Abstract
The capacity of species to respond adaptively to warming temperatures will be key to their survival in the Anthropocene. The embryos of egg-laying species such as sea turtles have limited behavioural means for avoiding high nest temperatures, and responses at the physiological level may be critical to coping with predicted global temperature increases. Using the loggerhead sea turtle (Caretta caretta) as a model, we used quantitative PCR to characterise variation in the expression response of heat-shock genes (hsp60, hsp70 and hsp90; molecular chaperones involved in cellular stress response) to an acute non-lethal heat shock. We show significant variation in gene expression at the clutch and population levels for some, but not all hsp genes. Using pedigree information, we estimated heritabilities of the expression response of hsp genes to heat shock and demonstrated both maternal and additive genetic effects. This is the first evidence that the heat-shock response is heritable in sea turtles and operates at the embryonic stage in any reptile. The presence of heritable variation in the expression of key thermotolerance genes is necessary for sea turtles to adapt at a molecular level to warming incubation environments.
Keywords: adaptation, heat shock, heritability, sea turtle, embryo, climate change
1. Introduction
Survival in a warming world depends on the ability of a species to move or remain within their current distributions and respond to changing environmental conditions by evolutionary and/or plastic responses [1–3]. Responses to changing environmental conditions include modification of life-history traits such as the timing of reproduction [3], changes in thermoregulatory behaviour and microhabitat use [4], and/or changes at a physiological level by acclimation or evolving tolerance to warmer climates [2,5]. Comparatively little attention has been paid to the capacity of embryos to survive thermal challenges, despite the fact that most embryos develop in fixed locations where stressful conditions are difficult to avoid [6]. Embryonic tolerance of thermal stress should be under strong selection, and plasticity of their physiological responses should further maximize their survival in altered environments (e.g. [7]).
Physiological responses to thermal stress begin at the molecular level, frequently with the activation of genes such as those that code for heat-shock proteins (Hsps) that mitigate damage to membranes, proteins and DNA [8–10]. Hsps in the Hsp60, Hsp70 and Hsp90 families increase expression in response to heat stress in a range of taxa (e.g. insects, fish, frogs and reptiles), and maintain protein folding and degradation and myelination of neurons to avoid apoptosis [11–17]. Because the upregulation of heat-shock protein genes (hsp) in response to thermal stress is highly conserved across taxa, it is a candidate mechanism for adaptation of embryos to higher temperatures. Consistent with this view, many studies have shown improved heat resistance after Hsp expression [8,10], and several studies have reported clinal variation in hsp genes across latitudinal [18,19] and altitudinal transects [20–22], indicating natural selection is acting on these genes. However, many factors influence Hsp expression levels (e.g. other environmental stressors, inbreeding, age) and the Hsp expression level in each species will be a balance between the costs and benefits of upregulation of Hsps [10].
The mechanisms by which sea turtle embryos respond to heat stress are of particular interest, as their lineage arose in the Mesozoic and has persisted through many instances of global heating and cooling [23–25]. The strong fidelity of nesting females to natal beaches [26] means that major shifts in rookery locations occurs slowly [27]. Consequently, rapid rises in beach temperatures due to anthropogenically forced climate change could be a novel challenge for embryos relative to more gradual thermal changes experienced throughout their evolutionary history.
Microevolution of the thermally sensitive genome of sea turtles is only possible if two key expectations are met. First, and taking heat shock genes as an example, the optimal level of hsp expression should vary among populations experiencing thermal differences in the nesting environment [28]. Second, the variation in expression of hsps in response to thermal stress must be heritable [28,29]. In general, there have been few studies on the adaptive potential of wild populations to heat stress, and laboratory evolution experiments have focused on the heritability of expression of hsp70 in most Drosophila species [30,31]. Given that Drosophila have short generation intervals (a few weeks), low levels of heritability may be sufficient for adaptation to rapid warming through evolutionary means. By contrast, sea turtles have long generation intervals of approximately 20–30 years [32], which constrains the rate of microevolution. Furthermore, if changes in female nesting behaviour cannot compensate for warmer incubation temperatures, sea turtle embryos are left with few options to avoid heat stress. The extent to which expression of hsps is heritable, or can be modified through plastic responses, will affect their ability to change their critical thermal limits.
Here, we investigated phenotypic and genetic variation of hsp gene expression in the embryos of loggerhead sea turtles (Caretta caretta), which are vulnerable to reduced fitness and higher mortality when exposed to high temperatures in terrestrial nests [33,34]. We first determined whether hsp expression differed between embryos from a temperate and a sub-tropical rookery. Although hsp sequences are generally highly conserved within and between species [11–17], we expected to find regional variation in hsp expression profiles. Secondly, we tested for and estimated the heritability of hsp expression, and the plasticity of hsp expression, in response to thermal stress. Our results provide some key parameters needed for understanding whether long-lived reptiles could adapt to the unprecedented pace of contemporary climate change through plastic and/or evolutionary adaptation.
2. Material and methods
Full methods and associated references are available in the electronic supplementary material, Materials and methods.
