Abstract
Infectious diseases are economically detrimental to aquaculture, and with continued expansion and intensification of aquaculture, the importance of managing infectious diseases will likely increase in the future. Here, we use evolution of virulence theory, along with examples, to identify aquaculture practices that might lead to the evolution of increased pathogen virulence. We identify eight practices common in aquaculture that theory predicts may favor evolution toward higher pathogen virulence. Four are related to intensive aquaculture operations, and four others are related specifically to infectious disease control. Our intention is to make aquaculture managers aware of these risks, such that with increased vigilance, they might be able to detect and prevent the emergence and spread of increasingly troublesome pathogen strains in the future.
Keywords: aquaculture, evolution of virulence, infectious diseases
Introduction
The emergence of highly virulent pathogens has devastated many food production industries, including examples such as Irish potato culture in the mid‐1800s and Taiwanese prawn culture in the 1980s (Bourke 1964; Lin 1989; Strange and Scott 2005). In aquaculture, infectious disease is already a substantial cause of economic loss (Meyer 1991). Given the rapid growth and dynamic nature of aquaculture worldwide (Food and Agricultural Organization of the United Nations 2014), it seems likely that even without evolution, epidemiological changes will lead to increases in the disease burden of aquaculture. Strong evidence, nevertheless, suggests that pathogen evolution, including evolution of virulence, is also playing a role in the emergence of some diseases in aquaculture (Walker and Winton 2010). Continued pathogen emergence is unavoidable as aquaculture intensifies. Here, we consider how current management practices may make aquaculture vulnerable to the evolutionary emergence of high virulence pathogen strains.
We define “virulence” as the deleterious health effects of pathogen infection on a host. As others have pointed out (Murray and Peeler 2005; Day and Prince 2007; Mennerat et al. 2010; Pulkkinen et al. 2010), aquaculture, like all farming industries, can create conditions that may favor the development of highly virulent pathogens. We survey various aquaculture practices that could lead to those conditions. Our discussion is grounded in the extensive body of theory that deals with evolution of virulence. This theory posits that pathogen virulence traits can evolve if these traits are directly or indirectly linked to pathogen fitness (Anderson and May 1982; Bull 1994; Ewald 1994; Read 1994; Ebert and Herre 1996; Frank 1996; Alizon et al. 2009; Brown et al. 2012; Cressler et al. 2015). For example, correlations between virulence and other aspects relating to pathogen fitness, such as transmission and replication rates, could drive virulence evolution. By studying how aquaculture practices alter pathogen ecology, insight can be gained into the likely direction of this evolution. Many basic predictions of this theory have been observed in biological systems. Nevertheless, the details of how virulence is linked to pathogen fitness matter, and so it is crucial to recognize that details are important (Cressler et al. 2015). Our discussion is thus intended to provoke thought rather than provide definitive predictions. Our goal is to draw attention to situations where vigilance may allow for the detection of troublesome evolutionary trajectories before they result in overly problematic pathogens.
To organize our discussion, we begin with practices related to intensive aquaculture operations that may have incidental impacts on the evolution of virulence. We then turn to aquaculture practices that are used specifically to control infectious disease in the short‐term that may facilitate pathogen virulence evolution in the long‐term.
Practices related to intensive aquaculture operations
Rearing at high densities
A large branch of evolution of virulence theory is based on an assumption, observed to be true in several systems (Fenner and Ratcliffe 1965; MacKinnon and Read 1999; de Roode et al. 2008; Atkins et al. 2011), that pathogen strains with high virulence tend to have higher transmission rates while hosts are alive than strains with low virulence. Nevertheless, high virulence strains tend to truncate infectious periods by killing their hosts, and so pathogen fitness may be evolutionarily optimal at intermediate virulence levels (Fig. 1). Under these assumptions, the strain that is most fit may change depending on the ecology of the host‐pathogen interaction.
Figure 1.
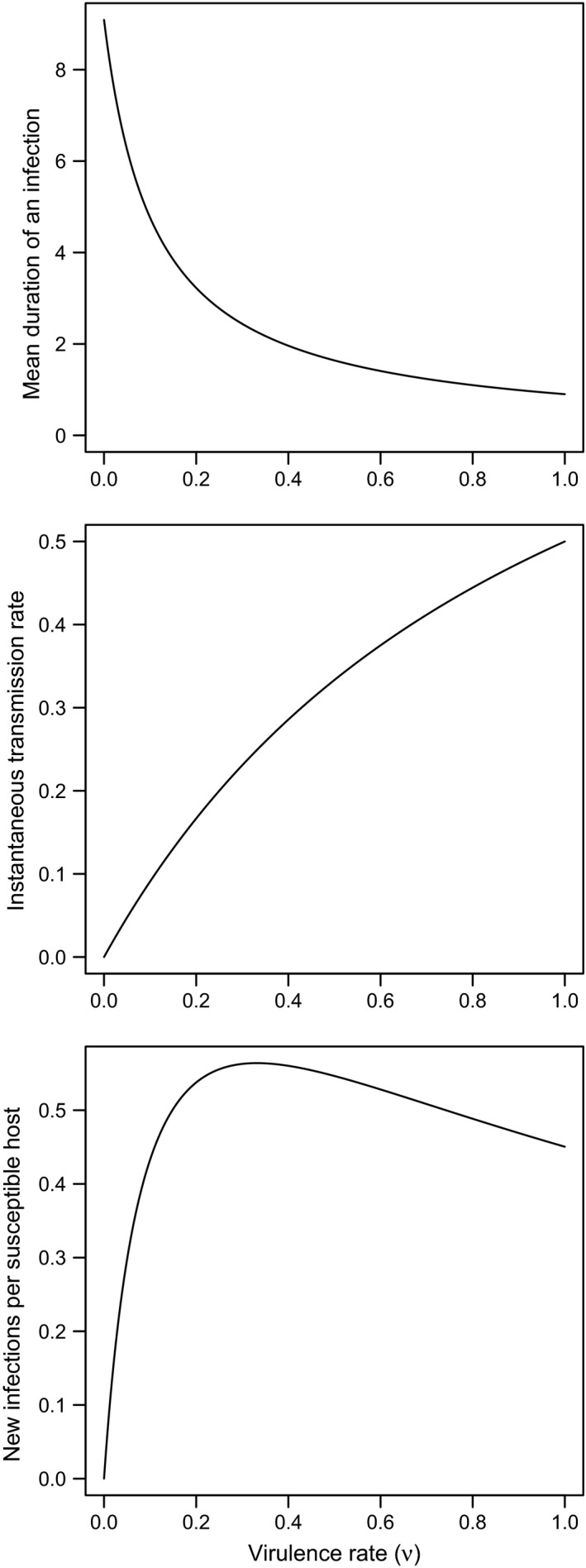
Illustration of a posited tradeoff between virulence and transmission. Virulence induced host mortality shortens the duration of an infection (top), while simultaneously increasing the instantaneous transmissibility of infection (middle). The tradeoff in these two components of pathogen fitness can generate situations where pathogen fitness is maximized at intermediate levels of virulence (bottom). Understanding how a management practice alters these curves is key to understanding how it might affect evolution of virulence, although other factors must also be considered. Mean infection duration above was calculated as the inverse of the sum of natural host mortality rate (μ), host recovery rate (γ), and virulence rate (ν). Instantaneous transmission rate was assumed to be ν/(1 + ν). New infections per susceptible host were calculated as the product of the mean infection duration and the instantaneous transmission rate. Above μ = 0.01 and γ = 0.1.
Disease modeling has demonstrated that the availability of susceptible hosts alters optimal virulence. This is because the fitness gain of increased infectiousness increases with the number of susceptible hosts, but the fitness cost of truncating infection does not (Day and Proulx 2004; Bolker et al. 2010; Borovkov et al. 2013). Thus, evolution of virulence theory predicts that increases in host densities can lead to evolutionary increases in virulence. Even in the absence of a tradeoff between infectiousness and virulence, high host densities can allow for the maintenance of pathogens that would otherwise kill hosts too quickly to persist (Anderson and May 1982).
