Abstract
Sleep abnormalities are highly correlated with neurodevelopmental disorders, and the severity of behavioral abnormalities correlates with the presence of sleep abnormalities. Given the importance of sleep in developmental plasticity, we sought to determine the effects of chronic sleep-restriction during development on subsequent adult behavior. We sleep-restricted developing wild-type mice from P5-P42 for three hours per day by means of gentle handling (n=30) and compared behavioral outputs to controls that were handled ten min daily (n=33). We assayed activity in the open field, social behavior, repetitive behavior, and anxiety immediately following sleep restriction and after four weeks recovery. At six weeks of age, immediately following chronic sleep-restriction, mice were less active in an open field arena. Sociability was increased, but repetitive behaviors were unchanged in both males and females. After a 4-week period of recovery, some behavioral abnormalities persisted and some became apparent. Sleep-restricted mice had decreased activity in the beginning of an open field test. Female mice continued to have increased sociability and, in addition, increased preference for social novelty. In contrast, male mice demonstrated decreased sociability with medium effect sizes. Repetitive behavior was decreased in sleep-restricted female mice and increased in males. Measures of anxiety were not affected in the sleep-restricted mice. These results indicate that chronic sleep restriction during development can lead to long-lasting behavioral changes that are modulated by sex. Our study may have implications for a role of disrupted sleep in childhood on the unfolding of neurodevelopmental disorders.
Keywords: Sleep Restriction, Gentle Handling, Social Behavior, Open Field Activity, Repetitive Behavior
1 Introduction
Sleep has an important role in brain development and synaptic plasticity [1]. Chronic sleep deprivation may result in an allostatic load contributing to cognitive problems [2] and disrupted plasticity [3]. The normal functions of sleep are thought to affect cellular processes important for plasticity, including: myelination, synapse formation/function, cellular detoxification/ cell stress reduction, and protein synthesis [3].
Children with disrupted sleep often display an increased prevalence of neurobehavioral issues such as hyperactivity, emotional lability, aggressiveness, and deficits in socialization [4-8] and disrupted sleep is a prevalent finding in many patients with neurodevelopmental disorders.[3]
Given the importance of sleep on plasticity, and the prevalence of disturbed sleep in children with neurodevelopmental disorders, we hypothesized that altered sleep during a critical period in development will result in alterations in plasticity leading to long-lasting behavioral changes. In this study, we examined the behavioral effects, both immediate and long-term, of chronic sleep restriction throughout development and adolescence in otherwise normal mice.
2 Materials and Methods
2.1 Animals
Litters of wild-type (WT) mice were produced from harem bred male and female C57Bl/6J mice obtained from Jackson Laboratories (Bar Harbor, ME). Once a female gave birth, the dam and her pups were separated from the other adults in the cage. Litters were randomly assigned to either the sleep-restriction (five litters: 13 male, 18 female offspring) or the control group (eight litters: 11 male, 22 female offspring). Six pups in the control group were cannibalized prior to determination of sex. One female pup in the sleep-restriction group was cannibalized, so the total number of females used for studies in that group was 17. Pups were weaned at P21. All mice were held in a climate-controlled facility with standard alternating 12 hr periods of light and darkness (lights on, 6:00AM-6:00PM). Food and water were available to mice ad libitum. Animal procedures were carried out in accordance with the National Institutes of Health Guidelines on the Care and Use of Animals and an animal study protocol approved by the National Institute of Mental Health Animal Care and Use Committee.
2.2 Sleep Restriction
When pups were five days of age, we began sleep-restriction by gentle handling [13]. Each litter was monitored daily between 11:00AM and 2:00PM. Mice showing inactivity or twitching behavior were gently prodded with a paintbrush until a response was elicited. A response was defined as a large movement and if the animal continued moving, we considered it to be awake. If the mother was on top of the pups, blocking them from view, then she was gently prodded away so that pups could be observed and sleep-restricted. Control mice were gently handled in the same manner (regardless of suspected sleep) for ten min a day, to control for the stress of the prodding.
Sleep restriction occurred through P42. Behavior testing was conducted on three consecutive days beginning the day after cessation of sleep-restriction. Behavior testing was conducted in the light phase. After a 4-week recovery from sleep-restriction (at P73), behavior testing was repeated on five consecutive days. We monitored sleep behavior for 72 consecutive hours, beginning 69-80 days following cessation of sleep-restriction. The timeline of testing is presented in Table 1. We assessed behavior in all animals; however, in several cases data were lost due to computer malfunction accounting for variations in the number per group across tests. No animal was excluded for any other reason.
Table 1.
Timeline of testing. Sleep restriction began at five days of age, continuing to 42 days. Behavioral testing was conducted following sleep restriction. Following one month of recovery, behavior testing was repeated.
| Age (days) | Procedure |
|---|---|
| 5-42 | Sleep Restriction |
| 43 | Social Behavior Test |
| 44 | Open Field Test |
| 45 | Marble Burying Test |
| 72 | Social Behavior Test |
| 73 | Open Field Test |
| 74 | Marble Burying Test |
| 75 | RotaRod Test |
| 76 | Elevated Plus Maze Test |
| 111-122 | Sleep Testing Initiation |
2.3 Social Behavior
Mice were tested for social behavior by means of a three-chambered apparatus [14]. Briefly, mice were tested in three phases, each lasting ten min. 1.) Habituation: While the doors were open, mice were placed in the center chamber and allowed to freely explore. 2.) Sociability: The test mouse was isolated to the center chamber while a sex/age matched stranger mouse was placed inside a social enclosure (Noldus, Leesburg, VA) in either Chamber 1 or Chamber 2. In the other chamber, an empty social enclosure (object) was placed. The doors were opened and the test mouse was allowed to freely explore. The time spent in each chamber was recorded. Video-recording of the testing allowed for subsequent recording of sniffing time which was determined by close proximity (< 4cm) to the enclosure in conjunction with head orientation toward the enclosure. 3.) Preference for social novelty: Immediately following the second phase, test mice were isolated back to the center chamber. A novel sex/age – matched stranger mouse was placed in the previously empty social enclosure. Doors were opened and the test mouse was allowed to freely explore. Measures were taken as in Phase 2. When the mice were retested at ten weeks of age, different stranger mice were used.
2.4 Open Field
Open field testing was used to determine levels of general activity, as well as anxiety. Activity was measured for 30 min (in five min epochs) by means of photobeam detection (Coulbourn Instruments, Whitehall, PA). Total horizontal distance traveled and ratio of center to total distance traveled were determined.
2.5 Marble Burying
Marble burying was used as an assay for repetitive behaviors. Mice were placed in a standard-sized clean cage with corncob bedding 4.5 cm in depth overlain with 20 glass marbles arranged in a grid. Mice were allowed to explore the cage for 30 min, after which the number of marbles buried (>50% coverage) were counted [15].
2.6 RotaRod
Mice were placed on an accelerating rotarod (Columbus Instruments, Columbus, OH). Acceleration was set at 0.1 rpm / second. The amount of time that the mouse was able to stay on the rotarod was recorded (max 180s). Two trials, one hour apart, were conducted and the average time was used for analysis.
2.7 Elevated Plus Maze
Mice were tested for general anxiety by means of the elevated plus maze (EPM). Mice were placed in the center of the apparatus facing one of the open arms. The times spent in the open arms, closed arms, and the center were recorded for five min.
