Abstract
Numerous studies have documented declines in the abundance of reef-building corals over the last several decades and in some but not all cases, phase shifts to dominance by macroalgae have occurred. These assessments, however, often ignore the remainder of the benthos and thus provide limited information on the present-day structure and function of coral reef communities. Here, using an unprecedentedly large dataset collected within the last 10 years across 56 islands spanning five archipelagos in the central Pacific, we examine how benthic reef communities differ in the presence and absence of human populations. Using islands as replicates, we examine whether benthic community structure is associated with human habitation within and among archipelagos and across latitude. While there was no evidence for coral to macroalgal phase shifts across our dataset we did find that the majority of reefs on inhabited islands were dominated by fleshy non-reef-building organisms (turf algae, fleshy macroalgae and non-calcifying invertebrates). By contrast, benthic communities from uninhabited islands were more variable but in general supported more calcifiers and active reef builders (stony corals and crustose coralline algae). Our results suggest that cumulative human impacts across the central Pacific may be causing a reduction in the abundance of reef builders resulting in island scale phase shifts to dominance by fleshy organisms.
Keywords: human impact, community structure, macroalgae, turf algae, reef builders
1. Introduction
Over the last several decades, coral reef ecosystems have suffered significant impacts from both local and global stressors. Locally, overfishing, pollution, deforestation, sedimentation and coastal development are the key drivers of reef decline [1,2]. The extraction of important herbivores [2,3] and increases in inorganic nutrient concentrations can negatively affect coral abundance by promoting the success of faster growing organisms such as reef algae [4,5]. Global stressors such as rising seawater temperatures have caused large-scale coral bleaching events and subsequent mortality at numerous locations around the world [6]. Recent evidence suggests that coral growth and/or calcification rates may also be declining in response to ocean acidification [7]. Disease outbreaks have caused large coral mortality events in certain regions around the globe [8]. Cumulative human impact has led to progressive ‘flattening’ or loss of three-dimensional habitat on reefs across the Caribbean [9] and an increase in microbialization or a shift in productivity from macrobial to microbial organisms on reefs across the Pacific [10]. In most cases, there is evidence to suggest that anthropogenic activities result in either direct coral mortality or indirect losses in coral cover by promoting the abundance of coral competitors.
Several recent syntheses have documented significant declines in coral cover across different regions and ocean basins. In the Caribbean, coral cover has declined by an estimated 80% over three decades from an average of 50% in the 1970s to 10% cover in the early 2000s [11]. In the Pacific, estimates suggest that coral cover has declined from an average of 43% in the 1980s to 22% in 2003 [12]. More detailed assessments on the Great Barrier Reef show significant reductions in coral cover across the whole system but larger declines (approx. 40% loss) on inshore reefs closer to human populations [13]. There is general consensus that most well-studied coral populations situated close to large human populations around the tropics have suffered significant losses in recent decades (and probably over much longer time scales) owing to historic human impacts [1].
With a human footprint evident on most coral reefs that have been well studied, it is difficult to determine what is or was a natural baseline state for these ecosystems [14]. A common metric for documenting reef ‘health’ is some measure of reef-building coral abundance (per cent cover, colony number, size-frequency distribution) where sites with higher coral cover are generally indicative of ‘healthier’ reefs. Early accounts of reef systems before they experienced the degree of degradation seen today suggest reef-building corals regularly accounted for greater than 50% of the space on the benthos in many habitats [15,16]. However, it is now rare to find reefs or even isolated patches of a given reef that support such abundant coral populations [11,12]. Further, given the number of natural stressors (storm damage, crown of thorns outbreaks, disease) and the increased frequency of bleaching events even in remote unpopulated areas, it is clear that coral populations are often in flux and a single snapshot of coral cover alone is not a good representation of the health or resilience of a particular reef.
When coral cover declines, it is often assumed that corals are replaced by macroalgae, resulting in a phase-shift [17]. However, a recent synthesis identified that macroalgae are rarely the ecological dominant on the reef benthos [18] aside from in some severely degraded habitats, in upwelling regions, or on high latitude reefs [17,19]. Further, data from across several reefs in the Florida Keys, the broader Caribbean, the Great Barrier Reef and the Indo-Pacific show that coral and macroalgae together account for only 19–55% of the reef benthos [20], highlighting the general lack of information for the remaining 45–81% of the benthos. A more holistic view of the coral reef benthos can provide greater insight into the structure, and ultimately, the function of a given reef community.
Many of the ecological services that coral reefs provide derive from their capacity to build carbonate reefs [21]. Thus, here we consider the abundance of reef-building organisms as an indication of the potential for a reef to build carbonate structure. In addition to hermatypic corals, crustose coralline algae (CCA) are among the most significant contributors to reef framework development. CCA are known for their capacity to bind carbonate sediments, fuse and cement reef components together, fill in interstitial spaces and ultimately stabilize reef structure as discovered by geologists in the early part of the twentieth century [22]. CCA are also critical for reef resilience since the larvae of many coral species preferentially settle and disproportionately survive on certain CCA taxa [23,24]. Many other species of calcified algae and invertebrates (Halimeda, foraminifera, bivalves) also contribute to carbonate production [25] but are generally not categorized as active reef builders (e.g. carbonate from these groups fill in the interstices between the framework formed by corals and CCA). Most of the other sessile benthic taxa or functional groups act in a more bioerosive capacity. Turf algae (generally less than 2 cm tall) consist of a diverse consortium of highly productive, largely fleshy, filamentous algae and cyanobacteria that rapidly colonize dead coral skeletons. Turf algae have been shown to reduce or prevent coral settlement via microbial activity [26] and negatively affect the survivorship of coral recruits [27]. Some species of turf algae can also be highly competitive and are able to colonize and even kill live coral tissue [28,29]. Further, turf algae are known to release large amounts of dissolved organic carbon which can fuel microbial activity [30] resulting in hypoxia [31]. Similarly fleshy, non-calcified macroalgae, a group of highly diverse seaweed species can be benign competitors or can differentially affect coral health through direct or indirect competition, allelopathy and/or microbial interactions [28,29,32]. Several non-calcifying invertebrate taxa can be common on reefs with varying competitive strategies and ecological roles. When considering the structure and function of benthic coral reef communities, surprisingly few studies have examined the entire benthos (but see [19,33]), yet the relative abundance of these different functional groups will largely dictate how a given reef functions or responds to and recovers from disturbance events.
