Abstract
Decoupling of the upper jaw bones—jaw kinesis—is a distinctive feature of the ray-finned fishes, but it is not clear how the innovation is related to the extraordinary diversity of feeding behaviours and feeding ecology in this group. We address this issue in a lineage of ray-finned fishes that is well known for its ecological and functional diversity—African rift lake cichlids. We sequenced ultraconserved elements to generate a phylogenomic tree of the Lake Tanganyika and Lake Malawi cichlid radiations. We filmed a diverse array of over 50 cichlid species capturing live prey and quantified the extent of jaw kinesis in the premaxillary and maxillary bones. Our combination of phylogenomic and kinematic data reveals a strong association between biting modes of feeding and reduced jaw kinesis, suggesting that the contrasting demands of biting and suction feeding have strongly influenced cranial evolution in both cichlid radiations.
Keywords: evolutionary innovation, kinematics, ultraconserved elements
1. Introduction
The history of life on earth has periodically involved major evolutionary transitions, such as the evolution of multicellularity, flight and active predation [1–3]. To understand how these transitions have shaped evolutionary history, it is important to understand both their phenotypic basis and ecological consequences. However, many major transitions occurred only a few times in evolutionary history [4], which makes it challenging to draw strong conclusions regarding function, particularly if multiple traits changed at once across a transition [5]. Additionally, many important innovations evolved far in the past, complicating our ability to infer their functional benefits, because both the innovating clade and its sister lineage may have changed during the intervening time for reasons unrelated to the transition of interest [6].
Sometimes, these issues can be surmounted by studying species flocks that have recently undergone an adaptive radiation [7]. These radiations can contain multiple transitions to new niches, improving the power to detect trait associations using phylogenetic comparative methods. Additionally, because these radiations are often relatively young, two divergent sister clades are less likely to have changed for reasons unrelated to the transition of interest.
Here, we focus on transitions between the two dominant feeding modes in vertebrates, biting and suction feeding. Each feeding mode imposes contrasting biomechanical demands on the trophic apparatus. When a predator bites its prey, force is applied to the prey directly via the predator's jaws, creating corresponding stress on the jaws themselves. By contrast, suction feeders create a pulse of subambient pressure by rapidly expanding the mouth cavity to pull water and the prey item directly into the mouth, often without touching the predator's jaws [8]. Transitions between biting and suction feeding have occurred in nearly every major group of aquatic vertebrates, with an especially large number of transitions having occurred within the ray-finned fishes [9].
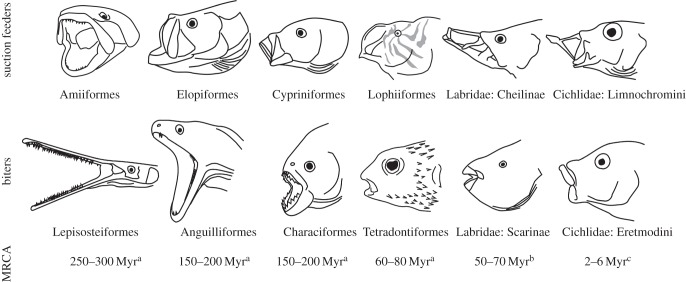
The ray-finned fishes are distinctive among vertebrates by virtue of an extremely large number of mobile bony elements in the skull, especially in the jaws. One particularly novel jaw feature involves decoupling of the upper jaw bones [10]. Some fishes and most tetrapods possess premaxillary and maxillary bones that are firmly connected to each other and to other skull bones, but in most ray-finned fishes, these bones show a range of mobility, and are more loosely connected by ligaments [11]. This configuration allows for novel movements and linkages not possible with an immobile jaw and may help to explain the extraordinary diversity seen in ray-finned fish skulls (figure 1). Most ray-finned fishes possess a movable maxilla, and a movable premaxilla has evolved independently at least five times in this group, including in Acanthomorpha and Cypriniformes, and is found in over half of all living species of ray fins [9].
Figure 1.