2.8 Homecage Assessment of Sleep
Mice were singly housed in a standard mouse cage and allowed an acclimatization period of four hours. The homecage was placed into a rectangular arena of photobeams (Comprehensive Laboratory Animal Monitoring System, Columbus Instruments). Photobeams were spaced 0.5 inches apart on both the × and the y axes in order to assess movement on a high-resolution grid. Bedding was placed below the level of the photobeams. Beam breaks were detected in epochs of 10s and Oxymax software (Columbus Instruments) was used to analyze the data. A mouse was considered inactive if there was no xy movement over the 10s epoch, and four consecutive epochs of such inactivity was recorded as sleep. These parameters as a measure of sleep were validated by comparison with electroencephalography in C57BL/6J mice [16]. The amount of time asleep was separated into light phase (time asleep between 6:00AM-6:00PM) or dark phase (time asleep between 6:00PM - 6:00AM) and reported as a percent time asleep in each phase. Due to the logistical limitations of testing, there was a range (of 11 days) over which testing was initiated and not all animals were able to be tested.
2.9 Statistical Analysis
Data from the marble burying and rotarod tests were analyzed by means of a two-way ANOVA with sex (male, female) and condition (sleep-restricted, control) as between subjects variables. Open field, social behavior, sleep, and EPM behavior were analyzed by means of mixed-model repeated measures three-way ANOVA with sex (male, female) and condition (sleep-restricted, control) as between subjects variables and epoch (open field), chamber (social behavior), phase (sleep), or arm (EPM) as within subjects variables. By chance, we had more female mice in the litters than male mice so statistical power was greater in the data from the female cohort. Separate analyses were carried out for data obtained immediately after sleep restriction at six weeks and after the period of recovery sleep at ten weeks. Effects with p≤0.05 were considered to be statistically significant (*), though values ≤0.10 are also reported here, and noted on figures with a “~”. Tables reporting F-values, corresponding p-values for interactions and main effects, and effect sizes in terms of Cohen’s f2 (interpretations of effect sizes, as presented by Cohen, are denoted with a “‡”for medium and “‡‡”for large) [17] are presented for all tests (Tables 2-6).
TABLE 2. Repeated Measures ANOVA Results Activitv/Anxiety.
| BEHAVIOR | TIME POINT | EFFECT | F(df, error) VALUE | P-VALUE | COHEN’S F2 |
|---|---|---|---|---|---|
| OPEN FIELD | Pre-Recovery | ||||
| Total distance moved | Sex × Condition × Epoch | F(5,208)= 0.530 | 0.724 | 0.011 | |
| Condition × Epoch | F(5,208)= 1.356 | 0.249 | 0.028 ‡ | ||
| Sex × Epoch | F(5,208)= 1.395 | 0.235 | 0.029 ‡ | ||
| Sex × Condition | F(1,49)= 0.718 | 0.401 | 0.014 | ||
| Sex | F(1,49)= 0.397 | 0.532 | 0.008 | ||
| Condition | F(1,49)= 7.774 | 0.008* | 0.159 ‡ | ||
| Epoch | F(5,208)= 89.497 | <0.001* | 1.825 ‡ ‡ | ||
| Total distance moved | Post-Recovery | Sex × Condition × Epoch | F(4,236)= 1.476 | 0.208 | 0.027 ‡ |
| Condition × Epoch | F(4,236)= 6.440 | <0.001* | 0.115 ‡ | ||
| Sex × Epoch | F(4,236)= 6.476 | <0.001* | 0.116 ‡ | ||
| Sex × Condition | F(1,56)= 0.488 | 0.488 | 0.009 | ||
| Sex | F(1,56)= 0.703 | 0.405 | 0.012 | ||
| Condition | F(1,56)= 1.446 | 0.234 | 0.026 ‡ | ||
| Epoch | F(4,236)= 100.900 | <0.001* | 1.801 ‡ ‡ | ||
| Center/Total Ratio | Pre-Recovery | Sex × Condition × Epoch | F(5,245)= 1.506 | 0.188 | 0.031 ‡ |
| Condition × Epoch | F(5,245)= 0.605 | 0.696 | 0.012 | ||
| Sex × Epoch | F(5,245)= 0.606 | 0.695 | 0.012 | ||
| Sex × Condition | F(1,49)= 0.497 | 0.484 | 0.010 | ||
| Sex | F(1,49)= 1.902 | 0.174 | 0.038 ‡ | ||
| Condition | F(1,49)= 0.095 | 0.759 | 0.002 | ||
| Epoch | F(5,245)= 11.62 | <0.001* | 0.238 ‡ | ||
| Center/Total Ratio | Post-Recovery | Sex × Condition × Epoch | F(5,278)= .895 | 0.484 | 0.016 |
| Condition × Epoch | F(5,278)= 3.290 | 0.055~ | 0.058 ‡ | ||
| Sex × Epoch | F(5,278)= 0.613 | 0.689 | 0.011 | ||
| Sex × Condition | F(1,56)= 0.145 | 0.705 | 0.003 | ||
| Sex | F(1,56)= 1.284 | 0.262 | 0.023 ‡ | ||
| Condition | F(1,56)= 0.026 | 0.872 | 0.000 | ||
| Epoch | F(5,278)= 4.33 | <0.001* | 0.078 ‡ | ||
| ELEVATED PLUS MAZE | Post-Recovery | Sex × Condition × Arm | F(1,59)= 0.333 | 0.566 | 0.006 |
| Condition × Arm | F(1,59)= 0.286 | 0.595 | 0.005 | ||
| Sex × Arm | F(1,59)= 0.012 | 0.914 | 0.000 | ||
| Sex × Condition | F(1,59)= 0.692 | 0.409 | 0.012 | ||
| Sex | F(1,59)= 0.014 | 0.908 | 0.000 | ||
| Condition | F(1,59)= 0.014 | 0.908 | 0.000 | ||
| Arm | F(1,59)= 1775.114 | <0.001* | 30.250 ‡ ‡ | ||
| ROTAROD | Post-Recovery | Sex × Condition | F(1,58)= 0.000 | 0.989 | 0.000 |
| Sex | F(1,58)= 0.631 | 0.430 | 0.011 | ||
| Condition | F(1,58)= 0.743 | 0.392 | 0.013 |
TABLE 6. Repeated Measures ANOVA Results Sleep.
| BEHAVIOR | TIME POINT | EFFECT | F(df, error) VALUE | P-VALUE | COHEN’S F2 |
|---|---|---|---|---|---|
| TOTAL SLEEP TIME | Post-Recovery | Sex × Condition × Phase | F(1,41)= 1.321 | 0.257 | 0.032 ‡ |
| Condition × Phase | F(1,41)= 0.043 | 0.837 | 0.001 | ||
| Sex × Phase | F(1,41)= 4.345 | 0.043* | 0.106 ‡ | ||
| Sex × Condition | F(1,41)= 0.007 | 0.933 | 0.000 | ||
| Sex | F(1,41)= 5.609 | 0.023* | 0.136 ‡ | ||
| Condition | F(1,41)= 2.954 | 0.093~ | 0.072 ‡ | ||
| Phase | F(1,41)= 753.843 | <0.001* | 18.231 ‡ ‡ |
3 Results
3.1 Decreased Exploratory Behavior in Sleep-Restricted Mice
Two days following completion of the 38 days of sleep-restriction, male and female mice were tested for activity in the open field. In all four groups, the total distance traveled was highest immediately following introduction into the open field and decreased thereafter (Figure 1A, Table 2) indicating habituation to the novel environment. Compared to controls, sleep-restricted mice traversed significantly less distance over the 30 min test period. This statistically significant main effect was regardless of sex, indicating that males and females had a similar response to sleep-restriction.
