Abstract
Introduction
Hypogonadism is one of the most common male endocrine problems. Although many treatments are currently available, unmet need exists for new testosterone (T) replacement therapies that are simple to administer and use, are safe, and mimic physiologic T levels.
Aim
The study aim was to determine the pharmacokinetics (PK), safety, and tolerability of T enanthate (TE) administered via a novel single‐use autoinjector system, which was designed to eject high‐viscosity solutions from a prefilled syringe fitted with a five‐eighths‐inch 27‐gauge needle.
Methods
Thirty‐nine men with hypogonadism entered this dose‐finding, open‐label, parallel‐group study. Patients were washed out of their topical T regimens and randomized to receive 50 or 100 mg of subcutaneous (SC) TE weekly. The reference group were patients with hypogonadism who were maintained on standard 200‐mg intramuscular (IM) TE.
Main Outcome Measure
The primary outcome measure was the PK profile of SC TE, analyzed in reference to T levels used by the Food and Drug Administration to approve T products. Secondary outcome measures were safety and tolerability assessments.
Results
Both doses of SC TE achieved normal average concentrations of serum T within a 168‐h dosing interval after injection. Concentration ranges were similar at all time points following 50‐mg SC TE injections and following the third injection in the 100‐mg arm. Mean steady‐state T concentration at week 6 was 422.4 and 895.5 ng/dL for the 50‐ and 100‐mg SC TE arms, respectively. SC TE demonstrated PK dose proportionality. SC TE restored normal serum T with low variation relative to 200‐mg IM without clinically significant adverse events.
Conclusions
Administration of TE via this novel injection system restored T levels to normal range in men with hypogonadism. SC TE dosed weekly demonstrated steady, dose‐proportional measures of exposure and was well‐tolerated. Kaminetsky J, Jaffe JS, Swerdloff RS. Pharmacokinetic profile of subcutaneous testosterone enanthate delivered via a novel, prefilled single‐use autoinjector: A phase II study. Sex Med 2015;3:263–273.
Keywords: Subcutaneous Injection, Testosterone, Autoinjector, Viscosity, Hypogonadism, Male, Clinical Trial, Phase II
Introduction
Hypogonadism in men is a deficiency in serum testosterone (T) levels with symptoms secondary and responsive to T replacement [1, 2]. Diagnosis may be made in men of any age [3]. Prevalence varies according to different reports, as it is influenced by the nature of the populations used in the study as well as the criteria used to define both the study and reference populations. According to one estimate, up to five million men are afflicted with this disorder in the United States and this number may reach 6.5 million by 2025 [3, 4]. Use of T replacement therapy (TRT) has increased in recent years [5]. Available TRTs in the United States include intramuscular injections (IM), transdermal, transbuccal and intranasal applications, and implantable pellets. Selection of treatment modality is often influenced by convenience and cost of therapy [6, 7, 8]. TRT benefits patients with hypogonadism by restoring T to physiologic levels, consequently improving mood, increasing bone and muscle mass, reducing adiposity, and improving libido and sexual function [9].
While TRT may benefit many patients, there are important class safety factors to consider. TRT is contraindicated for patients with prostate and male breast cancer [1]. T gels carry a warning due to the potential for secondary exposure to those in close contact with the patient [10]. A large volume, long‐acting formulation of T in oil injected IM through a large bore needle carries a boxed warning for pulmonary oil embolism [11]. Uncertainty exists as to whether or not patients may suffer adverse cardiovascular effects. Some outcome studies have indicated that TRT is associated with an increased risk of cardiovascular adverse events (AEs) [12, 13]. Meanwhile, other outcome studies find no effect or reductions in cardiovascular events related to TRT [14, 15], or rather that decrease in T levels (T deficiency) is associated with increased mortality and risk of cardiovascular disease [16, 17].
Drawbacks exist with each of the currently available T delivery mechanisms. For example, IM injections can be painful [18, 19]; the discomfort associated with large needle bore and length required for manual IM injection of viscous oil solutions can negatively impact patient compliance [20]. Because office visits are commonly recommended by providers, there is inconvenience to users. In addition, IM injection of T enanthate (TE) in sesame oil (e.g., Delatestryl®) is often administered in 200–400 mg doses every 2–4 weeks leading to peak and trough T levels outside of physiologic range [21, 22]. Resulting fluctuations may lead to mood swings and disturbances in energy level [9, 23]. Transdermal patches are commonly associated with skin reactions, which can lead to discontinuation of therapy [24]. Gels carry a risk of transference to women and children and are considered messy and may have an unpleasant odor to some users [10]. Nasally administered T and oral TRT in development appear to require multiple daily doses [25, 26]. Oral TRT can cause gastrointestinal side effects. Because there are limitations to these delivery systems, patient compliance with treatment is an issue and TRT discontinuation rates are high [27]. Therefore, weekly subcutaneous (SC) administration of TE in oil solution, via a device optimized to inject highly viscous solutions through a five‐eighths‐inch 27‐gauge needle (Supplemental Figure S1), is proposed as a viable alternative to other routes of administration, such as IM delivery systems. The objective of our phase II study was to assess the steady‐state pharmacokinetics (PK) of two strengths of TE administered SC via a drug‐device combination, as a multiple‐dose regimen, to evaluate the possible utility of this modality for chronic replacement therapy.
Aims
This was a multicenter, phase II, three‐arm, open‐label, multi‐dose, parallel‐group study of two dosing levels of TE to determine the PK profile of a novel drug‐device combination product to administer SC TE in oil once weekly.