Stocking density is a critical consideration in aquaculture to maximize productivity within constraints of space, water availability, and operating costs. The relationship between total productivity and rearing density is typically hump‐shaped (Refstie 1977; Holm et al. 1990; Zonneveld and Fadholi 1991; Hengsawat et al. 1997), because at very high densities, growth and survival are reduced due to stress and disease (Andrews et al. 1971). Nevertheless, rearing densities in aquaculture are almost always higher than in wild populations. For example, Rachycentron canadum (cobia), a marine fish of the order Perciformes, can be reared at densities as high as 30 kg/m3 with no loss of productivity in recirculating aquaculture systems (Riche et al. 2013), whereas in nature cobia are solitary or travel in small pods of 2–8 fish when not spawning (Shaffer and Nakamura 1989). Even fish that assemble at high density in the wild during spawning and after hatching only experience these densities for short periods of time. The consistently high rearing densities of aquaculture are thus novel environments for pathogens that could facilitate evolution of increased pathogen virulence.
Compression of rearing cycle
Evolution of virulence theory predicts that virulence levels depend on the natural lifespan of hosts, because virulence that results in a truncation of the infectious period of a host is more costly in long‐lived hosts than short‐lived hosts (Anderson and May 1982; Day 2002). Shortening the effective host lifespan, for example by compressing the rearing cycle duration, may thus favor evolution of increased pathogen virulence (May and Anderson 1983; Choo et al. 2003; Nidelet et al. 2009). This evolutionary mechanism may partially explain the increase in virulence observed in the chicken pathogen, Marek's disease virus, that co‐occurred with a compression of the chicken rearing cycle (Atkins et al. 2013). Consistent with the Marek's disease example, the pathogens that theory predicts are most likely to evolve higher virulence due to generational compression are those that can induce chronic, persistent infections with lifelong potential for pathogen transmission, such as the koi herpesvirus Cyprinid herpesvirus‐3 in koi and carp (Ilouze et al. 2011), infectious pancreatic necrosis virus in salmonids (Yamamoto 1975), and white spot syndrome virus in shrimp (Tsai et al. 1999).
Optimal harvest time is an important economic consideration in aquaculture, particularly in facilities where rearing can occur year round. To maximize profit, optimal cycle lengths are often intermediate values (Karp et al. 1986; Bjørndal 1988; Arnason 1992). However, tremendous improvements to aquaculture growth rates can be achieved through selective breeding (Gjedrem and Thodesen 2005; Gjedrem et al. 2012), and as growth rates increase, optimal cycle lengths are likely to decrease. Optimal cycle lengths are thus likely to decrease in the future, which may favor pathogen evolution toward increased virulence.
Use of broodstock with limited host genetic diversity
Pathogens that replicate quickly within their hosts, for example by evading detection by the immune system, are often assumed to be selectively favored, but high host genetic diversity is thought to mitigate this specialization (Lenski and Levin 1985; Ladle 1992; Ebert and Hamilton 1996; Jokela et al. 2009; Morran et al. 2011). For instance, serial passage of pathogens through one animal host type often results in increased virulence in the passage host and reduced virulence in other host types (Ebert 1998). When host populations have high genetic diversity, chains of pathogen transmission are likely to involve a diverse set of hosts, and so specialization on any single host genotype is unlikely. Host diversity might therefore prevent specialization, in turn mitigating pathogen virulence. Nevertheless, pathogen strains that specialize on low diversity populations may have high virulence in those populations, and low virulence in more genetically diverse wild populations, because of tradeoffs between generalism and specialism (Woolhouse et al. 2001; Gandon 2004; Garamszegi 2006; Poisot et al. 2011).
Aquaculture populations frequently have limited genetic diversity because of selective breeding, founder effects, and inbreeding in broodstock populations. Although breeding for traits beneficial to aquaculture, such as enhanced growth, disease resistance, and feed conversion, has the potential to greatly improve aquaculture production (Hershberger 1990; Gjedrem and Thodesen 2005), it may also result in a loss of heritable diversity (Whitt et al. 2002). Similarly, during broodstock formation, substantial diversity is often lost due to population bottlenecks and the subsequent domestication process (Hedrick et al. 2000; Perez‐Enriquez et al. 2009). Consequently, reduced genetic diversity has been observed across several aquaculture systems (Norris et al. 1999; Xu et al. 2001; Li et al. 2007).
Lack of host diversity is expected to drive virulence evolution in systems where there is variation in virulence across different host‐pathogen combinations, and such interactions have been observed in many aquatic and aquaculture systems. The presence of MHC diversity within aquatic finfish (Xia et al. 2002; Dionne et al. 2007), the observation that particular MHC alleles correlate with disease resistance in these systems (Palti et al. 2001; Xu et al. 2008; Dionne et al. 2009; Gómez et al. 2011), and the observation that MHC diversity increases with bacterial diversity in nature are indirect evidence of such interactions (Dionne et al. 2007). Direct evidence of specialization in hosts has also been observed within a single host species for Quahog Parasite Unknown (QPX) in clams (Dahl et al. 2008). It has also been observed between host species for infectious hematopoietic necrosis virus (IHNV) (Garver et al. 2006) and Gyrodactylus salaris (Bakke et al. 1990; Bakke 1991) in salmonids, and for viral hemorrhagic septicemia virus (VHSV) across five finfish species (Emmenegger et al. 2013). These observations suggest that for some aquatic pathogens, virulence is associated with specialization on particular hosts, and thus reduced host diversity could lead to the evolution of increased virulence as described above.
Accepting endemic disease in cultured populations
When endemic disease is maintained in a host population, pathogens have opportunities to adapt to the specifics of that situation. This might occur for example through specialization on a particular host species or lineage, on a particular host developmental stage, or on other factors such as water temperature. As pathogens become better adapted to replication in a particular setting, virulence in that setting will often increase for reasons similar to those described above relating to limited host genetic diversity.
Within aquaculture there are many diseases for which the cost of eradication is prohibitively expensive or control options are unavailable. Pathogen exchange between wild and cultured populations reared in close proximity can also make eradication of disease economically infeasible (Kurath and Winton 2011). In New York oyster and clam culture, seven protozoan parasites have been endemic since at least the 1970s (Meyers 1981). In Norway salmon culture, the disease infectious salmon anemia (ISA) has never been tolerated, but low virulence strains of the causative virus can be found by PCR in many production sites (Nylund et al. 2007; Lyngstad et al. 2011). In southern Idaho rainbow trout culture in the United States, IHNV has been endemic since the late 1970s (Wolf 1988). Phylogenetic analyses of IHNV have shown that the virus in this region has diverged into a new major genogroup with higher genetic diversity than the other genogroups (Troyer et al. 2000; Troyer and Kurath 2003). Consistent with theory, this phylogenetic divide is associated with host species specialization (Garver et al. 2006).
Specializing lineages can frequently become more virulent with serious downstream consequences. This is especially obvious following host species jumps, where evolution to higher virulence often occurs in the new host species. Consider for example, the fish rhabdoviruses IHNV and VHSV. These pathogens jumped host species several times, as evidenced by written descriptions of changes in host specificity and virulence of known pathogens, and in phylogenetic analyses of hundreds of field isolates (Kurath et al. 2003; Einer‐Jensen et al. 2004). These host jumps were followed by adaptation of the virus to the new host, resulting in increased virulence for the new host (Garver et al. 2006; Mochizuki et al. 2009; Kurath and Winton 2011). These examples all illustrate that tolerating low virulence infections or managing around the disease impacts of problematic pathogens may allow infectious agents to better adapt to local farming conditions, resulting in increased virulence.
Moreover, evolution of virulence theory predicts that pathogen competition in mixed infections can lead to evolution toward increased virulence. This is because the costs of virulence, such as truncating the infectious period by killing the host, are felt by both high and low virulence pathogen strains during co‐infection, but the benefits of virulence, such as fast pathogen growth and increased competitive ability, are likely to be experienced by only the more virulent strain (Bremermann and Pickering 1983; Nowak and May 1994). In several systems including the salmonid virus IHNV (Wargo et al. 2010; Wargo and Kurath 2011) and a mouse malaria model (de Roode et al. 2005; Bell et al. 2006), parasite competitive ability within hosts was positively correlated with parasite virulence. Accepting endemic disease increases opportunities for co‐infection, in turn potentially selecting for evolution of increased virulence.