Iconic suction feeders and biters across the ray-finned fishes, plus one of the oldest biting–suction feeding cichlid comparisons used in this study. Each comparison is reciprocally monophyletic, with age estimates for the most recent common ancestor derived from the 95% posterior density estimates of node age from a[12], b[13] and c[14]. Note the maxillary kinesis present in all six suction feeding clades and the premaxillary kinesis present in Cypriniformes, Lophiiformes, cheiline labrids and limnochromine cichlids.
Because the velocity of suction flow degrades exponentially from the mouth opening, it is often beneficial for a suction feeder to project its mouth as close to the prey as possible. Mobile upper jaw bones allow for the formation of a planar mouth opening, which allows for a more efficient and focused flow field [15], and mobile upper jaws are more easily projected towards prey, increasing fluid acceleration and reducing the window in which the prey can escape [16]. However, increased upper jaw kinesis may make the decoupled jaw bones less robust, reducing their ability to withstand stresses such as those produced by biting [17,18]. While the above evidence does suggest that jaw kinesis is important for suction feeding, it is currently unclear whether these patterns suggested by simulation studies relate to patterns of ecological divergence in the ray-finned fishes.
The cichlid radiations of East Africa's Rift Valley [19,20] offer us an excellent system to test the phenotypic consequences of transitions between suction feeding and biting because of the huge variety of trophic strategies within each lake. Many of these cichlids use suction feeding strategies to capture plankton, buried invertebrates and other fishes, whereas other cichlids use biting strategies to scrape algae, pick external parasites from other fishes or gouge scales from the sides of other cichlids. Although these cichlid radiations offer us a unique system in which to examine feeding mode transitions, the lack of well-resolved phylogenetic trees has impeded efforts to confidently estimate macroevolutionary patterns across these radiations [21–27].
Here, we use a phylogeny inferred from ultraconserved elements [28,29], measures of jaw kinesis derived from a large dataset of cichlid feeding kinematics, and feeding mode information to test whether changes in jaw kinesis are strongly associated with transitions in feeding mode across these two classic examples of adaptive radiation.
2. Methods
(a). Feeding kinematics
We obtained 96 individuals of 56 cichlid species (electronic supplementary material, table S1) and classified each species as a biter or a non-biter based on published field observations (electronic supplementary material). Species were defined as biting if their foraging mode requires direct contact between the fish's oral jaws and its prey (electronic supplementary material, table S2 and Supplementary Methods). We filmed each species capturing live evasive Culex larvae at 2000 frames per second and gathered at least eight kinematic sequences per individual, in order to calculate landmark positions on the premaxilla and maxilla (figure 2). We provide more detail on live animal work in the electronic supplementary material, Supplementary Methods. After filming, we euthanized fish with an overdose of MS-222 and preserved tissue samples in ethanol prior to DNA extraction.
Figure 2.

Landmarks used for quantifying premaxillary and maxillary kinesis, shown using a suction feeding Lepidiolamprologus lemairii from Lake Tanganyika: (1) distal tip of the arm of the maxilla (1), (2) joint between the articular and the quadrate, (3) anterior-most tip of the premaxilla and (4) proximal tip of the maxilla. Points 1 and 2 remain stable during cranial elevation; points 3 and 4 are affected by premaxillary and maxillary kinesis, respectively.
(b). Phylogenomics
To prepare and enrich ultraconserved element (UCE) libraries, we followed modified versions (available from http://ultraconserved.org) of the enrichment approach initially described in [28,29]. Following UCE identification, alignment and alignment trimming steps, we created two datasets: one that was 95% complete for the 56 species (939 loci) and another that was 75% complete (1043 loci). We partitioned UCE loci with PartitionFinder [30], and inferred trees using ExaBayes v. 1.4.1 [31] and RAxML v. 8.0.19 [32] for both the 75% and 95% complete datasets with and without partitioning. For a subset of the comparative analyses, we converted these phylogenies into ultrametric trees via the ‘chronos’ function in the R package ‘ape’ [33]. We provide a detailed description of the phylogenetic data collection and analysis in the electronic supplementary material, Supplementary Methods.