Because we lack many relevant baselines for how benthic reef communities were structured prior to widespread human disturbance, it is difficult to know what our current expectations for management should be [34]. Presently, our best opportunity to examine what ‘healthy’ reefs may have looked like in the past is to use a space for time comparison. By comparing benthic coral reef communities on extremely remote, uninhabited islands with those of populated islands we can determine if and how benthic communities differ in the presence and absence of local human populations [34]. We examined these questions in detail using an unprecedentedly large dataset across the central Pacific. We specifically determined if the common metrics previously used to define reef health and degradation (coral and macroalgal cover, respectively) are inversely related to one another, and related to human habitation. Taking a broader perspective, we then examined whether there was evidence to suggest that the benthic functional groups known to be important for reef health, growth and resilience (the reef builders: coral and CCA) were more common in the absence of humans while fleshy algal taxa (turf and fleshy macroalgae) were more common on inhabited islands. Finally, we used data from the entire benthos to determine if and how these communities as a whole varied across latitude, among archipelagos, and between inhabited and uninhabited islands.
2. Material and methods
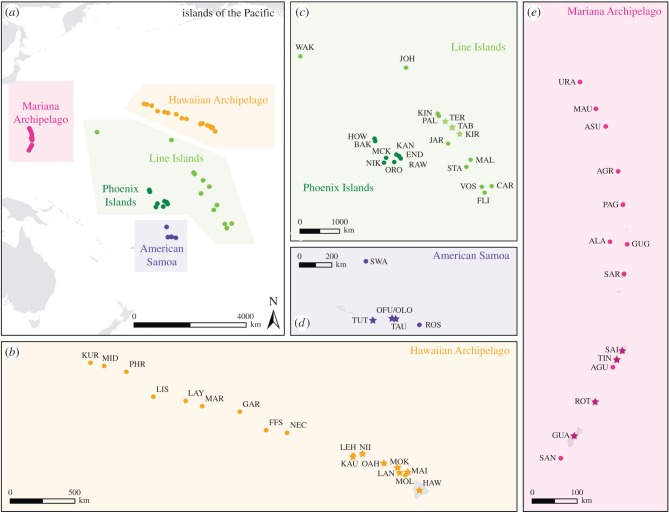
Fifty-six islands spanning 50° of latitude from five archipelagos and several unassociated islands in the central Pacific Ocean (the Hawaiian Islands, Mariana Islands, Line Islands, American Samoa, Wake and Johnston Atolls, and the Phoenix Islands) were surveyed between 2002 and 2009 (figure 1). All archipelagos contained both inhabited and uninhabited islands aside from the Phoenix Islands which are all functionally uninhabited. Many of the reefs surrounding the unpopulated islands receive high levels of protection and fall under the jurisdiction of the Papahānaumokuākea Marine National Monument (PMNM), the Pacific Remote Islands Marine National Monument (PRIMNM), the Phoenix Islands Protected Area (PIPA) and several United States Fish and Wildlife Refuges.
Figure 1.
(a) Map of the five archipelagos and 56 islands included in this analysis; (b) data were collected from 17 islands in the Hawaiian Archipelago, (c) 21 islands in the Line and Phoenix Islands, and Wake and Johnston Atolls, (d) six islands in American Samoa and (e) 14 islands in the Marianas. Circles designate uninhabited islands, while stars designate inhabited islands. Three letter codes indicate island names which are defined in the electronic supplementary material, table S1.
(a). Data collection
At each island, benthic data were collected on SCUBA using standard photoquadrat surveys at 10–12 m depth on the forereef (electronic supplementary material, Methods). In summary, over 6500 photographs were analysed from 450 sites across 56 islands. Photographs were analysed using the image analysis software Photogrid 1.0 by overlaying 100 stratified random points on each image and identifying taxa that occurred under each point. Taxonomic resolution varied by group but included genus-level identification for coral, macroalgae and macro-invertebrates and functional group-level identification for CCA and turf algae. Site means were averaged to determine island level mean functional group per cent cover. All islands were designated as ‘inhabited’ or ‘uninhabited’ based upon current human population densities at each island. Islands with very small or ephemeral populations were designated as functionally uninhabited while some uninhabited islands historically possessed military populations that may have residual effects on the reef biota (e.g. Palmyra, Wake and Johnston Atolls) making these designations conservative. Because the purpose of this study was to examine whether benthic reef communities showed consistent similarities in the presence or the absence of local human populations, we did not quantify the level or magnitude of human impact at these locations.
(b). Data analysis
To determine whether specific benthic functional groups differed in abundance among archipelago and on inhabited versus uninhabited islands, we used a two-way fixed factor analysis of variance (ANOVA). To account for large-scale geographical and/or oceanographic differences among archipelagos, latitude was used as a covariate. All proportional cover data were arcsine-square root transformed prior to analysis, and tests for normality and homogeneity of variances satisfied assumptions. First, we examined whether per cent cover of hard coral and macroalgae (figure 2a) differed between uninhabited and inhabited islands, among archipelagos and across latitude. Subsequently, we used a broader more inclusive approach to determine whether reef-building organisms (coral and CCA) and fleshy algae (turf and fleshy macroalgae) differed in per cent cover in the presence or the absence of human populations, among archipelago and across latitude (figure 2b). We used this approach because dominance by reef builders probably indicates a community that is actively accreting carbonate while dominance by fleshy algae probably indicates net reef loss.