Practices specific to control of infectious disease
Vaccination
Vaccines that protect hosts from disease symptoms, but allow for some level of pathogen infection and onward transmission can lead to the evolution of increased virulence (Gandon et al. 2001; MacKinnon and Read 2004; Gandon and Day 2008; MacKinnon et al. 2008). This may result in a decline in vaccine efficacy and more severe disease in unvaccinated individuals for two reasons. First, for vaccines that prevent host death but do not prevent infection or transmission, the infectious periods of highly virulent strains tend to be extended because infected hosts live longer. Second, pathogen traits that often correlate with virulence, such as immune suppression or rapid replication, may enhance pathogen fitness in vaccinated hosts. Patterns consistent with the first have been seen in Marek's disease (Witter 1997; Read et al. 2015) and infectious bursal disease in chickens (van den Berg 2000), and in feline calicivirus in cats (Radford et al. 2006). Patterns consistent with the second have been seen in experiments with a rodent malaria in laboratory mice (MacKinnon and Read 2004; Barclay et al. 2012).
Vaccination to control disease has been used successfully in finfish aquaculture for many decades (Gudding and Van Muiswinkel 2013), and vaccine use has increased substantially in recent years (Bravo and Midtlyng 2007). Commercial vaccines are available for many of the major aquatic diseases of finfish (Sommerset et al. 2005). Vaccination‐like strategies can also induce disease protection in crustaceans (reviewed in Johnson et al. 2008), and so development of vaccines for these systems is an active area of research (Teunissen et al. 1998; Witteveldt et al. 2004; Vaseeharan et al. 2006). In addition to commercially available vaccines, autogenous vaccines, defined as vaccines developed using a locally derived pathogen strain for application within a specific location, are also used in aquaculture (Toranzo et al. 2009). Most aquaculture vaccine development is focused on preventing disease symptoms that slow host growth or induce mortality, as opposed to preventing infection and transmission. Many aquaculture vaccines are thus precisely those that are predicted to prompt the evolution of more virulent strains. This evolution can lead both to waning vaccine efficacy, and to more severe disease in spillover populations, such as wild populations, or populations on neighboring farms in which vaccination is not being used.
Whether vaccines have already driven evolution of virulence in aquaculture is presently unknown. Nonetheless, vaccine‐associated pathogen change has been documented in at least one aquaculture pathogen, Yersinia ruckeri, the bacteria that causes enteric redmouth disease in salmonid fish. In this system, vaccine escape strains have evolved at least four separate times by the generation of a new biotype of Y. ruckeri that lacked flagella and was no longer sensitive to the immunity conferred by the vaccine (Welch et al. 2011). Whether this is a case of virulence evolution is inconclusive because although Fouz et al. (2006) found that a vaccine sensitive strain was less virulent than vaccine escape strains, Davies (1991) failed to find such a pattern with a larger sample of strains. Regardless, this system demonstrates that vaccination can lead to pathogen evolution in aquaculture. From the perspective of virulence evolution management, shifting the focus of aquaculture vaccine development from those that block disease to those that block infection may thus be beneficial.
Breeding for disease resistance
When disease resistance exists without completely blocking the potential for infection and transmission, pathogen evolution can occur in disease resistant hosts. Theory predicts that evolution of pathogens in disease resistant hosts can lead to the evolution of increased virulence (Fenner and Ratcliffe 1965; Fenner 1983; Gandon and Michalakis 2000; Ebert and Bull 2003) for the same reasons as listed above for vaccines. Indeed this pattern has been observed in plants (Thrall and Burdon 2003), rabbits (Fenner and Fantini 1999), and house finches (Hawley et al. 2013). Breeding for disease resistance in aquaculture populations may thus have important consequences on the evolution of pathogen virulence.
Selectively breeding for disease resistance has been used widely in aquaculture (Embody and Hayford 1925; Chevassus and Dorson 1990; Dorson et al. 1991; Gjedrem et al. 1991; Kirpichnikov et al. 1993; Dorson et al. 1995; Gjedrem 1997; Gjøen and Bentsen 1997; Argue et al. 2002; Nell and Hand 2003; Gitterle et al. 2005; Moss et al. 2005; Gitterle et al. 2006; Kettunen et al. 2007; Cock et al. 2009; Guo 2009; Lallias et al. 2009; Overturf et al. 2010; Purcell et al. 2010; Zhang et al. 2011; LaFrentz et al. 2012; Moss et al. 2012). For example, breeding for resistance to infectious pancreatic necrosis (IPN) has been particularly effective in salmonids in Norway where the number of IPN outbreaks has consistently declined in recent years (Hjeltnes 2014). In most cases, disease reduction has been the primary focus of these campaigns, with relatively less importance placed on whether selective breeding stops pathogen infection and onward transmission (for example, Quillet et al. 2007). Similar to the case of vaccination, the selective advantage of high virulence would likely be reduced if selective breeding programs were focused on preventing pathogen infection as opposed to reducing disease.
Chemotherapy
Chemotherapy, defined as the use of antibiotic drugs, might also select for the evolution of increased virulence if the mechanism that confers drug resistance is linked to virulence. For example, in experiments with the mouse malarial parasite, Plasmodium chabaudi, more virulent parasite strains were less sensitive to drug treatment than less virulent parasite strains (Schneider et al. 2008, 2012), potentially providing selective advantages to highly virulent strains during chemotherapy. Similarly, the highly studied bacterial plasmid, IncI1, found in both human and animal pathogenic bacterial species contains virulence factors, adhesion proteins and type IV pili systems, and a gene for beta‐lactamase resistance (Carattoli 2008; Garćıa‐Fernández et al. 2008) that simultaneously confers antibiotic resistance and high virulence. While several examples of antibiotic resistance have been reported in aquaculture systems (Miranda et al. 2013), to our knowledge, linkages between virulence and antibiotic resistance have yet to be identified in an aquaculture setting. Nevertheless, selection for increased virulence might also occur through a different route. In finfish aquaculture, the vast majority of chemotherapeutic drugs are administered orally as medicated feed (Burridge et al. 2010). By definition, high virulence pathogen strains cause severe infection, and one could speculate that the most severely affected fish would be those least likely to feed. By feeding less, these fish would be unlikely to receive adequate doses of drug, and high virulence might thus be selectively favored.
Chemotherapy is a valuable tool for the management of infectious diseases in aquaculture. Without eliminating use of chemotherapy, alternative ways to target antibiotics toward only those fish with the most severe disease symptoms might mitigate the evolutionary consequences of chemotherapy. We can speculate that the advantages of a targeted approach might be twofold. First, the overall strength of selection for drug resistance would be reduced, thus reducing the strength of indirect selection for increased virulence. Second, by targeting high virulence pathogen strains, low virulence strains would be selectively favored, potentially reducing and possibly reversing the direct selection for increased virulence. The practicality of employing a targeted chemotherapy approach, however, is an open question.
Reducing vertical transmission of pathogens
Whether pathogens are transmitted vertically, meaning from parent to offspring, or horizontally, meaning between conspecifics, is predicted to have important effects on the evolution of virulence. This is because new infections from a strictly vertically transmitted pathogen can only occur during host reproduction, and so a vertically transmitted pathogen that kills its host before reproduction could not persist, whereas an equally virulent horizontally transmitted pathogen may be able to (Ewald 1987; Lipsitch et al. 1996; Messenger et al. 1999). Thus, evolution of high virulence is unlikely for vertically transmitted pathogens.
Vertical transmission has been reduced in many types of aquaculture. For some pathogens, contamination on the surface of eggs can be reduced by submerging eggs in a chemical bath such as iodine for several minutes (McFadden 1969; Salvesen and Vadstein 1995). Pathogen contamination within eggs for intra‐ovum transmitted pathogens can sometimes be reduced by administration of antibiotics such as erythromycin treatment in broodstock during oogenesis (Klontz 1983; Evelyn et al. 1984; Lee and Evelyn 1994), or by the selective culling of eggs from pathogen‐positive broodstock (Munson et al. 2010). These methods are largely restricted to finfish rearing, but other methods are available to reduce vertical transmission in other systems, such as PCR screening to verify absence of pathogen in broodstock in the aquaculture of shrimp (Motte et al. 2003). As a result of these efforts, vertical transmission of some important pathogens has been greatly reduced. Most aquaculture pathogens that are transmitted vertically are also transmitted horizontally under favorable conditions. By reducing vertical transmission, the relative importance of horizontal transmission increases. Theory predicts that this may lead to virulence increases.