(a). Study species and sample collection
The Western Australia population of Caretta caretta nest at Turtle Bay on Dirk Hartog Island (DHI; 25.49827° S, 112.98719° E) and at smaller mainland rookeries including Bungelup Beach (BB; 22.282331° S, 113.831570° E) (figure 1). We collected a total of 1280 eggs from both nesting sites during peak nesting activity in January of 2013 and 2014. Eggs were collected, transported and monitored according to University of Western Australia ethics protocols. Details of these protocols can be found in the electronic supplementary material, Material and methods.
Figure 1.
Dirk Hartog Island (DHI) and Bungelup Beach (BB) are two range-edge C. caretta rookeries in Western Australia, isolated by approximately 520 km of coastline [35] and 3° latitude. DHI is located within a temperate zone while BB is a located within a sub-tropical zone [36]. Map adapted from [37].
(b). Incubation and heat-shock experiments
Eggs from each female were randomly distributed among 1.5 l plastic containers to reduce clutch effects (DHI: 80 containers each with 15 eggs, BB: 20 containers each with four eggs). All eggs were held at a constant 29°C (±0.3°C) until time of heat-shock treatment. A heat shock of 36°C for 3 h was applied to the treatment group 45 days into the incubation period, following previously established protocols for inducing thermal stress in C. caretta [17]. After a 1-h cool down period, embryos were removed from the egg, weighed and given a lethal injection of MS-222 (50 mg kg−1, Sigma) [17,38] followed by decapitation to ensure death.
(c). RNA extraction and RT-qPCR
Two independent RNA extractions of heart tissue from up to five embryos per treatment group were done using the FavorPrep™ Total Tissue Mini RNA Kit (FATRK-300, Fisher Biotec) without DNase treatments, following the manufacturer's instructions. Total RNA was eluted in nuclease-free water and quantified using a Qubit v. 2.0 Fluorometer (Invitrogen). Two hundred nanograms of total RNA was reverse-transcribed into cDNA using the oligo (dT)16 primer with MultiScribe™ MuLV Reverse Transcriptase in a High Capacity RNA-to-cDNA Kit (4387406, Invitrogen), following the manufacturer's protocol. Quantitative real-time PCR was performed in triplicate using the iTaq Universal SYBR® Green Supermix (172–5121, BioRad) on a Step-One Plus PCR System (Applied Biosystems) with the following program: 95°C for 10 min; 40 cycles of 95°C for 10 s, 60°C for 60 s. RT-qPCR was performed in a 10 µl reaction with 10 ng of cDNA and final primer concentration of 200 nM. The gene-specific primers used for RT-qPCR are listed in the electronic supplementary material, table S1. Target gene mRNA expression levels were normalized to reference gene 18s mRNA expression levels [17].
(d). Statistical analyses
Cycle threshold (Cq) values were converted to relative gene expression values ΔCq and ΔΔCq using the methods described in similar studies [17,39]. Details of our calculations are provided in the electronic supplementary material, Material and methods.
A linear mixed-effects model was used to estimate variance components between and within rookeries. The model included rookery and clutch nested within rookery as random factors. Comparing total variances explained by the full model with a model having one factor removed tested the significance of each level in the analysis. Variance components were calculated using REML, and the VarCorr function in the R package nlme [40]. All ΔCq and ΔΔCq values were log-transformed with 2–X, where X is the mean ΔCq or mean ΔΔCq value, prior to analysis.
Broad- and narrow-sense heritabilities of ΔCq (within-treatment) and ΔΔCq values (across-treatment) for hsp60, hsp70 and hsp90 were estimated using an ‘animal model’ in ASReml 3.0 [41,42], fitting separate animal models to each treatment (control and heat shock), with rookery, offspring identity and dam as random factors. REML likelihood-ratio tests (REML LRT [42]) were used to find the best fitting model, by starting with a fully saturated model and then systematically reducing the number of parameters. Spearman's rank correlation was used to estimate genetic correlation between the expression levels of the genes under each treatment (see also electronic supplementary material, Materials and methods). The statistical power (B) of the correlation tests were calculated using the R package pwr [43].
3. Results and discussion
(a). Clutch is the most important component of variation in basal and increased levels of expression for hsp60, hsp70 and hsp90
We detected significant variation in the hsp expression in response to heat stress. For both the procedural control (29°C, 3 h) and the heat-shock (36°C, 3 h) treatments, the variance components analysis revealed no significant differences in expression between rookeries (table 1). However, there was a significant proportion of variation in hsp expression among clutches within rookeries for all target genes (table 1). Approximately, 30–50% of all variation in hsp expression was due to clutch effects. This suggests that the phenotypic variance explained by clutch may be due to the genetic constitution of the offspring, as well as maternal effects such as yolk quality or the thermal environment during early embryogenesis (e.g. the environment in utero immediately prior to oviposition) [44]. Female C. caretta have been shown to alternate ‘active and stay warm’ and ‘passive and stay cool’ thermoregulation strategies to optimize reproductive output [45,46]. While any flow-on effects of thermoregulation during embryogenesis are yet to be documented in sea turtles, thermoregulatory behaviours in oviparous lizards (Bassiana duperreyi) can have significant effects on hatchling phenotypes, notably on offspring sex [47,48].
