Abstract
Growing evidence suggests that plant secondary compounds (PSCs) ingested by mammals become more toxic at elevated ambient temperatures, a phenomenon known as temperature-dependent toxicity. We investigated temperature-dependent toxicity in the desert woodrat (Neotoma lepida), a herbivorous rodent that naturally encounters PSCs in creosote bush (Larrea tridentata), which is a major component of its diet. First, we determined the maximum dose of creosote resin ingested by woodrats at warm (28–29°C) or cool (21–22°C) temperatures. Second, we controlled the daily dose of creosote resin ingested at warm, cool and room (25°C) temperatures, and measured persistence in feeding trials. At the warm temperature, woodrats ingested significantly less creosote resin; their maximum dose was two-thirds that of animals at the cool temperature. Moreover, woodrats at warm and room temperatures could not persist on the same dose of creosote resin as woodrats at the cool temperature. Our findings demonstrate that warmer temperatures reduce PSC intake and tolerance in herbivorous rodents, highlighting the potentially adverse consequences of temperature-dependent toxicity. These results will advance the field of herbivore ecology and may hone predictions of mammalian responses to climate change.
Keywords: detoxification, endotherms, climate change, thermal neutral zone, maximum dose, temperature-dependent toxicity
1. Introduction
The ability of herbivores to tolerate plant toxins plays a crucial role in herbivore feeding behaviour and diet selection [1]. Plant secondary compounds (PSCs) are produced by plants as a defence against herbivory [2]. In turn, herbivores have evolved behavioural and physiological countermeasures to avoid, metabolize or tolerate PSCs [3–5]. The capacity of herbivores to consume PSCs has traditionally been predicted by the detoxification limitation hypothesis [1,6], which posits that all herbivores have upper limits for PSCs. These thresholds are largely influenced by intrinsic factors, such as hepatic biotransformation enzyme activity [7,8]. Laboratory studies have established that mammalian herbivores ranging from domesticated goats and sheep [9,10] to wild marsupials [11,12] and rodents [13,14] significantly reduce food intake to avoid over-ingestion of PSCs, thereby remaining below PSC threshold doses (i.e. the amount of PSCs willingly ingested by an herbivore [13]).
Extrinsic factors are rarely considered in detoxification limits of herbivores, yet ambient temperature could be greatly impacting PSC tolerance in mammalian herbivores. Evidence from pharmacological [15,16] and agricultural studies [17–19] suggests that chemical toxicity is temperature-dependent, whereby compounds became more toxic at warmer temperatures. The mechanism, referred to as temperature-dependent toxicity, is the likely result of reduced liver function through changes in toxin clearance time, enzymatic reactions or gene expression of crucial detoxification pathways [20,21]. In addition to artificial systems, there is evidence for temperature-dependent toxicity from an ecologically and evolutionarily relevant context. Data from two species of herbivorous woodrats (genus Neotoma) revealed that woodrats decreased food intake of toxic plants and demonstrated reduced liver function at warmer ambient temperatures [22–24]. The interaction between temperature and liver function could have critical implications for mammalian herbivores that must balance PSC detoxification with thermoregulation, particularly in a warming environment.
Here, we expand upon previous studies of temperature-dependent toxicity by investigating the effect of temperature-dependent toxicity on PSC tolerance in the desert woodrat (Neotoma lepida). We chose this species because woodrats from the Mojave Desert can ingest large quantities of creosote bush (Larrea tridentata; up to 75% of diet [25,26]). The resin from creosote leaves contains a complex mixture of hundreds of PSCs with the most abundant component being nordihydroguaiaretic acid, a phenolic ligand and known feeding deterrent [27]. Desert woodrats can consume doses of these PSCs that cause kidney cysts and death in laboratory rodents [28,29].
The objective of this study was to determine the effect of ambient temperature on PSC tolerance, as defined by creosote resin intake, in desert woodrats. We hypothesized that woodrats at warmer temperatures would display a lower tolerance to PSCs than woodrats at cooler temperatures. We provided ecologically relevant PSCs from creosote resin in experimental diets to N. lepida housed at different temperatures. PSC tolerance was measured by determining the maximum dose of creosote resin at cool (22°C) and warm (28°C) temperatures. We predicted that animals at the warm temperature would have a lower maximum dose than animals at the cool temperature. In a second experiment, we controlled for the dose (g per day) of creosote resin ingested by animals and measured PSC tolerance at cool (21°C), warm (29°C) and room (25°C) temperatures. We predicted a gradient result, with animals at the warm temperature demonstrating low tolerances, animals at 25°C demonstrating intermediate tolerances, and animals at the cool temperature demonstrating high tolerances for creosote resin.