Figure 2.
(a) The two benthic functional groups commonly used to evaluate reef health: hard coral and macroalgae. (b) Images showing a landscape perspective of reef communities where many other organisms are present that can help to elucidate reef condition such as reef builders (CCA and hard coral) and fleshy algae (turf and macroalgae).
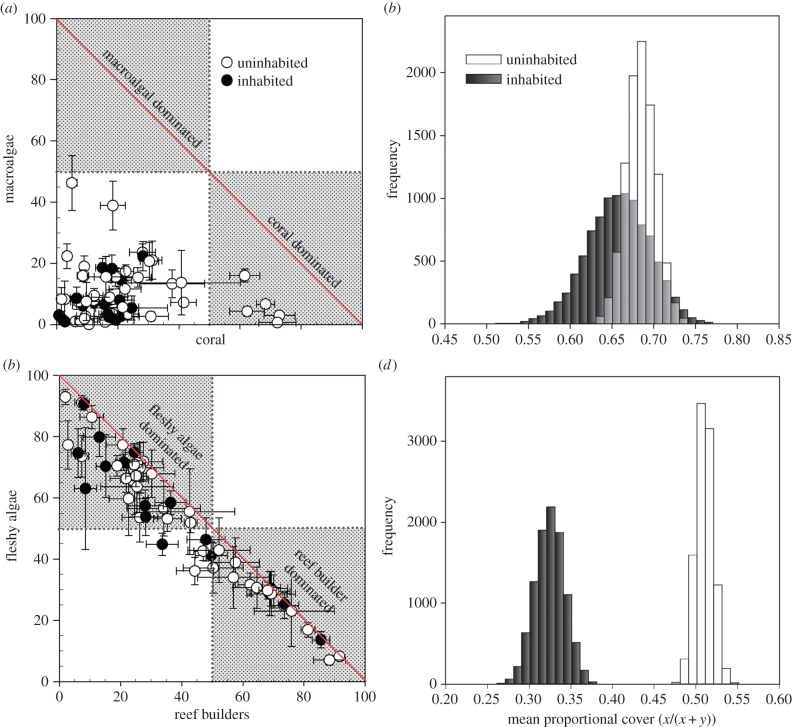
Using Pearson's correlation, we examined whether there was a negative relationship between total macroalgal cover and coral cover, which would suggest that as coral cover declines macroalgae become more abundant. We also determined whether there was a negative relationship between per cent cover of reef builders and that of fleshy algae, suggesting that reefs shift along a continuum of these two benthic groups. To account for differences in sample size among islands, we used a resampling approach to randomly select three sites per island without replacement to calculate the overall mean coral, macroalgae, fleshy algae and reef-builder cover by island and then by habitation. This process was repeated 10 000 times to estimate the frequency distribution of the mean proportional cover of coral to macroalgae as well as reef builders to fleshy algae by habitation.
To examine how the benthos as a whole varied among islands, we calculated Bray–Curtis similarity values for all functional group per cent cover data. We then used a permutation-based multivariate analysis (PERMANOVA) of covariance to test whether benthic community composition differed within and among archipelagoes and between populated and unpopulated islands (99 999 permutations). We used a SIMPER procedure in Primer-e to identify which benthic functional groups accounted for the differences between factors in the PERMANOVA. To visualize multivariate benthic community structure among islands, we used a principal components analysis (PCA) on the covariance matrix of the arcsine square root transformed functional group data. Finally, we calculated pairwise correlation coefficients and associated p-values between all of the different benthic functional groups to examine possible relationships between different taxa not specifically assessed in the above analyses.
3. Results
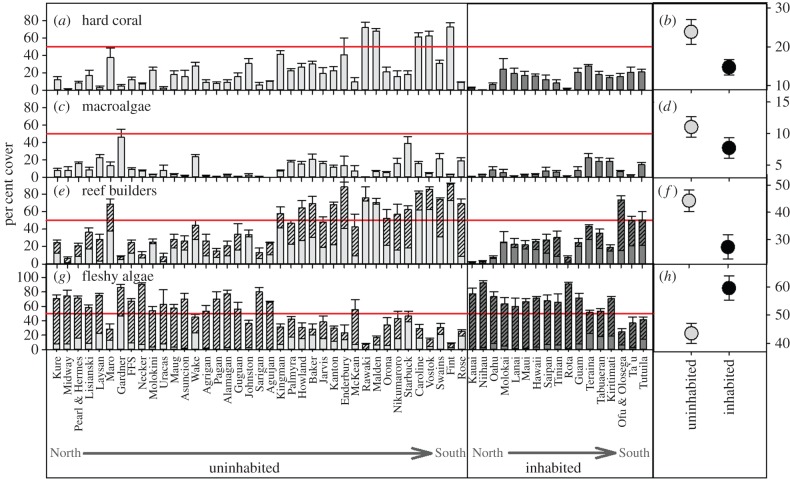
Using islands as replicates across the central Pacific, live coral accounted for an average of 21.15% (±2.35 s.e.) of the benthic substrate and exhibited an island-wide range of 1–74%. While coral cover appears higher on uninhabited islands (figure 3a,b), these differences were not significant (electronic supplementary material, table S2). Latitude was the only significant factor that explained variability in coral cover with cover declining with increasing latitude (electronic supplementary material, table S2 and figure S1a). Total macroalgal cover accounted for 10.05% (±1.24) of the benthos across all islands (figure 2c,d) and ranged from 1 to 46% cover at the island scale. There was a significant interaction between habitation and archipelago (electronic supplementary material, table S2) with the Hawaiian Islands and American Samoa having greater macroalgal cover on uninhabited islands than inhabited islands, and the Line and Mariana Islands having lower macroalgal cover on uninhabited islands (electronic supplementary material, table S2) than inhabited islands. There was no significant relationship between latitude and macroalgal cover (electronic supplementary material, figure S1b). In summary, coral and macroalgae together account for an average of 31% of the benthos (between 4 and 78% cover) across these 56 islands. Further, no significant correlations were found between coral and macroalgal cover (figure 4a; r = −0.034, p = 0.80) suggesting that these two functional groups do not covary. Based upon our resampling approach, the mean proportional cover of coral versus total coral and macroalgal cover was not different on inhabited and uninhabited islands (figure 4b).