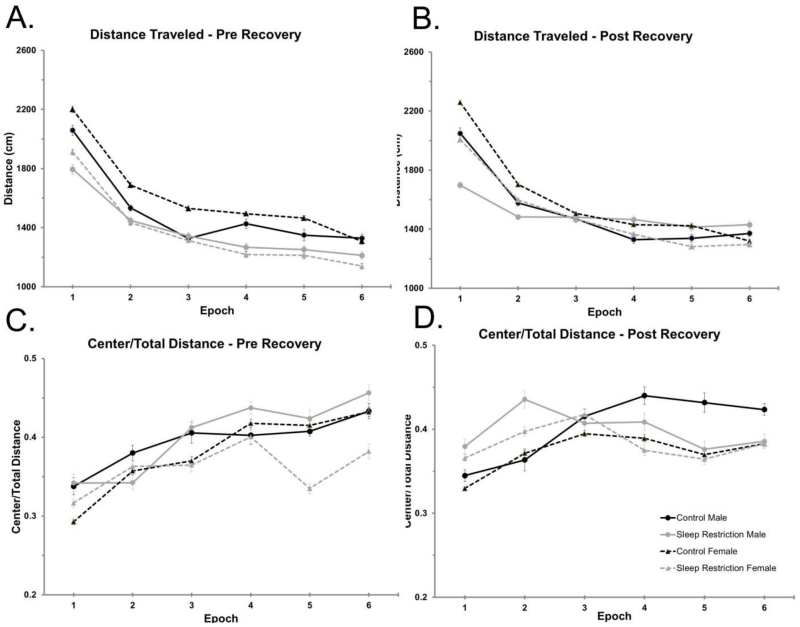
Figure 1.
Decreased activity in the open-field arena following chronic sleep-restriction. (A) Horizontal distance traveled in the open-field (cm) is plotted by epoch (five min each) for a 30 minute test period for animals two days following completion of chronic sleep restriction. Main effects of condition and epoch were statistically significant indicating that sleep-restricted mice had reduced exploratory activity compared to controls and that all groups had higher levels of activity at first followed by evidence of habituation. (B) Distance traveled in the open field following four weeks recovery sleep. The Condition × Epoch and Sex × Epoch interactions were statistically significant, indicating that sleep-restricted mice reacted less to the novel environment than controls and that females reacted more to the novel environment than males. All mice showed some tendency to habituate to the novel conditions over the 30 min test (main effect of epoch). (C) The ratio of distance traveled in the center to total distance traveled two days following completion of chronic sleep restriction. The main effect of epoch, was the only statistically significant effect. (D) The ratio of distance traveled in the center to total distance traveled after four weeks of recovery sleep. The main effect of epoch was the only statistically significant effect, and the Condition × Epoch interaction approached statistical significance (p=0.055). Data plotted are the means ± SEMs for (A&C) 10 control male, 11 sleep-restricted male, 17 control female, and 15 sleep-restricted female mice, and for (B&D) 11 control male, 11 sleep-restricted male, 22 control female, and 16 sleep-restricted female mice. Error bars are largely within the confines of the symbol.
After four weeks of recovery sleep, mice were again tested in the open field. We found statistically significant Condition × Epoch and Sex × Epoch interactions (Table 2). Compared to controls, sleep-restricted mice showed reduced exploratory activity in the beginning of the open field test and a tendency to habituate to the novel environment more quickly (Figure 1B). Additionally, female mice, regardless of sleep-restriction, had the greatest change in exploratory activity over time. Our results indicate that novelty-induced exploratory activity is decreased in mice that have undergone chronic sleep-restriction, even after a significant period of recovery sleep.
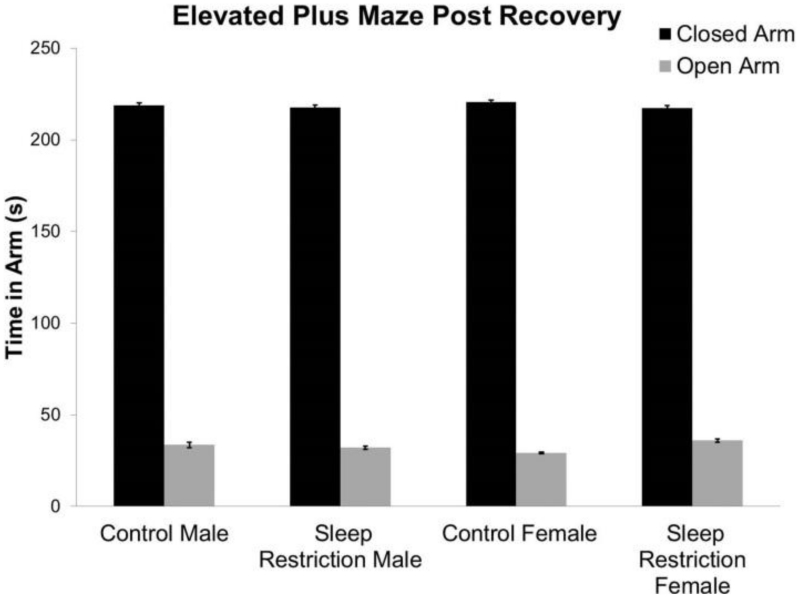
We determined the ratio of distance traveled in the center to total distance traveled as an index of anxiety-like behavior. Immediately following the period of sleep-restriction, there were no statistically significant interactions or main effects of condition or sex (Figure 1C, Table 2). There were also no significant interactions or main effects of condition or sex after four weeks of recovery sleep, though the Condition × Epoch interaction did approach statistical significance (p=0.055) (Figure 1D, Table 2). To further study effects on anxiety-like behavior, we tested mice in the EPM after four weeks of recovery sleep (Figure 2, Table 2). Our results indicate that anxiety-like behavior is similar in males and females and is not altered by chronic sleep-restriction.
Figure 2.
Behavior in the elevated plus maze four weeks post-recovery. Anxiety-like behavior as indicated by time in the open arms of the maze was not affected by chronic developmental sleep-restriction. Bars represent the means ± SEMs for 11 control male, 13 sleep-restricted male, 22 control female, and 17 sleep-restricted female mice.
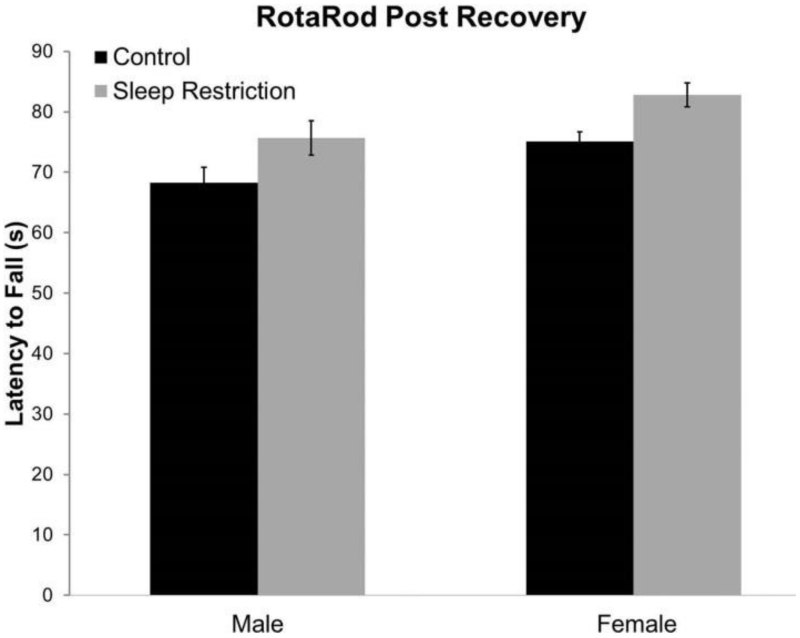
We also investigated the effects of sleep-restriction on motor function by means of the rotarod test, administered after four weeks of recovery sleep. Function on the rotarod test was not affected by sleep-restriction and was similar in males and females (Figure 3, Table2).