Table 1.
Results from the variance components analysis of relative gene expression within treatments (ΔCq) and across treatments (ΔΔCq). Significant variance components at the p < 0.05 level are highlighted in bold text.
|
hsp60 |
hsp70 |
hsp90 |
||||
|---|---|---|---|---|---|---|
| % of total variance | p-value | % of total variance | p-value | % of total variance | p-value | |
| ΔCq 29°C | ||||||
| rookery | 22.7 | 0.215 | 0.0 | 0.999 | 0.0 | 0.999 |
| clutch | 33.3 | <0.001 | 28.0 | 0.002 | 23.0 | 0.009 |
| ΔCq 36°C | ||||||
| rookery | 14.6 | 0.446 | 3.2 | 0.792 | 28.6 | 0.143 |
| clutch | 48.6 | <0.001 | 30.4 | <0.001 | 33.9 | <0.001 |
| ΔΔCq | ||||||
| rookery | 21.3 | 0.264 | 2.9 | 0.683 | 30.4 | 0.037 |
| clutch | 39.3 | <0.001 | 4.5 | 0.442 | 11.8 | 0.041 |
(b). There is geographical variation in the plasticity of expression for hsp90 in response to heat shock
Gene expression assays were conducted on 14 clutches from a temperate rookery (DHI: offspring N = 78) and four clutches from a sub-tropical rookery (BB: offspring N = 18). All offspring increased expression for all target genes in response to an acute heat stress, consistent with a previous study [17]. Expression of hsp90 increased 50.8-fold in embryos from the temperate rookery in response to the heat shock, and 19.3-fold in embryos from the sub-tropical rookery (figure 2). These fold change differences were reflected by the significant proportion of total variance in ΔΔCq between rookeries for hsp90 (table 1). Large fold-changes were also evident for hsp60 and hsp70 (figure 2), but the differences between rookeries for these genes were non-significant (table 1). As found in the within-treatment analyses for all genes, there was a significant proportion of variance in relative gene expression between clutches within rookeries for hsp60 and hsp90.
Figure 2.
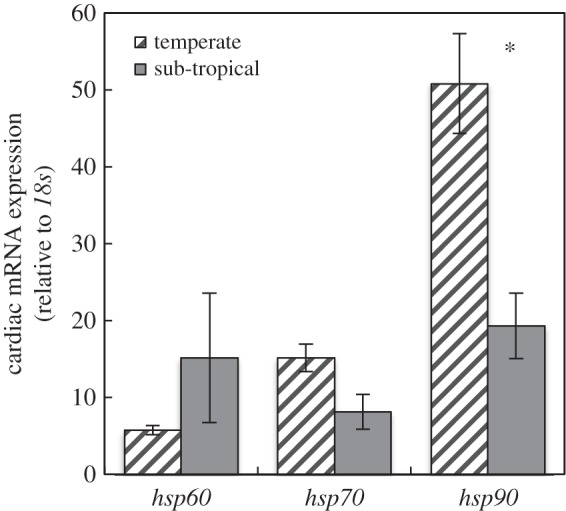
Expression levels of hsp60, hsp70 and hsp90 by C. caretta embryos from a temperate (DHI, hatched bars; n = 129–130 individuals per gene) and sub-tropical (BB, grey bars; n = 30 individuals per gene) rookery in response to an acute heat shock. All mRNA data are normalized to the reference gene 18s (values are clutch mean ± s.e.m.; N = 18). Significant differences (p < 0.05) between rookeries denoted with an asterisk.
Higher relative gene expression in hsp90 in the sub-tropical rookery is consistent with the nest temperature differences between the two study sites [37,49], and suggests that embryos developing on these beaches may be locally adapted to different nest temperatures. On Ascension Island in the South Atlantic Ocean, the black sand of Northeast Bay (NEB) averages 2.6°C warmer than the white sand of Long Beach (LB), and a common garden experiment revealed that offspring of green sea turtles (Chelonia mydas) nesting on these beaches were locally adapted at a fine spatial scale [50]. Although offspring from the NEB rookery exhibited greater tolerance to warmer temperatures, hatching success was significantly lower than at the LB rookery, which led the authors to suggest that embryonic thermotolerance had not evolved in concert with the 0.5°C warming documented at these rookeries over the past 150 years [50].
In our study, stronger evidence for local adaptation would require replication at the rookery level and knowledge of the genetic relationships between nesting females [51,52]. Nevertheless, that hsp90 has significant heritable plasticity in expression may provide C. caretta embryos with a mechanism for increasing their thermotolerance as a response to climate change. Further, the degree to which plasticity allows mean phenotypes to track changes in the thermal environment will determine whether nesting populations evolve to tolerate changes in nest temperature [53]. It should also be noted that studies on Drosophila suggest that populations that evolved in high temperature environments do not upregulate Hsps to the same degree when exposed to heat stress compared to populations from cooler habitats [54]. This suggests mechanisms other than Hsp expression are more important for coping with high temperatures in permanently high temperature environments in some taxa.