Figure 3.
Mean per cent cover by island (left) and in total (right) across inhabited and uninhabited islands for (a,b) hard coral, (c,d) total macroalgae, (e,f) reef-building organisms (hard corals and CCA (in hashed bars)), and (g,h) total fleshy algae (fleshy macroalgae and turf algae (hashed bars)). Data presented are the mean per cent cover ±1 s.e. from all sites within island (a,c,e,g) and across all islands (b,d,f,h) with and without local human populations. The horizontal line shows the 50% cover mark for reference. (Online version in colour.)
Figure 4.
(a) Relationship between mean coral and macroalgal cover across all islands showing no correlation between these two groups (±1 s.e.). (b) Histograms of the resampled means for all inhabited and uninhabited islands showing the proportion of coral cover (x) relative to total cover of coral and macroalgae (x + y) summed. (c) Relationship between reef builders versus fleshy algae for all 56 islands examined showing strong correlation between these groups. (d) Resampled means of the proportional cover of reef builders (x) relative to total cover of reef builders plus fleshy algae (x + y) showing no overlap in the distribution of means across inhabited and uninhabited islands. (Online version in colour.)
The average per cent cover of active reef builders (figure 3e,f) across all islands was 39.18% (±3.30 s.e.; range 1.8–91.6) and was significantly greater (electronic supplementary material, table S2) on uninhabited islands than inhabited islands (45.18 versus 27.26% cover, respectively). Reef-builder cover was also negatively correlated with latitude, but did not vary significantly among archipelago (electronic supplementary material, table S2). By contrast, the average per cent cover of fleshy algae (figure 3g,h) was 48.35% (±3.20 s.e.; range 6.5–91.5) across all islands. Fleshy algae were significantly more abundant on inhabited islands than uninhabited islands (59.28% versus 43.59% cover, respectively), and there were significant archipelagic effects (electronic supplementary material, table S2; Tukey posthoc test Mariana Islands > Line Islands) but no pattern with latitude. There was a strong negative relationship between per cent cover of reef builders and that of fleshy algae (figure 3c) regardless of human habitation (r = −0.942, p < 0.001). Most inhabited islands (82%) were dominated by fleshy algae whereas uninhabited islands showed more variability and thus a more even distribution of islands dominated by either group. The lack of overlap between the frequency distributions for the mean relative abundance of reef builders versus reef builders plus fleshy algae indicate that uninhabited islands have a consistently greater mean cover (figure 4d) of reef builders in comparison to inhabited islands.
There were significant differences in benthic communities as a whole across the 56 islands, among archipelagos, between inhabited and uninhabited islands and across latitude (electronic supplementary material, table S3). However, no significant interactions were observed suggesting there are consistent differences in community structure with and without local human populations regardless of island group. The biggest differences observed in benthic community composition were between higher latitude northern archipelagos (the Mariana and Hawaiian Islands) and reefs from American Samoa, the Line and Phoenix Islands. Multivariate posthoc comparisons showed that the only archipelagos that were not different from one another were Hawaii and the Marianas (p = 0.56), American Samoa and the Phoenix Islands (p = 0.29) and the Line and Phoenix Islands (p = 0.72). Both Hawaii and the Marianas exhibited higher per cent cover of turf algae and lower per cent cover of coral and CCA than the other archipelagos (electronic supplementary material, figure S2). Further, the lower latitude archipelagos contained a greater abundance of reef builders, but even here reef builders were more abundant on uninhabited islands (electronic supplementary material, figures S1 and S2). SIMPER results showed that turf algae, hard coral, CCA and fleshy macroalgae explained 87% of the dissimilarity between inhabited and uninhabited islands in order of decreasing importance (electronic supplementary material, table S4).
In general, inhabited islands were more similar in community structure to one another than to uninhabited islands. Turf algae largely dominated these benthic communities while the uninhabited islands showed more variability but generally a greater proportion of CCA cover (figure 3). Other functional groups contributed in abundance but much less to the overall variation in community similarity (electronic supplementary material, figure S2). These trends are shown in the PCA that explored covariance among different benthic functional groups for all 56 islands where the first two PCs explained greater than 80% of the variation in the dataset (figure 5). The first PC largely describes differences in per cent cover of the dominant reef builders and that of turf algae with turf algal per cent cover increasing in the direction of most of the inhabited islands and per cent cover of both CCA and coral increasing in the direction of many of the uninhabited islands. The second PC largely describes differences between coral and CCA cover. When examining correlations among all benthic functional groups the only patterns that emerged were significant negative relationships between turf algae and both coral and CCA (electronic supplementary material, table S5).
Figure 5.
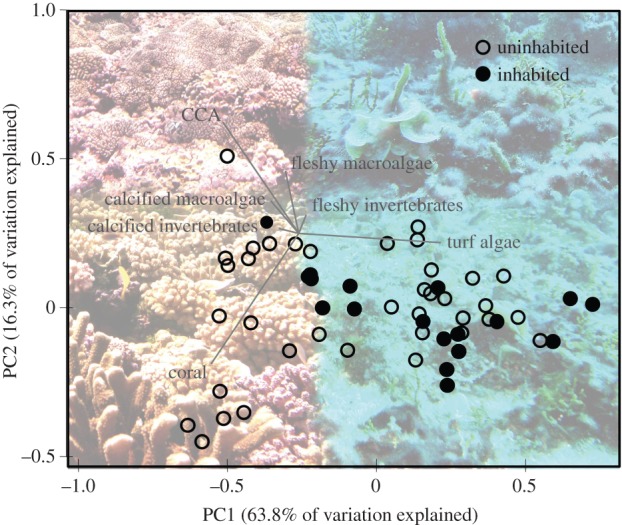
Principal components analysis (PCA) of benthic reef communities across all 56 islands surveyed. The first two principal components account for greater than 80% of the variation in the data. (Online version in colour.)