(c). Comparative methods
We used stochastic character mapping to reconstruct a distribution of transitions in feeding mode across consensus trees for each lake radiation. To quantify jaw kinesis, we calculated the Procrustes distance, a common measure of shape difference [34], based on movement of multiple landmarks on the premaxilla, maxilla and skull between each closed-mouth/open-mouth pair of images (figure 2). We analysed jaw kinesis and feeding mode data on the ExaBayes consensus ultrametric phylogeny for each lake radiation with a phylogenetic ANOVA implemented in the ‘phytools’ R package [35]. We provide more detail on morphometric and comparative methods in the electronic supplementary material, Supplementary Methods.
3. Results
We produced a highly resolved phylogenomic tree for the 56 species of East African cichlids (figure 3). All nodal support values were above 0.99 posterior probability in the ExaBayes trees generated from both the 75% and 95% complete datasets, with and without partitioning (electronic supplementary material, figures S2–S3). Stochastic character mapping revealed that both Malawi and Tanganyika contain multiple transitions in feeding mode, with at least eight recovered in each simulation (electronic supplementary material, table S7). Our phylogenetic ANOVA revealed that biting species exhibit less jaw kinesis of both the premaxilla (p < 0.01) and the maxilla (p < 0.01) than non-biting taxa. This result was similar across the 75% and 95% complete datasets as well as for the partitioned and unpartitioned loci (electronic supplementary material, Supplementary Methods). Additionally, these results are not sensitive to how strictly we define a taxon as biting or suction feeding (electronic supplementary material, Supplementary Methods and table S2).
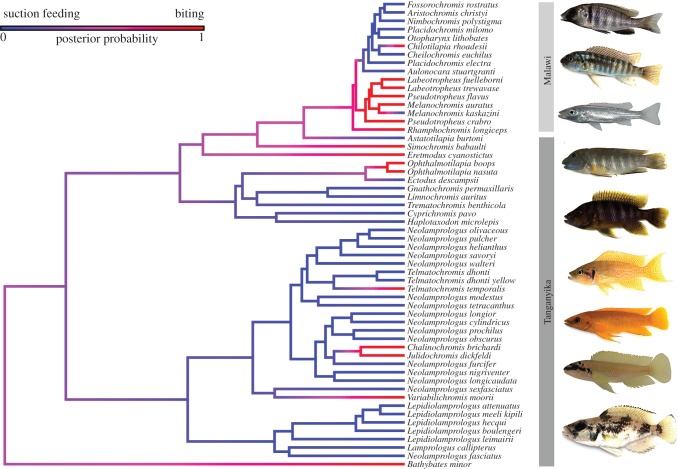
Figure 3.
Transitions between biting and suction feeding generated using stochastic character mapping on a phylogeny of 56 Tanganyika and Malawi cichlids. Individual cichlid images are, from top: Placidochromis electra (suction feeder), Labeotropheus trewavasae (biter), Rhamphochromis longiceps (biter), Eretmodus cyanostictus (biter), Trematochromis benthicola (suction feeder), Neolamprologus helianthus (suction feeder), Neolamprologus longior (suction feeder), Chalinochromis brichardi (biter), Lepidiolamprologus lemairii (suction feeder). Each node in this phylogeny is fully supported (posterior probability >0.99). (Online version in colour.)
4. Discussion
We provide, to our knowledge, the first strongly supported phylogenomic tree of all the Tanganyikan cichlid tribes as well as the major lineages within the Malawi cichlid radiation. Our phylogenetic and kinematic data show strong associations between feeding mode and the evolution of jaw kinesis within these species flocks (figure 3). We recover many transitions between biting and suction feeding across the phylogeny, suggesting that transitions in feeding mode are partially responsible for the large diversity of trophic traits within both radiations. Cichlids that transition to a biting strategy exhibit very little shape change in the upper jaw during the strike, whereas suction feeders exhibit more premaxillary protrusion and maxillary rotation.