(c). Variation in hsp expression is heritable in Caretta caretta
The animal models used to estimate heritability (see Material and methods) considered the effects of rookery, offspring identity and dam on hsp expression. Rookery had no significant effect on phenotype and was thereafter excluded from all models. The REML LRT showed that offspring was the best fitting pedigree factor in the animal model for hsp70, and so narrow-sense heritability was estimated for this gene. For hsp60, narrow-sense heritability was estimable in the heat shock treatment at 36°C, but not in the procedural control treatment at 29°C (table 2). The estimates of narrow-sense heritability for expression of hsp60 and hsp70 were high relative to those expected of physiological traits for ectotherms [55]. In general, physiological traits (e.g. O2 consumption, resistance to heat stress) have mid-level heritabilities (approx. 0.33), similar to those for behavioural traits (e.g. approx. 0.30), greater than those for life-history traits (e.g. approx. 0.26), but much lower than those for morphological traits (e.g. approx. 0.46) [55].
Table 2.
Heritability estimates for each heat shock gene within each treatment (ΔCq) and across treatments (ΔΔCq). Heritability values are listed with ±s.e.; all are narrow-sense heritabilities, with the exception of values denoted with a superscript, which are broad-sense heritabilities.
| hsp60 | hsp70 | hsp90 | |
|---|---|---|---|
| ΔCq 29°C | 0.51 ± 0.11a | 0.88 ± 0.23 | 0.32 ± 0.12a |
| ΔCq 36°C | 0.79 ± 0.28 | 0.75 ± 0.27 | 0.21 ± 0.13a |
| ΔΔCq | 0.38 ± 0.21 | 0.20 ± 0.10 | 0.37 ± 0.10a |
aBroad sense heritability (i.e. ‘dam’) was the only pedigree factor included in the best fitting model. Significance of heritability estimates determined using REML likelihood-ratio tests [42] are shown in bold.
Although the heritability estimates of the plastic response of all three hsp genes were lower than the heritability estimates of the relative expression of each gene within treatments, they were nevertheless significant for hsp70 and hsp90 (table 2). This is not an unexpected result, as heritability estimates for plastic responses of a trait are typically lower than the heritability estimate of the trait itself (reviewed by [12,56]). Taken together, our results show that populations have the potential to adapt through selection to increase the baseline expression levels of hsps (ΔCq) and also through selection on plasticity on hsp70 and hsp90 (ΔΔCq). Such a capacity to adapt to warmer and more variable climates is not universal, even in species with large population sizes. For example, low additive genetic variance and a non-significant response to selection for upper thermal limits was found in a marine copepod [57]; and in a freshwater live-bearing fish [58]. Rainforest restricted Drosophila also appear to lack genetic variance for desiccation [59], though significant evolutionary responses in desiccation resistance has been reported under mild desiccation stress [60].
The differences in heritability estimates between relative expression and plastic expression could be due to a cost–benefit trade-off [56,61] in which expression of hsp60 and hsp70 are regulated to minimize any detrimental effects of overexpression [13]. Overexpression of hsp70 compromises the fitness and survival of Drosophila larvae [61], but the rate at which developmental temperatures rise may affect how relative expression of hsp70 evolves in natural populations [62]. Hence, it is possible that moderate increases in relative expression of hsp60 and hsp70 (figure 2) produce a short-term thermotolerance response in embryonic C. caretta, but maximal levels of expression are tightly controlled to ensure embryonic survival.
For hsp90, only broad-sense heritability was detected. Relative to hsp60 and hsp70, the low, but significant heritability estimates for hsp90 were similar to estimates for other physiological traits in other species [55,56,62]. The broad-sense heritabilities we estimated do not exclude the possibility of additive genetic variance in the expression of these genes, but do suggest significant maternal, environmental or dominance effects. Clearly, model species such as Drosophila would be more amenable to separating these effects. However, as the heritability of relative expression of hsp90 has not been previously estimated in reptiles, future studies might examine whether increased expression levels of this hsp are consistent across clutches of individual females. For example, sampling from the multiple clutches laid by females within a single season could reveal if the heritability of plasticity in expression of hsp90 is characterized more by maternal or environmental effects.
Relative gene expression in the heat-shock treatment was positively correlated with relative gene expression in the procedural control treatment for hsp60 (Spearman's rank correlation coefficient rs = 0.911, n = 18, p ≤ 0.001), hsp70 (rs = 0.624, n = 18, p ≤ 0.05) and hsp90 (rs = 0.515, n = 18, p ≤ 0.05). Thus, clutches that express highly in the control treatment also express highly in the heat-shock treatment. These correlations suggest genes that increase expression in one thermal environment will also increase expression in another.