Figure 3.
Results of rotarod testing four weeks post recovery showed normal motor function. Bars represent means ± SEMs for 11 control male, 13 sleep-restricted male, 21 control female, and 17 sleep-restricted female mice. Each mouse was tested twice for latency to fall off a rotarod accelerating at 0.1 rpm/second.
3.2 Social Behavior Differences in Sleep-Restricted Mice
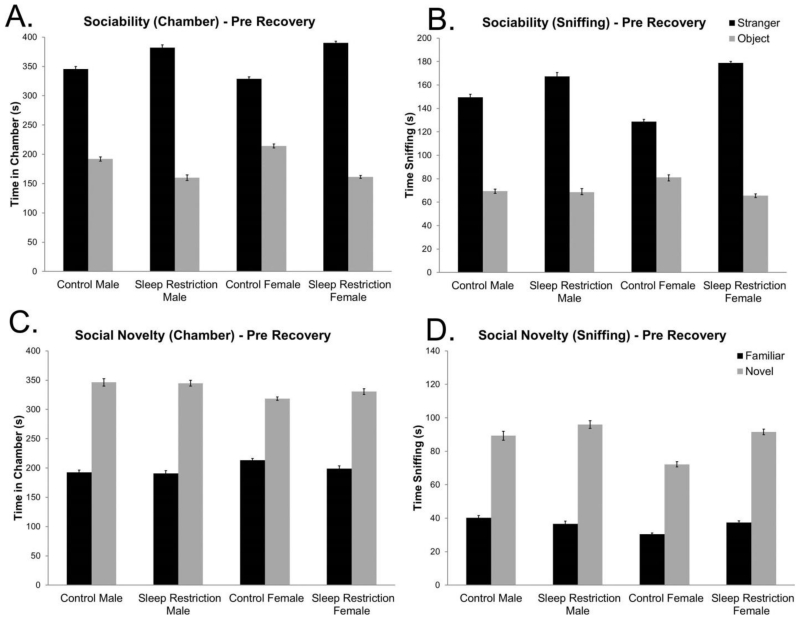
Mice were tested in the three chambered apparatus on the day following completion of chronic sleep-restriction (Figure 4 A&B). In the sociability phase of the test, we found a statistically significant Condition × Chamber interaction (Table 3), indicating that sleep-restricted mice (of both sexes) had a stronger preference for the chamber with the stranger mouse than controls. We also recorded time spent sniffing either the stranger mouse or the object. Similar to the time in chamber analysis, we found a statistically significant Chamber × Condition interaction (Table 3) further indicating that sleep-restricted mice had a stronger preference for the stranger mouse. Analysis of both variables; time in chamber and time spent sniffing; indicate that sleep-restricted mice manifested an increase in sociability.
Figure 4.
Social behavior is altered in mice one day following chronic sleep restriction. (A) Sociability test: time spent in the chamber measured at one day following completion of chronic sleep-restriction (pre-recovery) shows a statistically significant Condition × Chamber interaction and a simple effect of chamber. Sleep-restricted mice regardless of sex spent more time in the chamber with the stranger mouse than did control mice. (B) Sociability test: sniffing time measured one day following chronic sleep-restriction (pre-recovery) reveals a statistically significant Condition × Chamber interaction. Main effects of condition and chamber were also statistically significant. Sleep-restricted mice had increased sociability compared to control mice one day following chronic sleep-restriction (pre-recovery). (C) Social novelty test: time spent in the chamber one day following chronic sleep restriction (pre-recovery) shows a statistically significant main effect of chamber that does not change depending on condition. (D) Social novelty test: sniffing time measured one day following chronic sleep-restriction (pre-recovery) reveals a statistically significant main effect of chamber and a near statistically significant main effect of sex, but no statistically significant effect of condition. Bars represent the means ± SEMs in (A&C) 11 control male, 13 sleep-restricted male, 22 control female, and 17 sleep-restricted female mice, and in (B&D) 10 control male, 13 sleep-restricted male, 21 control female, and 17 sleep-restricted female mice.
TABLE 3. Repeated Measures ANOVA Results Social Behavior Pre Recovery.
| BEHAVIOR | TIME POINT | EFFECT | F(df, error) VALUE | P-VALUE | COHEN’S F2 |
|---|---|---|---|---|---|
| SOCIAL BEHAVIOR | |||||
| Sociability | |||||
| Chamber time | Pre-Recovery | Sex × Condition × Chamber | F(1,59)= 0.532 | 0.469 | 0.009 |
| Condition × Chamber | F(1,59)= 8.428 | 0.005* | 0.143 ‡ | ||
| Sex × Chamber | F(1,59)= 0.253 | 0.617 | 0.004 | ||
| Sex × Condition | F(1,59)= 0.071 | 0.791 | 0.001 | ||
| Sex | F(1,59)= 0.968 | 0.329 | 0.016 | ||
| Condition | F(1,59)= 0.761 | 0.386 | 0.013 | ||
| Chamber | F(1,59)= 130.618 | <0.001* | 2.215 ‡ ‡ | ||
| Sniffing time | Pre-Recovery | Sex × Condition × Chamber | F(1,57)= 2.218 | 0.142 | 0.038 |
| Condition × Chamber | F(1,57)= 7.142 | 0.010* | 0.125 ‡ | ||
| Sex × Chamber | F(1,57)= 0.323 | 0.572 | 0.006 | ||
| Sex × Condition | F(1,57)= 0.778 | 0.381 | 0.013 | ||
| Sex | F(1,57)= 0.000 | 0.986 | 0.000 | ||
| Condition | F(1,57)= 6.993 | 0.011* | 0.122 ‡ | ||
| Chamber | F(1,57)= 115.945 | <0.001* | 2.030 ‡ ‡ | ||
| Social novelty | |||||
| Chamber time | Pre-Recovery | Sex × Condition × Chamber | F(1,59)= 0.144 | 0.705 | 0.002 |
| Condition × Chamber | F(1,59)= 0.150 | 0.700 | 0.003 | ||
| Sex × Chamber | F(1,59)= 1.082 | 0.303 | 0.018 | ||
| Sex × Condition | F(1,59)= 0.002 | 0.967 | 0.000 | ||
| Sex | F(1,59)= 0.418 | 0.521 | 0.007 | ||
| Condition | F(1,59)= 0.074 | 0.786 | 0.001 | ||
| Chamber | F(1,59)= 62.855 | <0.001* | 1.066 ‡ ‡ | ||
| Sniffing time | Pre-Recovery | Sex × Condition × Chamber | F(1,57)= 0.013 | 0.909 | 0.000 |
| Condition × Chamber | F(1,57)= 1.482 | 0.229 | 0.026 ‡ | ||
| Sex × Chamber | F(1,57)= 0.446 | 0.507 | 0.008 | ||
| Sex × Condition | F(1,57)= 1.685 | 0.199 | 0.030 ‡ | ||
| Sex | F(1,57)= 2.947 | 0.091~ | 0.052 ‡ | ||
| Condition | F(1,57)=2.745 | 0.103 | 0.048 ‡ | ||
| Chamber | F(1,57)= 118.912 | <0.001* | 2.086 ‡ ‡ |
In the second phase of the test of social behavior, a novel stranger mouse was introduced in the previously empty enclosure, and we tested for preference for social novelty (Figure 4C&D, Table 3). We found no statistically significant interactions among the factors for the times spent in each chamber or for the time spent sniffing each stranger. Regardless of sex and sleep status, mice demonstrated a preference for the novel mouse.