(a). Resolution of major clades within the Tanganyika and Malawi cichlid radiations
Using ultraconserved elements, we recover unprecedented resolution within both the Lake Tanganyika and Lake Malawi cichlid radiations. Our results are the first, to our knowledge, to strongly support monophyly for a large clade of mouthbrooding Tanganyikan cichlid tribes which includes Perissodini, Cyprichromini, Limnochromini, Cyphotilapini and Ectodini (figure 3), resolving a century's worth of uncertainty [36]. In Lake Malawi, our UCE phylogenies strongly support monophyly of the ‘mbuna’ species flock, a clade of colourful rock-dwelling cichlids [37]. Both Lake Tanganyika and Lake Malawi comprise a wide array of species, and more sampling is required to conclusively establish evolutionary relationships among all species and genera within the radiation. However, our results suggest that targeted enrichment of UCE loci offers promise for resolving relationships among lineages in both lakes, most notably within the more recent Lake Malawi radiation where previous phylogenetic studies have shown limited ability to recover well-supported phylogenetic relationships [24,25].
(b). Feeding mode transitions across cichlids
Biting–suction feeding transitions occurred within every major tribe of Tanganyikan cichlids and occurred with particular frequency in the rock-dwelling lamprologines, which have independently transitioned to biting strategies at least three times in Chalinochromis, Telmatochromis and Variabilichromis. In Malawi, the split between the two largest species flocks, the mbuna and the non-mbuna ‘haps’, involves a transition between biting and suction feeding. There are a handful of suction feeding mbuna, like Cynotilapia, Abactochromis and some Melanochromis, as well as a few genera of non-mbuna that exhibit biting strategies, such as the scale-eating Docimodus and the herbivorous Hemitilapia [37], but each clade predominantly consists of either biters or suction feeders, respectively.
Suction feeding cichlids exhibit a strong pattern of enhanced premaxillary protrusion and maxillary rotation relative to biters across our dataset. We observed some of the highest Procrustes distances for premaxillary kinesis in the plankton feeder Cyprichromis pavo and the shrimp feeder Neolamprologus prochilus. These two species specialize on highly evasive prey like copepods and shrimp, which can rapidly escape from an incoming suction flow [16]. The enhanced premaxillary protrusion of these species probably allows them to rapidly project their jaws in front of a prey item, reducing the possibility of prey escape. We see some of the lowest Procrustes distances in rock-dwelling herbivorous taxa that feed primarily by algae scraping rather than by suction.
For maxillary kinesis, we observe high values for Cyprichromis pavo, but also for two fish-eating species, Aristochromis christyi and Lepidiolamprologus lemairii. Enhanced maxillary kinesis allows these species to produce large planar mouth opening, which enhances performance when capturing visually evasive prey like small fishes [38,39]. Our phylogenomic and kinematic data show that cichlid lineages which transition to biting behaviours rapidly reduce the amount of maxillary rotation. In some of these cichlids, such as Eretmodus cyanostictus (figure 1), this loss of maxillary kinesis has obviously compromised the fish's ability to form a planar mouth opening. Our results strongly suggest that maxillary rotation in the ray-finned fishes probably favoured more effective suction feeding.
(c). Evolution of fish feeding modes
Although it is widely assumed that the evolution of jaw kinesis in the ray-finned fishes was an innovation to enhance suction feeding performance, the lack of phylogenetic replication of this event across fishes has hindered direct tests of this hypothesis. Our results reveal that within the East African cichlid radiations, multiple transitions between biting and suction feeding have occurred, and that these functional transitions are accompanied by evolutionary reduction of jaw kinesis over short evolutionary timescales of 4 Myr or less (figure 3). These rapid and repeated shifts are consistent with the hypothesis that high jaw kinesis is selectively maintained in suction feeding fishes and provides evidence supporting upper jaw protrusion as a major evolutionary innovation for ray-finned fishes.