2. Material and methods
(a). Animal and plant collection
Woodrats and creosotes leaves were collected from the Mojave Desert in southwestern Utah (37°06′ N, 133°58′ W) in July 2011 and May 2012. Animals were transported to an animal facility at the University of Utah and maintained in the laboratory for three weeks before experiments. Plant material was stored at −20°C, and creosote resin was extracted from leaves as described previously. For further details, see electronic supplementary material.
(b). Temperature treatments
Animals were housed at cool (21–22°C), room (25°C) and warm (28–29°C) ambient temperatures during experiments. These temperatures regimes were chosen based upon previous work investigating temperature-dependent responses in Neotoma [22–24,30], the ecological relevance for Mojave Desert N. lepida [24] and the metabolic physiology of N. lepida. In endotherms, there is a range of intermediate ambient temperatures over which metabolic rate is at its lowest and where mammals can maintain thermal homeostasis without additional energy expenditure called the thermal neutral zone (TNZ) [31]. The TNZ for N. lepida ranges 25–34°C; thus, the warm (28–29°C) and room (25°C) treatments are within the TNZ of N. lepida, whereas the cool (21–22°C) treatment is just below the TNZ [24]. None of the ambient temperatures used herein are considered thermally stressful to N. lepida, and similar food intakes at 25°C and 29°C confirmed equivalent energetic costs at these temperatures (electronic supplementary material, figure S1). Temperatures were regulated with thermostats and space heaters (DeLonghi, USA), when necessary, and were measured every 10 min with HOBO data loggers.
(c). Maximum dose of creosote resin at two temperatures
The effect of temperature on the maximum dose of PSCs ingested by N. lepida was determined in a feeding trial by gradually increasing the concentration of creosote resin added to the diet. The maximum dose was defined as the greatest dose of creosote resin consumed by each animal within a 24 h period (g resin ingested per day) during the trial [13]. Woodrats (n = 16) were acclimated to cool or warm temperatures for 14–21 days before the trial was conducted. Woodrats were housed in metabolic cages (Lab Products Inc., USA), and were provided ad libitum food and water throughout the experiment, whereas the dietary concentration of creosote resin slowly increased from 0% to 12% over 21 days (electronic supplementary material, table S3). Total food, creosote resin and water intake, and body mass were recorded daily. Woodrats were removed from the experiment when they lost more than 10% of their starting body mass, because further loss could be lethal. Intake data were log + 1-transformed. Maximum dose and average intake values were analysed with one-way ANOVAs across temperatures.
(d). Controlled dose of creosote resin at three temperatures
We conducted a second feeding trial to control for ingested doses (g per day) of creosote resin to ensure all woodrats consumed the same daily amount of creosote resin. The effect of temperature on food intake, body mass maintenance and persistence in the trial was determined for woodrats acclimated to one of three temperatures (cool, warm and room; n = 10 per group) for 14–21 days. Woodrats entered a 10-day feeding trial and the amount of creosote resin ingested was controlled at a constant, daily dose of 0.36 g resin per day, which is below the maximum dose determined for warm and cool temperatures in the first experiment (electronic supplementary material, table S4). Woodrats were provided ad libitum food and water, and were housed in shoebox cages with feeder hoods (Lab Products Inc.) to permit accurate estimates of food intake. Body mass and intakes of food, creosote resin and water were measured daily. Woodrats were removed from the trial if they lost more than 10% of starting body mass, and the number of days the animals remained in the trial were measured as persistence. Intake data were log-transformed. Daily average intake data were analysed with one-way ANOVAs across temperature treatments with Tukey's honest significance difference post hoc tests. Persistence in the trial was analysed with Kaplan–Meier survival curves and pairwise comparisons with Bonferroni corrections were conducted across temperatures. Statistical analyses were performed in R (R Core Team).