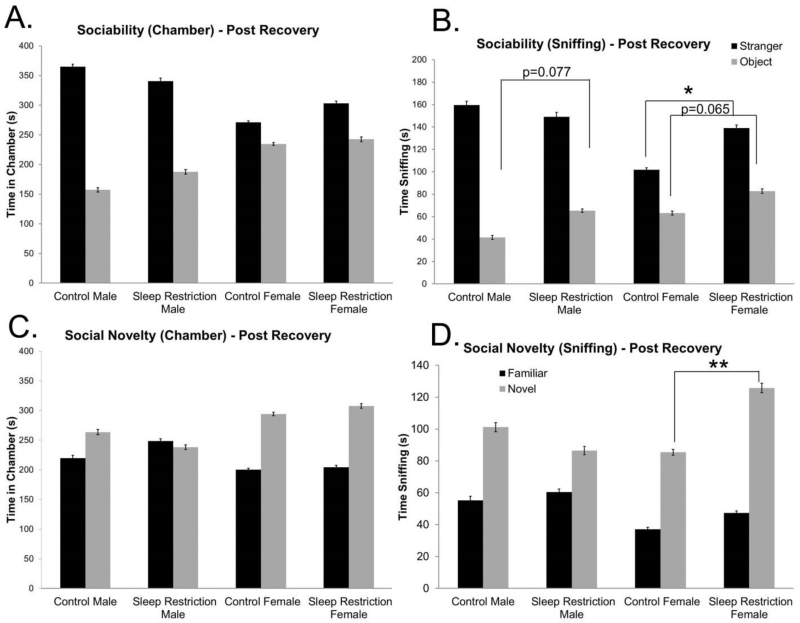
We repeated these tests following four weeks of recovery sleep. For the sociability phase of the test, the Sex × Chamber interaction was statistically significant, indicating that, regardless of sleep status, male mice showed a greater preference for the chamber with the stranger mouse than females (Figure 5A, Table 4). With respect to time spent sniffing, the Sex × Condition × Chamber interaction was nearly statistically significant (p=0.051), so we probed for pairwise effects (Table 4). Sleep-restricted female mice spent significantly more time sniffing the stranger mouse than female controls. This was not the case for male mice. Sleep-restricted male mice tended to spend more time sniffing the object than male controls (p=0.077) (Figure 5B). In the preference for social novelty phase of the test, we found a statistically significant Sex × Chamber interaction, indicating that female mice, regardless of sleep status, spent more time in the chamber with the novel stranger; male mice showed less preference for social novelty (Figure 5C, Table 4). With respect to time sniffing, we found a statistically significant Sex × Condition × Chamber interaction (Table 4). We probed for pairwise differences and found that sleep-restriction in female mice increased the preference for social novelty. This was not the case for male mice (Figure 5D). These data suggest that chronic sleep-restriction during development induced long lasting sex-specific behavioral effects in social behavior such that female mice demonstrated increased sociability and increased preference for social novelty; whereas male mice showed reduced sociability with a medium effect size.
Figure 5.
Social behavior is altered even after four weeks of recovery from chronic sleep-restriction. (A) Sociability test: time spent in the chamber after four weeks of recovery sleep (post-recovery) shows a statistically significant Sex × Chamber interaction and a near significant Sex × Condition interaction, indicating a sex-specific differential response to sociability post-recovery. Main effects of condition and chamber were statistically significant. (B) Sociability test: time spent sniffing following four weeks of recovery sleep (post-recovery) reveals a statistically significant Sex × Chamber interaction. Main effects of condition and chamber were also statistically significant. There was a close to statistically significant Condition × Sex × Chamber interaction. A post-hoc pairwise analysis reveals that sleep-restricted male mice had a trend toward statistically significant increase in time spent sniffing the object compared to controls. Sleep-restricted female mice had a statistically significant increase in time spent sniffing the stranger and a near significant increase in time spent sniffing the object compared to controls. These data show abnormalities in sociability following chronic sleep restricted that is sex dependent. (C) Social novelty test: time spent in the chamber following four weeks of recovery sleep (post-recovery) shows a statistically significant Sex × Chamber interaction and a statistically significant main effect of chamber. (D) Social novelty test: time spent sniffing measured four weeks after recovery sleep (post-recovery) reveals a statistically significant main effect of chamber, and a statistically significant Sex × Chamber, Sex × Condition, and Sex × Condition × Chamber interaction. A post-hoc pairwise analysis shows that sleep-restricted female mice had a statistically significant increase in time spent sniffing the novel mouse compared to controls. Bars represent the means ± SEMs in 11 control male, 13 sleep-restricted male, 22 control female, and 17 sleep-restricted female mice.
TABLE 4. Repeated Measures ANOVA Results Social Behavior Post Recovery.
| BEHAVIOR | TIME POINT | EFFECT | F(df, error) VALUE | P-VALUE | COHEN’S F2 |
|---|---|---|---|---|---|
| SOCIAL BEHAVIOR | |||||
| Sociability | |||||
| Chamber time | Post-Recovery | Sex × Condition × Chamber | F(1,59)= 1.915 | 0.172 | 0.032 ‡ |
| Condition × Chamber | F(1,59)= 0.302 | 0.584 | 0.005 | ||
| Sex × Chamber | F(1,59)= 21.279 | <0.001* | 0.361 ‡ ‡ | ||
| Sex × Condition | F(1,59)= 3.013 | 0.088~ | 0.052 ‡ | ||
| Sex | F(1,59)= 0.002 | 0.962 | 0.000 | ||
| Condition | F(1,59)= 5.560 | 0.022* | 0.094 ‡ | ||
| Chamber | F(1,59)= 64.491 | <0.001* | 1.092 ‡ ‡ | ||
| Sniffing time | Post-Recovery | Sex × Condition × Chamber | F(1,59)= 3.964 | 0.051~ | 0.067 ‡ |
| Condition × Chamber | F(1,59)= 0.402 | 0.528 | 0.007 | ||
| Sex × Chamber | F(1,59)= 16.536 | <0.001* | 0.280 ‡ | ||
| Sex × Condition | F(1,59)= 2.063 | 0.156 | 0.035 ‡ | ||
| Sex | F(1,59)= 0.884 | 0.351 | 0.015 | ||
| Condition | F(1,59)= 5.268 | 0.025* | 0.089 ‡ | ||
| Chamber | F(1,59)= 128.101 | <0.001* | 2.175 ‡ ‡ | ||
| Social novelty | |||||
| Chamber time | Post-Recovery | Sex × Condition × Chamber | F(1,59)= 1.413 | 0.239 | 0.024 ‡ |
| Condition × Chamber | F(1,59)= 0.687 | 0.411 | 0.012 | ||
| Sex × Chamber | F(1,59)= 9.362 | 0.003* | 0.159 ‡ | ||
| Sex × Condition | F(1,59)= 0.401 | 0.529 | 0.007 | ||
| Sex | F(1,59)= 2.667 | 0.108 | 0.045 ‡ | ||
| Condition | F(1,59)= 0.926 | 0.340 | 0.015 | ||
| Chamber | F(1,59)= 18.462 | <0.001* | 0.312 ‡ | ||
| Sniffing time | Post-Recovery | Sex × Condition × Chamber | F(1,59)= 4.554 | 0.037* | 0.078 ‡ |
| Condition × Chamber | F(1,59)= 0.190 | 0.664 | 0.003 | ||
| Sex × Chamber | F(1,59)= 5.495 | 0.022* | 0.093 ‡ | ||
| Sex × Condition | F(1,59)= 4.907 | 0.031* | 0.083 ‡ | ||
| Sex | F(1,59)= 0.081 | 0.777 | 0.001 | ||
| Condition | F(1,59)=2.263 | 0.138 | 0.038 ‡ | ||
| Chamber | F(1,59)= 72.088 | <0.001* | 1.222 ‡ ‡ |
3.3 Marble Burying Differences in Sleep Restricted Mice
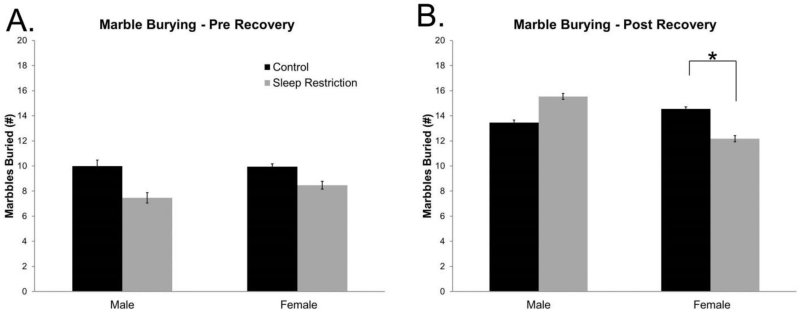
Immediately following sleep restriction, marble burying tended to be diminished in the sleep-restricted mice of both sexes, but the main effect of condition was not statistically significant (Figure 6A, Table 5). After recovery sleep, the Sex × Condition interaction was statistically significant (Table 5). We probed for pairwise interactions and found that sleep-restricted female mice buried fewer marbles than controls (p=0.034) (Figure 6B). These data indicate a sex-specific effect on repetitive behavior discernable after four weeks of recovery sleep.