The importance of vertical transmission in maintaining low pathogen virulence may have already been observed in aquaculture. In Atlantic salmon culture in Norway, low virulence strains of ISAV appear to be pervasive (Nylund et al. 2007), but outbreaks of ISA disease caused by high virulence ISAV are relatively rare and sporadic. A proposed explanation for this pattern, consistent with phylogenetic data (Nylund et al. 2007), is that vertical transmission of ISAV favors the maintenance of low virulence strains in broodstock, but when fish are moved to marine production sites where fish densities are high, horizontal transmission becomes relatively more important, and high virulence strains sometimes emerge. A corollary to the impact of these practices is that by reducing vertical transmission, exposure to pathogen will likely occur at an older age. Since fish often develop increased disease resistance as they age and grow (Tatner 1997; Zapata et al. 2006), resistance to disease will be greater, and so these practices might influence virulence in much the same way as described above for vaccination or breeding for resistance. This consideration also suggests that reducing vertical transmission may thus favor evolution of higher virulence.
Conclusions
Mitigating infectious diseases is one of many challenges to aquaculture. We have identified several aquaculture practices that might drive evolution of virulence and thus alter future disease risk. This is particularly concerning because many wild and cultured populations co‐exist in the same geographic areas, and the potential for transmission between them is high (Kurath and Winton 2011). Ultimately, more research is needed to make conclusive statements about virulence evolution in aquaculture diseases and its impacts on both wild and aquaculture populations. Our hope is that this synthesis of theoretical predictions and observations from the practice of aquaculture may stimulate consideration of these ideas, future investigation, and where appropriate, development of potential mitigation strategies.
Although we focused our discussion on pathogen virulence, it is worth mentioning that many other pathogen traits in addition to virulence can evolve in aquaculture settings. We already mentioned evolution of resistance to antibiotics, or to vaccines, but other life history traits can evolve as well. For example, in the sea louse Lepeophtheirus salmonis, which has a tradeoff between mean egg diameter and total eggs produced in an egg string (Heuch et al. 2000), the evolutionarily optimal egg size might very well differ between wild and aquaculture populations for reasons unrelated to virulence on hosts. These traits could nevertheless impact disease severity. It is thus unreasonable to expect all evolutionary changes in disease severity to be explained by evolution of virulence theory alone.
For the sake of brevity, and because the evolution of reduced virulence is not troublesome, we have not discussed aquaculture practices that might drive evolution of pathogens toward decreased virulence. Nevertheless, theoretical arguments similar to those presented above can be used to predict that some aquaculture practices might lead to the evolution of decreased pathogen virulence. For example, culling strategies that selectively target diseased populations or individuals may favor low virulence pathogen strains over high virulence strains, thereby driving evolution toward reduced virulence. Such practices are not our focus here though, because they present no conflict between short‐term and long‐term costs.
In general, economic considerations in aquaculture tend to favor managing for reduced impacts of disease today rather than considering avoidance of potentially increased cost in the future. As a result, among aquaculture professionals the potential risks associated with evolution of virulence due to farm practices are not widely recognized. However, previous work on virulence evolution has revealed that changes to virulence can occur on the order of several years (Fenner and Ratcliffe 1965; Witter 1997; Hawley et al. 2013), a timescale that could be highly relevant to aquaculture professionals. Altering rearing practices in the interest of preventing pathogen evolution could potentially give a long‐term benefit with short‐term costs. Whether these costs would be acceptable to current aquaculture managers, however, is an open question that requires further study.
A great deal of virulence evolution theory is based on only a handful of case studies. Investigating whether aquaculture practices are driving virulence evolution could therefore also be a valuable source of case studies for fundamental questions arising from the theory, such as: (i) Will emerging pathogens become more or less virulent over time? (ii) Why does variation in virulence exist, and how does natural selection act on this variation? (iii) Do certain ecological patterns result in evolution of higher virulence, and if so can this evolution be prevented? With increasing interest and interaction between experts in virulence evolution, fish health, and aquaculture, there is potential to explore a broad range of concepts in virulence evolution theory, and this research could have direct economic relevance to aquaculture.
Acknowledgement
DAK was funded by the RAPIDD Program of the Science and Technology Directorate, Department of Homeland Security and Fogarty International Center, National Institutes of Health, and by the Institute of General Medical Sciences (R01GM105244), National Institutes of Health as part of the joint NSF‐NIH‐USDA Ecology and Evolution of Infectious Diseases Program. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We thank Tiffany Bogich, Mike Boots, Rachel Breyta, Marine Brieuc, Troy Day, Greg Dwyer, Gary Fornshell, Caroline Friedman, Kyle Garver, Alison Kell, Shannon Ladeau, Scott LaPatra, Jennie Lavine, Jamie Lloyd‐Smith, Kerry Naish, Kim Pepin and Mark Zwart for discussions that helped shape this manuscript.
Literature cited
- Alizon, S. , Hurford A., Mideo N., and van Baalen M. 2009. Virulence evolution and the trade‐off hypothesis: history, current state of affairs and the future. Journal of Evolutionary Biology 22:245–259. [DOI] [PubMed] [Google Scholar]
- Anderson, R. M. , and May R. M. 1982. Coevolution of hosts and parasites. Parasitology 85:411–426. [DOI] [PubMed] [Google Scholar]
- Andrews, J. W. , Knight L. H., Page J. W., Matsuda Y., and Brown E. E. 1971. Interactions of stocking density and water turnover on growth and food conversion of channel catfish reared in intensively stocked tanks. The Progressive Fish‐Culturist 33:197–203. [Google Scholar]
- Argue, B. J. , Arce S. M., Lotz J. M., and Moss S. M. 2002. Selective breeding of Pacific white shrimp (Litopenaeus vannamei) for growth and resistance to Taura Syndrome Virus. Aquaculture 204:447–460. [Google Scholar]
- Arnason, R. 1992. Optimal feeding schedules and harvesting time in aquaculture. Marine Resource Economics 7:15–35. [Google Scholar]
- Atkins, K. E. , Read A. F., Savill N. J., Renz K. G., Walkden‐Brown S. W., and Woolhouse M. E. J. 2011. Modelling Marek's disease virus (MDV) infection: parameter estimates for mortality rate and infectiousness. BMC Veterinary Research 7:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins, K. E. , Read A. F., Savill N. J., Renz K. G., Islam A. F. M., Walkden‐Brown S. W., and Woolhouse M. E. J. 2013. Vaccination and reduced cohort duration can drive virulence evolution: Marek's disease virus and industrialized agriculture. Evolution 67:851–860. [DOI] [PubMed] [Google Scholar]
- Bakke, T. A. 1991. A review of the inter‐and intraspecific variability in salmonid hosts to laboratory infections with Gyrodactylus salaris Malmberg. Aquaculture 98:303–310. [Google Scholar]
- Bakke, T. A. , Jansen P. A., and Hansen L. P. 1990. Differences in the host resistance of Atlantic salmon, Salmo salar L., stocks to the monogenean Gyrodactylus salaris Malmberg, 1957. Journal of Fish Biology 37:577–587. [Google Scholar]
- Barclay, V. C. , Sim D., Chan B. H., Nell L. A., Rabaa M. A., Bell A. S., Anders R. F. et al. 2012. The evolutionary consequences of blood‐stage vaccination on the rodent malaria Plasmodium chabaudi . PLoS Biology 10:e1001368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell, A. S. , Roode J. C., Sim D., and Read A. F. 2006. Within‐host competition in genetically diverse malaria infections: parasite virulence and competitive success. Evolution 60:1358–1371. [PubMed] [Google Scholar]
- van den Berg, T. P. 2000. Acute infectious bursal disease in poultry: a review. Avian Pathology 29:175–194. [DOI] [PubMed] [Google Scholar]
- Bjørndal, T. 1988. Optimal harvesting of farmed fish. Marine Resource Economics 5:139–159. [Google Scholar]
- Bolker, B. M. , Nanda A., and Shah D. 2010. Transient virulence of emerging pathogens. Journal of The Royal Society Interface 7:811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borovkov, K. , Day R., and Rice T. 2013. High host density favors greater virulence: a model of parasite–host dynamics based on multi‐type branching processes. Journal of Mathematical Biology 66:1123–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke, P. M. A. 1964. Emergence of potato blight, 1843–46. Nature 203:805–808. [Google Scholar]
- Bravo, S. , and Midtlyng P. J. 2007. The use of fish vaccines in the Chilean salmon industry 1999–2003. Aquaculture 270:36–42. [Google Scholar]
- Bremermann, H. J. , and Pickering J. 1983. A game‐theoretical model of parasite virulence. Journal of Theoretical Biology 100:411–426. [DOI] [PubMed] [Google Scholar]
- Brown, S. P. , Cornforth D. M., and Mideo N. 2012. Evolution of virulence in opportunistic pathogens: generalism, plasticity, and control. Trends in Microbiology 20:336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull, J. J. 1994. Perspective: virulence. Evolution 48:1423–1437. [DOI] [PubMed] [Google Scholar]
- Burridge, L. , Weis J. S., Cabello F., Pizarro J., and Bostick K. 2010. Chemical use in salmon aquaculture: a review of current practices and possible environmental effects. Aquaculture 306:7–23. [Google Scholar]
- Carattoli, A. 2008. Evolution of plasmids and evolution of virulence and antibiotic resistance plasmids In Baquero F., Nombela G., Cassel H., and Gutierrez J., eds. Evolutionary Biology of Bacterial and Fungal Pathogens, pp. 155–165. ASN Press, Washington, DC. [Google Scholar]
- Chevassus, B. , and Dorson M. 1990. Genetics of resistance to disease in fishes. Aquaculture 85:83–107. [Google Scholar]
- Choo, K. , Williams P. D., and Day T. 2003. Host mortality, predation and the evolution of parasite virulence. Ecology Letters 6:310–315. [Google Scholar]
- Cock, J. , Gitterle T., Salazar M., and Rye M. 2009. Breeding for disease resistance of Penaeid shrimps. Aquaculture 286:1–11. [Google Scholar]
- Cressler, C. E. , McLeod D. V., Rozins C., van den Hoogen J., and Day T. 2015. The adaptive evolution of virulence: a review of theoretical predictions and empirical tests. Parasitology. doi:10.1017/S003118201500092X [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl, S. F. , Perrigault M., and Allam B. 2008. Laboratory transmission studies of QPX disease in the hard clam: Interactions between different host strains and pathogen isolates. Aquaculture 280:64–70. [Google Scholar]
- Davies, R. L. 1991. Virulence and serum‐resistance in different clonal groups and serotypes of Yersinia ruckeri . Veterinary Microbiology 29:289–297. [DOI] [PubMed] [Google Scholar]
- Day, T. 2002. On the evolution of virulence and the relationship between various measures of mortality. Proceedings of the Royal Society of London. Series B: Biological Sciences 269:1317–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day, R. , and Prince J. 2007. A review of the effects on fishery stocks of pathogens released by aquaculture. Victorian Abalone Divers Association Inc, (VADA). Report to the Abalone Council of Australia.