The basal expression levels of different genes within environments were not significantly correlated in the control treatment for hsp60 and hsp70 (rs = 0.385, n = 18, p = 0.114), hsp60 and hsp90 (rs = 0.259, n = 18, p = 0.300) or hsp70 and hsp90 (rs = 0.424, n = 18, p = 0.080). In the heat-shock treatment, expression levels for hsp60 and hsp70 were significantly correlated (rs = 0.488, n = 18, p = 0.040). Increased expression levels hsp60 and hsp90 were marginally non-significant (rs = 0.451, n = 18, p = 0.060). Finally, increased expression levels of hsp70 and hsp90 were not significantly correlated (rs = 0.152, n = 18, p = 0.550). These correlations are all positive and suggest that selection for increased basal expression levels in one gene does, in general, give rise to increased expression in other genes (although here our sample sizes were too low to detect significant effects in most cases; statistical power ranged from 0.092 to 0.487 for non-significant correlations). Stress-induced transcription of hsp genes is regulated by heat-shock transcription factors (HSFs), which bind to the heat shock element sequences present in the promoters of hsp genes [63,64]. There are multiple HSFs in vertebrates (HSF1–4), which may provide redundancy and specialization of stress signals, though HSF1 appears to be the primary regulator of heat-inducible hsp gene expression in most vertebrate cells [64,65].
4. Conclusion
This is the first study to examine geographical and genetic variation in a physiological response to heat stress in sea turtle embryos, and the heritability of that response at the molecular level for any reptile species. Evolutionary adaptation to changing environments requires traits to be heritable, and we have shown heritability of expression of heat shock genes under both control and heat-stress conditions, and that the plasticity of expression of these heat shock genes is also heritable. Expression levels of hsp60 and hsp70 were attributed to both maternal and additive effects; expression levels of hsp90 were characterized by maternal and environmental effects. Additionally, we found strong correlations between expression levels of all target genes and incubation environments, suggesting that an elevated level of baseline expression results in relatively higher expression under thermal stress. While the relevance of our results to survival of embryos under field conditions are equivocal, they provide strong evidence of molecular mechanisms for tolerating and/or adapting to higher incubation temperatures. Of pressing concern is that no matter how many routes to adaption can be demonstrated, the pace of contemporary climate change may outstrip the evolutionary potential of slow-breeding species.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank David Holley and Chris McMonagle (Western Australian Department of Parks and Wildlife, DPaW) in Shark Bay, and Peter Barnes and Keely Markovina (DPaW, Ningaloo Turtle Program in Cape Range National Park) for facilitating site access and fieldwork support. Blair Bentley, Adelaide Bevilaqua, Christie Craig, Kasey Darts, Frankie Hoult and Frances Leng provided invaluable support in the field. Additionally, we are grateful to Sherralee Lukehurst and Yvette Hitchen for assistance with the molecular work.
Ethics
All research described here adhered to local guidelines, and all appropriate ethical approval and licences were obtained.
Data accessibility
The datasets supporting this article have been uploaded onto DRYAD and as part of the electronic supplementary material.
Author's contributions
J.N.T., W.J.K. and N.J.M. conceived and designed the research, and S.W. provided substantial funding. J.N.T. carried out the molecular work and data analysis, the latter with assistance from W.J.K. J.L.T. performed heritability estimates. J.N.T., W.J.K., J.L.T., O.B., S.W., M.G.M. and N.J.M. wrote the manuscript. All authors approved the manuscript for publication.
Competing interests
The authors have no competing interests.
Funding
This project was funded by the Western Australian Department of Parks and Wildlife (to J.N.T.), the ANZ Holsworth Wildlife Trust (to N.J.M and J.N.T.) and the School of Animal Biology at the University of Western Australia. Funding from the Australian Institute of Marine Science and the Commonwealth Scientific and Industrial Research Organisation also provided scholarship support to J.N.T.).
References
- 1.Skelly DK, Joseph LN, Possingham HP, Freidenburg LK, Farrugia TJ, Kinnison MT, Hendry AP. 2007. Evolutionary responses to climate change. Conserv. Biol. 21, 1353–1355. () [DOI] [PubMed] [Google Scholar]