4. Discussion
Assessments of coral reef ecosystem health are often made by examining coral cover across space and over time, inside and outside of Marine Protected Areas, before and after a disturbance or across gradients of human population density [11,12,16,33]. However, given the variability in the frequency and intensity of natural and anthropogenic disturbances, coral cover alone is not a reliable indicator of reef health or resilience [35]. At any given point in time coral cover may be in flux, and low coral cover alone does not indicate low resilience or low recovery potential [36]. Thus, there is an urgent need to begin assessing additional parameters that are known to be important indicators of reef replenishment processes [2]. Coral growth rates and recruitment success are clearly important but are not routinely or easily measured in monitoring programmes. The abundance of other key benthic functional groups known to be indicators of important ecosystem processes can provide insight into reef condition [37]. Here, taking a comprehensive view of benthic reef communities at an island scale across the central Pacific, we see striking differences between inhabited and remote, unpopulated islands regardless of archipelago, suggesting that the human presence may have consistent effects on the reef benthos. Reefs in the presence of local human populations were less variable and generally dominated by fleshy turf and macroalgae, while reef communities on uninhabited islands were more variable but commonly dominated by calcifying, reef-building organisms. These results suggest that benthic habitats on islands with local human populations either never had (owing to geomorphology, island age), or may be losing, one of the most significant ecological services that they are known for, their capacity to build carbonate reefs.
There is growing evidence that suggests phase-shifts or large-scale changes in benthic community structure have occurred and continue to occur on coral reefs around the globe [2,4,38]. Whether any given change represents a transient state following a disturbance event or is actually an alternate stable state remains unclear owing to the lack of consistent long-term monitoring data. To date most discussions of coral reef phase shifts have focused on shifts away from coral ‘dominance’ (often arbitrarily defined) to dominance by macroalgae (but see, Norstrom et al. [39]). Several syntheses have clearly identified declines in coral cover [11,12,17,38], but patterns in macroalgal cover have been less clear [18]. Here we show no correlation between coral and fleshy macroalgae, identifying that there is no evidence for coral to macroalgal phase shifts in this dataset. Rather, this and other recent studies have documented the ubiquity of turf algae on reefs [40], but this inconspicuous group of algae are often ignored or lumped into categories such as ‘dead coral’ or ‘rubble’, making benthic comparisons across studies difficult. Similarly, CCA are often lumped with ‘bare space’ or ignored entirely. Given their importance and functional roles, these different benthic taxa need to be incorporated consistently into future benthic reef assessments.
It is unclear what proportion of the benthos needs to be dominated by reef builders and other calcifiers to maintain a reef in a state of net accretion. Ultimately, this will depend upon the balance between growth and calcification rates of key taxa and dissolution and bioerosion processes which will all be based upon local oceanographic conditions, community structure (including macrobes and microbes [10]), and anthropogenic influences at a particular site [41–43]. However, it seems logical that reefs with a greater abundance of reef builders will have higher rates of net reef growth and accretion than reefs dominated by fleshy organisms. Thus, reef-builder abundance could serve as an important indicator of current reef state and future trajectory.
If reef-building corals suffer mortality either at small scales owing to predation or abrasion or at larger scales resulting from warming events and subsequent mass bleaching events, storm damage or crown of thorns outbreaks, the likelihood of recovery will be dependent upon the other members of the reef benthos [37,36]. In particular, some species of CCA provide settlement cues for coral larvae [23,24] and are often benign or neutral competitors with corals [44]. CCA abundance is tied to a number of physical and biological factors such as high flow and herbivory which may also help to promote coral recovery. Further, because CCA help to stabilize the reef matrix itself, reefs with high CCA cover are likely to be more resistant to storm damage. By contrast, if turf algae are abundant there will be less suitable substrate for corals to settle and those that do may not survive [27,45]. Turf algae are also known to be aggressive competitors with adult corals via smothering and allelopathy [44,46] in addition to releasing large amounts of dissolved organic carbon that enhances microbial communities that are known to harm corals [30]. Turf algae are opportunistic and are often the first to colonize open space following a disturbance event but given the considerable variability that exists in turf height, density and taxonomic composition [47] the ultimate impacts of turfs on corals will probably be driven by local conditions. Lastly, macroalgae, which include a highly diverse group of both fleshy and calcified taxa, have a variety of positive, neutral and negative effects on larval settlement and survival, and on competitive interactions with adult corals [37,44,48,49]. Based upon what is known about coral–algal interactions, it is likely that reef communities with a greater abundance of CCA will be more resilient and recover more quickly from large-scale disturbances than communities dominated by turf and/or macroalgae.