- Day, T. , and Proulx S. R. 2004. A general theory for the evolutionary dynamics of virulence. The American Naturalist 163:E40–E63. [DOI] [PubMed] [Google Scholar]
- Dionne, M. , Miller K. M., Dodson J. J., Caron F., and Bernatchez L. 2007. Clinal variation in MHC diversity with temperature: evidence for the role of host–pathogen interaction on local adaptation in Atlantic salmon. Evolution 61:2154–2164. [DOI] [PubMed] [Google Scholar]
- Dionne, M. , Miller K. M., Dodson J. J., and Bernatchez L. 2009. MHC standing genetic variation and pathogen resistance in wild Atlantic salmon. Philosophical Transactions of the Royal Society B: Biological Sciences 364:1555–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorson, M. , Chevassus B., and Torhy C. 1991. Comparative susceptibility of three species of char and of rainbow trout X char triploid hybrids to several pathogenic salmonid viruses. Diseases of Aquatic Organisms 11:217–224. [Google Scholar]
- Dorson, M. , Quillet E., Hollebecq M. G., Torhy C., and Chevassus B. 1995. Selection of rainbow trout resistant to viral haemorrhagic septicaemia virus and transmission of resistance by gynogenesis. Veterinary Research 26:361–368. [PubMed] [Google Scholar]
- Ebert, D. 1998. Experimental evolution of parasites. Science 282:1432–1436. [DOI] [PubMed] [Google Scholar]
- Ebert, D. , and Bull J. J. 2003. Challenging the trade‐off model for the evolution of virulence: is virulence management feasible? Trends in Microbiology 11:15–20. [DOI] [PubMed] [Google Scholar]
- Ebert, D. , and Hamilton W. D. 1996. Sex against virulence: the coevolution of parasitic diseases. Trends in Ecology & Evolution 11:79–82. [DOI] [PubMed] [Google Scholar]
- Ebert, D. , and Herre E. A. 1996. The evolution of parasitic diseases. Parasitology Today 12:96–101. [DOI] [PubMed] [Google Scholar]
- Einer‐Jensen, K. , Ahrens P., Forsberg R., and Lorenzen N. 2004. Evolution of the fish rhabdovirus viral haemorrhagic septicaemia virus. Journal of General Virology 85:1167–1179. [DOI] [PubMed] [Google Scholar]
- Embody, G. C. , and Hayford C. O. 1925. The advantage of rearing brook trout fingerlings from selected breeders. Transactions of the American Fisheries Society 55:135–148. [Google Scholar]
- Emmenegger, E. J. , Moon C. H., Hershberger P. K., and Kurath G. 2013. Virulence of viral hemorrhagic septicemia virus (VHSV) genotypes Ia, IVa, IVb, IVc, in five fish species. Diseases of Aquatic Organisms 107:99–111. [DOI] [PubMed] [Google Scholar]
- Evelyn, T. P. T. , Ketcheson J. E., and Prosperi‐Porta L. 1984. Further evidence for the presence of Renibacterium salmoninarum in salmonid eggs and for the failure of povidone‐iodine to reduce the intra‐ovum infection rate in water‐hardened eggs. Journal of Fish Diseases 7:173–182. [Google Scholar]
- Ewald, P. W. 1987. Transmission modes and evolution of the parasitism‐mutualism continuum. Annals of the New York Academy of Sciences 503:295–306. [DOI] [PubMed] [Google Scholar]
- Ewald, P. W. 1994. Evolution of Infectious Disease. Oxford University Press, Oxford. [Google Scholar]
- Fenner, F. 1983. The Florey Lecture, 1983: biological control, as exemplified by smallpox eradication and myxomatosis. Proceedings of the Royal Society of London. Series B. Biological Sciences 218:259–285. [DOI] [PubMed] [Google Scholar]
- Fenner, F. , and Fantini B. 1999. Biological Control of Vertebrate Pests: The History of Myxomatosis, An Experiment in Evolution. CABI Publishing, Wallingford, UK. [Google Scholar]
- Fenner, F. , and Ratcliffe F. N. 1965. Myxomatosis. Cambridge University Press, Cambridge, UK. [Google Scholar]
- Food and Agricultural Organization of the United Nations 2014. The State of World Fisheries and Aquaculture: Opportunities and Challenges. FAO, Rome. [Google Scholar]
- Fouz, B. , Zarza C., and Amaro C. 2006. First description of nonmotile Yersinia ruckeri serovar I strains causing disease in rainbow trout, Oncorhynchus mykiss (Walbaum), cultured in Spain. Journal of Fish Diseases 29:339–346. [DOI] [PubMed] [Google Scholar]
- Frank, S. A. 1996. Models of parasite virulence. Quarterly Review of Biology 71:37–78. [DOI] [PubMed] [Google Scholar]
- Gandon, S. 2004. Evolution of multihost parasites. Evolution 58:455–469. [PubMed] [Google Scholar]
- Gandon, S. , and Day T. 2008. Evidences of parasite evolution after vaccination. Vaccine 26:C4–C7. [DOI] [PubMed] [Google Scholar]
- Gandon, S. , and Michalakis Y. 2000. Evolution of parasite virulence against qualitative or quantitative host resistance. Proceedings of the Royal Society of London. Series B: Biological Sciences 267:985–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandon, S. , MacKinnon M. J., Nee S., and Read A. F. 2001. Imperfect vaccines and the evolution of pathogen virulence. Nature 414:751–756. [DOI] [PubMed] [Google Scholar]
- Garamszegi, L. Z. 2006. The evolution of virulence and host specialization in malaria parasites of primates. Ecology Letters 9:933–940. [DOI] [PubMed] [Google Scholar]
- Garćıa‐Fernández, A. , Chiaretto G., Bertini A., Villa L., Fortini D., Ricci A., and Carattoli A. 2008. Multilocus sequence typing of IncI1 plasmids carrying extended‐spectrum β‐lactamases in Escherichia coli and Salmonella of human and animal origin. Journal of Antimicrobial Chemotherapy 61:1229–1233. [DOI] [PubMed] [Google Scholar]
- Garver, K. A. , Batts W. N., and Kurath G. 2006. Virulence comparisons of infectious hematopoietic necrosis virus U and M genogroups in sockeye salmon and rainbow trout. Journal of Aquatic Animal Health 18:232–243. [DOI] [PubMed] [Google Scholar]
- Gitterle, T. , Salte R., Gjerde B., Cock J., Johansen H., Salazar M., Lozano C. et al. 2005. Genetic (co)variation in resistance to White Spot Syndrome Virus (WSSV) and harvest weight in Penaeus (Litopenaeus) vannamei . Aquaculture 246:139–149. [Google Scholar]
- Gitterle, T. , Gjerde B., Cock J., Salazar M., Rye M., Vidal O., Lozano C. et al. 2006. Optimization of experimental infection protocols for the estimation of genetic parameters of resistance to White Spot Syndrome Virus (WSSV) in Penaeus (Litopenaeus) vannamei . Aquaculture 261:501–509. [Google Scholar]
- Gjedrem, T. 1997. Selective breeding to improve aquaculture production. World Aquaculture 28:33–45. [Google Scholar]
- Gjedrem, T. , and Thodesen J. 2005. Selection In Gjedrem T., ed. Selection and Breeding Programs in Aquaculture, pp. 89–111. Springer, Dordrecht, The Netherlands. [Google Scholar]
- Gjedrem, T. , Salte R., and Gjøen H. M. 1991. Genetic variation in susceptibility of Atlantic salmon to furunculosis. Aquaculture 97:1–6. [Google Scholar]
- Gjedrem, T. , Robinson N., and Rye M. 2012. The importance of selective breeding in aquaculture to meet future demands for animal protein: a review. Aquaculture 350:117–129. [Google Scholar]
- Gjøen, H. M. , and Bentsen H. B. 1997. Past, present, and future of genetic improvement in salmon aquaculture. ICES Journal of Marine Science: Journal du Conseil 54:1009–1014. [Google Scholar]