Figure 6.
Marble burying differences are differentially regulated by sex following chronic sleep restriction. (A) No statistically significant differences were found in marble burying behavior three days following chronic sleep-restriction (pre-recovery). (B) After four weeks of recovery sleep (post-recovery), there was a statistically significant Sex × Condition interaction. A post-hoc pairwise analysis showed that female sleep-restricted mice buried significantly fewer marbles than control mice. These data show sex-specific changes in repetitive behaviors following chronic sleep restriction. Bars represent the means ± SEMs in (A) 9 control male, 13 sleep-restricted male, 19 control female, and 17 sleep-restricted female mice, and in (B) 11 control male, 13 sleep-restricted male, 22 control female, and 17 sleep-restricted female mice.
TABLE 5. Repeated Measures ANOVA Results Marble Burying.
| BEHAVIOR | TIME POINT | EFFECT | F(df, error) VALUE | P-VALUE | COHEN’S F2 |
|---|---|---|---|---|---|
| MARBLE BURYING | Pre-Recovery | Sex × Condition | F(1,54)= 0.159 | 0.691 | 0.003 |
| Sex | F(1,54)= 0.129 | 0.746 | 0.002 | ||
| Condition | F(1,54)= 2.277 | 0.137 | 0.042 ‡ | ||
| Post-Recovery | Sex × Condition | F(1,59)= 6.361 | 0.014* | 0.107 ‡ | |
| Sex | F(1,59)= 1.655 | 0.203 | 0.028 ‡ | ||
| Condition | F(1,59)= 0.026 | 0.872 | 0.000 |
3.4 Sleep Behavior Following Chronic Sleep-Restriction
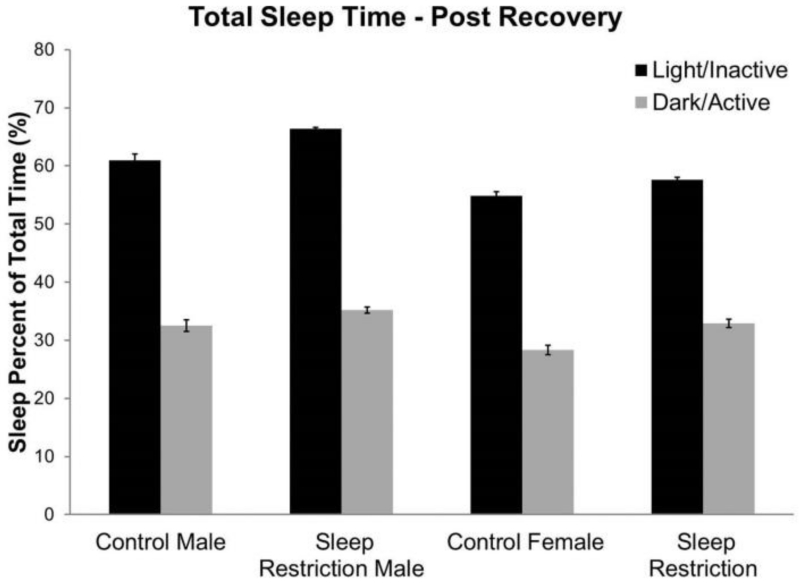
We tested sleep behavior in mice approximately seven weeks after the period of recovery sleep (Figure 7, Table 6). Percent time asleep in the active (dark) and inactive phases (light) was analyzed as a repeated measure. We found a statistically significant Sex × Phase interaction, indicating that male mice spent more time sleeping than female mice in the light phase. As expected, all groups spent more time sleeping during the inactive phase (Figure 7). Moreover, sleep-restricted mice tended to spend more time sleeping than controls regardless of phase or sex (p=0.093).
Figure 7.
Sleep behavior following chronic sleep restriction. Averaged percent time asleep, after 11 weeks of recovery sleep (17 weeks of age), showed a statistically significant main effect of sex and phase, and a significant interaction between Sex × Phase. There was a trend toward an effect of condition (p=0.093), in which sleep-restricted mice showed increased time sleeping compared to control mice. Bars represent the means ± SEMs in 9 control male, 11 sleep-restricted male, 14 control female, and 11 sleep-restricted female mice.
4 Discussion
Sleep is known to have important roles in brain function including developmental plasticity [1]. In our study, we sought to determine whether sleep-restriction during a time window in which the brain is still developing could have long lasting effects on behavior in otherwise normal mice. We report that even after recovery sleep, behavioral changes persisted. Moreover, behavioral changes were sex-specific. In females, changes included decreased activity in a novel environment, increased sociability, and decreased repetitive behaviors. In males, changes included decreased activity in a novel environment and a trend toward decreased sociability and increased repetitive behaviors.
4.1 Gentle Handling
We chose to initiate chronic sleep-restriction at five days of age because we wanted to determine if chronic sleep-restriction during development has long-term effects on behavioral outputs. We chose five days, because at that point, pups were large enough that they would be safe from cannibalization from the stressed mothers. Death of pups did not occur any more frequently in sleep-restricted mice (one pup) than in control mice (six pups). We extended the period of sleep-restriction beyond the period of brain development to mimic the lifelong sleep restriction seen in patients with neurodevelopmental disorders. We left the dams in the cage to eliminate the extraneous effects of maternal separation. Dams were often disturbed by our prodding the pups, and we cannot rule out possible effects of sleep-restriction on maternal hormones in lactating dams. In a separate study (unpublished results), we determined the growth curves of sleep-restricted and control mice and did not find any differences in the curves during the pre-weaning phase of growth, suggesting that maternal care in both groups was similar. Preliminary results indicate that after weaning growth curves are flatter in sleep-restricted mice.