- 2.Hoffmann AA, Sgrò CM. 2011. Climate change and evolutionary adaptation. Nature 470, 479–485. ( 10.1038/nature09670) [DOI] [PubMed] [Google Scholar]
- 3.Bellard C, Bertelsmeier C, Leadley P, Thuiller W, Courchamp F. 2012. Impacts of climate change on the future of biodiversity. Ecol. Lett. 15, 365–377. () [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adolph SC. 1990. Influence of behavioral thermoregulation on microhabitat use by two Sceloporus lizards. Ecology 71, 315–327. ( 10.2307/1940271) [DOI] [Google Scholar]
- 5.Urban MC, Richardson JL, Freidenfelds NA. 2014. Plasticity and genetic adaptation mediate amphibian and reptile responses to climate change. Evol. Appl. 7, 88–103. ( 10.1111/eva.12114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du W-G, Zhao B, Chen Y, Shine R. 2011. Behavioral thermoregulation by turtle embryos. Proc. Natl Acad. Sci. USA 108, 9513–9515. ( 10.1073/pnas.1102965108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradshaw AD. 1965. Evolutionary significance of phenotypic plasticity in plants. Adv. Genet. 13, 115–155. ( 10.1016/S0065-2660(08)60048-6) [DOI] [Google Scholar]
- 8.Feder ME, Hofmann GE. 1999. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu. Rev. Physiol. 61, 243–282. ( 10.1146/annurev.physiol.61.1.243) [DOI] [PubMed] [Google Scholar]
- 9.Kültz D. 2005. Molecular and evolutionary basis of the cellular stress response. Annu. Rev. Physiol. 67, 225–257. ( 10.1146/annurev.physiol.67.040403.103635) [DOI] [PubMed] [Google Scholar]
- 10.Sorensen JG, Kristensen TN, Loeschcke V. 2003. The evolutionary and ecological role of heat shock proteins. Ecol. Lett. 6, 1025–1037. () [DOI] [Google Scholar]
- 11.Mailhos C, Howard MK, Latchman DS. 1994. Heat shock proteins hsp90 and hsp70 protect neuronal cells from thermal stress but not from programmed cell death. J. Neurochem. 63, 1787–1795. ( 10.1046/j.1471-4159.1994.63051787.x) [DOI] [PubMed] [Google Scholar]
- 12.Krebs RA, Feder ME, Lee J. 1998. Heritability of expression of the 70KD heat-shock protein in Drosophila melanogaster and its relevance to the evolution of thermotolerance. Evolution (NY) 52, 841–847. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann AA, Sorensen JG, Loeschcke V. 2003. Adaptation of Drosophila to temperature extremes: bringing together quantitative and molecular approaches. J. Therm. Biol. 28, 175–216. () [DOI] [Google Scholar]
- 14.Fangue NA, Hofmeister M, Schulte PM. 2006. Intraspecific variation in thermal tolerance and heat shock protein gene expression in common killifish, Fundulus heteroclitus. J. Exp. Biol. 209, 2859–2872. ( 10.1242/jeb.02260) [DOI] [PubMed] [Google Scholar]
- 15.Sorensen JG, Pekkonen M, Lindgren B, Loeschcke V, Laurila A, Merila J. 2009. Complex patterns of geographic variation in heat tolerance and Hsp70 expression levels in the common frog Rana temporaria. J. Therm. Biol. 34, 49–54. ( 10.1016/j.jtherbio.2008.10.004) [DOI] [Google Scholar]
- 16.Stecyk JAW, Couturier CS, Fagernes CE, Ellefsen S, Nilsson GE. 2012. Quantification of heat shock protein mRNA expression in warm and cold anoxic turtles (Trachemys scripta) using an external RNA control for normalization. Comp. Biochem. Physiol. D Genomics Proteomics 7, 59–72. ( 10.1016/j.cbd.2011.11.001) [DOI] [PubMed] [Google Scholar]
- 17.Tedeschi JN, Kennington WJ, Berry O, Whiting S, Meekan M, Mitchell NJ. 2014. Increased expression of Hsp70 and Hsp90 mRNA as biomarkers of thermal stress in loggerhead turtle embryos (Caretta caretta). J. Therm. Biol. 47, 42–50. ( 10.1016/j.jtherbio.2014.11.006) [DOI] [PubMed] [Google Scholar]
- 18.Frydenberg J, Hoffmann AA, Loeschcke V. 2003. DNA sequence variation and latitudinal associations in hsp23, hsp26 and hsp27 from natural populations of Drosophila melanogaster. Mol. Ecol. 12, 2025–2032. () [DOI] [PubMed] [Google Scholar]
- 19.Hallas R, Schiffer M, Hoffmann AA. 2002. Clinal variation in Drosophila serrata for stress resistance and body size. Genet. Res. 79, 141–148. ( 10.1017/S0016672301005523) [DOI] [PubMed] [Google Scholar]
- 20.McColl G, McKechnie SW. 1999. The Drosophila heat shock hsr-omega gene: an allele frequency cline detected by quantitative PCR. Mol. Biol. Evol. 16, 1568–1574. ( 10.1093/oxfordjournals.molbev.a026069) [DOI] [PubMed] [Google Scholar]
- 21.Bettencourt BR, Kim I-Y, Hoffmann AA, Feder ME. 2002. Response to natural and laboratory selection at the Drosophila hsp70 genes. Evolution (NY) 56, 1796–1801. () [DOI] [PubMed] [Google Scholar]