Methods
Study Population
Male patients (18–75 years) with a history of physician‐diagnosed hypogonadism of any etiology and with serum total T (TT) levels <300 ng/dL recorded on two occasions at least 1 week apart were eligible for this study. Patients were required to be in good general health without significant comorbidities and with a body mass index (BMI) between 18 and 32 kg/m2. All patients were provided complete information of all AEs related to T and were subsequently required to provide written informed consent to be screened for all study requirements and restrictions. Patients were excluded if they had normal T levels (>300 ng/dL) or if they were deemed to have any clinically significant medical condition, which, in the opinion of the investigator, made the patient an unsuitable candidate for enrollment in the study. All 39 patients enrolled were included in the safety and PK populations. Investigators are listed in Supplemental Table S1. Summary of prior T treatment is provided in Supplemental Table S2.
Study Design
This was a three‐arm, open‐label, multi‐dose, parallel‐group, phase II study (ClinicalTrials.gov Identifier: NCT01887418) of the PK, safety, and tolerability of TE administered SC via autoinjector or IM via needle and syringe. The study design is summarized in Figure 1. Of the 86 patients screened between August 28, 2013, and November 1, 2013, 39 were dosed. Reasons for screen failure are provided in Supplemental Table S3. Patients randomized to 50 or 100 mg SC TE must have never previously received TRT or had to be washed out of any prior therapy before first dose. Any patient that had received buccal or transdermal T at the time of the screening visit were washed out of therapy for at least 2 weeks, assessed for study eligibility, and randomized to treatment with SC TE (50 or 100 mg in 0.5 mL). Patients receiving IM TE treatment at time of study entry were assessed for study eligibility for the IM TE reference group. The SC arms received their dose of the preservative‐free formulation of TE via an autoinjector device designed to deliver high‐viscosity solutions through a small bore needle. All study medication was administered by study personnel. Patients in the IM group, who were already at steady‐state, received a single 1‐mL IM injection of 200 mg/mL of TE in sesame oil (West‐Ward Pharmaceutical Corp, Eatontown, NJ, USA) via needle and syringe. During the treatment period, the SC treatment arms received their respective dose once weekly over 6 weeks. Institutional review boards of participating centers approved the study design. The study was conducted in accordance with the Declaration of Helsinki and with all applicable laws and regulations of the locale and country where the study was conducted, and was in compliance with Good Clinical Practice Guidelines. Informed consent was obtained from the patients.
Figure 1.
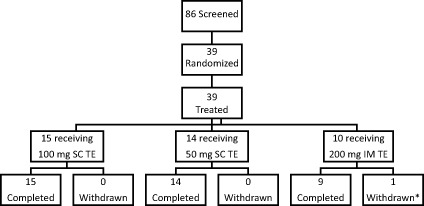
Study design. *One person in the 200 mg intramuscular (IM) testosterone enanthate (TE) arm discontinued due to personal reasons unrelated to study medication. SC = subcutaneous
PK Profile Assessment
Seven‐day PK profiles were collected for each patient in the SC TE treatment arms at weeks 1 and 5 and the full PK profile following the sixth dose. Pre‐dose trough and 24 hours post‐dose samples were collected at each of the 6 weekly treatment visits. For patients in the IM TE group, a PK profile was collected through week 4.
Laboratory Tests, Biomarker Analyses, and Safety Analyses
Sensitive and specific liquid chromatography–tandem mass spectrometry (LC–MS/MS) assays for the quantification of T, dihydrotestosterone (DHT), and estradiol were developed, validated and performed by Medpace Bioanalytical Laboratories (Cincinnati, OH, USA) [28, 29].
Safety data collected included treatment‐emergent AEs (TEAEs), vital signs, injection site assessments (ISA), prostate exam, 12‐lead electrocardiogram, and laboratory assessments. The collection of all treatment TEAEs and determination of frequency of TEAEs for each organ system allowed for deciphering of any treatment‐related patterning of TEAEs. AEs were coded using MedDRA version 16.0. An AE was considered to be a TEAE if the event started on or after the first dose of study medication. ISA were performed 0.5, 1.0, and 24.0 hours post‐dose after each injection. Parameters for documentation included erythema, induration, bleeding, hematoma, ecchymosis, pinprick/needle mark, device pressure mark, and the presence of itching and pain. For patients who reported pain or itch on injection, pain/itch was rated using a visual analog scale (VAS) (1, barely noticeable, to 10, worst they have ever experienced). For the erythema, induration, hematoma, ecchymosis, and pressure mark, measurements of the diameter at the widest point were measured to the nearest mm. An injection site adverse reaction was reported for observations measuring ≥25 mm or persisting ≥24 hours. Routine safety laboratory and prostate‐specific antigen testing were performed. Clinical laboratory evaluations included hematology, biochemistry, and urinalysis. These safety laboratory tests were performed at screening, week 3 for the SC treatment arms, week 4 for the IM treatment group, and at the end of the study.
Determination of Sample Size and Statistic Analyses
Sample size was determined by study objectives, as opposed to statistic power calculations, and was planned to ensure that at least 24 patients completed the study. Up to 45 patients could be enrolled and the final population included 14 patients in the 50‐mg SC TE arm, 15 in the 100‐mg SC TE treatment arm, and 10 in the IM treatment group. PK and safety analyses were performed in those patients who received at least one dose of study medication. For evaluation of PK parameters, relative bioavailability was determined from point estimates of the geometric least‐squares mean ratios and associated 90% confidence intervals. Creation of datasets and statistic analyses were performed using SAS® version 9.3 (SAS Institute Inc., USA).