- Gómez, D. , Conejeros P., Consuegra S., and Marshall S. H. 2011. MHC mediated resistance to Piscirickettsia salmonis in salmonids farmed in Chile. Aquaculture 318:15–19. [Google Scholar]
- Gudding, R. , and Van Muiswinkel W. B. 2013. A history of fish vaccination: science‐based disease prevention in aquaculture. Fish & Shellfish Immunology 35:1683–1688. [DOI] [PubMed] [Google Scholar]
- Guo, X. 2009. Use and exchange of genetic resources in molluscan aquaculture. Reviews in Aquaculture 1:251–259. [Google Scholar]
- Hawley, D. M. , Osnas E. E., Dobson A. P., Hochachka W. M., Ley D. H., and Dhondt A. A. 2013. Parallel patterns of increased virulence in a recently emerged wildlife pathogen. PLoS Biology 11:e1001570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick, P. W. , Dowling T. E., Minckley W. L., Tibbets C. A., Demarais B. D., and Marsh P. C. 2000. Establishing a captive broodstock for the endangered bonytail chub (Gila elegans). Journal of Heredity 91:35–39. [DOI] [PubMed] [Google Scholar]
- Hengsawat, K. , Ward F. J., and Jaruratjamorn P. 1997. The effect of stocking density on yield, growth and mortality of African catfish (Clarias gariepinus Burchell 1822) cultured in cages. Aquaculture 152:67–76. [Google Scholar]
- Hershberger, W. K. 1990. Selective breeding in aquaculture. Food Reviews International 6:359–372. [Google Scholar]
- Heuch, P. A. , Nordhagen J. R., and Schram T. A. 2000. Egg production in the salmon louse [Lepeophtheirus salmonis (Krøyer)] in relation to origin and water temperature. Aquaculture Research 31:805–814. [Google Scholar]
- Hjeltnes, B. 2014. Fish Health Report 2013. Nowegian Veterinary Institute, Oslo. [Google Scholar]
- Holm, J. C. , Refstie T., and Bø S. 1990. The effect of fish density and feeding regimes on individual growth rate and mortality in rainbow trout (Oncorhynchus mykiss). Aquaculture 89:225–232. [Google Scholar]
- Ilouze, M. , Davidovich M., Diamant A., Kotler M., and Dishon A. 2011. The outbreak of carp disease caused by CyHV‐3 as a model for new emerging viral diseases in aquaculture: a review. Ecological Research 26:885–892. [Google Scholar]
- Johnson, K. N. , van Hulten M. C. W., and Barnes A. C. 2008. “Vaccination” of shrimp against viral pathogens: phenomenology and underlying mechanisms. Vaccine 26:4885–4892. [DOI] [PubMed] [Google Scholar]
- Jokela, J. , Dybdahl M. F., and Lively C. M. 2009. The maintenance of sex, clonal dynamics, and host‐parasite coevolution in a mixed population of sexual and asexual snails. The American Naturalist 174:S43–S53. [DOI] [PubMed] [Google Scholar]
- Karp, L. , Sadeh A., and Griffin W. L. 1986. Cycles in agricultural production: the case of aquaculture. American Journal of Agricultural Economics 68:553–561. [Google Scholar]
- Kettunen, A. , Serenius T., and Fjalestad K. T. 2007. Three statistical approaches for genetic analysis of disease resistance to vibriosis in Atlantic cod (Gadus morhua L.). Journal of Animal Science 85:305–313. [DOI] [PubMed] [Google Scholar]
- Kirpichnikov, V. S. , Ilyasov J. I., Shart L. A., Vikhman A. A., Ganchenko M. V., Ostashevsky A. L., Simonov V. M. et al. 1993. Selection of Krasnodar common carp (Cyprinus carpio L.) for resistance to dropsy: principal results and prospects. Aquaculture 111:7–20. [Google Scholar]
- Klontz, G. W. 1983. Bacterial kidney disease in salmonids: an overview In Anderson D. P., and Dorson M., eds. Antigens of Fish Pathogens: Development and Production for Vaccines and Serodiagnostics, pp. 177–200. Collection Foundation Marcel Merieux, Lyon. [Google Scholar]
- Kurath, G. , and Winton J. 2011. Complex dynamics at the interface between wild and domestic viruses of finfish. Current Opinion in Virology 1:73–80. [DOI] [PubMed] [Google Scholar]
- Kurath, G. , Garver K. A., Troyer R. M., Emmenegger E. J., Einer‐Jensen K., and Anderson E. D. 2003. Phylogeography of infectious haematopoietic necrosis virus in North America. Journal of General Virology 84:803–814. [DOI] [PubMed] [Google Scholar]
- Ladle, R. J. 1992. Parasites and sex: catching the Red Queen. Trends in Ecology & Evolution 7:405–408. [DOI] [PubMed] [Google Scholar]
- LaFrentz, B. R. , Shoemaker C. A., Booth N. J., Peterson B. C., and Ourth D. D. 2012. Spleen index and mannose‐binding lectin levels in four channel catfish families exhibiting different susceptibilities to Flavobacterium columnare and Edwardsiella ictaluri . Journal of Aquatic Animal Health 24:141–147. [DOI] [PubMed] [Google Scholar]
- Lallias, D. , Gomez‐Raya L., Haley C., Arzul I., Heurtebise S., Beaumont A., Boudry P. et al. 2009. Combining two‐stage testing and interval mapping strategies to detect QTL for resistance to bonamiosis in the European flat oyster Ostrea edulis . Marine Biotechnology 11:570–584. [DOI] [PubMed] [Google Scholar]
- Lee, E. H. , and Evelyn T. P. T. 1994. Prevention of vertical transmission of the bacterial kidney disease agent Renibacterium salmoninarum by broodstock injection with erythromycin. Diseases of Aquatic Organisms 18:1–4. [Google Scholar]
- Lenski, R. E. , and Levin B. R. 1985. Constraints on the coevolution of bacteria and virulent phage: a model, some experiments, and predictions for natural communities. The American Naturalist 125:585–602. [Google Scholar]
- Li, Q. , Shu J., Yu R., and Tian C. 2007. Genetic variability of cultured populations of the Pacific abalone (Haliotis discus hannai Ino) in China based on microsatellites. Aquaculture Research 38:981–990. [Google Scholar]
- Lin, C. K. 1989. Prawn culture in Taiwan. What went wrong? World Aquaculture 20:19–20. [Google Scholar]
- Lipsitch, M. , Siller S., and Nowak M. A. 1996. The evolution of virulence in pathogens with vertical and horizontal transmission. Evolution 50:1729–1741. [DOI] [PubMed] [Google Scholar]
- Lyngstad, T. M. , Hjortaas M. J., Kristoffersen A. B., Markussen T., Karlsen E. T., Jonassen C. M., and Jansen P. A. 2011. Use of molecular epidemiology to trace transmission pathways for infectious salmon anaemia virus (ISAV) in Norwegian salmon farming. Epidemics 3:1–11. [DOI] [PubMed] [Google Scholar]
- MacKinnon, M. J. , and Read A. F. 1999. Genetic relationships between parasite virulence and transmission in the rodent malaria Plasmodium chabaudi . Evolution 53:689–703. [DOI] [PubMed] [Google Scholar]
- MacKinnon, M. J. , and Read A. F. 2004. Immunity promotes virulence evolution in a malaria model. PLoS Biology 2:e230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon, M. J. , Gandon S., and Read A. F. 2008. Virulence evolution in response to vaccination: the case of malaria. Vaccine 26:C42–C52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May, R. M. , and Anderson R. M. 1983. Epidemiology and genetics in the coevolution of parasites and hosts. Proceedings of the Royal Society of London. Series B: Biological Sciences 219:281–313. [DOI] [PubMed] [Google Scholar]
- McFadden, T. W. 1969. Effective disinfection of trout eggs to prevent egg transmission of Aeromonas liquefaciens . Journal of the Fisheries Board of Canada 26:2311–2318. [Google Scholar]