- 22.Otsuka Y, Takano TS, Yamazaki T. 1997. Genetic variation in the expression of the six hsp genes in the presence of heat shock in Drosophila melanogaster. Genes Genet. Syst. 72, 19–24. ( 10.1266/ggs.72.19) [DOI] [PubMed] [Google Scholar]
- 23.Pritchard PCH. 1997. Evolution, phylogeny, and current status. In The biology of sea turtles, vol. I (eds Lutz PL, Musick JA), pp. 1–28. Boca Raton, FL: CRC Press. [Google Scholar]
- 24.Poloczanska ES, Limpus CJ, Hays GC. 2009. Vulnerability of marine turtles to climate change. In Advances in marine biology (ed. Sims D.), vol. 56, pp. 151–211. London, UK: Academic Press: ( 10.1016/S0065-2881(09)56002-6) [DOI] [PubMed] [Google Scholar]
- 25.Pyenson ND, Kelley NP, Parham JF. 2014. Marine tetrapod macroevolution: physical and biological drivers on 250 Ma of invasions and evolution in ocean ecosystems. Palaeogeogr. Palaeoclimatol. Palaeoecol. 400, 1–8. ( 10.1016/j.palaeo.2014.02.018) [DOI] [Google Scholar]
- 26.Lohmann KJ, Lohmann CMF, Brothers JR, Putman NF. 2013. Natal homing and imprinting in sea turtles. In The biology of sea turtles vol. III (eds Wyneken J, Lohmann KJ, Musick JA), pp. 59–78. Boca Raton, FL: CRC Press. [Google Scholar]
- 27.Dethmers KEM, et al. 2006. The genetic structure of Australasian green turtles (Chelonia mydas): exploring the geographical scale of genetic exchange. Mol. Ecol. 15, 3931–3946. () [DOI] [PubMed] [Google Scholar]
- 28.Feder ME. 1999. Organismal, ecological, and evolutionary aspects of heat-shock proteins and the stress response: established conclusions and unresolved issues. Am. Zool. 39, 857–864. ( 10.1093/icb/39.6.857) [DOI] [Google Scholar]
- 29.Howard R, Bell I, Pike DA. 2014. Thermal tolerances of sea turtle embryos: current understanding and future directions. Endanger. Species Res. 26, 75–86. ( 10.3354/esr00636) [DOI] [Google Scholar]
- 30.McColl G, Hoffmann AA, McKechnie SW. 1996. Response of two heat shock genes to selection for knockdown heat resistance in Drosophila melanogaster. Genetics 143, 1615–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bettencourt BR, Feder ME, Cavicchi S. 1999. Experimental evolution of Hsp70 expression and thermotolerance in Drosophila melanogaster. Evolution (NY) 53, 484–492. ( 10.2307/2640784) [DOI] [PubMed] [Google Scholar]
- 32.Heppell SS, Snover ML, Crowder LB. 2003. Sea turtle population ecology. In The biology of sea turtles vol. II (eds Lutz J, Musick PL, Wyneken JA), pp. 287–306. Boca Raton, FL: CRC Press. [Google Scholar]
- 33.Reece SE, Broderick AC, Godley BJ, West SA. 2002. The effects of incubation environment, sex and pedigree on the hatchling phenotype in a natural population of loggerhead turtles. Evol. Ecol. Res. 4, 737–748. [Google Scholar]
- 34.Fisher LR, Godfrey MH, Owens DW. 2014. Incubation temperature effects on hatchling performance in the loggerhead sea turtle (Caretta caretta). PLoS ONE 9, e114880 ( 10.1371/journal.pone.0114880) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reinhold L, Whiting A. 2014. High-density loggerhead sea turtle nesting on Dirk Hartog Island, Western Australia. Mar. Turt. Newsl. 141, 7–10. [Google Scholar]
- 36.Pike DA. 2013. Climate influences the global distribution of sea turtle nesting. Glob. Ecol. Biogeogr. 22, 555–566. ( 10.1111/geb.12025) [DOI] [Google Scholar]
- 37.Trocini S. 2013. Health assessment and hatching success of two Western Australian loggerhead turtle (Caretta caretta) populations. PhD Thesis, Murdoch University, Perth, Australia.
- 38.Conroy CJ, Papenfuss T, Parker J, Hahn NE. 2009. Use of tricaine methanesulfonate (MS222) for euthanasia of reptiles. J. Am. Assoc. Lab. Anim. Sci. 48, 28–32. [PMC free article] [PubMed] [Google Scholar]
- 39.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25, 402–408. ( 10.1006/meth.2001.1262) [DOI] [PubMed] [Google Scholar]
- 40.Pinheiro J, Bates D, DebRoy S, Sarkar D, orpR Core Team 2014. nlme: linear and nonlinear mixed effects models. R package version 3.1–117. See https://cran.r-project.org/web/packages/nlme/index.html.
- 41.Gilmour AR, Thompson R, Cullis BR. 1995. Average information REML: an efficient algorithm for variance parameter estimation in linear mixed models. Biometrics 51, 1440–1450. ( 10.2307/2533274) [DOI] [Google Scholar]
- 42.Gilmour AR, Gogel BJ, Cullis BR, Thompson R. 2009. ASReml user guide release 3.0. Hemel Hempstead, UK: VSN Int. Ltd. [Google Scholar]
- 43.Champely S, Ekstrom C, Dalgaard P, Gill J, De Rosario H, orpR Core Team 2015. Pwr: basic functions for power analysis. R package version 1.1–2. See https://cran.r-project.org/web/packages/pwr/pwr.pdf.
