice of early discharge was implemented although a shift from gram-negative to gram-positive organisms was noted in the outpatient cohort.24 In contrast, in our study, ED patients experienced a higher number of bacteremias than inpatient controls, with most bacteremias in ED patients being due to gram-positive organisms. Consistent with the findings of the study by Halim et al.,24 this observation suggests that with increased use of outpatient care, there might be an increase in the frequency of gram-positive infections. The large number of staphylococcal and streptococcal infections in the discharged patient population suggests the value of closer attention to patient education regarding care of indwelling catheters. It may also be influenced by the use of levofloxacin, which has broad gram-negative coverage, as outpatient prophylaxis, although the approach to antimicrobial prophylaxis did not differ between the two groups. Because of the size of our cohort and the occurrence of only 41 documented bloodstream infections in our study, we were relatively limited in our ability to identify the covariates associated with bloodstream infection. However, in a multivariate analysis, treatment for relapsed/refractory AML/MDS was independently associated with the diagnosis of bloodstream infection. It is therefore also possible that the increased number of bacteremias was due to an imbalance in the stage of treatment (newly diagnosed vs. relapsed/refractory disease; Table 1) rather than the place of treatment (in- vs. outpatient). Despite more positive blood cultures, ED patients received fewer days of IV antibiotics during the study period. Since the days of IV antibiotics did not differ between ED patients or controls when positive blood cultures were found, it is interesting to speculate that the overall reduction in IV antibiotic days during the study period for ED patients was driven by differences in the management of culture-negative neutropenic fevers and/or a tendency toward more rapid conversion from IV to oral antibiotics after clinical stabilization in patients who were considered for early hospital discharge.
Although our ED patients spent a median of >60% of the study time as outpatients, hospital readmissions were common. While we restricted study eligibility to a group of medically fit patients, it is plausible that a subset could be identified that is at particularly high risk of readmission and medical complications and thus not well suited for early hospital discharge. To begin addressing this question, we investigated the relationship between re-hospitalization time and older age, treatment intensity, and disease stage (newly diagnosed vs. relapsed/refractory disease), but none of these factors was associated with increased time spent as inpatient or higher treatment charges, suggesting that early hospital discharge should not be withheld based on any of these factors.
One limitation to our study was the inability to account for the influence of certain disease and patient-related factors on early mortality, given the limited number of events. As a second limitation, we did not collect data on certain expenses incurred such as lodging, transportation, caregiver time, home care cost (including cost for home administration for antimicrobials), prescription cost, nursing and child care expenses, etc.; such cost are higher for out- than inpatients and may disproportionally burden outpatients directly as they may not be covered by their health care insurance. Finally, the non-randomized nature of this study should be acknowledged as a limitation. Theoretically, a randomized assessment between early hospital discharge strategy and conventional hospital care for selected patients after completion of (re)-induction chemotherapy for MDS/AML is feasible and would be ideal to further test this care concept in this patient population; besides assessment of safety and direct health care utilization and associated cost, such a study could also investigate effects on quality of life and “indirect” cost (e.g. lodging, transportation, etc.) and their immediate financial impact on patients and caregivers. However, such a trial might be difficult to conduct: in our experience, most AML/MDS patients were highly interested in an outpatient care approach if there were no logistics hurdles such as lack of caregiver or housing and would not have been willing to remain hospitalized if randomized to the control group. Moreover, some physicians may feel strongly about one care approach. With this, we suspect that a randomized study including a large number of eligible patients may be challenging to complete and potentially subject to significant selection bias.
CONCLUSIONS
Our findings suggest that an early discharge policy following intensive AML chemotherapy in selected adult patients allows a shift toward a greater portion of care being delivered in the outpatient setting. This care strategy may reduce health care cost and the duration of the use of IV antibiotics; however, close follow-up in well-equipped and well-staffed outpatient facilities are required, and specific outpatient support and readmission procedures should be put in place to ensure maximal patient safety.
Acknowledgments
Funding/Support: J.E.V. was supported by a fellowship training grant from the National Heart, Lung, and Blood Institute/National Institutes of Health (NHLBI/NIH: T32-HL007093). R.B.W. is a Leukemia & Lymphoma Society Scholar in Clinical Research.
Role of Funder/Sponsor: The study sponsors play no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
AUTHOR CONTRIBUTIONS:
Dr. Vaughn had full access to all of the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
Study concept and design: Vaughn, Estey, Walter.
Acquisition, analysis and interpretation of data: Vaughn, Powell, Othus, Gardner, Estey and Walter.
Drafting of the Manuscript: Vaughn, Walter.
Critical revision of the manuscript for all important intellectual content: all authors.
Statistical analysis: Othus.
Study supervision: Walter.
Conflict of Interest Disclosures: None reported.