Main Outcome Measures
The primary endpoint was to quantify measures of exposure to two dose levels of a SC TE formulation over 6 weeks, analyzed in reference to T levels used by the Food and Drug Administration (FDA) to approve T products. Blood sampling following dosing allowed for determination of the PK parameters of the regimen including area under the concentration–time curve from time zero to immediately prior to the next weekly dose (AUC0–168 h), from which the average concentration over the 7‐day dosing interval (Cavg0–168 h), and maximum observed plasma concentration (Cmax) was derived. Secondary endpoints included safety and tolerability parameters over the 6‐week treatment period.
Results
Patient Disposition and Baseline Demographics/Clinical Characteristics
Thirty‐nine patients were enrolled (Table 1) and evaluated for safety and PK analyses. Thirty‐eight of 39 (97.4%) patients completed this study; one patient in the IM treatment group withdrew from the study after dose administration for reasons unrelated to the study. Mean age of the study population was 52.9 years. The majority of patients were Caucasian (89.7%) and the remainder of the population included African descent (7.7%) and multiple races (2.6%). At screening, average BMI was 28.91 kg/m2. Dosing groups were generally comparable with respect to demographics and baseline characteristics. There was no significant difference in baseline mean TT levels between SC treatment arms; baseline mean TT was 214.64 and 201.50 ng/dL for patients in the 50 and 100 mg arms, respectively. Baseline mean TT was 735.10 ng/dL for the 200 mg IM TE group as a result of these patients being maintained on their IM regimen at study entry.
Table 1.
Patient disposition and baseline demographics/clinical characteristics
| Category | Treatment arm | |||
|---|---|---|---|---|
| 50 mg SC TE | 100 mg SC TE | 200 mg IM TE | Total | |
| Enrolled—n (%) | 14 (100) | 15 (100) | 10 (100) | 39 (100) |
| Mean age (SD) | 54.0 (12.4) | 54.7 (12.8) | 48.9 (10.9) | 52.9 (12.1) |
| Race—n (%) | ||||
| Caucasian | 14 (100) | 13 (86.7) | 8 (80.0) | 35 (89.7) |
| African descent | 0 (0.0) | 2 (13.3) | 1 (10.0) | 3 (7.7) |
| Multiple | 0 (0.0) | 0 (0.0) | 1 (10.0) | 1 (2.6) |
| Ethnicity—n (%) | ||||
| Hispanic or Latino | 0 (0.0) | 0 (0.0) | 2 (20.0) | 2 (5.1) |
| Not Hispanic or Latino | 14 (100.0) | 15 (100.0) | 8 (80.0) | 37 (94.9) |
| Enrollment status—n (%) | ||||
| Treatment naïve | 13 (92.9) | 13 (86.7) | ||
| Requiring washout | 1 (7.1) | 2 (13.3) | ||
| Mean BMI—kg/m2 at screening (SD) | 28.51 (2.17) | 29.19 (2.87) | 29.05 (2.21) | 28.91 (2.43) |
| Mean weight—kg at screening (SD) | 91.75 (10.27) | 93.16 (12.27) | 90.35 (12.03) | 91.93 (11.27) |
| Mean height—cm at screening (SD) | 179.21 (4.98) | 178.50 (8.39) | 176.02 (8.02) | 178.12 (7.16) |
| Mean baseline TT—ng/dL (SD) | 214.64 (59.05) | 201.50 (71.53) | 735.10 (187.96) | |
| Safety population*—n (%) | 14 (100) | 15 (100) | 10 (100) | 39 (100) |
| Pharmacokinetic population † —n (%) | 14 (100) | 15 (100) | 10 (100) | 39 (100) |
| Completed the study—n (%) | 14 (100) | 15 (100) | 9 (90) | 38 (97.4) |
| Discontinued from the study—n (%) | 0 (0.0) | 0 (0.0) | 1 (10.0) | 1 (2.6) |
| Primary reason for discontinuation: Other—n (%) | 0 (0.0) | 0 (0.0) | 1 (10.0) | 1 (2.6) |
*The safety population consisted of all patients who were randomized to treatment and received at least one dose of study medication. †The pharmacokinetic population consisted of all patients from the safety population who did not have a major protocol deviation that would impact the integrity of the PK data following dose administration and had at least one valid plasma concentration value post‐dose. For SC TE treatments, baseline TT was the measurement at SV2 for treatment‐naïve patients and the average of measurements at SV2 and SV3 for patients receiving any buccal or transdermal T treatment. If retest of TT happened because of a sample being ≥300 ng/dL, the average of two of the three samples with TT levels <300 ng/dL was used to calculate the baseline. For the 200 mg IM TE arm, baseline TT was the measurement at QV. If retest of the QV was needed, the retest value was used for baseline. BMI = body mass index; IM = intramuscular; PK = pharmacokinetic; QV = qualifying visit; SC = subcutaneous; SD = standard deviation; SV = screening visit; T = testosterone; TE = testosterone enanthate; TT = total testosterone
PK Profile for T
Figure 2 shows pre‐dose and 24 hours post‐dose TT levels collected during the dosing interval. Patients achieved mean TT levels within the predetermined reference range (300–1,100 ng/dL) within 24 hours following the first dose with either 50 or 100 mg of TE delivered via the self‐administration system. The 50 mg dose of SC TE provided a temporary increase to T levels, which fell to baseline between doses (Figure 2). PK curves in the 50 mg SC group were similar between weeks 1, 5, and 6 (Figure 3A). Unlike the 50‐mg group, T levels rose following the first three doses of 100 mg SC TE. Pre‐dose and 24 hours post‐dose concentration ranges overlapped at week 4 and beyond, which is consistent with having approached steady‐state exposure (Figure 2). At weeks 5 and 6, PK curves for the 100‐mg group overlapped and provided greater T exposure than the exposure observed at week 1 (Figure 3B).