3. Results
(a). Maximum dose of creosote resin
Ambient temperature significantly affected the maximum tolerated dose of creosote resin ingested by desert woodrats. Warm-acclimated (28°C) animals had a maximum dose that was only two-thirds that of cool animals (22°C; figure 1; F1,14 = 9.1, p = 0.009). Temperature also influenced food, and creosote resin intake averaged across all days. Woodrats at 22°C ingested on average 63% more food (figure 2a; F1,14 = 60.1, p < 0.001) and 29% more creosote resin (figure 2b; F1,14 = 6.7, p = 0.021) per day compared with animals at 28°C. Daily and cumulative intake values also reflected this pattern (electronic supplementary material, figures S2 and S3). Average daily water intake did not differ between temperatures (electronic supplementary material, table S5; F1,14 = 0.8, p = 0.395).
Figure 1.
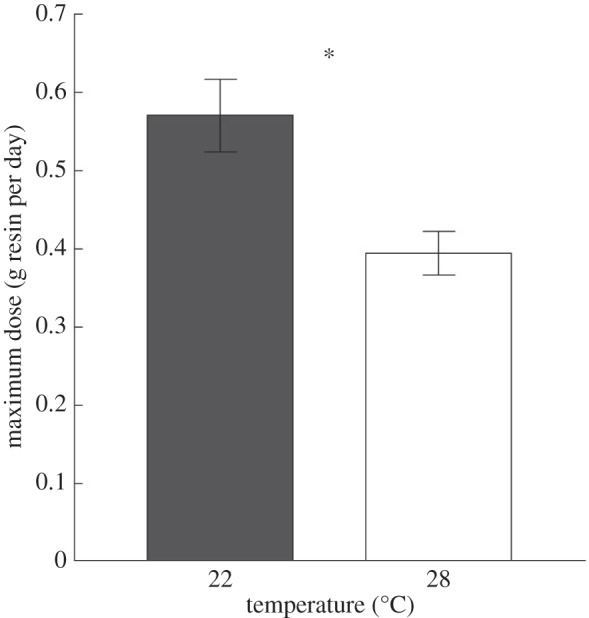
Maximum dose (mean ± s.e.) of creosote resin for woodrats acclimated to two ambient temperatures. Asterisk indicates significance.
Figure 2.
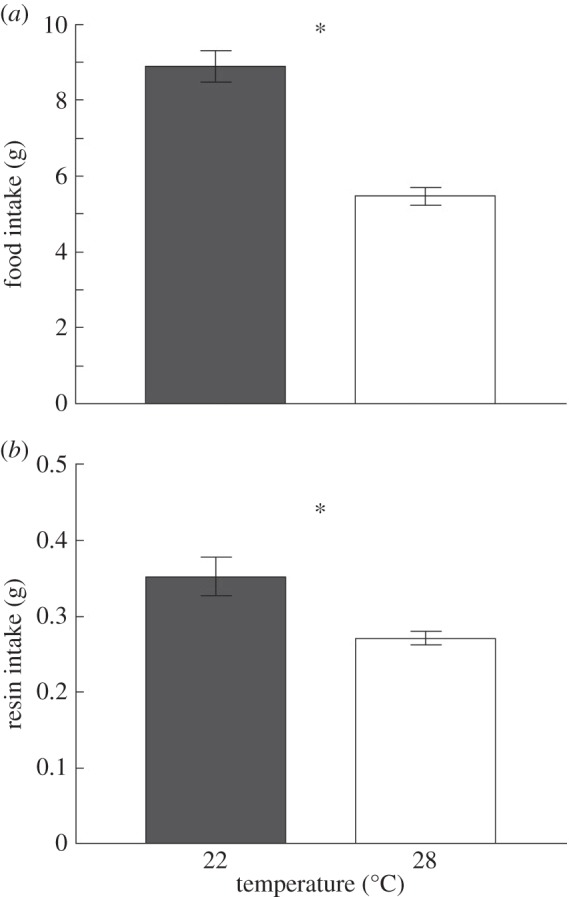
(a) Food and (b) creosote resin intake (mean ± s.e.) of woodrats at two temperatures averaged per individual (n = 9 at cool; n = 7 at warm) during 21 day trial. Asterisk indicates significance.
(b). Controlled dose of creosote resin
Creosote resin intake did not differ between temperature treatments during the second feeding trial (figure 3a; F2,27 = 1.7, p = 0.206); all woodrats ingested on average 0.36 g resin per day. There was no significant difference in food intake between warm (29°C) and room (25°C) temperatures (figure 3b; p = 0.346), but woodrats at the cool (21°C) temperature ingested three times more food than animals at other temperatures (figure 3b; F2,27 = 87.7, p < 0.001). Initial body mass (F2,57 = 1.0, p = 0.37) and water intake (electronic supplementary material, table S5; F2,27 = 1.0, p = 0.37) did not differ across temperatures.
