Abstract
Clustered regularly interspaced palindromic repeats (CRISPR)-CRISPR-associated (Cas9) technology has proven a formidable addition to our armory of approaches for genomic editing. Derived from pathways in archaea and bacteria that mediate the resistance to exogenous genomic material, the CRISPR-Cas9 system utilizes a short single guide RNA (sgRNA) to direct the endonuclease Cas9 to virtually anywhere in the genome. Upon targeting, Cas9 generates DNA double strand breaks (DSBs) and facilitates the repair or insertion of mutations, insertion of recombinase recognition sites or large DNA elements. Here, we discuss the practical advantages of the CRISPR-Cas9 system over conventional and other nuclease-based targeting technologies and provide suggestions for the use of this technology to address immunological questions.
Introduction
The use of genetically modified mice provides critical insights into the organization and function of the immune system. For the past three decades, gene targeting by homologous recombination in embryonic stem cells has been the method of choice for genome modification in mice (Capecchi, 2005). However, this approach is tedious, time-consuming, expensive, and cannot be applied to other mammalian species or mouse strains with complex genetic backgrounds due to the lack of established embryonic stem cells. To overcome these limitations, targetable nuclease technologies such as zinc finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs) and, more recently, clustered regularly interspaced palindromic repeats (CRISPR)-CRISPR-associated (Cas9) have been developed. These endonucleases can be targeted virtually anywhere in the genome of any species to introduce DNA double strand breaks (DSBs). Resolution of these DSBs by either non-homologous end joining (NHEJ) or homology directed repair (HDR) then facilitates the introduction of random or specific mutations, repair of endogenous mutations, or insertion of DNA elements. Unlike conventional targeting, nuclease-based genome engineering does not require in vitro single cell clonal expansion and prolonged drug selection, which may introduce genetic mutations, nor does it leave behind undesired exogenous DNA elements. Moreover, nuclease-based technologies can be performed directly in zygotes, accelerating the generation of animal models from 12 months for conventional gene targeting in ES cells to less than a few months using CRISPR-Cas9 technology. In this review, we briefly describe CRISPR-Cas9 and related technologies for genome engineering and provide guidelines for their use in generating mouse models. We also discuss recent advances in these technologies and their use for immunological studies.
CRISPR-Cas9 technology
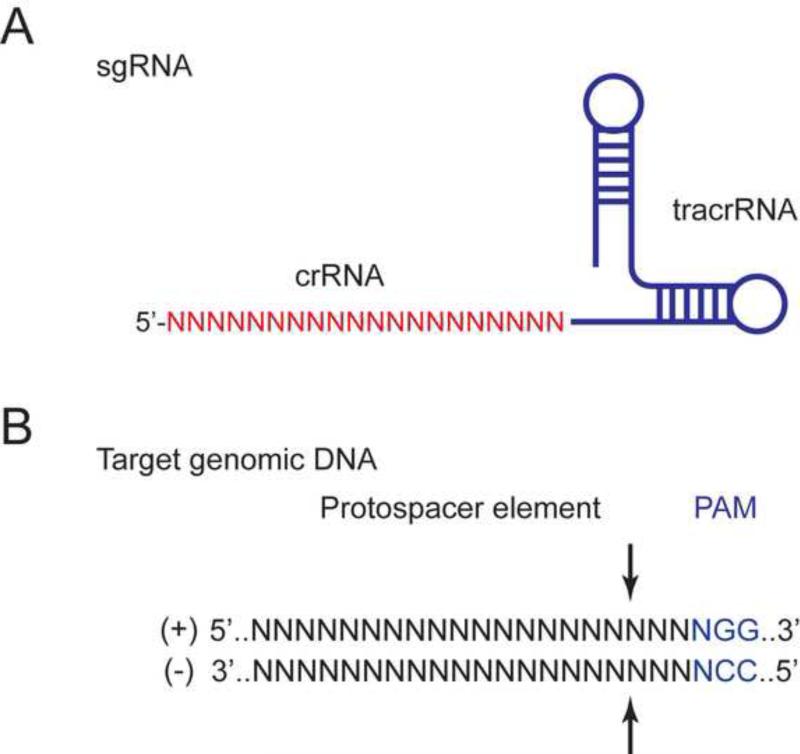
Because of its simplicity, the CRISPR-Cas9 system is rapidly becoming the method of choice for gene targeting. Unlike ZFNs and TALENs, both of which require the complex engineering of highly-specific DNA binding domains for their proper targeting to genomic loci, the endonuclease Cas9 relies solely on a small artificial RNA molecule termed single guide RNA (sgRNA) (Jinek et al., 2012). The sgRNA is a 100-nucleotide molecule generated from the fusion of a 20-nucleotide CRISPR RNA (crRNA) with a transactivating CRISPR RNA (tracrRNA) (Fig. 1). The crRNA confers sequence specificity by forming an RNA-DNA complex with a region called the protospacer element on the target DNA, while the tracrRNA interacts with Cas9 to form the ribonucleoprotein. The genomic sequences receptive to CRISPR-Cas9 targeting require the presence of a short nucleotide sequence located 3′ of the protospacer element, termed the protospacer adjacent motif (PAM) (Fig. 1B). For example, Streptococcus pyogenes Cas9, the most commonly used Cas endonuclease for genome editing, requires a PAM sequence consisting of 5’-NGG-3’ (where N represents any nucleotide) for optimal activity (Hsu et al., 2013). A variant of this sequence, 5’-NAG-3’, confers limited activity (20%) whereas any other combinations do not (Hsu et al., 2013). Although the requirement for PAM sequences may be viewed as a limitation to the technology, S. pyogenes Cas9 PAM sequences are found, on average, as frequently as every 10 nucleotides in the genome (+ and – strand combined, Table 1). Moreover, several additional CRISPR-Cas systems from Streptococcus mutans, Streptococcus thermophilus (CRISPR3), Streptococcus thermophilus (CRISPR1), Campylobacter jejuni, Neisseria meningitidis, Pasteurella multocida, Francisella novicida and Treponema denticola (Table 2) have also been developed into tools for genome engineering (Esvelt et al., 2013; Fonfara et al., 2014; Hou et al., 2013; Zhang et al., 2013). These systems have distinct PAM requirements and may serve as a collection of tools to cover virtually every nucleotide within genomes from different species. The orthogonality of these systems also allows simultaneous and independent targeted-gene regulation and editing in the same cell (Esvelt et al., 2013).
Figure 1. DNA sequence elements recognized by CRISPR-Cas9.
A) Schematic representation of a single guide RNA, a synthetic RNA molecule resulting from the fusion of crRNA (N20, red) and the scaffolding tracrRNA (black). sgRNAs are required and sufficient to target the endonuclease Cas9 to specific sequences in the genome. B) Schematic representation of a target DNA sequence which comprises the protospacer element (N20, black) upstream of the protospacer adjacent motif (PAM, NGG, blue). Arrows indicate the location of the double strand break introduce by the Cas9 endonuclease.
Table 1.
Number and frequency of N20NGG, unique N20NGG, N13NGG and unique N12NGG sequence in the mouse genome.
| Sequence | Number of site | Frequency in mouse genome (number of sites/number of nucleotides) |
|---|---|---|
| N20NGG | 274,072,490 | 1/10 |
| Unique N20NGG | 204,035,213 | 1/13 |
| Unique N13NGG | 11,369,632 | 1/240 |
| Unique N12NGG | 5,274,838 | 1/517 |
Mouse genome length is 2,725,765,481 nucleotides. This table was generated by downloading the mouse genome (mm9, NCBI Build 37) in FASTA format (chromFa.tar.gz) from http://hgdownload.soe.ucsc.edu/goldenPath/mm9/bigZips/. All possible 20-mers followed by a PAM sequence (NGG) were extracted with the fuzznuc tool from the EMBOSS Suite (Rice et al., 2000). A Python script was used to convert the fuzznuc outputs to BED files as well as extracting all 20-, 13- or 12-mers to separate files, with one site per line. A pipeline of the UNIX command “sort” and “uniq” was used to return unique lines.