Figure 2.
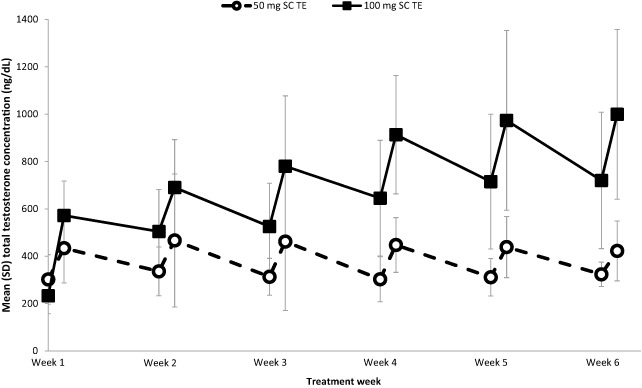
Mean pre‐dose and 24 hours post‐dose total testosterone concentration. Mean total testosterone (TT) concentrations for 50 (open circles) and 100 (closed squares) mg subcutaneous (SC) testosterone enanthate (TE) measured pre‐dose (0 hour) and 24 hours post‐dose at weeks 1–6. SD = standard deviation
Figure 3.
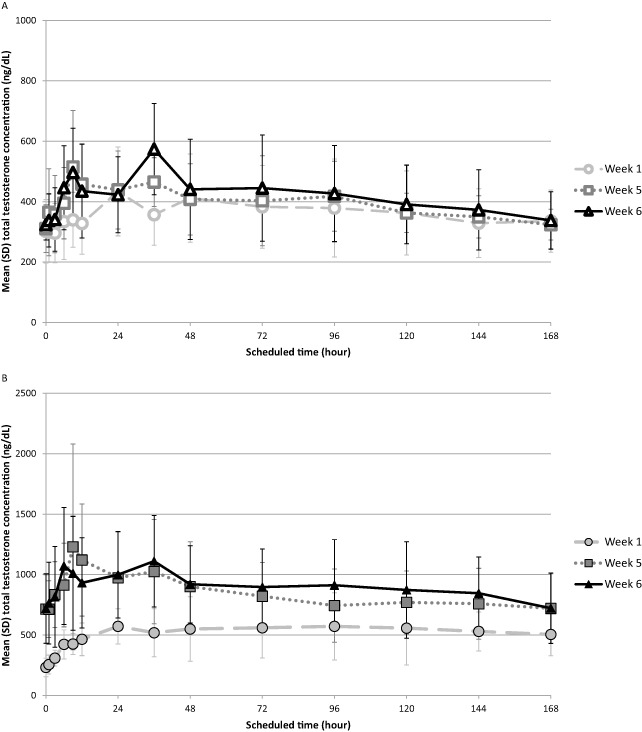
Mean total testosterone concentration vs. time. Mean total testosterone (TT) concentration across weeks 1, 5, and 6 for subcutaneous (SC) testosterone enanthate (TE) treatment arms. (A) 50 mg SC TE (open shapes). (B) 100 mg SC TE (closed shapes). SD = standard deviation
Table 2 summarizes PK parameters by treatment. Both doses of SC TE produced average steady‐state concentrations of T within the normal range over the dosing interval of 168 hours (7 days). In contrast, 200 mg IM TE produced supra‐physiologic levels the first week after dosing. At week 6, the 50 mg SC TE treatment group achieved a Cavg0–168 h (standard deviation [SD] ) of 422.4 ng/dL (123.9), while the 100 mg SC TE treatment group achieved a Cavg0–168 h (SD) of 895.5 ng/dL (279.8). The IM TE treatment group achieved a Cavg0–168 h (SD) of 1658.7 ng/dL (1001.8). Because of rising T levels at later time points in the 50 mg arm, reliable estimation of half‐life (T1/2) of this dose was not possible. The apparent T1/2 (SD) was 239.63 hours (59.93) for 100 mg SC TE and 172.57 hours (34.74) for 200 mg IM TE.
Table 2.