- Mennerat, A. , Nilsen F., Ebert D., and Skorping A. 2010. Intensive farming: evolutionary implications for parasites and pathogens. Evolutionary Biology 37:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messenger, S. L. , Molineux I. J., and Bull J. J. 1999. Virulence evolution in a virus obeys a trade off. Proceedings of the Royal Society of London. Series B: Biological Sciences 266:397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, F. P. 1991. Aquaculture disease and health management. Journal of Animal Science 69:4201–4208. [DOI] [PubMed] [Google Scholar]
- Meyers, T. R. 1981. Endemic diseases of cultured shellfish of Long Island, New York: adult and juvenile American oysters (Crassostrea virginica) and hard clams (Mercenaria mercenaria). Aquaculture 22:305–330. [Google Scholar]
- Miranda, C. D. , Tello A., and Keen P. L. 2013. Mechanisms of antimicrobial resistance in finfish aquaculture environments. Frontiers in Microbiology 4:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki, M. , Kim H. J., Kasai H., Nishizawa T., and Yoshimizu M. 2009. Virulence change of infectious hematopoietic necrosis virus against rainbow trout Oncorhynchus mykiss with viral molecular evolution. Fish Pathology 44:159–165. [Google Scholar]
- Morran, L. T. , Schmidt O. G., Gelarden I. A., Parrish R. C., and Lively C. M. 2011. Running with the Red Queen: host‐parasite coevolution selects for biparental sex. Science 333:216–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss, S. M. , Doyle R. W., and Lightner D. V. 2005. Breeding shrimp for disease resistance: challenges and opportunities for improvement In Walker P. J., Lester R. G., and Bondad‐Reantaso M. G., eds. Diseases in Asian Aquaculture V. Proceedings of the Fifth Symposium on Diseases in Asian Aquaculture, pp. 379–393. Asian Fisheries Society, Manila. [Google Scholar]
- Moss, S. M. , Moss D. R., Arce S. M., Lightner D. V., and Lotz J. M. 2012. The role of selective breeding and biosecurity in the prevention of disease in penaeid shrimp aquaculture. Journal of Invertebrate Pathology 110:247–250. [DOI] [PubMed] [Google Scholar]
- Motte, E. , Yugcha E., Luzardo J., Castro F., Leclercq G., Rodrıguez J., Miranda P. et al. 2003. Prevention of IHHNV vertical transmission in the white shrimp Litopenaeus vannamei . Aquaculture 219:57–70. [Google Scholar]
- Munson, A. D. , Elliott D. G., and Johnson K. 2010. Management of bacterial kidney disease in Chinook salmon hatcheries based on broodstock testing by enzyme‐linked immunosorbent assay: a multiyear study. North American Journal of Fisheries Management 30:940–955. [Google Scholar]
- Murray, A. G. , and Peeler E. J. 2005. A framework for understanding the potential for emerging diseases in aquaculture. Preventive Veterinary Medicine 67:223–235. [DOI] [PubMed] [Google Scholar]
- Nell, J. A. , and Hand R. E. 2003. Evaluation of the progeny of second‐generation Sydney rock oyster Saccostrea glomerata (Gould, 1850) breeding lines for resistance to QX disease Marteilia sydneyi . Aquaculture 228:27–35. [Google Scholar]
- Nidelet, T. , Koella J. C., and Kaltz O. 2009. Effects of shortened host life span on the evolution of parasite life history and virulence in a microbial host‐parasite system. BMC Evolutionary Biology 9:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris, A. T. , Bradley D. G., and Cunningham E. P. 1999. Microsatellite genetic variation between and within farmed and wild Atlantic salmon (Salmo salar) populations. Aquaculture 180:247–264. [Google Scholar]
- Nowak, M. A. , and May R. M. 1994. Superinfection and the evolution of parasite virulence. Proceedings of the Royal Society of London. Series B: Biological Sciences 255:81–89. [DOI] [PubMed] [Google Scholar]
- Nylund, A. , Plarre H., Karlsen M., Fridell F., Ottem K. F., Bratland A., and Saether P. A. 2007. Transmission of infectious salmon anaemia virus (ISAV) in farmed populations of Atlantic salmon (Salmo salar). Archives of Virology 152:151–179. [DOI] [PubMed] [Google Scholar]
- Overturf, K. , LaPatra S., Towner R., Campbell N., and Narum S. 2010. Relationships between growth and disease resistance in rainbow trout, Oncorhynchus mykiss (walbaum). Journal of Fish Diseases 33:321–329. [DOI] [PubMed] [Google Scholar]
- Palti, Y. , Nichols K. M., Waller K. I., Parsons J. E., and Thorgaard G. H. 2001. Association between DNA polymorphisms tightly linked to MHC class II genes and IHN virus resistance in backcrosses of rainbow and cutthroat trout. Aquaculture 194:283–289. [Google Scholar]
- Perez‐Enriquez, R. , Hernández‐Martınez F., and Cruz P. 2009. Genetic diversity status of White shrimp Penaeus (Litopenaeus) vannamei broodstock in Mexico. Aquaculture 297:44–50. [Google Scholar]
- Poisot, T. , Bever J. D., Nemri A., Thrall P. H., and Hochberg M. E. 2011. A conceptual framework for the evolution of ecological specialisation. Ecology Letters 14:841–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulkkinen, K. , Suomalainen L.‐R., Read A. F., Ebert D., Rintamäki P., and Valtonen E. T. 2010. Intensive fish farming and the evolution of pathogen virulence: the case of columnaris disease in Finland. Proceedings of the Royal Society B: Biological Sciences 277:593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell, M. K. , LaPatra S. E., Woodson J. C., Kurath G., and Winton J. R. 2010. Early viral replication and induced or constitutive immunity in rainbow trout families with differential resistance to Infectious hematopoietic necrosis virus (IHNV). Fish & Shellfish Immunology 28:98–105. [DOI] [PubMed] [Google Scholar]
- Quillet, E. , Dorson M., Le Guillou S., Benmansour A., and Boudinot P. 2007. Wide range of susceptibility to rhabdoviruses in homozygous clones of rainbow trout. Fish & Shellfish Immunology 22:510–519. [DOI] [PubMed] [Google Scholar]
- Radford, A. D. , Dawson S., Coyne K. P., Porter C. J., and Gaskell R. M. 2006. The challenge for the next generation of feline calicivirus vaccines. Veterinary Microbiology 117:14–18. [DOI] [PubMed] [Google Scholar]
- Read, A. F. 1994. The evolution of virulence. Trends in Microbiology 2:73–76. [DOI] [PubMed] [Google Scholar]
- Read, A. F. , Baigent S. J., Powers C., Kgosana L. B., Blackwell L., Smith L. P., Kennedy D. A. et al. 2015. Imperfect vaccination can enhance the transmission of highly virulent pathogens. PLoS Biology 13:e1002198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refstie, T. 1977. Effect of density on growth and survival of rainbow trout. Aquaculture 11:329–334. [Google Scholar]
- Riche, M. A. , Weirich C. R., Wills P. S., and Baptiste R. M. 2013. Stocking density effects on production characteristics and body composition of market size cobia, Rachycentron canadum, reared in recirculating aquaculture systems. Journal of the World Aquaculture Society 44:259–266. [Google Scholar]