Table 2.
CRISPR-Cas systems developed for genome editing and their cognate PAM requirements.
| Species | PAM | References |
|---|---|---|
| Streptococcus pyogenes | NGG | (Hsu et al., 2013) |
| Streptococcus mutans | NGG | (van der Ploeg, 2009) |
| Streptococcus thermophilus (CRISPR3) | NGGNG | (Deveau et al., 2008; Fonfara et al., 2014; Horvath et al., 2008) |
| Streptococcus thermophilus (CRISPR1) | NNAAAAW | (Fonfara et al., 2014) |
| Campylobacter jejuni | NNNNACA | (Fonfara et al., 2014) |
| Neisseria meningitidis | NNNNGATT | (Hou et al., 2013; Zhang et al., 2013) |
| Pasteurella multocida | GNNNCNNA | (Fonfara et al., 2014) |
| Francisella novicida | NG | (Fonfara et al., 2014) |
| Treponema denticola | NAAAAN | (Esvelt et al., 2013) |
Limitations associated with CRISPR-Cas9 system
Although the CRISPR-Cas9 system provides great advantages over conventional targeting, several potential issues must be addressed when considering using this technology. As with any other nuclease-based technology, it is necessary to take appropriate precautions to ensure target specificity. Several studies have demonstrated that mismatches between crRNA and targeted sequences can be tolerated by the Cas9 endonuclease (Fu et al., 2013; Hsu et al., 2013; Jinek et al., 2012; Lin et al., 2014; Mali et al., 2013a; Pattanayak et al., 2013). Although there are no definitive rules for Cas9 specificity, the number and location of mismatches play a critical role. As an approximation, DNA-RNA duplex mismatches in close proximity to the PAM sequence impair Cas9 activity whereas mismatches distal to the PAM sequence are tolerated. While a single nucleotide mismatch does not affect Cas9 activity, two or more mismatches, depending on their location with respect to the PAM sequence, decrease Cas9 activity (Fu et al., 2013; Hsu et al., 2013; Lin et al., 2014; Mali et al., 2013a; Pattanayak et al., 2013). Several strategies have been proposed to minimize the number of off-target cleavage events. These include the use of double nickases (Mali et al., 2013a; Ran et al., 2013), shorter sgRNAs (Fu et al., 2014), and the use of a fusion protein containing a catalytically inactive Cas9 and the endonuclease Fok1 (Guilinger et al., 2014; Tsai et al., 2014). Although these strategies proved to be helpful for gene targeting in cell lines, the careful selection of sgRNAs is sufficient to avoid off-target cleavage in vivo (see sgRNA selection below).
Developed for its high efficiency, CRISPR-Cas9 technology can readily target several loci simultaneously, including both alleles of the same gene (Wang et al., 2013; Yang et al., 2013a; Zhou et al., 2014). Although this represents a remarkable attribute when modification of multiple genes is desired, it carries with it obvious limitations when the targeted genes of choice are essential for embryonic or germ cell development. This problem may be overcome by limiting the concentration of Cas9 mRNA transcript for zygotic injections, thereby reducing CRISPR-Cas9 activity to favor heterozygosity over homozygosity.
Another limitation associated with nuclease-based technology for genome modification is the generation of mice mosaic for the target gene. Mosaicism may occur when more than two alleles serve as substrates for the nuclease. To avoid this problem, injections should be performed as early as possible after fertilization to increase the chances of targeting alleles at the single cell stage. Although the rate of mosaicism has remained relatively low in our experience, studies have reported up to 40% mosaicism using this technology (Yang et al., 2013a).
Gene targeting in mice using CRISPR-Cas9 technology
The basic principles of conventional gene targeting also apply to CRISPR-Cas9 technology (Nagy, 2003). For example, the generation of a conditional allele requires careful analysis of the gene structure for optimal insertion of loxP sites. Similarly, insertion of transgenic DNA elements, point mutations, or deletion of chromosomal segments must be carefully planned with appropriate implementation of precautionary measures. Below, we present examples of different types of targeting strategies (Fig. 2).
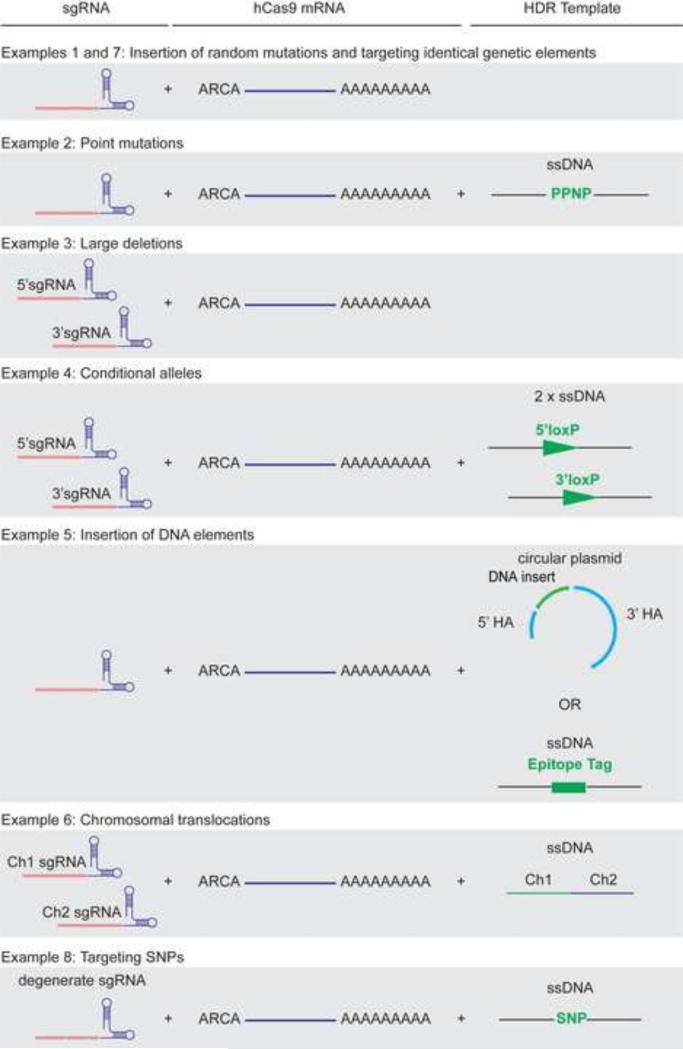
Figure 2. Targeting strategies used to introduce various types of mutations using CRIPSR-Cas9 technology.
Example 1 and 7 (Insertion of random mutations and targeting identical genetic elements) make use of a single sgRNA together with Cas9 to target a single copy or multiple copy number genes. Resolution of the DSB(s) by NHEJ will result in the generation of several genetic modifications, including nonsense, missense or various deletions. Example 2 (Point mutations) makes use of a sgRNA together with Cas9 and an ssDNA oligonucleotide to facilitate HDR. Resolution of the DSB by HDR will result in the introduction of the desired mutation (P). Example 3 (Large deletions) makes use of two sgRNAs flanking a region of interest. Resolution of the DSBs by NHEJ will result in the deletion of the region of interest. Example 4 (Conditional alleles) also makes use of two sgRNAs flanking a region of interest, Cas9 and 2 ssDNA oligonucleotides to facilitate HDR. Resolution of DSBs by HDR will introduce loxP sites at the desired locations. Example 5 (Insertion of DNA elements) makes use of one sgRNA, Cas9 and a circular plasmid or ssDNA oligonucleotide. Resolution of DSBs by homologous recombination will introduce the DNA elements. Example 6 (Chromosomal translocations) makes use of two sgRNAs designed to introduce DSBs on separate Chromosome (Ch1 and Ch2) together with Cas9 and a single ssDNA with sequence complementarity flanking the translocation break. Example 8 (Targeting SNPs) makes use of a single degenerate sgRNA containing 1 or 2 mismatches with its target sequence. Resolution of the DSB by HDR will introduce a single nucleotide mutation. The newly form sites now having an extra mismatch may no longer be recognized by the degenerate sgRNA-Cas9 ribonucleoprotein complex and cleaved again allowing the insertion of a single nucleotide polymorphism.