REFERENCES
- 1.Ferrara F, Schiffer CA. Acute myeloid leukaemia in adults. Lancet. 2013;381(9865):484–495. doi: 10.1016/S0140-6736(12)61727-9. [DOI] [PubMed] [Google Scholar]
- 2.Ades L, Itzykson R, Fenaux P. Myelodysplastic syndromes. Lancet. 2014;383(9936):2239–2252. doi: 10.1016/S0140-6736(13)61901-7. [DOI] [PubMed] [Google Scholar]
- 3.Walter RB, Taylor LR, Gardner KM, Dorcy KS, Vaughn JE, Estey EH. Outpatient management following intensive induction or salvage chemotherapy for acute myeloid leukemia. Clin Adv Hematol Oncol. 2013;11(9):571–577. [PMC free article] [PubMed] [Google Scholar]
- 4.Redaelli A, Botteman MF, Stephens JM, Brandt S, Pashos CL. Economic burden of acute myeloid leukemia: a literature review. Cancer Treat Rev. 2004;30(3):237–247. doi: 10.1016/j.ctrv.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Nerich V, Lioure B, Rave M, et al. Induction-related cost of patients with acute myeloid leukaemia in France. Int J Clin Pharm. 2011;33(2):191–199. doi: 10.1007/s11096-010-9462-1. [DOI] [PubMed] [Google Scholar]
- 6.Leunis A, Blommestein HM, Huijgens PC, Blijlevens NM, Jongen-Lavrencic M, Uyl-de Groot CA. The costs of initial treatment for patients with acute myeloid leukemia in the Netherlands. Leuk Res. 2013;37(3):245–250. doi: 10.1016/j.leukres.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 7.Meisenberg BR, Miller WE, McMillan R, et al. Outpatient high-dose chemotherapy with autologous stem-cell rescue for hematologic and nonhematologic malignancies. J Clin Oncol. 1997;15(1):11–17. doi: 10.1200/JCO.1997.15.1.11. [DOI] [PubMed] [Google Scholar]
- 8.Meisenberg BR, Ferran K, Hollenbach K, Brehm T, Jollon J, Piro LD. Reduced charges and costs associated with outpatient autologous stem cell transplantation. Bone Marrow Transplant. 1998;21(9):927–932. doi: 10.1038/sj.bmt.1701191. [DOI] [PubMed] [Google Scholar]
- 9.Rizzo JD, Vogelsang GB, Krumm S, Frink B, Mock V, Bass EB. Outpatient-based bone marrow transplantation for hematologic malignancies: cost saving or cost shifting? J Clin Oncol. 1999;17(9):2811–2818. doi: 10.1200/JCO.1999.17.9.2811. [DOI] [PubMed] [Google Scholar]
- 10.Summers N, Dawe U, Stewart DA. A comparison of inpatient and outpatient ASCT. Bone Marrow Transplant. 2000;26(4):389–395. doi: 10.1038/sj.bmt.1702534. [DOI] [PubMed] [Google Scholar]
- 11.Stiff P, Mumby P, Miler L, et al. Autologous hematopoietic stem cell transplants that utilize total body irradiation can safely be carried out entirely on an outpatient basis. Bone Marrow Transplant. 2006;38(11):757–764. doi: 10.1038/sj.bmt.1705525. [DOI] [PubMed] [Google Scholar]
- 12.Solomon SR, Matthews RH, Barreras AM, et al. Outpatient myeloablative allo-SCT: a comprehensive approach yields decreased hospital utilization and low TRM. Bone Marrow Transplant. 2010;45(3):468–475. doi: 10.1038/bmt.2009.234. [DOI] [PubMed] [Google Scholar]
- 13.McDiarmid S, Hutton B, Atkins H, et al. Performing allogeneic and autologous hematopoietic SCT in the outpatient setting: effects on infectious complications and early transplant outcomes. Bone Marrow Transplant. 2010;45(7):1220–1226. doi: 10.1038/bmt.2009.330. [DOI] [PubMed] [Google Scholar]
- 14.Gutiérrez-Aguirre CH, Ruiz-Argüelles G, Cantú-Rodríguez OG, et al. Outpatient reduced-intensity allogeneic stem cell transplantation for patients with refractory or relapsed lymphomas compared with autologous stem cell transplantation using a simplified method. Ann Hematol. 2010;89(10):1045–1052. doi: 10.1007/s00277-010-0986-1. [DOI] [PubMed] [Google Scholar]
- 15.Preussler JM, Denzen EM, Majhail NS. Costs and cost-effectiveness of hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18(11):1620–1628. doi: 10.1016/j.bbmt.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martino M, Montanari M, Bruno B, et al. Autologous hematopoietic progenitor cell transplantation for multiple myeloma through an outpatient program. Expert Opin Biol Ther. 2012;12(11):1449–1462. doi: 10.1517/14712598.2012.707185. [DOI] [PubMed] [Google Scholar]