Our power to detect associations between jaw kinesis and transitions in feeding mode is directly connected to the sheer number of dietary transitions that have occurred in these cichlid radiations, highlighting the importance of cichlid adaptive radiations in the study of adaptation and speciation [20]. Other studies have examined divergence between biting and suction feeding fishes [17,40] but few have done so in a way that incorporates multiple recent transitions (but see [41]). A previous study of New World cichlids shows an association between enhanced jaw protrusion and foraging on evasive prey, though only some of the non-evasive prey specialists in that study were biting taxa [42]. It is also likely that our results reflect changes in the anterior four-bar linkage, which governs movements of the premaxilla and maxilla in cichlids and other fishes [43].
The differences we observe between biting and suction feeding cichlids mirror those seen in other fish groups, particularly the diversity of taxa inhabiting coral reefs. Many biting lineages, like tetraodontiform fishes, have re-evolved less-mobile premaxillae and maxillae, which contributes to the high observed bite forces in this group [44]. In the diverse fish family Labridae, biting specialists will often have relatively non-protrusible and robust jaws that do not form a circular opening when feeding, unlike suction feeding labrids [45].
One potential limitation of our study involves the fact that many fishes are known to modulate their kinematic patterns depending on the type of prey consumed [46], and we filmed all taxa consuming evasive midwater prey. Evasive midwater prey are appropriate for a ram-biter like Bathybates minor or Rhamphochromis longiceps, but they are not necessarily representative prey for a herbivore like many of our Lake Malawi mbuna or E. cyanostictus of Lake Tanganyika. However, if fish were modulating kinematics on midwater prey, then we would expect to see less of a difference between biters and suction feeders. The fact that we still observe a large difference suggests that our results are probably robust to modularity in strike kinematics.
Our results suggest that the study of rapid radiations is a critical component of biodiversity research. The rapid speciation and ecological transitions within these radiations can provide a framework to more rigorously examine the consequences of innovation. In our case, we observe a strong association between transitions in feeding mode and jaw kinesis evolution within cichlid lake radiations. The strong relationship we identify in these young radiations suggests that the biomechanical demands of suction feeding could have plausibly influenced the original evolution of maxillary kinesis in ray-finned fishes and the evolution of premaxillary protrusion in spiny-finned fishes.
Acknowledgements
We thank members of the American Cichlid Association for access to live cichlids, particularly R. Bireley, L DeMason, J. Ellenberger, P. Chin, S. Lundblad and D. Schumacher. We thank C.L. Berg, J.S. Chow, N. Cholst, K. Distor, S. Faiq, A. Goodman, M. Hymes, J. Lagarbo, K. Long, J.R. Reustle, D. Stirling, D. Turner and A. Yu for assistance filming fish.
Ethics
All maintenance and experimental procedures used in this research followed a protocol reviewed by the University of California, Davis Institutional Animal Care and Use Committee.
Authors' contributions
M.D.M. and M.E.A. designed the study. M.D.M. and S.R.B. collected kinematic data and tissue samples. J.Z. and B.C.F. performed laboratory work. B.C.F. analysed the molecular data. M.D.M. analysed the kinematic and comparative data. M.D.M. and B.C.F. wrote the manuscript, with comments from S.R.B., J.Z., C.D.H., P.C.W. and M.E.A. All authors reviewed the final version of the text.
Data accessibility
UCE raw read and contig data are available from NCBI SRA291316 (BioProject PRJNA293437) and NCBI GenBank (KT634321-KT692534). Kinematic data, R code, sequence assemblies, sequence alignments and phylogenetic trees are available from Dryad: http://dx.doi.org/10.5061/dryad.9pg8d.
Competing interests
We declare we have no competing interests.
Funding
Live animal work at UC Davis (IACUC 16956) was supported by NSF IOS-0924489, DEB-0717009, and DEB-061981 to P.C.W. Phylogenomics supported by UCLA Faculty Core Grant to M.E.A. NSF DEB-1242260 (to B.C.F.) supported computational portions (data processing) of this work. Portions of this research (data analysis) were conducted with high performance computing resources provided by Louisiana State University (http://www.hpc.lsu.edu).