Summary of steady‐state pharmacokinetic parameters by treatment
| PK parameters at week 6 | 50 mg SC TE (n = 14) | 100 mg SC TE (n = 15) | 200 mg IM TE (n = 10) | Ratio of SC TE 100/SC TE 50 | Ratio of 200 mg IM TE/100 mg SC TE |
|---|---|---|---|---|---|
| Testosterone | |||||
| Mean AUC0–168h—ng × h/dL (SD) | 70955.7 (20815.2) | 150445.2 (46998.8) | 278657.9 (168295.5) | 2.13 | 1.85 |
| Cavg0–168h | |||||
| Mean—ng/dL (SD) | 422.4 (123.9) | 895.5 (279.8) | 1658.7 (1001.8) | 2.12 | 1.85 |
| Range—ng/dL | 257–673 | 406–1,368 | 681–3,758 | ||
| Number of patients with Cavg0–168h within 300–1,100 ng/dL | 12 | 10 | 3 | ||
| Cavg0–336h | |||||
| Mean—ng/dL (SD) | 409.6 (96.1) | 868.5 (256.5) | 1267.5 (724.5) | 1.4 † | |
| Number of patients with Cavg0–336h within 300–1,100 ng/dL | 12 | 11 | 5 | ||
| Cmax—ng/dL | |||||
| Mean (SD) | 622.4 (129.5) | 1345.6 (435.6) | 2261.9 (1310.3) | 2.17 | 1.7 |
| Range | 388–825 | 624–2,120 | 787–4,840 | ||
| Cmin—ng/dL | |||||
| Mean (SD) | 272.9 (54.7) | 568.3 (196.4) | 466.1 (199.1) | 2.08 | 0.82 |
| Range | 182–372 | 236–860 | 203–780 | ||
| Mean Tmax—h (SD) | 45.43 (33.60) | 35.54 (30.40) | 33.30 (24.52) | ||
| Mean T½—h (SD) | ND ‡ | 239.63 (59.93) | 172.57 (34.74) | ||
| DHT | |||||
| Mean AUC0–168h—ng × h/dL (SD) | 5173.8 (1641.4) | 8718.4 (2436.8) | 19668.6 (11145.0) | ||
| Mean DHT/T ratio § | 0.0750 | 0.0609 | 0.0732 | ||
| Mean Cavg0–168h—ng/dL (SD) ¶ | 30.8 (9.8) | 51.9 (14.5) | 117.1 (66.3) | ||
| E2 | |||||
| Mean AUC0–168h—ng × h/dL (SD) | 429.2 (139.4) | 811.0 (344.9) | 839.4 (478.1) | ||
| Mean E2/T ratio § | 0.0063 | 0.0055 | 0.0032 | ||
| Mean Cavg0–168h—pg/mL (SD) ¶ | 25.6 (8.3) | 48.3 (20.5) | 50.0 (28.5) |
| Testosterone PK parameters (combined weeks 5 and 6) | 50 mg SC TE | 100 mg SC TE | 200 mg IM TE | Ratio of SC TE 100/SC TE 50 | Ratio of 200 mg IM/100 mg SC TE |
|---|---|---|---|---|---|
| Mean AUC0–336h—ng × h/dL (SD) | 137620.1 (32282.2) | 291829.3 (86197.7) | 425879.5 (243433.7) | 2.13 | 1.45 |
| Mean AUC0‐inf—ng × h/dL (SD) | 376238.6 ‡ | 541221.9 (140895.3) | 517679.0 (193305.0) | 1.45 | 0.95 |
†Calculation for the 168‐h dosing interval for SC TE and 336‐h dosing interval for IM TE. ‡n = 1 due to rising T levels at later time points in 13/14 patients. §Ratio of AUC0–168h of metabolite to AUC0–168h of T. ¶Reference range was defined as 300–1,100 ng/dL for T, 4–57.5 ng/dL for DHT, and 10–50 pg/mL for E2. AUC = area under the concentration–time curve; DHT = dihydrotestosterone; E2 = estradiol; IM = intramuscular; ND = not determined; PK = pharmacokinetic; SC = subcutaneous; SD = standard deviation; T = testosterone; TE = testosterone enanthate
SC TE demonstrated dose proportionality as AUC0–168 h, Cavg0–168 h, Cmax, and minimum observed plasma concentration (Cmin) of the 100 mg dose of SC TE were approximately twice those of the 50 mg dose (Table 2). Relative to 200 mg IM TE, the two doses of 100 mg SC TE (week 5 and week 6 combined) demonstrated similar AUC0‐inf, suggesting that the bioavailability of TE is similar whether administered SC or IM.
PK Profile for DHT and Estradiol
A summary of the main PK parameters for DHT and estradiol (E2) can be found in Table 2. Overall, the Cavg0–168 h for each metabolite was within the reference range (DHT: 4–57.5 ng/dL; E2: 10–50 pg/mL) for both doses of SC TE. The Cavg0–168 h for DHT was 30.8 ng/dL and for E2 was 25.6 pg/mL for the 50 mg SC TE group. For the 100 mg SC TE group, Cavg0–168 h for DHT was 51.9 ng/dL and for E2 was 48.3 pg/mL. The Cavg0–168h for DHT for the 200 mg IM TE group was 117.1 ng/dL. The Cavg0–168 h for E2 for the 200 mg IM TE group was comparable with that of the 100 mg SC TE group, reaching 50.0 pg/mL. The ratios of T to metabolites were similar, suggesting that the conversion rate of T to its metabolites was similar across groups.
Safety Profile
AEs that occurred during this trial are summarized in Table 3. No deaths or serious TEAEs were reported during the course of this study. No study patient during the course of the study experienced any cardiovascular events. Thirteen of 39 patients (33.3%) enrolled in the study reported at least one TEAE during the course of this trial. Generally, these TEAEs were of mild to moderate intensity and only four were reported related to study drug (Table 4). Two patients in the SC TE arms experienced insomnia. One patient in the 100 mg SC TE group reported acne. No AEs were reported from IM TE administration. No TEAE led to discontinuation from the study. All TEAEs resolved by the end of the study.
Table 3.
Overview of adverse events*
| Patients with AEs | 50 mg SC TE (n = 14) | 100 mg SC TE (n = 15) | 200 mg IM TE (n = 10) |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Patients with a TEAE | |||
| Any TEAE | 6 (42.9) | 7 (46.7) | 0 (0.0) |
| Any drug‐related TEAE | 2 (14.3) | 2 (13.3) | 0 (0.0) |
| Patients with an SAE | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Treatment–emergent SAE | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Drug‐related SAE | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Deaths | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Discontinuations because of TEAE | 0 (0.0) | 0 (0.0) | 0 (0.0) |
*An AE was considered to be a TEAE if the AE started on or after the first dosing of study medication. Percentage was calculated using the number of patients in the column heading as the denominator. AE = adverse event; IM = intramuscular; SAE = serious adverse event; SC = subcutaneous; TE = testosterone enanthate; TEAE = treatment‐emergent adverse event
Table 4.