Example 1: Insertion of random mutations
The most common and straight-forward type of mutation generated using CRISPR-Cas9 technology is the insertion of random mutations caused by the resolution of DSBs by NHEJ. Although this approach has been widely used to target single or multiple genes at once (Wang et al., 2013; Yang et al., 2013a; Zhou et al., 2014), it is important to note that random insertion or deletion of DNA elements do not always result in gene disruption and may produce proteins with undesired and/or artificial properties.
Example 2: Point mutations
To generate a point mutation, a single DSB is introduced near the location of the desired mutation using a single sgRNA. Resolution of the DSB by homology directed repair is then facilitated by the co-injection of an HDR template. In this case, the HDR template consists of a 200-nucleotide-long single stranded DNA (ssDNA) molecule that contains the desired mutation flanked by two homology arms in addition to sequence modifications enabling detection of the altered locus and preventing subsequent cleavage of the repaired locus by Cas9 (Fig. 3). The latter is particularly important when the desired mutation lies outside of the protospacer element or PAM sequence. Excessive cleavage by CRISPR-Cas9 and repair by NHEJ will introduce undesired mutations such as small deletions or insertions in addition to the desired one. Although 120-170-nucleotide-long ssDNA oligonucleotides are typically used for genome editing in mice (Wang et al., 2013; Yang et al., 2013a), ssDNA oligonucleotides of various lengths (ranging from 70-200 nucleotides) have been used successfully in human iPSCs, and ssDNA oligonucleotides shorter than 200 nucleotides demonstrated similar and sometimes better efficiencies (Yang et al., 2013b).
Figure 3. Homology directed repair (HDR) templates.
A) 200-nucleotide-long ssDNA oligonucleotides are used to introduce point mutations using CRISPR-Cas9 technology. The ssDNA contains the desired mutations (P, green) flanked by 60-90-nucleotide-long sequences homologous to the genomic target locus (not shown). Silent substitutions (X, red) are also introduced to facilitate the detection of the mutant allele, including mutations that disrupt or insert restriction enzyme sites. It is not always possible to insert the desired mutations within the protospacer element. Therefore, to avoid successive cleavage by Cas9 and introduction of internal deletion/insertion by NHEJ, additional silent mutations are introduced within the HDR oligonucleotide to alter the protospacer element or the PAM sequence. B) Insertion of recombinase recognition sites or epitope tags within the genome also relies on 200-nucleotide-long single stranded oligonucleotides containing the recombinase recognition sequence (loxP, green) and a restriction enzyme recognition sequence (green underlined) flanked by 80-nucleotide-long sequence homologous to the genomic target locus (not shown). The insertion of a restriction site will facilitate detection of the allele by PCR and Southern blot analyses. Recombinase recognition sites are inserted 3 nucleotides upstream of the PAM sequence, disrupting the protospacer element upon HDR and preventing further cleavage by Cas9. Because the insertion site of epitope tags may fall outside of the protospacer element or the PAM sequence, additional mutations within the protospacer element or the PAM sequence are introduced (not shown). C) Circular plasmids are used to introduce larger DNA elements such as green fluorescent proteins. These plasmids contain the DNA element flanked by large homology arms (HA) of 500 to >2,000 base pairs (blue). If the insertion site lies outside of the protospacer element or the PAM sequence, additional substitutions should be also introduced to prevent further cleavage by Cas9 and introduce undesirable mutations.
Example 3: Large deletions
Targeted disruption of one or multiple contiguous genes by intragenic or intergenic deletions is efficient using CRISPR-Cas9 technology. Introduction of two DSBs flanking the region of interest and their resolution by NHEJ facilitate chromosomal rearrangement and exclusion of the region of interest. Deletions up to 60kb have been generated in our hands and an apparently inverse correlation between the frequency and the length of the deletion was observed, although the experiments were not design to address this question specifically. Consistent with this, a recent study showed an inverse relationship between deletion frequency and deletion size (Canver et al., 2014). In this strategy, the precise location of the DSBs is less important than in those aimed at introducing point mutations. Nevertheless, careful attention should be given to the number and the location of potential off-target loci; avoiding off-target loci located on the same chromosome as the intended on-target sites.
Example 4: Conditional alleles
The generation of mice bearing conditional alleles, regardless of the recognition site (loxP or others), makes use of a combination of the two previous strategies. As detailed above, DSBs are generated on each side of a region of interest, except that in the case of conditional allele insertion, DSBs are instead resolved by HDR. To promote HDR over NHEJ at sites of DSBs, two HDR oligonucleotides each containing a loxP site and a restriction enzyme site flanked by arms of homology (Fig. 3B), are co-injected with CRISPR-Cas9. Design of the two sgRNAs depends on a combination of the aforementioned criteria, the options for which may be limited by accommodating the effective placement of the loxP sites. LoxP site locations are chosen to ensure appropriate disruption of the target gene upon Cre-mediated recombination and to limit the likelihood of potential simultaneous disruption of splice donors, acceptors and branch sites. As with the other approaches, appropriate consideration of sgRNA sequences with potential off-target events located on the on-target site chromosome, exons or introns of other genes should be avoided. Of note, resolution of the DSBs by NHEJ may produce a large deletion (example 3). Thus, from a single injection, both conditional and conventional knockout mice can be produced.
Example 5: Insertion of large DNA elements
Generation of mice with large DNA element insertions is also possible using the CRISPR-Cas9 system (Wang et al., 2013; Yang et al., 2013a). In this case, a single DSB is introduced at or near the site of insertion and HDR is stimulated by co-injection of a circular plasmid containing the DNA element(s) to be inserted, flanked by homology arms long enough to promote HDR (Fig. 3C). Cleavage of the targeting plasmid itself by CRISPR-Cas9 will prevent proper targeting and instead promote random integration of the linear DNA into the genome. Thus silent mutations should be incorporated into the DNA plasmid to avoid its recognition by CRISPR-Cas9. Moreover, in the event that proper targeting occurs, disruption of the protospacer element will also prevent further cleavage of the locus by CRISPR-Cas9 and generation of unwanted mutations. When preparing plasmid DNA for microinjection, special attention should be given to ensure that no residual RNAase remains in the preparation to avoid degradation of Cas9 mRNA and sgRNA transcripts.
Example 6: Chromosomal translocations
Chromosomal translocations have been clinically linked to various forms of cancer, including leukemias, lymphomas and sarcomas and mimicking such translocation in transgenic mice has proven invaluable to our understanding of cancer biology. CRISPR-Cas9 technology enables the generation of such clinically relevant translocations, although feasibility of this approach is limited. In this case, DSBs are introduced in sites within both chromosomes of interest. To promote chromosomal translocation, an HDR oligonucleotide with sequence homology to both chromosomal loci is then co-injected with CRISPR-Cas9. Although this approach has been successful in cell lines, only 2% of the cells exhibited the appropriate recombination event (Torres et al., 2014). We are not aware of this approach being attempted in vivo.