Numerous natural and anthropogenic drivers acting on local and global scales can influence the structure of a given coral reef community. In our analysis, using islands as replicates, we examined not why, but how these reef communities varied in the presence and absence of local human populations across the central Pacific. Looking at coral cover alone, clear latitudinal and archipelagic effects were evident with generally higher cover at lower, more equatorial latitudes. These differences are probably owing to numerous oceanographic factors such as temperature, nutrient availability, aragonite saturation state, frequency and intensity of warming events, storms, waves and hydrodynamics. The lack of a human habitation signal on coral cover is probably owing to the combined effects of warming and subsequent bleaching events that have occurred throughout the region regardless of human population density [50–52]. There were no latitudinal or habitation effects on macroalgal cover, suggesting that macroalgae may be ephemeral or be more strongly influenced by local-scale processes such as nutrient availability or herbivore abundance. Perhaps most interesting was the complete lack of correlation between coral and macroalgal cover in our dataset but a strong negative correlation between both coral and CCA cover and that of turf algae. These results identify that central Pacific reefs are either dominated by reef builders or by turf algae; all inhabited islands have greater than 50% of the benthos covered with turf algae. Algal turfs may be competitively dominant on these central Pacific islands in contrast to macroalgal dominance on Caribbean reefs [53] owing to different disturbance regimes (higher wave energy, greater grazing pressure, etc.) that favour opportunistic turfs over macroalgae. Given that herbivorous reef fish populations are reduced at many of the populated islands in our dataset [3], we might expect to see to a greater abundance of macroalgae at these locations [54,55]. However, the majority of fishing activities in these regions have focused on the herbivore feeding guilds that primarily consume turf algae (scraper/excavators and grazer/detritivores) rather than macroalgae helping to explain the patterns seen here. On the unfished and uninhabited islands, high grazing intensity on fleshy algae could also indirectly promote the abundance of CCA and facilitate coral recruitment [5], leading to a greater abundance of reef builders on these remote uninhabited islands.
This is, to our knowledge, the first study to comprehensively examine how benthic communities vary across the central Pacific in the presence and absence of local human populations. We provide data from some of the world's most remote and unpopulated islands and show that in the absence of local human populations these islands were often truly dominated (more than 50% cover) by reef-building corals and coralline algae. These results suggest that in the absence of local human impacts reefs may be more resistant or resilient to global change and provide incentive for local management action on more populated islands. However, we also show that coral cover alone is not the best indicator of the human presence or the absence on reefs across a broad geographical gradient. Because of the incredible diversity present in coral reef communities, it is more insightful to examine the structure of the benthos as a whole than to focus on a single taxon. While corals may have been dominant space occupiers decades or centuries ago, this is rarely the case now owing to global stressors that affect even the most remote regions of the planet. However, just because corals do not ‘dominate’ a habitat does not mean that it is not ‘healthy’. Here, we suggest new definitions of reef health based upon reef-building capacity: a healthy coral reef is actively growing and accreting a calcium carbonate framework and is therefore dominated by reef-building organisms, where a degraded or marginal reef is dominated by fleshy organisms. Of course, it is not just the reef state at any given point in time but the trajectory or change over time that will ultimately determine reef health and resilience. Future monitoring programmes should consider a more holistic perspective of the benthos, beyond abundance of coral and macroalgae, and should also measure key indicators of reef resilience such as coral recruitment, reef growth or accretion and herbivory, as these rates will help to elucidate future trends in benthic community structure.
Supplementary Material
Supplementary Material
Acknowledgements
We thank the staff of all research vessels that supported data collection including the Hi'ialikai, the Hanse Explorer, the Searcher, the Naia and the White Holly, and the Center for Marine Biodiversity and Conservation at Scripps, Randi Rotjan and the New England Aquarium for logistical support.
Data accessibility
Data are available in the electronic supplementary material.
Authors' contributions
J.E.S., P.S.V. and D.O. collected the data. A.C., C.E., J.H., L.L., S.D. and J.E.S. analysed the data. R.B., S.A.S., E.S. and D.O. organized, coordinated and fundraised for research cruises and all authors contributed to writing of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This work was funded by the National Science Foundation and NOAA through the Comparative Analysis of Marine Ecosystem Organization (CAMEO) award no. NSF OCE10417212 to J.E.S. and S.A.S. Funding for research cruises was provided by the National Geographic Society, the Scripps family, the Gordon and Betty Moore Foundation, the Moore Family Foundation, the Oak Foundation and the Marine Management Area Science Program at Conservation International.
References
- 1.Pandolfi JM, et al. 2003. Global trajectories of the long-term decline of coral reef ecosystems. Science 301, 955–958. ( 10.1126/science.1085706) [DOI] [PubMed] [Google Scholar]