Figure 3.
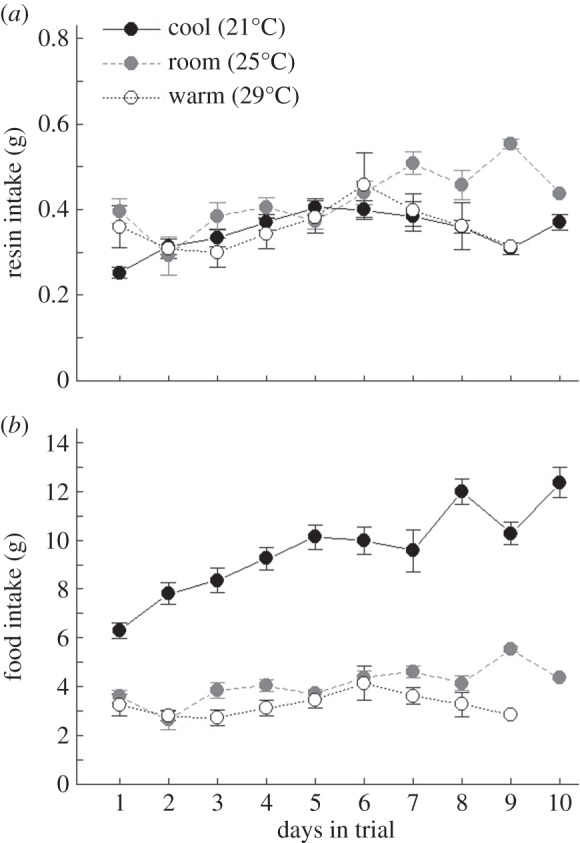
Daily (a) creosote resin and (b) food intake (mean ± s.e.) of woodrats at three temperatures (cool, black circles; room, grey circles; warm, white circles).
There was a significant effect of temperature on woodrat persistence in the trial. Woodrats at 25°C and 29°C lost more body mass and were removed from the trial sooner compared with woodrats at 21°C (figure 4; 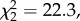 p < 0.001). More than half of the woodrats at 29°C were unable to maintain body mass through day 5, and no animals remained by day 10. Similarly, more than half of the woodrats at 25°C dropped out of the trial by day 7 and only one animal remained by day 10. In contrast, all animals at 21°C persisted through the end of the trial. However, there was no difference in persistence between 25°C and 29°C (figure 4;
p < 0.001). More than half of the woodrats at 29°C were unable to maintain body mass through day 5, and no animals remained by day 10. Similarly, more than half of the woodrats at 25°C dropped out of the trial by day 7 and only one animal remained by day 10. In contrast, all animals at 21°C persisted through the end of the trial. However, there was no difference in persistence between 25°C and 29°C (figure 4; 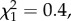 p > 0.5).
p > 0.5).
Figure 4.
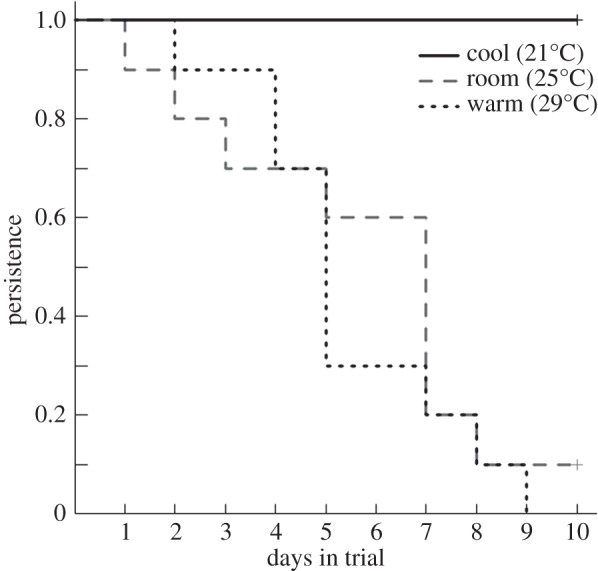
Proportion of woodrats remaining in the trial (persistence) while ingesting a controlled dose of creosote (0.36 g resin per day) at three temperatures (cool, solid line; room, grey dashed line; warm, dotted line).