Example 7: Targeting identical genetic elements
In addition to targeting single-copy genes, CRISPR-Cas9 technology can be applied to gene families in which genes share identity in some regions, such as those encoding major histocompatibility molecules, antigen receptors, and pattern recognition receptors. A single sgRNA-HDR template pair can be designed to disrupt several or all of the genes in a family or subfamily based on highly conserved sequences. Such a strategy has not been reported.
Example 8: insertion of single nucleotide polymorphisms
Single Nucleotide Polymorphisms (SNPs) are the most abundant genetic variations in individuals and are, in some instances, causally linked to disease susceptibility and severity, and responses to pharmacological treatments. When located within the coding region of a gene, SNPs can generate nonsense mutation or missense mutations that may directly affect the function of the gene product. When located in regulatory regions of a gene, SNPs may also have dramatic effects by influencing gene expression, mRNA conformation and stability, and/or localization within cells (Capon et al., 2004; Kimchi-Sarfaty et al., 2007; Nackley et al., 2006). Faithful reproduction of these variations in cells or animal models is therefore important for our understanding of diseases associated with specific SNPs.
Generation of SNPs using CRISPR-Cas9 technology, however, may prove to be difficult due to the tolerance of Cas9 for base-pairing mismatches between the sgRNA and its cognate protospacer element. The newly generated allele with only one base pair mismatch may continue to serve as a substrate for Cas9 and therefore be cleaved repeatedly until repaired by NHEJ. To circumvent this limitation, a degenerate sgRNA containing 1 or 2 mismatches for the target site can be substituted for the conventional, perfectly paired, sgRNA. Following the introduction of the DSB at the desired site and repair by HR using an ssDNA oligonucleotide, the repaired allele would now have an extra base pair mismatch. In principle this would prevent further cleavage of the target site. This strategy is a feasible approach to directly assess the role of particular SNPs in susceptibility in vivo.
sgRNA selection
The successful implementation of CRISPR-Cas9 technology is entirely dependent upon selecting the appropriate sgRNA(s) to achieve the modification of choice. The selection of sgRNAs depends on the type of mutation to be generated and the target specificity of the sgRNA. Several open-access websites now provide tools for the identification of on- and off-target loci. These include CRISPR Design (http://crispr.mit.edu/), E-CRISP (http://www.e-crisp.org/E-CRISP/) (Heigwer et al., 2014), ZiFit http://zifit.partners.org/ZiFiT/) and Cas-OFFinder (http://www.rgenome.net/) (Bae et al., 2014). These websites accept a wide range of inputs, identify potential on target sites and generate a list of potential off-target loci for each on-target locus. However, caution should be exercised as some of these web-based CRISPR tools fail to identify all possible off-target loci and/or to provide critical information such as their chromosomal location and position relative to known genes (exonic, intronic or intergenic).
While implementing CRISPR-Cas9 technology for mouse genome editing in our laboratory, we developed a strategy for the selection of sgRNAs allowing optimal flexibility and efficacy. Based on studies of CRISPR-Cas9 specificity in cell lines (Cong et al., 2013; Fu et al., 2013; Hsu et al., 2013; Jiang et al., 2013; Jinek et al., 2012; Mali et al., 2013b), our strategy combines the use of several web-based CRISPR-Cas9 tools and takes into account the original observations that several mismatches (up to 8) located at the 5’ end of the sgRNA can be tolerated by S. pyogenes Cas9 (Jinek et al., 2012). The details of our procedure are presented in Box 1.
As an alternative to this strategy, The CRISPR Design web tool can also be used for sgRNA target site identification. However, caution is advised as this web-based resource does not always return the complete list of all possible off-target sites associated with given sgRNA. Similarly, we do not recommend using a traditional algorithm such as BLAST (Altschul et al., 1990) for the identification of off-target loci as this fails to return all possible results. Instead, we recommend using Fuzznuc from EMBOSS Suite (Rice et al., 2000) or Bowtie (Langmead et al., 2009) which, unlike BLAST, search the input pattern base by base, returning all possible hits.
Preparation of reagents for zygotic injections
Injection of CRISPR-Cas9 directly into pronuclear stage zygotes has greatly accelerated the generation of genetically modified mice (Wang et al., 2013; Yang et al., 2014; Yang et al., 2013a). The approach consists of microinjecting human codon optimized Cas9 mRNA transcripts, sgRNA transcripts and HDR templates directly into pronuclear stage zygotes. Upon translation, sgRNA-loaded Cas9 translocates to the nucleus and introduce DSBs. The concentrations of reagents used are typically as follows: Cas9 mRNA transcript: 100ng/μl, sgRNA: 50ng/μl, HDR oligonucleotides; 200ng/μl and circular plasmids; 200ng/μl.
As an alternative to RNA transcript injection, recent studies showed that microinjection of circular plasmids encoding the human codon optimized Cas9 and sgRNA was equally efficient for introducing DSBs in zygotes (Mashiko et al., 2013). However, 2 out of the 46 pups also integrated the Cas9 encoding plasmid (Mashiko et al., 2013), which may have undesirable effects over the phenotype. As an alternative to Cas9 mRNA or cDNA injections, Cas9 protein can be used. In zebrafish, Cas9 protein microinjection in one cell stage embryos was equally effective in generating DSBs and, in some cases, increased mutagenic activity (Gagnon et al., 2014). The use of Cas9 protein in lieu of the Cas9 transcript for zygotic injections may prove to be useful to reduce mosaicism due to delayed translation of Cas9 mRNA into a functional entity. In the zygotes, transcription and translation are greatly attenuated until the first cell division (Oh et al., 2000) delaying even more Cas9 endonuclease translation and activity to the two-cell stage embryo at which point, four alleles are present. Second, it may facilitate insertion of large DNA elements by directly injecting the ribonucleoprotein complex together with large circular plasmids directly in the pronuclei. Third, it may limit off-target activity by reducing the duration of Cas9 activity. This approach is now being tested in mammalian zygotes.
To introduce point mutations or insertion of short DNA elements (e.g. loxP sites) and to avoid random integration into the genome, 200-nucleotide-long oligonucleotides, are co-injected with CRIPSR/Cas9. Insertion of larger DNA elements such as green fluorescent proteins makes use of circular plasmids to prevent random integration. Linear DNA molecules integrate the genome at higher frequency than circular DNA (Brinster et al., 1985).
Both cytosolic and nuclear injections of reagents in zygotes have been shown to be efficient for introducing DSBs (Horii et al., 2014; Wang et al., 2013; Yang et al., 2013a; Zhou et al., 2014). For mouse strains that are not amenable to superovulation or mice with complex genetic backgrounds, injection can also be performed in in vitro fertilized eggs as demonstrated recently (Li et al., 2014).
Characterization of CRISPR-Cas9-engineered animals
The genome of mice obtained from zygotic injections is characterized using a combination of approaches. The on-target locus is PCR amplified using primers flanking the locus and directly sequenced and/or digested with the specified restriction enzyme (when restriction sites are inserted or deleted by HDR). The desired restriction pattern, however, does not necessarily indicate the presence of the intended mutation. Additional frame shift mutations or reorganizations may occur. Sequencing is therefore required to ascertain that the repair occurred as planned. Moreover, direct sequencing of the PCR product may yield inconclusive results due to the presence of multiple alleles. Thus PCR amplicons are TOPO cloned and a minimum of 30 clones sequenced for each mouse. TOPO cloning of PCR fragments also provide information on possible mosaicism at the target locus. For large deletions, primers flanking the region of interest are used to detect a recombined allele. Direct sequencing of this PCR product also provides information regarding the nature and location of the DNA breaks and rearrangements. One of the challenges in generating mice bearing a conditional allele generated by CRISPR-Cas9 technology is to ensure the successful introduction of recombinase recognition sequences (i.e loxP sites) on the same allele. To ascertain that loxP sites are indeed present on the desired allele, Southern blot analyses may be required (especially when loxP sites are located several thousands of base pairs apart). In addition, in some cases, analysis of the F1 progeny may be required to determine that the two loxP sites are chromosomally linked.