- 44.Shine R, Elphick MJ, Harlow PS. 1997. The influence of natural incubation environments on the phenotypic traits of hatchling lizards. Ecology 78, 2559–2568. () [DOI] [Google Scholar]
- 45.Schofield G, Bishop CM, Katselidis KA, Dimopoulos P, Pantis JD, Hays GC. 2009. Microhabitat selection by sea turtles in a dynamic thermal marine environment. J. Anim. Ecol. 78, 14–21. () [DOI] [PubMed] [Google Scholar]
- 46.Fossette S, Schofield G, Lilley MKS, Gleiss AC, Hays GC. 2012. Acceleration data reveal the energy management strategy of a marine ectotherm during reproduction. Funct. Ecol. 26, 324–333. () [DOI] [Google Scholar]
- 47.Shine R, Elphick MJ, Harlow PS. 1995. Sisters like it hot. Nature 378, 451–452. ( 10.1038/378451a0) [DOI] [Google Scholar]
- 48.Robert KA, Thompson MB. 2006. Sex determination: viviparous lizard selects sex of embryos. Nature 412, 698–699. ( 10.1038/35089135) [DOI] [PubMed] [Google Scholar]
- 49.Woolgar L, Trocini S, Mitchell N. 2013. Key parameters describing temperature-dependent sex determination in the southernmost population of loggerhead sea turtles. J. Exp. Mar. Biol. Ecol. 449, 77–84. ( 10.1016/j.jembe.2013.09.001) [DOI] [Google Scholar]
- 50.Weber SB, Broderick AC, Groothuis TGG, Ellick J, Godley BJ, Blount JD. 2012. Fine-scale thermal adaptation in a green turtle nesting population. Proc. R. Soc. B 279, 1077–1084. ( 10.1098/rspb.2011.1238) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ghalambor CK, McKay JK, Carroll SP, Reznick DN. 2007. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol. 21, 394–407. () [DOI] [Google Scholar]
- 52.Chown SL, Hoffmann AA, Kristensen TN, Angilletta MJ, Stenseth NC, Pertoldi C. 2010. Adapting to climate change: a perspective from evolutionary physiology. Clim. Res. 43, 3–15. ( 10.3354/cr00879) [DOI] [Google Scholar]
- 53.Mousseau TA, Roff DA. 1987. Natural selection and the heritability of fitness components. Heredity (Edinb.) 59, 181–197. ( 10.1038/hdy.1987.113) [DOI] [PubMed] [Google Scholar]
- 54.Sorensen JG, Dahlgaard J, Loeschcke V. 2001. Genetic variation in thermal tolerance among natural populations of Drosophila buzzatii: down regulation of Hsp70 expression and variation in heat stress resistance traits. Funct. Ecol. 15, 289–296. () [DOI] [Google Scholar]
- 55.Scheiner SM. 1993. Genetics and evolution of phenotypic plasticity. Annu. Rev. Ecol. Syst. 24, 35–68. ( 10.1146/annurev.es.24.110193.000343) [DOI] [Google Scholar]
- 56.Scheiner SM, Lyman RF. 1989. The genetics of phenotypic plasticity I. Heritability. J. Evol. Biol. 107, 95–107. () [DOI] [Google Scholar]
- 57.Kelly MW, Sanford E, Grosberg RK. 2012. Limited potential for adaptation to climate change in a broadly distributed marine crustacean. Proc. R. Soc. B 279, 349–356. ( 10.1098/rspb.2011.0542) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baer CF, Travis J. 2000. Direct and correlated responses to artificial selection on acute thermal stress tolerance in a livebearing fish. Evolution 54, 238–244. () [DOI] [PubMed] [Google Scholar]
- 59.Hoffmann AA, Hallas RJ, Dean JA, Schiffer M. 2003. Low potential for climatic stress adaptation in a rainforest Drosophila species. Science (80-.). 301, 100–102. ( 10.1126/science.1084296) [DOI] [PubMed] [Google Scholar]
- 60.van Heerwaarden B, Sgrò CM. 2014. Is adaptation to climate change really constrained in niche specialists? Proc. R. Soc. B 281, 20140396 ( 10.1098/rspb.2014.0396) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krebs RA, Feder ME. 1997. Nautral variation in the expression of the heat shock protein Hsp70 in a population of Drosophila melanogaster and its correlation with tolerance of ecologically relevant thermal stress. Evolution (NY) 51, 173–179. [DOI] [PubMed] [Google Scholar]
- 62.Feder ME, Blair N, Figueras H. 1997. Natural thermal stress and heat-shock protein expression in Drosophila larvae and pupae. Funct. Ecol. 11, 90–100. () [DOI] [Google Scholar]
- 63.Morimoto RI. 1998. Regulation of the heat-shock transcriptional response: cross talk between a family of heat-shock factors, molecular chaperones, and negative regulators. Genes Dev. 12, 3788–3796. ( 10.1101/gad.12.24.3788) [DOI] [PubMed] [Google Scholar]
- 64.Voellmy R. 2004. On mechanisms that control heat shock transcription factor activity in metazoan cells. Cell Stress Chaperones 9, 122–133. ( 10.1379/CSC-14R.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shamovsky I, Nudler E. 2008. New insights into the mechanism of heat shock response activation. Cell. Mol. Life Sci. 65, 855–861. () [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded onto DRYAD and as part of the electronic supplementary material.