References
- 1.Paul GS. 2002. Dinosaurs of the air: the evolution and loss of flight in dinosaurs and birds. Baltimore, MD: JHU Press. [Google Scholar]
- 2.Long JA, Gordon MS. 2004. The greatest step in vertebrate history: a paleobiological review of the fish-tetrapod transition. Phys. Biochem. Zool. 77, 700–719. ( 10.1086/425183) [DOI] [PubMed] [Google Scholar]
- 3.Kuratani S. 2004. Evolution of the vertebrate jaw: comparative embryology and molecular developmental biology reveal the factors behind evolutionary novelty. J. Anat. 205, 335–347. ( 10.1111/j.0021-8782.2004.00345.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vermeij GJ. 2006. Historical contingency and the purported uniqueness of evolutionary innovations. Proc. Natl Acad. Sci. USA 103, 1804–1809. ( 10.1073/pnas.0508724103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maddison WP, FitzJohn RG. 2015. The unsolved challenge to phylogenetic correlation tests for categorical characters. Syst. Biol. 64, 127–136. ( 10.1093/sysbio/syu070) [DOI] [PubMed] [Google Scholar]
- 6.Garland T, Adolph SC. 1994. Why not to do two-species comparative studies: limitations on inferring adaptation. Phys. Zool. 67, 797–828. [Google Scholar]
- 7.Schluter D. 2000. The ecology of adaptive radiation. Oxford, UK: Oxford University Press. [Google Scholar]
- 8.Wainwright P, Carroll AM, Collar DC, Day SW, Higham TE, Holzman RA. 2007. Suction feeding mechanics, performance, and diversity in fishes. Integr. Comp. Biol. 47, 96–106. ( 10.1093/icb/icm032) [DOI] [PubMed] [Google Scholar]
- 9.Wainwright PC, McGee MD, Longo SJ, Hernandez LP. 2015. Origins, innovations, and diversification of suction feeding in vertebrates. Integ. Comp. Biol. 55 , 134–145. ( 10.1093/icb/icv026) [DOI] [PubMed] [Google Scholar]
- 10.Westneat MW. 2004. Evolution of levers and linkages in the feeding mechanisms of fishes. Integr. Comp. Biol. 44, 378–389. ( 10.1093/icb/44.5.378) [DOI] [PubMed] [Google Scholar]
- 11.Gregory WK. 1993. Fish skulls: a study of the evolution of natural mechanisms. Trans. Am. Phil. Soc. 23, 75–481. [Google Scholar]
- 12.Near TJ, et al. 2013. Phylogeny and tempo of diversification in the superradiation of spiny-rayed fishes. Proc. Natl Acad. Sci. USA 110, 12 738–12 743. ( 10.1073/pnas.1304661110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kazancıoğlu E, Near TJ, Hanel R, Wainwright PC. 2009. Influence of sexual selection and feeding functional morphology on diversification rate of parrotfishes (Scaridae). Proc. R. Soc. B 276, 3439–3446. ( 10.1098/rspb.2009.0876) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman M, Keck BP, Dornburg A, Eytan RI, Martin CH, Hulsey CD, Wainwright PC, Near TJ. 2013. Molecular and fossil evidence place the origin of cichlid fishes long after Gondwanan rifting. Proc. R. Soc. B 280, 20131733 ( 10.1098/rspb.2013.1733) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skorczewski T, Cheer A, Wainwright PC. 2012. The benefits of planar circular mouths on suction feeding performance. J. R. Soc. Interface 9, 1767–1773. ( 10.1098/rsif.2011.0904) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holzman R, Day SW, Mehta RS, Wainwright PC. 2008. Jaw protrusion enhances forces exerted on prey by suction feeding fishes. J. R. Soc. Interface 5, 1445–1457. ( 10.1098/rsif.2008.0159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barel CDN. 1982. Towards a constructional morphology of cichlid fishes (Teleostei, Perciformes). Neth. J. Zool. 33, 357–424. ( 10.1163/002829683X00183) [DOI] [Google Scholar]