Mice with the desired modifications are then analyzed for potential off-target alterations. To identify all potential off-target loci, we recommend using E-CRISP or Cas-OFFinder searching for N20NRG sequence with 1-3 mismatches because these web tools use Bowtie (Langmead et al., 2009) which returns all possible hits. Also, as mentioned above, if non-unique N13NGG target sequences are used as template for sgRNAs, these additional target sites should be considered as potential off-target loci. Although there is no evidence that off-target loci having 3 or more mismatches are cleaved in vivo (Mashiko et al., 2013; Wang et al., 2013; Yang et al., 2013a), these potential off-target sequences should, nonetheless, be analyzed by PCR amplification and direct sequencing. The number of off-target cleavage sites has remained very low in studies using zygotic injection of CRISPR-Cas9 for mouse genome engineering. Only off-target loci with 1 or 2 mismatches contained mutations Mice with off-target modifications are either discarded or bred to wild type mice to eliminate the undesired mutation(s). In this case, careful analysis of the F1 progeny is required prior to establishing the colony to ensure that non-desirable mutations are no longer present.
The successful generation of mice with appropriate mutations using zygotic injections of CRISPR-Cas9 varies depending upon the type of mutation generated. The rate of random insertion/deletions (NHEJ) at any particular site is astonishingly high; reaching up to 100% of the alleles in some cases. Introduction of point mutations by HDR occurs in 10-70% of the alleles. Large deletion frequency using pairs of sgRNAs are found in 15-65% of mice and this strategy has become our standard method for gene ablation. Introduction of LoxP sites or large DNA elements however is less efficient, with less than 20% of the mice having the proper recombination events (Yang et al., 2013a).
When ssDNA oligonucleotides are used to induce HDR, additional mutations in the vicinity of the desired mutations are sometimes found. It is unclear whether these additional mutations come from the ssDNA oligonucleotide itself or from microdeletions/insertions/mutations that occur during the DNA repair process. Nevertheless, this observation underscores the critical importance of performing thorough analyses of founder mice.
Mouse mutation nomenclature
Once new mouse lines have been generated they should be named in accordance to the Guidelines for Nomenclature of Genes, Genetic Markers, Alleles, and Mutations in Mouse and Rat from the International Committee on Standardized Genetic Nomenclature for Mice http://www.informatics.jax.org/mgihome/nomen/index.shtml. We also propose that the nature and the precise location of the mutation be clearly described according to the guidelines of the Human Genome Variation Society (http://www.hgvs.org/mutnomen/) (den Dunnen and Antonarakis, 2000).
Use of CRISPR-Cas9 technology for immunological studies
Amenable to all species, CRISPR-Cas9 technology can introduce mutation(s) in any mouse strain, avoiding expensive and lengthy backcrossing and problems inherent to backcrossing such as the presence of single nucleotide polymorphisms or other genomic variants located in the vicinity of the mutation of choice. One such example is the Casp11/Casp1 conundrum in innate immunity. Casp1 which encodes Caspase-1 was targeted in 129 embryonic stem cells which already contained a inactivating mutation in the Casp11 gene encoding Caspase-11, which is located downstream of Casp1 and could not be segregated by recombination. Thus, some of the functions originally attributed to Casp1 are now known to be Casp11-dependent (Kayagaki et al., 2011). Similarly, strains with complex genetic backgrounds, such as the non-obese diabetic (NOD)- Rag1−/−; Il2rg−/− (NRG) mouse, and the NOD-SCID; Il2rg−/− (NSG) mouse can be directly targeted (Li et al., 2014). This is particularly useful for immunologic studies, where cell transfer experiments are often desirable and can produce artifacts if performed using mice with incomplete backcrossing. Genome sequences of several strains, including the non-obese diabetic (NOD) mouse genome, can be found at http://www.sanger.ac.uk/resources/mouse/genomes/.
CRISPR-Cas9 technology has been used to generate a variety of immunodeficient mouse strains by introducing small deletions in genes essential for the development and function of the immune system (Zhou et al., 2014). Because this approach can target multiple genes simultaneously (Wang, et al., 2013), several immunologic genes of interest can be deleted in a single zygote. For example, sgRNAs targeting one exon each of B2m, Il2rg, Prf1, Prkdc, and Rag1 were injected into zygotes, resulting in all combinations of deletions, including quintuple deletions of all five genes, with the expected effects on immune functions (Zhou et al., 2014). Clearly, the simultaneous targeting of multiple genes has direct application to the generation and investigation of complex genetic events, such as those invoked for many autoimmune syndromes. These syndromes are thought to result from either single causative allele to multi genic causes. Faithful reproduction of these mutations in mice or other species would greatly benefit our understanding of the origin and the progression of these diseases and perhaps the development of better therapeutics.
CRISPR-Cas9 technology can also be used to manipulate viral genomes, thereby facilitating the study of viral gene functions and perhaps generation of attenuated viruses for vaccination (Bi et al., 2014). The potential for therapeutic intervention against HIV-1 infections, by targeting C-C chemokine receptor type 5 gene, has also been tested in vitro and showed promising results (Cradick et al., 2013). More recently, researchers used CRISPR-Cas9 to target HIV1 in latently infected microglial, pro-monocytic and T cells (Hu et al., 2014). This may be used in combination with antiretroviral therapy to impair viral reactivation events from integrated HIV-1 genes.
In addition to simplifying and accelerating the process of gene targeting in vivo, CRISPR-Cas9 technology can be applied to large scale in vitro screens of diverse immunological phenomena with lentiviral vector-based sgRNA libraries for mouse and human genes being available (Sanjana et al., 2014). Moreover, the repurposing of the CRISPR-Cas9 system into RNA-guided transcriptional activators (CRISPRa) or repressors (CRISPRi) (Cheng et al., 2013; Gilbert et al., 2013) allows not only large scale loss-of-function studies but also gain-of-function studies. In these systems, an endonuclease-inactive Cas9 is fused to transcriptional activators or repressors and sgRNA guided tiling of these fusion proteins allows graded gene expression or suppression. A combination of these systems has been elegantly applied to identify genes that control cell growth and pathways governing responses to Cholera-Diphtheria fusion toxin (Gilbert et al., 2014).
Fusion of endonuclease-inactive Cas9 (dCas9) to RNA-guided DNA, histone, and chromatid modifying enzymes may to be useful for the study of epigenetics and its role in cancer prove, neurological disorders and autoimmune diseases (Portela and Esteller, 2010) including rheumatoid arthritis (Javierre et al., 2008; Karouzakis et al., 2009; Vanden Berghe et al., 2006), systemic lupus erythematosus (SLE) (Javierre et al., 2010), immunodeficiency, centromeric instability and facial anomalies (ICF) syndrome (Jin et al., 2008) and Type I diabetes (Miao et al., 2008)
Other applications of CRISPR-Cas9 can be envisioned. A light- or ligand-induced heterodimerization of split Cas9 could be developed based on previously established systems such as the photoactivatable CIB1-CRY2 dimerization system (Kennedy et al., 2010) or the ligand-induced heterodimerization AP21967-DmrA and -DmrC binding domains system. This would permit inducible activation of Cas9 in cells expressing suitable sgRNAs in order to evaluate effects of acute targeting in tissues of interest.