Drug‐related treatment‐emergent adverse events*
| System organ class (preferred term) | 50 mg SC TE (n = 14) | 100 mg SC TE (n = 15) | 200 mg IM TE (n = 10) |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Patients with any drug‐related TEAE | 2 (14.3) | 2 (13.3) | 0 (0.0) |
| General disorders and administration site conditions | 1 (7.1) | 0 (0.0) | 0 (0.0) |
| Injection site hemorrhage † | 1 (7.1) | 0 (0.0) | 0 (0.0) |
| Psychiatric disorders | 1 (7.1) | 1 (6.7) | 0 (0.0) |
| Insomnia | 1 (7.1) | 1 (6.7) | 0 (0.0) |
| Skin and subcutaneous tissue disorders | 0 (0.0) | 1 (6.7) | 0 (0.0) |
| Acne | 0 (0.0) | 1 (6.7) | 0 (0.0) |
*An AE was considered to be a TEAE if the AE started on or after the first dosing of study medication. Percentage was calculated using the number of patients in the column heading as the denominator. Although a patient may have had two or more TEAEs, the patient was counted only once within a system organ class category. The same patient may have contributed to two or more preferred term categories. †Injection site ecchymosis (reported term of “left abdominal injection site ecchymosis”, and coded as “injection site ecchymosis” as lower level term and “hemorrhage” as the preferred term because of MedDRA coding specifications). AE = adverse event; IM = intramuscular; SC = subcutaneous; TE = testosterone enanthate; TEAE = treatment‐emergent adverse event
ISA were performed for each injection. Eleven of 39 patients (28.2%) had no quantifiable ISA observations for all six injections. Almost all findings related to injections were transient and resolved. Only one patient in the 50 mg SC TE arm experienced an injection site reaction TEAE (ecchymosis). Thirty‐eight of 39 patients reported no pain on injection; a single patient rated one of six injections a two on the pain VAS.
Discussion
Although there have been only a few reports in the literature, these previous studies suggest that the SC route of administration achieves therapeutic T levels and is a viable alternative to IM administration [30, 31, 32]. Our article reports the results from a multicenter, multiple‐dose, phase II PK study and demonstrates that TE in oil‐administered SC via a prefilled single‐use disposable autoinjector was able to achieve serum T reliably within reference ranges over a 1‐week dosing interval. Normal T levels were achieved within hours after first dose and steady‐state was approached after the third dose. SC TE minimized variation in exposure relative to a chronic 200 mg IM TE dosing cohort. Mean steady‐state Cavg and Cmax T levels were in the reference range with both doses of SC TE. In the IM TE cohort, Cavg and Cmax were higher than normal in the week after dosing. The supra‐physiologic levels of T following 200 mg IM TE is consistent with prior reports [21, 22].
The 50‐mg dose exhibited no accumulation between doses. A similar result at this dose was demonstrated in healthy men, suggesting that clearance exceeds exposure by the end of the dosing interval [33]. The 100 mg SC TE dose achieved approximately twice the T exposure as the 50 mg dose; thus demonstrating dose proportionality. Accordingly, DHT and E2 levels increased with TE dose. The ratio of DHT to T was similar across the groups, suggesting a similar rate of metabolism. Overall, the results presented here suggest that an intermediate dose of 75 mg weekly will provide optimal T exposure in most patients.
The TEAEs reported were mild in nature and largely unrelated to T or the route of administration. No TEAEs resulted in discontinuation of the study. No safety signal emerged from assessment of TEAEs. Not only does SC administration of TE appear to be safe for clinical use, but the TE–autoinjector device combination was well‐tolerated. The TE–autoinjector combination was not associated with any serious injection reactions; only one injection site adverse reaction (ecchymosis) was reported. A property of our SC injection systems is reduced or absent sensation of fine‐needle entry and pain. This is likely due to distraction from pain sensation caused by the force applied against the skin to retract the safety collar [34]. Similar to our previous study, pain related to injection was not present for most patients in this study. This profile suggests that SC TE injections will be a well‐tolerated treatment for patients.
While the data show that SC TE has a reproducible and dose‐proportional PK profile, limitations to this study exist regarding the comparison with the IM regimen. Randomization of all three treatment arms, including the IM group, would have been a more conventional design. This approach was considered, but not pursued because of the protracted period of time required to achieve steady‐state with de novo IM TE treatment dosed every 2 weeks. The purpose of including an IM arm receiving chronic dosing was to provide reference PK data reflecting the on‐label regimen for TE in contemporary patients and not for formal statistic comparison.
The advantages of this novel SC TE autoinjector combination product are numerous. This delivery method effectively restores physiologic levels of T in patients with hypogonadism. The device is designed to produce accurate and rapid dosing, as a 0.5 mL injection of drug occurs in less than 5 seconds, and reduces the risk for medication abuse. While patients in this study did not self‐administer medication, in other (unpublished) device use studies, men with hypogonadism report that this device is easy to use, and home self‐administration will be assessed in long‐term therapeutic studies. Further, the risk of passive transference to women and children associated with topical gels is eliminated with SC TE [10]. As peak to trough variability is reduced with weekly SC TE, there may be less risk for treatment‐related mood swings seen with IM TE [9, 23]. A weekly regimen of SC TE, and the convenience and ease of use of at‐home self‐administration may lead to higher patient compliance than daily dosing regimens [35].