Many of the traditional sleep-deprivation methods are unsuitable for infant mice. We used gentle handling for sleep-restriction because it reduces the aspect of forced locomotor activity required in other traditional sleep-deprivation techniques [13]. Although gentle handling is considered one of the less stressful methods of achieving sleep deprivation, corticosterone levels are known to be elevated by this procedure [18]. In an effort to control for the stress of the handling, we also handled control mice. Future studies, perhaps, should compare corticosterone levels in these two groups. Nevertheless, sleep restriction is inherently stressful and separating out the effects of stress and sleep loss is not possible.
4.2 Effects of Age at Time of Testing
It is interesting that behavioral changes differ between the pre-recovery and post-recovery data, in some cases becoming more apparent following recovery sleep. It seems paradoxical; if sleep-restriction induced the behavioral change, then one might expect that change to be present immediately following sleep-restriction. An alternative explanation is that the initial round of testing altered performance on the second round of testing. We think this is unlikely in the case of open field behavior because control mice exhibited similar activity curves at both time points. With respect to social behavior, we used different stranger animals for each test so an effect of repeat testing is an unlikely confound. Furthermore, the three-chambered test of social behavior was designed to be repeated in the same animal[19]. Changes in marble burying behavior, however, likely do reflect the effects of repeat testing. In the present study, all groups buried more marbles at the second time point. In a separate study (unpublished results), we tested marble burying in WT mice twice with a one week interval between tests and found that animals tend to bury more marbles on the second trial. In the present study, we controlled for this effect by comparing control and sleep-restricted animals both having undergone repeat testing following recovery sleep. We cannot rule out the possibility that sleep-restriction might differentially alter the effect of repeat testing.
Another explanation of the change between pre-recovery and post-recovery data is the effect of developmental age on behavior. A post-hoc analysis of results in control mice revealed statistically significant Age × Measure interactions in the open field, sociability, preference for social novelty, and marble burying tests. We conjecture that sleep restricted mice had an alteration in the developmental trajectory of behavioral phenotype. As mice matured, the magnitude of differences between control and sleep-restricted groups increased. Group differences immediately following sleep-restriction may reflect direct results of sleep restriction, and behavioral differences detected post-recovery may indicate long-lasting changes in behavior due to the sleep-restriction during a critical period. Other studies in rodents have reported changes in adult behavior following neonatal sleep deprivation (during a shorter window than our present study). In one study, sleep deprivation of six hours in neonatal mice (by gentle handling) increased pain sensitivity in adolescent mice [20]. Additionally, REM sleep deprivation (by shaking) in neonatal rats increased depressive-like behaviors in adults [21].
4.3 Sex Differences
One interesting and consistent feature of our results is that males and females had different behavioral responses to chronic sleep restriction. We did not monitor the estrous state of post-recovery female mice, so we cannot comment on its influence on female behavior. Previously published data indicate that open field activity, in C57BL/6J mice, is stable across the estrous cycle [22]. Additionally, nonsexual social behavior did not alter social preference in rats [23] which is the rationale behind the sex matching of the stranger mice in the experimental design [24]. However, it has been shown that marble burying is affected by the estrous cycle [25]. Given that control and sleep-restricted groups likely were composed of females in all phases of the estrous cycle, differences in marble burying based on condition may still be interpreted as an effect of condition. Further, our data do not show increased variance in post-recovery female mice in comparison to pre-recovery female mice or male mice, indicating that the estrous cycle was not a significant cause of variability in the animals.
The differential effect of sleep restriction on behavior in the sexes is an interesting question, and one that should be pursued in subsequent studies. One possible explanation is the well characterized hormonal difference between males and females. Hormones, like prostaglandin E2 (PGE2), which is involved in the masculinization of the brain and behavior [28], activates the histaminergic system to induce wakefulness [29]. Perhaps the already higher levels of PGE2 in male mice predisposes them to the consequences of prolonged wakefulness.
In addition, there are also documented differences in other aspects that can be regulated by sleep. Microglia are part of the response to PGE2 in masculinized brains, and are at a higher density and are more activated in male rats [30]. They are also activated by sleep deprivation [31] and activated microglia are a feature commonly associated with autism [32, 33]. Perhaps male mice, with already higher numbers of activated microglia, are less able to handle the effects of further activation of microglia in response to sleep restriction. There are many additional cellular processes; such as synapse formation and activity, myelination, and cellular toxicity, that occur in response to sleep loss[3], and these processes may be differentially affected by sex. Further study of why there was a differential sex-dependent response to sleep restriction may help us gain insight into sex differences in the comorbidities of sleep loss. In particular, we plan to initiate future studies to determine if sleep restriction during development has a long-term effect on dendritic tree and spine complexity, microglia activation, ER stress, and myelination. As with this study, we plan to examine the brains of these mice following recovery sleep to determine what changes are long-lasting.
4.4 Conclusion
In this study, we investigated the behavioral effects of chronic sleep restriction throughout much of neonatal development and continuing through adulthood, modeling chronic sleep loss. Our results show that chronic sleep restriction (or stress) has long-lasting effects on behavior that are sex specific. These results highlight the importance of sleep on the development of behavior. Studies to determine the mechanisms by which this occurs may point to future treatment strategies for behavioral changes associated with sleep disturbance.
Highlights.