- 18.McGee MD, Wainwright PC. 2013. Sexual dimorphism in the feeding mechanism of threespine stickleback. J. Exp. Biol. 216, 835–840. ( 10.1242/jeb.074948) [DOI] [PubMed] [Google Scholar]
- 19.Fryer G, Iles TD. 1972. The cichlid fishes of the great lakes of Africa: their biology and evolution. Edinburgh, UK: Oliver and Boyd. [Google Scholar]
- 20.Seehausen O. 2006. African cichlid fish: a model system in adaptive radiation research. Proc. R. Soc. B 273, 1987–1998. ( 10.1098/rspb.2006.3539) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albertson RC, Markert JA, Danley PD, Kocher TD. 1999. Phylogeny of a rapidly evolving clade: the cichlid fishes of Lake Malawi, East Africa. Proc. Natl Acad. Sci. USA 96, 5107–5110. ( 10.1073/pnas.96.9.5107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schelly R, Salzburger W, Koblmüller S, Duftner N, Sturmbauer C. 2006. Phylogenetic relationships of the lamprologine cichlid genus Lepidiolamprologus (Teleostei: Perciformes) based on mitochondrial and nuclear sequences, suggesting introgressive hybridization. Mol. Phylogenet. Evol. 38, 426–438. ( 10.1016/j.ympev.2005.04.023) [DOI] [PubMed] [Google Scholar]
- 23.Sturmbauer C, Salzburger W, Duftner N, Schelly R, Koblmüller S. 2010. Evolutionary history of the Lake Tanganyika cichlid tribe Lamprologini (Teleostei: Perciformes) derived from mitochondrial and nuclear DNA data. Mol. Phylogenet. Evol. 57, 266–284. ( 10.1016/j.ympev.2010.06.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hulsey CD, Mims MC, Parnell NF, Streelman JT. 2010. Comparative rates of lower jaw diversification in cichlid adaptive radiations. J. Evol. Biol. 23, 1456–1467. ( 10.1111/j.1420-9101.2010.02004.x) [DOI] [PubMed] [Google Scholar]
- 25.Joyce DA, Lunt DH, Genner MJ, Turner GF, Bills R, Seehausen O. 2011. Repeated colonization and hybridization in Lake Malawi cichlids. Curr. Biol. 21, R108–R109. ( 10.1016/j.cub.2010.11.029) [DOI] [PubMed] [Google Scholar]
- 26.Genner MJ, Turner GF. 2012. Ancient hybridization and phenotypic novelty within Lake Malawi's cichlid fish radiation. Mol. Biol. Evol. 29, 195–206. ( 10.1093/molbev/msr183) [DOI] [PubMed] [Google Scholar]
- 27.Meyer BS, Matschiner M, Salzburger W. 2015. A tribal level phylogeny of Lake Tanganyika cichlid fishes based on a genomic multi-marker approach. Mol. Phylogenet. Evol. 83, 56–71. ( 10.1016/j.ympev.2014.10.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faircloth BC, Sorenson L, Santini F, Alfaro ME. 2013. A phylogenomic perspective on the radiation of ray-finned fishes based upon targeted sequencing of ultraconserved elements (UCEs). PLoS ONE 8, e65923 ( 10.1371/journal.pone.0065923) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faircloth BC, McCormack JE, Crawford NG, Harvey MG, Brumfield RT, Glenn TC. 2012. Ultraconserved elements anchor thousands of genetic markers spanning multiple evolutionary timescales. Syst. Biol. 61, 717–726. ( 10.1093/sysbio/sys004) [DOI] [PubMed] [Google Scholar]
- 30.Lanfear R, Calcott B, Ho SY, Guindon S. 2012. Partitionfinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol. Biol. Evol. 29, 1695–1701. ( 10.1093/molbev/mss020) [DOI] [PubMed] [Google Scholar]