Conclusion
Once approved by the FDA, the combination product of TE in oil‐administered SC via a novel, prefilled, disposable autoinjector, which was developed to deliver high‐viscosity solutions through a small‐gauge needle, will provide a simple and well‐tolerated method for SC self‐administration of TE. The safety profile was benign. The overall results of this study indicate that SC TE delivered in this fashion has acceptable tolerability with a reproducible and dose‐proportional PK profile. The outcomes of this phase II study warrant proceeding to a larger phase III study. Furthermore, dose proportionality supports the predictability of exposure to an intermediate dose of TE and suggests the feasibility of dose adjustment based on T levels.
Conflict of Interest: JK is a speaker and consultant for Auxilium Pharmaceuticals. He is also an investigator for Lipocine Inc., Clarus Therapeutics, Antares Pharma, Inc., Auxilium Pharmaceuticals, and Eli Lilly and Company. Furthermore, he serves as an investigator, speaker, and consultant for AbbVie Pharmaceuticals. JJ is an employee of Antares Pharma, Inc. RS is a paid consultant to Antares Pharma, Inc. He is also an investigator and consultant for Clarus Therapeutics, Antares Pharma, Inc., Besins Pharma, AbbVie Pharmaceuticals, and Novartis.
Supporting information
Figure S1 Subcutaneous testosterone enanthate autoinjector device. The autoinjector is designed to eject high‐viscosity solutions from a prefilled syringe fitted with a five‐eighths‐inch 27‐gauge needle
Table S1 List of investigators and study sites
Table S2 Summary of prior testosterone treatment
Table S3 Summary of screen failure patients
Source of Funding: This work was supported by Antares Pharma, Inc..
References
- 1. Petak SM, Nankin HR, Spark RF, Swerdloff RS, Rodriguez‐Rigau LJ. American association of clinical endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hypogonadism in adult male patients—2002 update. Endocr Pract 2002;8:440–456. [PubMed] [Google Scholar]
- 2. Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM. Testosterone therapy in men with androgen deficiency syndromes: An endocrine society clinical practice guideline. J Clin Endocrinol Metab 2010;95:2536–2559. [DOI] [PubMed] [Google Scholar]
- 3. Araujo AB, Esche GR, Kupelian V, O'Donnell AB, Travison TG, Williams RE, Clark RV, McKinlay JB. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab 2007;92:4241–4247. [DOI] [PubMed] [Google Scholar]
- 4. Araujo AB, O'Donnell AB, Brambilla DJ, Simpson WB, Longcope C, Matsumoto AM, McKinlay JB. Prevalence and incidence of androgen deficiency in middle‐aged and older men: Estimates from the Massachusetts Male Aging Study. J Clin Endocrinol Metab 2004;89:5920–5926. [DOI] [PubMed] [Google Scholar]
- 5. Baillargeon J, Urban RJ, Ottenbacher KJ, Pierson KS, Goodwin JS. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med 2013;173:1465–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pfeil E, Dobs AS. Current and future testosterone delivery systems for treatment of the hypogonadal male. Expert Opin Drug Deliv 2008;5:471–481. [DOI] [PubMed] [Google Scholar]
- 7. Kovac JR, Rajanahally S, Smith RP, Coward RM, Lamb DJ, Lipshultz LI. Patient satisfaction with testosterone replacement therapies: The reasons behind the choices. J Sex Med 2014;11:553–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Smith RP, Khanna A, Coward RM, Rajanahally S, Kovac JR, Gonzales MA, Lipshultz LI. Factors influencing patient decisions to initiate and discontinue subcutaneous testosterone pellets (Testopel) for treatment of hypogonadism. J Sex Med 2013;10:2326–2333. [DOI] [PubMed] [Google Scholar]
- 9. Surampudi PN, Wang C, Swerdloff R. Hypogonadism in the aging male diagnosis, potential benefits, and risks of testosterone replacement therapy. Int J Endocrinol 2012;2012:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Ronde W. Hyperandrogenism after transfer of topical testosterone gel: Case report and review of published and unpublished studies. Hum Reprod 2009;24:425–428. [DOI] [PubMed] [Google Scholar]
- 11. Wang C, Harnett M, Dobs AS, Swerdloff RS. Pharmacokinetics and safety of long‐acting testosterone undecanoate injections in hypogonadal men: An 84‐week phase III clinical trial. J Androl 2010;31:457–465. [DOI] [PubMed] [Google Scholar]
- 12. Finkle WD, Greenland S, Ridgeway GK, Adams JL, Frasco MA, Cook MB, Fraumeni JF Jr, Hoover RN. Increased risk of non‐fatal myocardial infarction following testosterone therapy prescription in men. PLoS ONE 2014;9:e85805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vigen R, O'Donnell CI, Baron AE, Grunwald GK, Maddox TM, Bradley SM, Barqawi A, Woning G, Wierman ME, Plomondon ME, Rumsfeld JS, Ho PM. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA 2013;310:1829–1836. [DOI] [PubMed] [Google Scholar]
- 14. Baillargeon J, Urban RJ, Kuo YF, Ottenbacher KJ, Raji MA, Du F, Lin YL, Goodwin JS. Risk of myocardial infarction in older men receiving testosterone therapy. Ann Pharmacother 2014;48:1138–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jones TH, Arver S, Behre HM, Buvat J, Meuleman E, Moncada I, Morales AM, Volterrani M, Yellowlees A, Howell JD, Channer KS, Investigators T. Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome (the TIMES2 study). Diabetes Care 2011;34:828–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shores MM, Matsumoto AM, Sloan KL, Kivlahan DR. Low serum testosterone and mortality in male veterans. Arch Intern Med 2006;166:1660–1665. [DOI] [PubMed] [Google Scholar]