- 2.Bellwood DR, Hughes TP, Folke C, Nystrom M. 2004. Confronting the coral reef crisis. Nature 429, 827–833. ( 10.1038/nature02691) [DOI] [PubMed] [Google Scholar]
- 3.Edwards CB, et al. 2014. Global assessment of the status of coral reef herbivorous fishes: evidence for fishing effects. Proc. R. Soc. B 281, 20131835 ( 10.1098/rspb.2013.1835) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCook LJ. 1999. Macroalgae, nutrients and phase shifts on coral reefs: scientific issues and management consequences for the Great Barrier Reef. Coral Reefs 18, 357–367. ( 10.1007/s003380050213) [DOI] [Google Scholar]
- 5.Smith JE, Hunter CL, Smith CM. 2010. The effects of top-down versus bottom-up control on benthic coral reef community structure. Oecologia 163, 497–507. ( 10.1007/s00442-009-1546-z) [DOI] [PubMed] [Google Scholar]
- 6.Hoegh-Guldberg O. 1999. Climate change, coral bleaching and the future of the world's coral reefs. Mar. Freshwater Res. 50, 839–866. ( 10.1071/MF99078) [DOI] [Google Scholar]
- 7.De'ath G, Lough JM, Fabricius KE. 2009. Declining coral calcification on the Great Barrier Reef. Science 323, 116–119. ( 10.1126/science.1165283) [DOI] [PubMed] [Google Scholar]
- 8.Aronson RB, Precht WF. 2001. White-band disease and the changing face of Caribbean coral reefs. Hydrobiologia 460, 25–38. ( 10.1023/A:1013103928980) [DOI] [Google Scholar]
- 9.Alvarez-Filip L, Dulvy NK, Gill JA, Côté IM, Watkinson AR. 2009. Flattening of Caribbean coral reefs: region-wide declines in architectural complexity. Proc. R. Soc. B 276, 3019–3025. ( 10.1098/rspb.2009.0339) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDole T, et al. 2012. Assessing coral reefs on a Pacific-wide scale using the microbialization score. PLoS ONE 7, e0043233 ( 10.1371/journal.pone.0043233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gardner TA, Cote IM, Gill JA, Grant A, Watkinson AR. 2003. Long-term region-wide declines in Caribbean corals. Science 301, 958–960. ( 10.1126/science.1086050) [DOI] [PubMed] [Google Scholar]
- 12.Bruno JF, Selig ER. 2007. Regional decline of coral cover in the Indo-Pacific: timing, extent, and subregional comparisons. PLoS ONE 2, e0000711 ( 10.1371/journal.pone.0000711) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De'ath G, Fabricius KE, Sweatman H, Puotinen M. 2012. The 27-year decline of coral cover on the Great Barrier Reef and its causes. Proc. Natl Acad. Sci. USA 109, 17 995–17 999. ( 10.1073/pnas.1208909109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson JBC. 1997. Reefs since Columbus. Coral Reefs 16, S23–S32. ( 10.1007/s003380050238) [DOI] [Google Scholar]
- 15.Done TJ. 1982. Patterns in the distribution of coral communities across the central Great Barrier Reef. Coral Reefs 1, 95–107. ( 10.1007/BF00301691) [DOI] [Google Scholar]
- 16.Connell JH, Hughes TP, Wallace CC. 1997. A 30-year study of coral abundance, recruitment, and disturbance at several scales in space and time. Ecol. Monogr. 67, 461–488. ( 10.1890/0012-9615(1997)067%5B0461:AYSOCA%5D2.0.CO;2) [DOI] [Google Scholar]
- 17.Hughes TP. 1994. Catastrophes, phase-shifts, and large-scale degradation of a Caribbean coral reef. Science 265, 1547–1551. ( 10.1126/science.265.5178.1547) [DOI] [PubMed] [Google Scholar]
- 18.Bruno JF, Sweatman H, Precht WF, Selig ER, Schutte VGW. 2009. Assessing evidence of phase shifts from coral to macroalgal dominance on coral reefs. Ecology 90, 1478–1484. ( 10.1890/08-1781.1) [DOI] [PubMed] [Google Scholar]
- 19.Vroom PS, Braun CL. 2010. Benthic composition of a healthy subtropical reef: baseline species-level cover, with an emphasis on algae, in the Northwestern Hawaiian Islands. PLoS ONE 5, e9733 ( 10.1371/journal.pone.0009733) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hughes TP, Graham NAJ, Jackson JBC, Mumby PJ, Steneck RS. 2010. Rising to the challenge of sustaining coral reef resilience. Trends Ecol. Evol. 25, 633–642. ( 10.1016/j.tree.2010.07.011) [DOI] [PubMed] [Google Scholar]
- 21.Moberg F, Folke C. 1999. Ecological goods and services of coral reef ecosystems. Ecol. Econ. 29, 215–233. ( 10.1016/S0921-8009(99)00009-9) [DOI] [Google Scholar]
- 22.Setchell WA. 1930. Biotic cementation in coral reefs. Proc. Natl Acad. Sci. USA 16, 781–783. ( 10.1073/pnas.16.12.781) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrington L, Fabricius K, De'ath G, Negri A. 2004. Recognition and selection of settlement substrata determine post-settlement survival in corals. Ecology 85, 3428–3437. ( 10.1890/04-0298) [DOI] [Google Scholar]
- 24.Price NN. 2010. Habitat selection, facilitation, and biotic settlement cues affect distribution and performance of coral recruits in French Polynesia. Oecologia 163, 747–758. ( 10.1007/s00442-010-1578-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chave KE, Smith SV, Roy KJ. 1972. Carbonate production by coral reefs. Mar. Geol. 12, 123–140. ( 10.1016/0025-3227(72)90024-2) [DOI] [Google Scholar]
- 26.Vermeij MJA, Smith J, Smith C, Vega Thurber R, Sandin S. 2009. Survival and settlement success of coral planulae: independent and synergistic effects of macroalgae and microbes. Oecologia 159, 325–336. ( 10.1007/s00442-008-1223-7) [DOI] [PubMed] [Google Scholar]
- 27.Vermeij MJA, Sandin SA. 2008. Density-dependent settlement and mortality structure the earliest life phases of a coral population. Ecology 89, 1994–2004. ( 10.1890/07-1296.1) [DOI] [PubMed] [Google Scholar]
- 28.McCook LJ, Jompa J, Diaz-Pulido G. 2001. Competition between corals and algae on coral reefs: a review of evidence and mechanisms. Coral Reefs 19, 400–417. ( 10.1007/s003380000129) [DOI] [Google Scholar]
- 29.Smith JE, et al. 2006. Indirect effects of algae on coral: algae-mediated, microbe-induced coral mortality. Ecol. Lett. 9, 835–845. ( 10.1111/j.1461-0248.2006.00937.x) [DOI] [PubMed] [Google Scholar]