4. Discussion
The interaction between ambient temperature and PSC tolerance is a relatively unexplored aspect of foraging behaviour, yet an interaction could have serious consequences for mammalian herbivores. Our results support the hypothesis that warm temperatures reduce ingestion of and tolerance to PSCs in the desert woodrat. The maximum dose of creosote resin was 30% lower with only a 6°C increase in temperature. Moreover, woodrats were unable to maintain body mass at temperatures within the TNZ (25°C and 29°C), whereas woodrats at a cooler temperature just below the TNZ (21°C) were able to maintain mass when ingesting the same daily dose of creosote resin. These results provide further support for temperature-dependent toxicity and highlight the potentially negative impacts of this phenomenon on mammalian herbivores.
A temperature-mediated decrease in PSC tolerance could alter woodrat foraging behaviour in nature. As demonstrated here, a critical food source for the Mojave Desert population (i.e. creosote bush) is more potent at warmer temperatures. Woodrats will probably reduce intake of creosote bush by changing meal size and frequency of meals [32]. Diet switching could also be a viable strategy for woodrats to avoid PSCs when annual forbs and grasses are available [25,26], but few alternatives to creosote bush exist during much of the year in the Mojave Desert, particularly during the hottest months [33,34]. Additionally, changes in foraging behaviour could increase risk of predation. Given these constraints, woodrats may be forced to find new habitats to cope with decreased tolerance to creosote bush at warmer temperatures. Indeed, several species of small mammals including Neotoma have shifted their ranges to higher latitudes and elevations over the last 30–50 years, probably owing to climate change [35–37].
Contrary to our predictions, there was no difference in persistence on a controlled dose of creosote resin at 25°C versus 29°C, which are both within the TNZ. PSC tolerance in woodrats was predicted to decrease as temperatures rise across the TNZ owing to decreased liver function, resulting from a reduced ability to dissipate heat at warmer temperatures [21]. It is possible that the animals made physiological changes (e.g. shunting blood to the periphery) or behavioural changes in conductance to increase heat dissipation without reducing liver function. In addition, several liver enzymes are thought to play a role in metabolizing creosote resin ingested by animals held at room temperature [38]. We are currently investigating whether the expression of liver enzymes differs in woodrats exposed to different ambient temperatures.
Our results not only have immediate consequences for Mojave Desert woodrats, but are probably also relevant to other species. Mammalian herbivores must balance the physiological challenges of detoxifying PSCs in their diet with thermoregulation to maintain homeostasis; both these functions are critical roles played by the liver [39]. Temperature-dependent toxicity may also impact other endotherms facing similar physiological challenges, such as omnivorous European starlings [40]. Furthermore, these effects could be size-dependent; smaller mammals inherently have higher energetic costs than their larger counterparts, but can dissipate heat more readily [31]. A broader investigation of temperature-dependent toxicity across endotherms is a promising avenue for future research.
Temperature-dependent toxicity has implications for herbivorous mammals experiencing increased temperatures as a function of global climate change. The frequency and severity of extreme events (e.g. droughts and heat waves) are expected to increase, particularly in desert ecosystems [41,42]. Equally important is the documented rise in temperature minima, which can result in higher average surface temperatures [42]. Recent work also predicts declines in food quality and availability with climate change [37,43]. Temperature-dependent toxicity could amplify the impacts of these changes. Herbivorous mammals are crucial components of many ecosystems; thus, understanding their responses to climate change is imperative to developing conservation strategies.
In summary, our work provides novel evidence for temperature-dependent toxicity in an ecologically and evolutionarily relevant system, and supports the hypothesis that PSC tolerance decreases with ambient temperature. Our results help to fill knowledge gaps concerning the importance of extrinsic factors and their interactions with plant toxins in the current literature. A better understanding of these interactions will be likely to add a new dimension to the field of mammalian herbivore ecology.
Supplementary Material
Acknowledgements
We thank C. McNamara, A. Schmidt and A. Stengel for experimental assistance; Lytle Ranch Preserve, K.D. Kohl, K. Luong and A. Miller for animal collection assistance; and J. Varner, K. Oakeson and C. McArthur for insightful comments on the manuscript.
Ethics
All procedures were approved by University of Utah Institutional Animal Care and Use Committee (12-12010) and Utah Division of Wildlife Resources (COR no. 1COLL5194).
Data accessibility
Data and R code: Dryad: http://dx.doi.org/10.5061/dryad.6q16d.