- 17. Malkin CJ, Pugh PJ, Morris PD, Asif S, Jones TH, Channer KS. Low serum testosterone and increased mortality in men with coronary heart disease. Heart 2010;96:1821–1825. [DOI] [PubMed] [Google Scholar]
- 18. Mackey MA, Conway AJ, Handelsman DJ. Tolerability of intramuscular injections of testosterone ester in oil vehicle. Hum Reprod 1995;10:862–865. [DOI] [PubMed] [Google Scholar]
- 19. Sartorius G, Fennell C, Spasevska S, Turner L, Conway AJ, Handelsman DJ. Factors influencing time course of pain after depot oil intramuscular injection of testosterone undecanoate. Asian J Androl 2010;12:227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gill HS, Prausnitz MR. Does needle size matter? J Diabetes Sci Technol 2007;1:725–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dobs AS, Meikle AW, Arver S, Sanders SW, Caramelli KE, Mazer NA. Pharmacokinetics, efficacy, and safety of a permeation‐enhanced testosterone transdermal system in comparison with bi‐weekly injections of testosterone enanthate for the treatment of hypogonadal men. J Clin Endocrinol Metab 1999;84:3469–3478. [DOI] [PubMed] [Google Scholar]
- 22. Partsch CJ, Weinbauer GF, Fang R, Nieschlag E. Injectable testosterone undecanoate has more favourable pharmacokinetics and pharmacodynamics than testosterone enanthate. Eur J Endocrinol 1995;132:514–519. [DOI] [PubMed] [Google Scholar]
- 23. Yassin AA, Haffejee M. Testosterone depot injection in male hypogonadism: A critical appraisal. Clin Interv Aging 2007;2:577–590. [PMC free article] [PubMed] [Google Scholar]
- 24. Parker S, Armitage M. Experience with transdermal testosterone replacement therapy for hypogonadal men. Clin Endocrinol (Oxf) 1999;50:57–62. [DOI] [PubMed] [Google Scholar]
- 25. Mattern C, Hoffmann C, Morley JE, Badiu C. Testosterone supplementation for hypogonadal men by the nasal route. Aging Male 2008;11:171–178. [DOI] [PubMed] [Google Scholar]
- 26. Yin AY, Htun M, Swerdloff RS, Diaz‐Arjonilla M, Dudley RE, Faulkner S, Bross R, Leung A, Baravarian S, Hull L, Longstreth JA, Kulback S, Flippo G, Wang C. Reexamination of pharmacokinetics of oral testosterone undecanoate in hypogonadal men with a new self‐emulsifying formulation. J Androl 2012;33:190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shortridge EF, Polzer P, Donga P, Blanchette CM, Fang Y, Burudpakdee C, Carswell B. Experiences and treatment patterns of hypogonadal men in a U.S. health system. Int J Clin Pract 2014;68:257–263. [DOI] [PubMed] [Google Scholar]
- 28. Zhou G, Huang R, Roenker N, Li Y‐X. Quantification of testosterone and dihydrotestosterone in human plasma using qtrap 6500 systems. Paper presented at: 2013 AAPS Annual Meeting and Exposition; November 10–14, 2013; San Antonio, TX Poster M1365; 2013.
- 29. Zhu A, Lu T‐S, Snider E, Castro J, Li Y‐X. A sensitive and rapid uplc‐ms/ms quantification of estradiol in human plasma. Paper presented at: 2013 AAPS Annual Meeting and Exposition; November 10–14, 2013; San Antonio, TX Poster M1356; 2013.
- 30. Al‐Futaisi AM, Al‐Zakwani IS, Almahrezi AM, Morris D. Subcutaneous administration of testosterone. A pilot study report. Saudi Med J 2006;27:1843–1846. [PubMed] [Google Scholar]
- 31. Olshan JS, Spack NP, Eimicke T, Savage C, Morris AH, Dedekian MA, Spratt DI. Evaluation of the efficacy of subcutaneous administration of testosterone in female to male transexuals and hypogonadal males. Paper presented at: ENDO 2013; June 15–18, 2013; San Francisco, CA Poster MON‐594; 2013.
- 32. Olson J, Schrager SM, Clark LF, Dunlap SL, Belzer M. Subcutaneous testosterone: An effective delivery mechanism for masculinizing young transgender men. LGBT Health 2014;1:165–167. [DOI] [PubMed] [Google Scholar]
- 33. Amory JK, Anawalt BD, Bremner WJ, Matsumoto AM. Daily testosterone and gonadotropin levels are similar in azoospermic and nonazoospermic normal men administered weekly testosterone: Implications for male contraceptive development. J Androl 2001;22:1053–1060. [DOI] [PubMed] [Google Scholar]
- 34. Freundlich B, Kivitz A, Jaffe JS. Nearly pain‐free self‐administration of subcutaneous methotrexate with an autoinjector: Results of a phase 2 clinical trial in patients with rheumatoid arthritis who have functional limitations. J Clin Rheumatol 2014;20:256–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jin J, Sklar GE, Min Sen Oh V, Chuen Li S. Factors affecting therapeutic compliance: A review from the patient's perspective. Ther Clin Risk Manag 2008;4:269–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Subcutaneous testosterone enanthate autoinjector device. The autoinjector is designed to eject high‐viscosity solutions from a prefilled syringe fitted with a five‐eighths‐inch 27‐gauge needle
Table S1 List of investigators and study sites
Table S2 Summary of prior testosterone treatment
Table S3 Summary of screen failure patients