- 30.Haas AF, Nelson CE, Wegley Kelly L, Carlson CA, Rohwer F, Leichter JJ, Wyatt A, Smith JE. 2011. Effects of coral reef benthic primary producers on dissolved organic carbon and microbial activity. PLoS ONE 6, e27973 ( 10.1371/journal.pone.0027973) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barott K, Smith J, Dinsdale E, Hatay M, Sandin S, Rohwer F. 2009. Hyperspectral and physiological analyses of coral-algal interactions. PLoS ONE 4, e8043 ( 10.1371/journal.pone.0008043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rasher DB, Hay ME. 2010. Chemically rich seaweeds poison corals when not controlled by herbivores. Proc. Natl Acad. Sci. USA 107, 9683–9688. ( 10.1073/pnas.0912095107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sandin SA, et al. 2008. Baselines and degradation of coral reefs in the northern Line Islands. PLoS ONE 3, e1548 ( 10.1371/journal.pone.0001548) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knowlton N, Jackson JBC. 2008. Shifting baselines, local impacts, and global change on coral reefs. PLoS Biol. 6, 215–220. ( 10.1371/journal.pbio.0060054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vroom PS. 2011. 'Coral dominance': a dangerous ecosystem misnomer? J. Mar. Biol. 2011, 1–8. ( 10.1155/2011/164127) [DOI] [Google Scholar]
- 36.Diaz-Pulido G, et al. 2009. Doom and boom on a resilient reef: climate change, algal overgrowth and coral recovery. PLoS ONE 4, e0005239 ( 10.1371/journal.pone.0005239) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Birrell CL, McCook LJ, Willis BL, Diaz-Pulido GA. 2008. Effects of benthic algae on the replenishment of corals and the implications for the resilience of coral reefs. Oceanogr. Mar. Biol. 46, 25. [Google Scholar]
- 38.Côté IM, Gill JA, Gardner TA, Watkinson AR. 2005. Measuring coral reef decline through meta-analyses. Phil. Trans. R. Soc. B 360, 385–395. ( 10.1098/rstb.2004.1591) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Norstrom AV, Nystrom M, Lokrantz J, Folke C. 2009. Alternative states on coral reefs: beyond coral-macroalgal phase shifts. Mar. Ecol.-Prog. Ser. 376, 295–306. ( 10.3354/meps07815) [DOI] [Google Scholar]
- 40.Jouffray J-B, Nyström M, Norström AV, Williams ID, Wedding LM, Kittinger JN, Williams GJ. 2014. Identifying multiple coral reef regimes and their drivers across the Hawaiian archipelago. Phil. Trans. R. Soc. B 370, 20130268 ( 10.1098/rstb.2013.0268) [DOI] [Google Scholar]
- 41.Gattuso JP, Frankignoulle M, Wollast R. 1998. Carbon and carbonate metabolism in coastal aquatic ecosystems. Annu. Rev. Ecol. Syst. 29, 405–434. ( 10.1146/annurev.ecolsys.29.1.405) [DOI] [Google Scholar]
- 42.Andersson AJ, Kuffner IB, Mackenzie FT, Jokiel PL, Rodgers KS, Tan A. 2009. Net loss of CaCO3 from a subtropical calcifying community due to seawater acidification: mesocosm-scale experimental evidence. Biogeosciences 6, 1811–1823. ( 10.5194/bg-6-1811-2009) [DOI] [Google Scholar]
- 43.Feely RA, Doney SC, Cooley SR. 2009. Ocean acidification: present conditions and future changes in a high-CO2 world. Oceanography 22, 36–47. ( 10.5670/oceanog.2009.95) [DOI] [Google Scholar]
- 44.Barott KL, Williams GJ, Vermeij MJA, Harris J, Smith JE, Rohwer FL, Sandin SA. 2012. Natural history of coral-algae competition across a gradient of human activity in the Line Islands. Mar. Ecol. Prog. Ser. 460, U1–U2. ( 10.3354/meps09874) [DOI] [Google Scholar]
- 45.Arnold SN, Steneck RS, Mumby PJ. 2010. Running the gauntlet: inhibitory effects of algal turfs on the processes of coral recruitment. Mar. Ecol. Prog. Ser. 414, 91–105. ( 10.3354/meps08724) [DOI] [Google Scholar]
- 46.Jompa J, McCook LJ. 2003. Contrasting effects of turf algae on corals: massive Porites spp. are unaffected by mixed-species turfs, but killed by the red alga Anotrichium tenue. Mar. Ecol. Prog. Ser. 258, 79–86. ( 10.3354/meps258079) [DOI] [Google Scholar]
- 47.Harris J, Lewis LS, Smith J. 2015. Quantifying scales of spatial variability in algal turf assemblages on coral reefs. Mar. Ecol. Prog. Ser. 532, 41–57. ( 10.3354/meps11344) [DOI] [Google Scholar]
- 48.Box SJ, Mumby PJ. 2007. Effect of macroalgal competition on growth and survival of juvenile Caribbean corals. Mar. Ecol. Prog. Ser. 342, 139–149. ( 10.3354/meps342139) [DOI] [Google Scholar]
- 49.Diaz-Pulido G, Harii S, McCook LJ, Hoegh-Guldberg O. 2010. The impact of benthic algae on the settlement of a reef-building coral. Coral Reefs 29, 203–208. ( 10.1007/s00338-009-0573-x) [DOI] [Google Scholar]
- 50.Aeby GS, Kenyon JC, Maragos JE, Potts DC. 2003. First record of mass coral bleaching in the northwestern Hawaiian Islands. Coral Reefs 22, 256 ( 10.1007/s00338-003-0309-2) [DOI] [Google Scholar]
- 51.Williams GJ, Knapp IS, Maragos JE, Davy SK. 2010. Modeling patterns of coral bleaching at a remote Central Pacific atoll. Mar. Pollut. Bull. 60, 1467–1476. ( 10.1016/j.marpolbul.2010.05.009) [DOI] [PubMed] [Google Scholar]
- 52.Obura D, Mangubhai S. 2011. Coral mortality associated with thermal fluctuations in the Phoenix Islands, 2002–2005. Coral Reefs 30, 607–619. ( 10.1007/s00338-011-0741-7) [DOI] [Google Scholar]
- 53.Roff G, Mumby PJ. 2012. Global disparity in the resilience of coral reefs. Trends Ecol. Evol. 27, 404–413. ( 10.1016/j.tree.2012.04.007) [DOI] [PubMed] [Google Scholar]
- 54.Williams ID, Polunin NVC. 2001. Large-scale associations between macroalgal cover and grazer biomass on mid-depth reefs in the Caribbean. Coral Reefs 19, 358–366. ( 10.1007/s003380000121) [DOI] [Google Scholar]
- 55.Friedlander AM, Brown E, Monaco ME. 2007. Defining reef fish habitat utilization patterns in Hawaii: comparisons between marine protected areas and areas open to fishing. Mar. Ecol. Prog. Ser. 351, 221–233. ( 10.3354/meps07112) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available in the electronic supplementary material.