- 31.Aberer AJ, Kobert K, Stamatakis A. 2014. ExaBayes: massively parallel Bayesian tree inference for the whole-genome era. Mol. Biol. Evol. 31, 2553–2556. ( 10.1093/molbev/msu236) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313. ( 10.1093/bioinformatics/btu033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paradis E, Claude J, Strimmer K. 2004. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290. ( 10.1093/bioinformatics/btg412) [DOI] [PubMed] [Google Scholar]
- 34.Zelditch ML, Sheets HD, Fink WL. 2003. The ontogenetic dynamics of shape disparity. Paleobiology 29, 139–156. () [DOI] [Google Scholar]
- 35.Revell LJ. 2012. Phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–223. ( 10.1111/j.2041-210X.2011.00169.x) [DOI] [Google Scholar]
- 36.Boulenger GA, Moore JE. 1898. Report on the collection of fishes made by Mr J. E. S. Moore in Lake Tanganyika during his expedition, 1895–1896. With an appendix by J. E. S. Moore. Trans. Zool. Soc. 15, 1–30. [Google Scholar]
- 37.Konings A. 2007. Malawi cichlids in their natural habitat, 4th edn El Paso, TX: Cichlid Press. [Google Scholar]
- 38.Holzman R, Collar DC, Mehta RS, Wainwright PC. 2012. An integrative modeling approach to elucidate suction-feeding performance. J. Exp. Biol. 215, 1–13. ( 10.1242/jeb.057851) [DOI] [PubMed] [Google Scholar]
- 39.Rasband WS. 1997. ImageJ. Bethesda, MD: US National Institutes of Health. [Google Scholar]
- 40.Alfaro ME, Janovetz J, Westneat MW. 2001. Motor control across trophic strategies: muscle activity of biting and suction feeding fishes. Am. Zool. 41, 1266–1279. ( 10.1093/icb/41.6.1266) [DOI] [Google Scholar]
- 41.Collar DC, Wainwright PC, Alfaro ME, Revell LJ, Mehta RS. 2014. Biting disrupts integration to spur skull evolution in eels. Nat. Commun. 5, 5505( 10.1038/ncomms6505) [DOI] [PubMed] [Google Scholar]
- 42.Hulsey CD, García de León FJ. 2005. Cichlid jaw mechanics: linking morphology to feeding specialization. Funct. Ecol. 19, 487–494. ( 10.1111/j.1365-2435.2005.00987.x) [DOI] [Google Scholar]
- 43.Westneat MW. 1990. Feeding mechanics of teleost fishes (Labridae; Perciformes): a test of four-bar linkage models. J. Morphol. 205, 269–295. ( 10.1002/jmor.1052050304) [DOI] [PubMed] [Google Scholar]
- 44.Korff WL, Wainwright PC. 2004. Motor pattern control for increasing crushing force in the striped burrfish (Chilomycterus schoepfi). Zoology 107, 335–346. ( 10.1016/j.zool.2004.09.001) [DOI] [PubMed] [Google Scholar]
- 45.Wainwright PC, Bellwood DR, Westneat MW, Grubich JR, Hoey AS. 2004. A functional morphospace for the skull of labrid fishes: patterns of diversity in a complex biomechanical system. Biol. J. Linn. Soc. 82, 1–25. ( 10.1111/j.1095-8312.2004.00313.x) [DOI] [Google Scholar]
- 46.van Wassenbergh S, De Rechter D. 2011. Piscivorous cyprinid fish modulates suction feeding kinematics to capture elusive prey. Zoology 114, 46–52. ( 10.1016/j.zool.2010.10.001) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
UCE raw read and contig data are available from NCBI SRA291316 (BioProject PRJNA293437) and NCBI GenBank (KT634321-KT692534). Kinematic data, R code, sequence assemblies, sequence alignments and phylogenetic trees are available from Dryad: http://dx.doi.org/10.5061/dryad.9pg8d.