Alternatively, the fusion of fluorescent proteins to orthogonal Cas proteins may allow multi-color/multi-locus capabilities and may allow real time imaging of the recombination events, such as those of the Igh, Igk, Igl, Tcra and Tcrb loci during B and T cell development. Technologies to directly modify or visualize RNA molecules in cells have been limited. Cas Repeat-associated mysterious proteins (RAMP) module proteins identified in Pyrococcus furious and Sulfolobus solfataricus, which target RNA molecules guided by small CRISPR RNAs could be modified to serve this purpose (Hale et al., 2008; Hale et al., 2012; Hale et al., 2009). This would permit modification and/or visualization of mRNAs in cells of interest.
Concluding remarks
In addition to becoming a method of choice for mouse genome engineering, CRISPR-Cas9 technology also holds great promise for gene therapy. Adoptive transfer of genetically engineered lymphocytes for the treatment of cancer, for example, could greatly benefit from the CRISPR-Cas9 technology as it is non-invasive and no residual pro-inflammatory exogenous DNA elements such as vector backbones are generated. Clearly, the successful clinical application of this technology must be carefully considered in the context of potential undesirable effects. This includes the obvious challenge of ensuring limited of off-target cleavage frequency. Perhaps mechanistic studies will provide key information regarding Cas9 specificity and suggest ways to improve potential clinical efficacy.
The potential applications of CRISPR-Cas9 technology are evolving rapidly. Clearly, the opportunities for disrupting, modifying, and visualizing genetic and epigenetic events hold incredible promise for advancing our understanding of fundamental biology, including but not limited to that of the immune system. As we have been empowered by advances in sequencing, the application of this astonishing new technology will open heretofore unimagined ways to explore, and ultimately engineer, biological events.
Supplementary Material
Box 1. sgRNA selection for mouse genome engineering.
The first step consists of identifying unique 12-nucleotide-long sequences followed by a PAM (N12NGG) in a given region of interest. Unique N12NGG can be easily visualized in the UCSC genome browser using the Older UCSC Genome Browser tracks http://www.genome-engineering.org/crispr/?page_id=41. Unique N12NGG sites, however, are relatively rare in genomes and may not always be found in the region of interest (Table 1). If no suitable N12NGG sites are identified, genomic sequences having unique 13-nucleotide-long sequences followed by a PAM sequence (unique N13NGG sites) are instead selected. Although N12NGG and, even more so, N13NGG sequences are not always predictive of Cas9 specificity (Fu et al., 2014; Hsu et al., 2013; Lin et al., 2014), the identification of these sequences provides more flexibility for the identification of potential on-target loci and avoids obvious off-target effects. To identify these unique N13NGG, we recommend using E-CRISP or Cas-OFFinder. In our experience, E-CRISP and Cas-OFFinder return lists of all potential off-target loci together with important information such as chromosome location and positioning of the loci relative to known genes. Again, if no unique N13NGG are identified in the vicinity of the intended target region, genomic sequences with non-unique N13NGG can instead be used as targets. Importantly however, these additional N13NGG loci may also serve as substrates for Cas9 and should be considered as potential off-target loci.
The second step in identifying suitable target sites consists of identifying, for each unique N12NGG or N13NGG sequence, all possible off-target loci in the context of the full-length target sites (N20NRG, where R represents A or G). Again this can be easily performed using E-CRIPSP or Cas-OFFinder. Putative on-target sites (N20NRG) having more than 90% homology (2 mismatches) with other genomic loci are discarded whenever possible. On-target sites with less than 85% homology (3 mismatches or more) with other genomic loci are conserved and suitable for sgRNA design. This cutoff has been selected based on previous analyses showing that off-target loci having 3 or more mismatches are rarely, if ever, targeted by CRISPR/Cas9 in vivo mutations (Mashiko et al., 2013; Wang et al., 2013; Yang et al., 2013a). These on-target sites are then used for the design of the sgRNAs. As mentioned above, each sgRNA is composed of a 20-nucleotide-long crRNA molecule with sequence homology to the on-target site (N20) fused to a tracrRNA. PAM sequences are uniquely found at the target sites and should not be incorporated into the sgRNA molecule itself.
The following describes the step-by step procedure used in our laboratory for the selection of sgRNAs using the Cas-OFFinder (www.rgenome.net) website.
-
1-
Copy and paste target region of interest into the Microhomology-associated score calculator query box and submit your request. This will return a list of all putative target sites within your target region. Copy this list to an excel spreadsheet and discard any information concerning the microhomology scores. Only keep the list of target sites.
-
2-
In a new column, copy and paste the list of potential target sequences and trim off the PAM (NGG) located at the 3’end of the sequences (N20).
-
3-
Copy and paste the N20 sequences in a new column titled N12NGG and replace the first 8 nucleotides of each sequence by N (which represents any nucleotides). These represent all possible N12NGG in the target sequence.
-
4-
Copy and paste the N12NGG list into the Query sequences. Chose the appropriate parameters. For example: SpCas9 from Streptococcus pyogenes: 5′-NGG-3′. Target genome: Mus musculus (mm10) – Mouse. Set the number of mismatches to 0 and press submit. This will return a list of all loci containing identical N12NGG sequences.
-
5-
Copy and paste this list in the excel spread sheet under a new Tab entitled “unique N12NGG”.
-
6-
Generate a pivot table from these data and identify the number of off-target sequences for each of the N12NGG sequences. Sequences with only 1 instance can be used for sgRNA design and continue with step 9.
-
7-
If no unique N12NGG sequences are identified, copy and paste the N20 sequences in a new column entitled N13NGG and replace the first 7 nucleotides of each sequence by N (which represents any nucleotides) and repeat steps 4, 5 and 6.
-
8-
If no unique N13NGG can be identified, a sgRNA with non-unique N13NGG sequence can be used. However, the additional sites with perfect N13NGG matches should be considered as potential off-target loci.
-
9-
Whenever possible, select sgRNAs having unique N12NGG or N13NGG.
To identify potential off-target loci of sgRNAs having unique or non-unique N12NGG or N13NGG, copy and paste their corresponding N20 sequences in the Cas-OFFinder application under query sequences. Choose the appropriate parameters, for example: SpCas9 from Streptococcus pyogenes: 5′-NRG-3′ (R = A or G); Target genome: Mus musculus (mm10) – Mouse. Set the number of mismatches to 4 and press submit. This will return a list of all potential off-target loci for a given sgRNA. Strategies for cloning and expression of Cas9 mRNA and sgRNAs are outlined in Supplemental Figure S1 and S2.