- de Roode, J. C. , Pansini R., Cheesman S. J., Helinski M. E. H., Huijben S., Wargo A. R., Bell A. S. et al. 2005. Virulence and competitive ability in genetically diverse malaria infections. Proceedings of the National Academy of Sciences 102:7624–7628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Roode, J. C. , Yates A. J., and Altizer S. 2008. Virulence‐transmission tradeoffs and population divergence in virulence in a naturally occurring butterfly parasite. Proceedings of the National Academy of Sciences of the United States of America 105:7489–7494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvesen, I. , and Vadstein O. 1995. Surface disinfection of eggs from marine fish: evaluation of four chemicals. Aquaculture International 3:155–171. [Google Scholar]
- Schneider, P. , Chan B. H. K., Reece S. E., and Read A. F. 2008. Does the drug sensitivity of malaria parasites depend on their virulence? Malaria Journal 7:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider, P. , Bell A. S., Sim D. G., O'Donnell A. J., Blanford S., Paaijmans K. P., Read A. F. et al. 2012. Virulence, drug sensitivity and transmission success in the rodent malaria, Plasmodium chabaudi . Proceedings of the Royal Society B: Biological Sciences 279:4677–4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer, R. V. , and Nakamura E. L. 1989. Synopsis of biological data on the cobia Rachycentron canadum (Pisces: Rachycentridae). Technical report, FAO Fisheries Synopsis. 153, National Marine Fisheries Service, Washington, DC. [Google Scholar]
- Sommerset, I. , Krossøy B., Biering E., and Frost P. 2005. Vaccines for fish in aquaculture. Expert Review of Vaccines 4:89–101. [DOI] [PubMed] [Google Scholar]
- Strange, R. N. , and Scott P. R. 2005. Plant disease: a threat to global food security. Phytopathology 43:83–116. [DOI] [PubMed] [Google Scholar]
- Tatner, M. F. 1997. 6 natural changes in the immune system of fish. Fish Physiology 15:255–287. [Google Scholar]
- Teunissen, O. S. P. , Faber R., Booms G. H. R., Latscha T., and Boon J. H. 1998. Influence of vaccination on vibriosis resistance of the giant black tiger shrimp Penaeus monodon (Fabricius). Aquaculture 164:359–366. [Google Scholar]
- Thrall, P. H. , and Burdon J. J. 2003. Evolution of virulence in a plant host‐pathogen metapopulation. Science 299:1735–1737. [DOI] [PubMed] [Google Scholar]
- Toranzo, A. E. , Romalde J. L., Magarin˜os B., and Barja J. L. 2009. Present and future of aquaculture vaccines against fish bacterial diseases In Rogers C., and Basurco B., eds. The Use of Veterinary Drugs and Vaccines in Mediterranean Aquaculture, pp. 155–176. CIHEAM, Zaragoza. [Google Scholar]
- Troyer, R. M. , and Kurath G. 2003. Molecular epidemiology of infectious hematopoietic necrosis virus reveals complex virus traffic and evolution within southern Idaho aquaculture. Diseases of Aquatic Organisms 55:175–185. [DOI] [PubMed] [Google Scholar]
- Troyer, R. M. , LaPatra S. E., and Kurath G. 2000. Genetic analyses reveal unusually high diversity of infectious haematopoietic necrosis virus in rainbow trout aquaculture. Journal of General Virology 81:2823–2832. [DOI] [PubMed] [Google Scholar]
- Tsai, M. F. , Kou G. H., Liu H. C., Liu K. F., Chang C. F., Peng S. E., Hsu H. C. et al. 1999. Long‐term presence of white spot syndrome virus (WSSV) in a cultivated shrimp population without disease outbreaks. Diseases of Aquatic Organisms 38:107–114. [Google Scholar]
- Vaseeharan, B. , Prem Anand T., Murugan T., and Chen J. C. 2006. Shrimp vaccination trials with the VP292 protein of white spot syndrome virus. Letters in Applied Microbiology 43:137–142. [DOI] [PubMed] [Google Scholar]
- Walker, P. J. , and Winton J. R. 2010. Emerging viral diseases of fish and shrimp. Veterinary Research 41:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wargo, A. R. , and Kurath G. 2011. In vivo fitness associated with high virulence in a vertebrate virus is a complex trait regulated by host entry, replication, and shedding. Journal of Virology 85:3959–3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wargo, A. R. , Garver K. A., and Kurath G. 2010. Virulence correlates with fitness in vivo for two M group genotypes of Infectious hematopoietic necrosis virus (IHNV). Virology 404:51–58. [DOI] [PubMed] [Google Scholar]
- Welch, T. J. , Verner‐Jeffreys D. W., Dalsgaard I., Wiklund T., Evenhuis J. P., Cabrera J. A. G., Hinshaw J. M. et al. 2011. Independent emergence of Yersinia ruckeri biotype 2 in the United States and Europe. Applied and Environmental Microbiology 77:3493–3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitt, S. R. , Wilson L. M., Tenaillon M. I., Gaut B. S., and Buckler E. S. 2002. Genetic diversity and selection in the maize starch pathway. Proceedings of the National Academy of Sciences of the United States of America 99:12959–12962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter, R. L. 1997. Increased virulence of Marek's disease virus field isolates. Avian Diseases 41:149–163. [PubMed] [Google Scholar]
- Witteveldt, J. , Vlak J. M., and van Hulten M. C. W. 2004. Protection of Penaeus monodon against white spot syndrome virus using a WSSV subunit vaccine. Fish & Shellfish Immunology 16:571–579. [DOI] [PubMed] [Google Scholar]
- Wolf, K. 1988. Fish Viruses and Fish Viral Diseases. Cornell University Press, Ithaca, NY. [Google Scholar]
- Woolhouse, M. E. J. , Taylor L. H., and Haydon D. T. 2001. Population biology of multihost pathogens. Science 292:1109–1112. [DOI] [PubMed] [Google Scholar]
- Xia, C. , Kiryu I., Dijkstra J. M., Azuma T., Nakanishi T., and Ototake M. 2002. Differences in MHC class I genes between strains of rainbow trout (Oncorhynchus mykiss). Fish & Shellfish Immunology 12:287–301. [DOI] [PubMed] [Google Scholar]
- Xu, Z. , Primavera J. H., de la Pena L. D., Pettit P., Belak J., and Alcivar‐Warren A. 2001. Genetic diversity of wild and cultured Black Tiger Shrimp (Penaeus monodon) in the Philippines using microsatellites. Aquaculture 199:13–40. [Google Scholar]
- Xu, T. J. , Chen S. L., Ji X. S., and Tian Y. S. 2008. MHC polymorphism and disease resistance to Vibrio anguillarum in 12 selective Japanese flounder (Paralichthys olivaceus) families. Fish & Shellfish Immunology 25:213–221. [DOI] [PubMed] [Google Scholar]
- Yamamoto, T. 1975. Frequency of detection and survival of infectious pancreatic necrosis virus in a carrier population of brook trout (Salvelinus fontinalis) in a lake. Journal of the Fisheries Board of Canada 32:568–570. [Google Scholar]
- Zapata, A. , Diez B., Cejalvo T., Gutierrez‐de Frias C., and Cortes A. 2006. Ontogeny of the immune system of fish. Fish & Shellfish Immunology 20:126–136. [DOI] [PubMed] [Google Scholar]
- Zhang, Z. , Niu C., Storset A., Bøgwald J., and Dalmo R. A. 2011. Comparison of Aeromonas salmonicida resistant and susceptible salmon families: a high immune response is beneficial for the survival against Aeromonas salmonicida challenge. Fish & Shellfish Immunology 31:1–9. [DOI] [PubMed] [Google Scholar]
- Zonneveld, N. , and Fadholi R. 1991. Feed intake and growth of red tilapia at different stocking densities in ponds in Indonesia. Aquaculture 99:83–94. [Google Scholar]