Study in mice of effects of chronic developmental sleep restriction on behavior
Effects were sex-dependent and long lasting
Sociability, response to social novelty, and repetitive behavior were affected
Acknowledgments
This work was funded by the Intramural Research Program of the National Institutes of Mental Health, a postdoctoral fellowship (#8679) from Autism Speaks awarded to R. Michelle Reith (Saré), and a summer fellowship from the Irene & Eric Simon Brain Research Foundation awarded to Christine Hildreth.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Peirano PD, Algarin CR. Sleep in brain development. Biological research. 2007;40:471–8. [PubMed] [Google Scholar]
- [2].McEwen BS. Sleep deprivation as a neurobiologic and physiologic stressor: Allostasis and allostatic load. Metabolism: clinical and experimental. 2006;55:S20–3. doi: 10.1016/j.metabol.2006.07.008. [DOI] [PubMed] [Google Scholar]
- [3].Picchioni D, Reith RM, Nadel JL, Smith CB. Sleep, Plasticity and the Pathophysiology of Neurodevelopmental Disorders: The Potential Roles of Protein Synthesis and Other Cellular Processes. Brain Sciences. 2014;4:150–201. doi: 10.3390/brainsci4010150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Owens-Stively J, Frank N, Smith A, Hagino O, Spirito A, Arrigan M, et al. Child temperament, parenting discipline style, and daytime behavior in childhood sleep disorders. Journal of developmental and behavioral pediatrics : JDBP. 1997;18:314–21. doi: 10.1097/00004703-199710000-00005. [DOI] [PubMed] [Google Scholar]
- [5].Sadeh A, Gruber R, Raviv A. Sleep, neurobehavioral functioning, and behavior problems in school-age children. Child development. 2002;73:405–17. doi: 10.1111/1467-8624.00414. [DOI] [PubMed] [Google Scholar]
- [6].O’Brien LM, Mervis CB, Holbrook CR, Bruner JL, Smith NH, McNally N, et al. Neurobehavioral correlates of sleep-disordered breathing in children. Journal of sleep research. 2004;13:165–72. doi: 10.1111/j.1365-2869.2004.00395.x. [DOI] [PubMed] [Google Scholar]
- [7].Rosen CL, Storfer-Isser A, Taylor HG, Kirchner HL, Emancipator JL, Redline S. Increased behavioral morbidity in school-aged children with sleep-disordered breathing. Pediatrics. 2004;114:1640–8. doi: 10.1542/peds.2004-0103. [DOI] [PubMed] [Google Scholar]
- [8].Beebe DW. Neural and neurobehavioral dysfunction in children with obstructive sleep apnea. PLoS medicine. 2006;3:e323. doi: 10.1371/journal.pmed.0030323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Richdale AL. Sleep problems in autism: prevalence, cause, and intervention. Developmental medicine and child neurology. 1999;41:60–6. doi: 10.1017/s0012162299000122. [DOI] [PubMed] [Google Scholar]
- [10].Schreck KA, Mulick JA, Smith AF. Sleep problems as possible predictors of intensified symptoms of autism. Research in developmental disabilities. 2004;25:57–66. doi: 10.1016/j.ridd.2003.04.007. [DOI] [PubMed] [Google Scholar]
- [11].Mayes SD, Calhoun SL. Variables related to sleep problems in children with autism. Research in Autism Spectrum Disorders. 2009;3:931–41. [Google Scholar]
- [12].Sikora DM, Johnson K, Clemons T, Katz T. The relationship between sleep problems and daytime behavior in children of different ages with autism spectrum disorders. Pediatrics. 2012;130(Suppl 2):S83–90. doi: 10.1542/peds.2012-0900F. [DOI] [PubMed] [Google Scholar]
- [13].Colavito V, Fabene PF, Grassi-Zucconi G, Pifferi F, Lamberty Y, Bentivoglio M, et al. Experimental sleep deprivation as a tool to test memory deficits in rodents. Front Syst Neurosci. 2013;7:106. doi: 10.3389/fnsys.2013.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, et al. Automated apparatus for quantitation of social approach behaviors in mice. Genes, brain, and behavior. 2004;3:303–14. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- [15].Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, Paylor R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology. 2009;204:361–73. doi: 10.1007/s00213-009-1466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Pack AI, Galante RJ, Maislin G, Cater J, Metaxas D, Lu S, et al. Novel method for high-throughput phenotyping of sleep in mice. Physiological genomics. 2007;28:232–8. doi: 10.1152/physiolgenomics.00139.2006. [DOI] [PubMed] [Google Scholar]
- [17].Cohen J. A power primer. Psychological bulletin. 1992;112:155–9. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- [18].Hairston IS, Ruby NF, Brooke S, Peyron C, Denning DP, Heller HC, et al. Sleep deprivation elevates plasma corticosterone levels in neonatal rats. Neuroscience letters. 2001;315:29–32. doi: 10.1016/s0304-3940(01)02309-6. [DOI] [PubMed] [Google Scholar]
- [19].Chadman KK, Yang M, Crawley JN. Criteria for validating mouse models of psychiatric diseases. American journal of medical genetics. Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2009;150B:1–11. doi: 10.1002/ajmg.b.30777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Araujo P, Tufik S, Anderson ML. Sleep and pain: a relationship that begins in early life. Pain physician. 2014;17:E787–98. [PubMed] [Google Scholar]
- [21].Feng P, Ma Y. Instrumental REM sleep deprivation in neonates leads to adult depression-like behaviors in rats. Sleep. 2003;26:990–6. doi: 10.1093/sleep/26.8.990. [DOI] [PubMed] [Google Scholar]
- [22].Meziane H, Ouagazzal AM, Aubert L, Wietrzych M, Krezel W. Estrous cycle effects on behavior of C57BL/6J and BALB/cByJ female mice: implications for phenotyping strategies. Genes, brain, and behavior. 2007;6:192–200. doi: 10.1111/j.1601-183X.2006.00249.x. [DOI] [PubMed] [Google Scholar]
- [23].Lukas M, Neumann ID. Social preference and maternal defeat-induced social avoidance in virgin female rats: sex differences in involvement of brain oxytocin and vasopressin. Journal of neuroscience methods. 2014;234:101–7. doi: 10.1016/j.jneumeth.2014.03.013. [DOI] [PubMed] [Google Scholar]
- [24].Yang M, Silverman JL, Crawley JN, Crawley Jacqueline N., et al. Current protocols in neuroscience. 2011. Automated three-chambered social approach task for mice. Chapter 8:Unit 8 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Schneider T, Popik P. Attenuation of estrous cycle-dependent marble burying in female rats by acute treatment with progesterone and antidepressants. Psychoneuroendocrinology. 2007;32:651–9. doi: 10.1016/j.psyneuen.2007.04.003. [DOI] [PubMed] [Google Scholar]
- [26].(CDC) C. f. D. C. a. P. Prevalence of Autism Spectrum Disorders - Autism and Developmental Disabilities Monitoring Network, 14 Sites, United States, 2008. In: Baio J, editor. Surveillance Summaries. Centers for Disease Control and Prevention; Atlanta, GA: 2012. [PubMed] [Google Scholar]
- [27].Jacquemont S, Coe BP, Hersch M, Duyzend MH, Krumm N, Bergmann S, et al. A higher mutational burden in females supports a “female protective model” in neurodevelopmental disorders. American journal of human genetics. 2014;94:415–25. doi: 10.1016/j.ajhg.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wright CL, McCarthy MM. Prostaglandin E2-induced masculinization of brain and behavior requires protein kinase A, AMPA/kainate, and metabotropic glutamate receptor signaling. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:13274–82. doi: 10.1523/JNEUROSCI.3603-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Huang ZL, Sato Y, Mochizuki T, Okada T, Qu WM, Yamatodani A, et al. Prostaglandin E2 activates the histaminergic system via the EP4 receptor to induce wakefulness in rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:5975–83. doi: 10.1523/JNEUROSCI.23-14-05975.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lenz KM, Nugent BM, Haliyur R, McCarthy MM. Microglia are essential to masculinization of brain and behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:2761–72. doi: 10.1523/JNEUROSCI.1268-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hsu JC, Lee YS, Chang CN, Chuang HL, Ling EA, Lan CT. Sleep deprivation inhibits expression of NADPH-d and NOS while activating microglia and astroglia in the rat hippocampus. Cells, tissues, organs. 2003;173:242–54. doi: 10.1159/000070380. [DOI] [PubMed] [Google Scholar]
- [32].Morgan JT, Chana G, Pardo CA, Achim C, Semendeferi K, Buckwalter J, et al. Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biological psychiatry. 2010;68:368–76. doi: 10.1016/j.biopsych.2010.05.024. [DOI] [PubMed] [Google Scholar]
- [33].Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Annals of neurology. 2005;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- [34].Riby DM, Hancock PJ. Viewing it differently: social scene perception in Williams syndrome and autism. Neuropsychologia. 2008;46:2855–60. doi: 10.1016/j.neuropsychologia.2008.05.003. [DOI] [PubMed] [Google Scholar]
- [35].Fishman I, Yam A, Bellugi U, Mills D. Language and sociability: insights from Williams syndrome. Journal of neurodevelopmental disorders. 2011;3:185–92. doi: 10.1007/s11689-011-9086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Stores G, Wiggs L. Sleep Disturbance in Children and Adolescents with Disorders of Development: its Significance and Management. Mac Keith Press; London, UK: 2001. [Google Scholar]
- [37].Karmiloff-Smith A. Perspectives on the dynamic development of cognitive capacities: insights from Williams syndrome. Current opinion in neurology. 2012;25:106–11. doi: 10.1097/WCO.0b013e3283518130. [DOI] [PubMed] [Google Scholar]