Acknowledgements
The authors thank the Transgenic/Gene Knockout Shared Resource (St. Jude Children's Research Hospital) for CRISPR/Cas9 injections and Dr. Helen Beere for helpful discussions. The authors received support from the U.S. National Institutes of Health and the American Lebanese Syrian Associated Charities.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. Journal of molecular biology. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Bae S, Park J, Kim JS. Cas-OFFinder: a fast and versatile algorithm that searches for potential off-target sites of Cas9 RNA-guided endonucleases. Bioinformatics. 2014;30:1473–1475. doi: 10.1093/bioinformatics/btu048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi Y, Sun L, Gao D, Ding C, Li Z, Li Y, Cun W, Li Q. High-efficiency targeted editing of large viral genomes by RNA-guided nucleases. PLoS pathogens. 2014;10:e1004090. doi: 10.1371/journal.ppat.1004090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster RL, Chen HY, Trumbauer ME, Yagle MK, Palmiter RD. Factors affecting the efficiency of introducing foreign DNA into mice by microinjecting eggs. Proceedings of the National Academy of Sciences of the United States of America. 1985;82:4438–4442. doi: 10.1073/pnas.82.13.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canver MC, Bauer DE, Dass A, Yien YY, Chung J, Masuda T, Maeda T, Paw BH, Orkin SH. Characterization of genomic deletion efficiency mediated by clustered regularly interspaced palindromic repeats (CRISPR)/Cas9 nuclease system in mammalian cells. The Journal of biological chemistry. 2014;289:21312–21324. doi: 10.1074/jbc.M114.564625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capecchi MR. Gene targeting in mice: functional analysis of the mammalian genome for the twenty-first century. Nature reviews. Genetics. 2005;6:507–512. doi: 10.1038/nrg1619. [DOI] [PubMed] [Google Scholar]
- Capon F, Allen MH, Ameen M, Burden AD, Tillman D, Barker JN, Trembath RC. A synonymous SNP of the corneodesmosin gene leads to increased mRNA stability and demonstrates association with psoriasis across diverse ethnic groups. Human molecular genetics. 2004;13:2361–2368. doi: 10.1093/hmg/ddh273. [DOI] [PubMed] [Google Scholar]
- Cheng AW, Wang H, Yang H, Shi L, Katz Y, Theunissen TW, Rangarajan S, Shivalila CS, Dadon DB, Jaenisch R. Multiplexed activation of endogenous genes by CRISPR-on, an RNA-guided transcriptional activator system. Cell research. 2013;23:1163–1171. doi: 10.1038/cr.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cradick TJ, Fine EJ, Antico CJ, Bao G. CRISPR/Cas9 systems targeting beta-globin and CCR5 genes have substantial off-target activity. Nucleic acids research. 2013;41:9584–9592. doi: 10.1093/nar/gkt714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Dunnen JT, Antonarakis SE. Mutation nomenclature extensions and suggestions to describe complex mutations: a discussion. Human mutation. 2000;15:7–12. doi: 10.1002/(SICI)1098-1004(200001)15:1<7::AID-HUMU4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Deveau H, Barrangou R, Garneau JE, Labonte J, Fremaux C, Boyaval P, Romero DA, Horvath P, Moineau S. Phage response to CRISPR-encoded resistance in Streptococcus thermophilus. Journal of bacteriology. 2008;190:1390–1400. doi: 10.1128/JB.01412-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esvelt KM, Mali P, Braff JL, Moosburner M, Yaung SJ, Church GM. Orthogonal Cas9 proteins for RNA-guided gene regulation and editing. Nature methods. 2013;10:1116–1121. doi: 10.1038/nmeth.2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonfara I, Le Rhun A, Chylinski K, Makarova KS, Lecrivain AL, Bzdrenga J, Koonin EV, Charpentier E. Phylogeny of Cas9 determines functional exchangeability of dual-RNA and Cas9 among orthologous type II CRISPR-Cas systems. Nucleic acids research. 2014;42:2577–2590. doi: 10.1093/nar/gkt1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Foden JA, Khayter C, Maeder ML, Reyon D, Joung JK, Sander JD. High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nature biotechnology. 2013;31:822–826. doi: 10.1038/nbt.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Sander JD, Reyon D, Cascio VM, Joung JK. Improving CRISPR-Cas nuclease specificity using truncated guide RNAs. Nature biotechnology. 2014;32:279–284. doi: 10.1038/nbt.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon JA, Valen E, Thyme SB, Huang P, Ahkmetova L, Pauli A, Montague TG, Zimmerman S, Richter C, Schier AF. Efficient mutagenesis by Cas9 protein-mediated oligonucleotide insertion and large-scale assessment of single-guide RNAs. PloS one. 2014;9:e98186. doi: 10.1371/journal.pone.0098186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert LA, Horlbeck MA, Adamson B, Villalta JE, Chen Y, Whitehead EH, Guimaraes C, Panning B, Ploegh HL, Bassik MC, et al. Genome-Scale CRISPR-Mediated Control of Gene Repression and Activation. Cell. 2014 doi: 10.1016/j.cell.2014.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert LA, Larson MH, Morsut L, Liu Z, Brar GA, Torres SE, Stern-Ginossar N, Brandman O, Whitehead EH, Doudna JA, et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell. 2013;154:442–451. doi: 10.1016/j.cell.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilinger JP, Thompson DB, Liu DR. Fusion of catalytically inactive Cas9 to FokI nuclease improves the specificity of genome modification. Nature biotechnology. 2014;32:577–582. doi: 10.1038/nbt.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale C, Kleppe K, Terns RM, Terns MP. Prokaryotic silencing (psi)RNAs in Pyrococcus furiosus. Rna. 2008;14:2572–2579. doi: 10.1261/rna.1246808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale CR, Majumdar S, Elmore J, Pfister N, Compton M, Olson S, Resch AM, Glover CV, 3rd, Graveley BR, Terns RM, Terns MP. Essential features and rational design of CRISPR RNAs that function with the Cas RAMP module complex to cleave RNAs. Molecular cell. 2012;45:292–302. doi: 10.1016/j.molcel.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale CR, Zhao P, Olson S, Duff MO, Graveley BR, Wells L, Terns RM, Terns MP. RNA-guided RNA cleavage by a CRISPR RNA-Cas protein complex. Cell. 2009;139:945–956. doi: 10.1016/j.cell.2009.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heigwer F, Kerr G, Boutros M. E-CRISP: fast CRISPR target site identification. Nature methods. 2014;11:122–123. doi: 10.1038/nmeth.2812. [DOI] [PubMed] [Google Scholar]
- Horii T, Arai Y, Yamazaki M, Morita S, Kimura M, Itoh M, Abe Y, Hatada I. Validation of microinjection methods for generating knockout mice by CRISPR/Cas-mediated genome engineering. Scientific reports. 2014;4:4513. doi: 10.1038/srep04513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath P, Romero DA, Coute-Monvoisin AC, Richards M, Deveau H, Moineau S, Boyaval P, Fremaux C, Barrangou R. Diversity, activity, and evolution of CRISPR loci in Streptococcus thermophilus. Journal of bacteriology. 2008;190:1401–1412. doi: 10.1128/JB.01415-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Z, Zhang Y, Propson NE, Howden SE, Chu LF, Sontheimer EJ, Thomson JA. Efficient genome engineering in human pluripotent stem cells using Cas9 from Neisseria meningitidis. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:15644–15649. doi: 10.1073/pnas.1313587110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu PD, Scott DA, Weinstein JA, Ran FA, Konermann S, Agarwala V, Li Y, Fine EJ, Wu X, Shalem O, et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nature biotechnology31. 2013:827–832. doi: 10.1038/nbt.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Kaminski R, Yang F, Zhang Y, Cosentino L, Li F, Luo B, Alvarez-Carbonell D, Garcia-Mesa Y, Karn J, et al. RNA-directed gene editing specifically eradicates latent and prevents new HIV-1 infection. Proceedings of the National Academy of Sciences of the United States of America. 2014 doi: 10.1073/pnas.1405186111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javierre BM, Esteller M, Ballestar E. Epigenetic connections between autoimmune disorders and haematological malignancies. Trends in immunology. 2008;29:616–623. doi: 10.1016/j.it.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Javierre BM, Fernandez AF, Richter J, Al-Shahrour F, Martin-Subero JI, Rodriguez-Ubreva J, Berdasco M, Fraga MF, O'Hanlon TP, Rider LG, et al. Changes in the pattern of DNA methylation associate with twin discordance in systemic lupus erythematosus. Genome research. 2010;20:170–179. doi: 10.1101/gr.100289.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Bikard D, Cox D, Zhang F, Marraffini LA. RNA-guided editing of bacterial genomes using CRISPR-Cas systems. Nature biotechnology. 2013;31:233–239. doi: 10.1038/nbt.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin B, Tao Q, Peng J, Soo HM, Wu W, Ying J, Fields CR, Delmas AL, Liu X, Qiu J, Robertson KD. DNA methyltransferase 3B (DNMT3B) mutations in ICF syndrome lead to altered epigenetic modifications and aberrant expression of genes regulating development, neurogenesis and immune function. Human molecular genetics. 2008;17:690–709. doi: 10.1093/hmg/ddm341. [DOI] [PubMed] [Google Scholar]
- Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karouzakis E, Gay RE, Gay S, Neidhart M. Epigenetic control in rheumatoid arthritis synovial fibroblasts. Nature reviews. Rheumatology. 2009;5:266–272. doi: 10.1038/nrrheum.2009.55. [DOI] [PubMed] [Google Scholar]
- Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- Kennedy MJ, Hughes RM, Peteya LA, Schwartz JW, Ehlers MD, Tucker CL. Rapid blue-light-mediated induction of protein interactions in living cells. Nature methods. 2010;7:973–975. doi: 10.1038/nmeth.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimchi-Sarfaty C, Oh JM, Kim IW, Sauna ZE, Calcagno AM, Ambudkar SV, Gottesman MM. A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science. 2007;315:525–528. doi: 10.1126/science.1135308. [DOI] [PubMed] [Google Scholar]
- Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome biology. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Cowley DO, Banner D, Holle E, Zhang L, Su L. Efficient genetic manipulation of the NOD-Rag1−/−IL2RgammaC-null mouse by combining in vitro fertilization and CRISPR/Cas9 technology. Scientific reports. 2014;4:5290. doi: 10.1038/srep05290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Cradick TJ, Brown MT, Deshmukh H, Ranjan P, Sarode N, Wile BM, Vertino PM, Stewart FJ, Bao G. CRISPR/Cas9 systems have off-target activity with insertions or deletions between target DNA and guide RNA sequences. Nucleic acids research. 2014;42:7473–7485. doi: 10.1093/nar/gku402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali P, Aach J, Stranges PB, Esvelt KM, Moosburner M, Kosuri S, Yang L, Church GM. CAS9 transcriptional activators for target specificity screening and paired nickases for cooperative genome engineering. Nature biotechnology. 2013a;31:833–838. doi: 10.1038/nbt.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE, Church GM. RNA-guided human genome engineering via Cas9. Science. 2013b;339:823–826. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashiko D, Fujihara Y, Satouh Y, Miyata H, Isotani A, Ikawa M. Generation of mutant mice by pronuclear injection of circular plasmid expressing Cas9 and single guided RNA. Scientific reports. 2013;3:3355. doi: 10.1038/srep03355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao F, Smith DD, Zhang L, Min A, Feng W, Natarajan R. Lymphocytes from patients with type 1 diabetes display a distinct profile of chromatin histone H3 lysine 9 dimethylation: an epigenetic study in diabetes. Diabetes. 2008;57:3189–3198. doi: 10.2337/db08-0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nackley AG, Shabalina SA, Tchivileva IE, Satterfield K, Korchynskyi O, Makarov SS, Maixner W, Diatchenko L. Human catechol-O-methyltransferase haplotypes modulate protein expression by altering mRNA secondary structure. Science. 2006;314:1930–1933. doi: 10.1126/science.1131262. [DOI] [PubMed] [Google Scholar]
- Nagy A. Manipulating the mouse embryo : a laboratory manual. 3rd edn Cold Spring Harbor Laboratory Press; Cold Spring Harbor, N.Y.: 2003. [Google Scholar]
- Oh B, Hwang S, McLaughlin J, Solter D, Knowles BB. Timely translation during the mouse oocyte-to-embryo transition. Development. 2000;127:3795–3803. doi: 10.1242/dev.127.17.3795. [DOI] [PubMed] [Google Scholar]
- Pattanayak V, Lin S, Guilinger JP, Ma E, Doudna JA, Liu DR. High-throughput profiling of off-target DNA cleavage reveals RNA-programmed Cas9 nuclease specificity. Nature biotechnology. 2013;31:839–843. doi: 10.1038/nbt.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portela A, Esteller M. Epigenetic modifications and human disease. Nature biotechnology. 2010;28:1057–1068. doi: 10.1038/nbt.1685. [DOI] [PubMed] [Google Scholar]
- Ran FA, Hsu PD, Lin CY, Gootenberg JS, Konermann S, Trevino AE, Scott DA, Inoue A, Matoba S, Zhang Y, Zhang F. Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell. 2013;154:1380–1389. doi: 10.1016/j.cell.2013.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice P, Longden I, Bleasby A. EMBOSS: the European Molecular Biology Open Software Suite. Trends in genetics : TIG. 2000;16:276–277. doi: 10.1016/s0168-9525(00)02024-2. [DOI] [PubMed] [Google Scholar]
- Sanjana NE, Shalem O, Zhang F. Improved vectors and genome-wide libraries for CRISPR screening. Nature methods. 2014;11:783–784. doi: 10.1038/nmeth.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres R, Martin MC, Garcia A, Cigudosa JC, Ramirez JC, Rodriguez-Perales S. Engineering human tumour-associated chromosomal translocations with the RNA-guided CRISPR-Cas9 system. Nature communications. 2014;5:3964. doi: 10.1038/ncomms4964. [DOI] [PubMed] [Google Scholar]
- Tsai SQ, Wyvekens N, Khayter C, Foden JA, Thapar V, Reyon D, Goodwin MJ, Aryee MJ, Joung JK. Dimeric CRISPR RNA-guided FokI nucleases for highly specific genome editing. Nature biotechnology. 2014;32:569–576. doi: 10.1038/nbt.2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ploeg JR. Analysis of CRISPR in Streptococcus mutans suggests frequent occurrence of acquired immunity against infection by M102-like bacteriophages. Microbiology. 2009;155:1966–1976. doi: 10.1099/mic.0.027508-0. [DOI] [PubMed] [Google Scholar]
- Vanden Berghe W, Ndlovu MN, Hoya-Arias R, Dijsselbloem N, Gerlo S, Haegeman G. Keeping up NF-kappaB appearances: epigenetic control of immunity or inflammation-triggered epigenetics. Biochemical pharmacology. 2006;72:1114–1131. doi: 10.1016/j.bcp.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Wang H, Yang H, Shivalila CS, Dawlaty MM, Cheng AW, Zhang F, Jaenisch R. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell. 2013;153:910–918. doi: 10.1016/j.cell.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Wang H, Jaenisch R. Generating genetically modified mice using CRISPR/Cas-mediated genome engineering. Nature protocols. 2014;9:1956–1968. doi: 10.1038/nprot.2014.134. [DOI] [PubMed] [Google Scholar]
- Yang H, Wang H, Shivalila CS, Cheng AW, Shi L, Jaenisch R. One-step generation of mice carrying reporter and conditional alleles by CRISPR/Cas-mediated genome engineering. Cell. 2013a;154:1370–1379. doi: 10.1016/j.cell.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Guell M, Byrne S, Yang JL, De Los Angeles A, Mali P, Aach J, Kim-Kiselak C, Briggs AW, Rios X, et al. Optimization of scarless human stem cell genome editing. Nucleic acids research. 2013b;41:9049–9061. doi: 10.1093/nar/gkt555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Heidrich N, Ampattu BJ, Gunderson CW, Seifert HS, Schoen C, Vogel J, Sontheimer EJ. Processing-independent CRISPR RNAs limit natural transformation in Neisseria meningitidis. Molecular cell. 2013;50:488–503. doi: 10.1016/j.molcel.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Shen B, Zhang W, Wang J, Yang J, Chen L, Zhang N, Zhu K, Xu J, Hu B, et al. One-step generation of different immunodeficient mice with multiple gene modifications by CRISPR/Cas9 mediated genome engineering. The international journal of biochemistry & cell biology. 2014;46:49–55. doi: 10.1016/j.biocel.2013.10.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.