- 17.Holbro A, Ahmad I, Cohen S, et al. Safety and cost-effectiveness of outpatient autologous stem cell transplantation in patients with multiple myeloma. Biol Blood Marrow Transplant. 2013;19(4):547–551. doi: 10.1016/j.bbmt.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Campbell P, Walker P, Avery S, et al. Safe and effective use of outpatient non-myeloablative allogeneic stem cell transplantation for myeloma. Blood Cancer J. 2014;4:e213. doi: 10.1038/bcj.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graff TM, Singavi AK, Schmidt W, et al. Safety of outpatient autologous hematopoietic cell transplantation for multiple myeloma and lymphoma. Bone Marrow Transplant. 2015 doi: 10.1038/bmt.2015.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruiz-Argüelles GJ, Apreza-Molina MG, Alemán-Hoey DD, Gómez-Almaguer D, Marín-López A, Mercado-Díaz L. Outpatient supportive therapy after induction to remission therapy in adult acute myelogenous leukaemia (AML) is feasible: a multicentre study. Eur J Haematol. 1995;54(1):18–20. doi: 10.1111/j.1600-0609.1995.tb01620.x. [DOI] [PubMed] [Google Scholar]
- 21.Gillis S, Dann EJ, Rund D. Selective discharge of patients with acute myeloid leukemia during chemotherapy-induced neutropenia. Am J Hematol. 1996;51(1):26–31. doi: 10.1002/(SICI)1096-8652(199601)51:1<26::AID-AJH5>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 22.Allan DS, Buckstein R, Imrie KR. Outpatient supportive care following chemotherapy for acute myeloblastic leukemia. Leuk Lymphoma. 2001;42(3):339–346. doi: 10.3109/10428190109064590. [DOI] [PubMed] [Google Scholar]
- 23.Savoie ML, Nevil TJ, Song KW, et al. Shifting to outpatient management of acute myeloid leukemia: a prospective experience. Ann Oncol. 2006;17(5):763–768. doi: 10.1093/annonc/mdl011. [DOI] [PubMed] [Google Scholar]
- 24.Halim TY, Song KW, Barnett MJ, et al. Positive impact of selective outpatient management of high-risk acute myelogenous leukemia on the incidence of septicemia. Ann Oncol. 2007;18(7):1246–1252. doi: 10.1093/annonc/mdm112. [DOI] [PubMed] [Google Scholar]
- 25.Møller T, Nielsen OJ, Welinder P, et al. Safe and feasible outpatient treatment following induction and consolidation chemotherapy for patients with acute leukaemia. Eur J Haematol. 2010;84(4):316–322. doi: 10.1111/j.1600-0609.2009.01397.x. [DOI] [PubMed] [Google Scholar]
- 26.Walter RB, Lee SJ, Gardner KM, et al. Outpatient management following intensive induction chemotherapy for myelodysplastic syndromes and acute myeloid leukemia: a pilot study. Haematologica. 2011;96(6):914–917. doi: 10.3324/haematol.2011.040220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2011;52(4):e56–e93. doi: 10.1093/cid/cir073. [DOI] [PubMed] [Google Scholar]
- 28.Othus M, Kantarjian H, Petersdorf S, et al. Declining rates of treatment-related mortality in patients with newly diagnosed AML given 'intense' induction regimens: a report from SWOG and MD Anderson. Leukemia. 2014;28(2):289–292. doi: 10.1038/leu.2013.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Himmelstein DU, Thorne D, Warren E, Woolhandler S. Medical bankruptcy in the United States, 2007: results of a national study. Am J Med. 2009;122(8):741–746. doi: 10.1016/j.amjmed.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 30.Fojo T, Mailankody S, Lo A. Unintended consequences of expensive cancer therapeutics-the pursuit of marginal indications and a me-too mentality that stifles innovation and creativity: the John Conley Lecture. JAMA Otolaryngol Head Neck Surg. 2014;140(12):1225–1236. doi: 10.1001/jamaoto.2014.1570. [DOI] [PubMed] [Google Scholar]
- 31.Himmelstein DU, Woolhandler S. Cost control in a parallel universe: Medicare spending in the United States and Canada. Arch Intern Med. 2012;172(22):1764–1766. doi: 10.1001/2013.jamainternmed.272. [DOI] [PubMed] [Google Scholar]
- 32.Experts in Chronic Myeloid L. The price of drugs for chronic myeloid leukemia (CML) is a reflection of the unsustainable prices of cancer drugs: from the perspective of a large group of CML experts. Blood. 2013;121(22):4439–4442. doi: 10.1182/blood-2013-03-490003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stalfelt AM, Brodin H. Costs over time in conventional treatment of acute myeloid leukaemia. A study exploring changes in treatment strategies over two decades. J Intern Med. 1994;236(4):401–409. doi: 10.1111/j.1365-2796.1994.tb00816.x. [DOI] [PubMed] [Google Scholar]
- 34.Craig BM, Rollison DE, List AF, Cogle CR. Diagnostic testing, treatment, cost of care, and survival among registered and non-registered patients with myelodysplastic syndromes. Leuk Res. 2011;35(11):1453–1456. doi: 10.1016/j.leukres.2011.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]