Authors' contributions
M.D.D. conceived of the study; P.K. and M.DD. designed the study; P.K. and N.D.M. participated in laboratory work and data analysis; P.K. wrote early drafts of the manuscript and all authors contributed substantially to revisions.
Competing interests
We have no competing interests.
Funding
The project was supported by the National Science Foundation (0817527 and 1256383 to M.D.D.), American Society of Mammalogists and Society for Integrative & Comparative Biology (GIARs to P.K.). P.K. was supported by the National Science Foundation K-12 Fellowship (DGE 08-41233).
References
- 1.Freeland WJ, Janzen DH. 1974. Strategies in herbivory by mammals: the role of plant secondary compounds. Am. Nat. 108, 269–289. ( 10.2307/2459891) [DOI] [Google Scholar]
- 2.Iason G. 2005. The role of plant secondary metabolites in mammalian herbivory: ecological perspectives. Proc. Nutr. Soc. 64, 123–131. ( 10.1079/PNS2004415) [DOI] [PubMed] [Google Scholar]
- 3.Fraenkel GS. 1959. The raison d'être of secondary plant substances: these odd chemicals arose as a means of protecting plants from insects and now guide insects to food Science 129, 1466–1470. [DOI] [PubMed] [Google Scholar]
- 4.Iason GR, Villalba JJ. 2006. Behavioral strategies of mammal herbivores against plant secondary metabolites: the avoidance–tolerance continuum. J. Chem. Ecol. 32, 1115–1132. ( 10.1007/s10886-006-9075-2) [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Feng Z, Swihart R, Bryant J, Huntly N. 2006. Modeling the impact of plant toxicity on plant–herbivore dynamics. J. Dyn. Diff. Equ. 18, 1021–1042. ( 10.1007/s10884-006-9029-y) [DOI] [Google Scholar]
- 6.Karen JM, Ian RW, Rose LA, William JF. 2006. The detoxification limitation hypothesis: where did it come from and where is it going? J. Chem. Ecol. 32, 1247–1266. ( 10.1007/s10886-006-9082-3) [DOI] [PubMed] [Google Scholar]
- 7.Dearing MD, Foley WJ, McLean S. 2005. The influence of plant secondary metabolites on the nutritional ecology of herbivorous terrestrial vertebrates. Annu. Rev. Ecol. Evol. Syst. 36, 169–189. [Google Scholar]
- 8.McArthur C, Hagerman AE, Robbins CT. 1991. Physiological strategies of mammalian herbivores against plant defenses. In Plant defenses against mammalian herbivory (eds RT Palo, CT Robbins), pp. 103–114. Boston, MA: CRC Press.
- 9.Mote TE, Villalba JJ, Provenza FD. 2007. Relative availability of tannin-and terpene-containing foods affects food intake and preference by lambs. J. Chem. Ecol. 33, 1197–1206. ( 10.1007/s10886-007-9305-2) [DOI] [PubMed] [Google Scholar]
- 10.Jansen DA, van Langevelde F, de Boer WF, Kirkman KP. 2007. Optimisation or satiation, testing diet selection rules in goats. Small Ruminant Res. 73, 160–168. ( 10.1016/j.smallrumres.2007.01.012) [DOI] [Google Scholar]
- 11.Boyle R, McLean S. 2004. Constraint of feeding by chronic ingestion of 1,8-cineole in the brushtail possum (Trichosurus vulpecula). J. Chem. Ecol. 30, 757–775. ( 10.1023/B:JOEC.0000028430.92739.83) [DOI] [PubMed] [Google Scholar]
- 12.DeGabriel JL, Moore BD, Shipley LA, Krockenberger AK, Wallis IR, Johnson CN, Foley WJ. 2009. Inter-population differences in the tolerance of a marsupial folivore to plant secondary metabolites. Oecologia 161, 539–548. ( 10.1007/s00442-009-1407-9) [DOI] [PubMed] [Google Scholar]
- 13.Mangione AM, Dearing MD, Karasov WH. 2000. Interpopulation differences in tolerance to creosote bush resin in desert woodrats (Neotoma lepida). Ecology 81, 2067–2076. ( 10.1890/0012-9658(2000)081%5B2067:IDITTC%5D2.0.CO;2) [DOI] [Google Scholar]
- 14.Skopec MM, Haley S, Dearing MD. 2007. Differential hepatic gene expression of a dietary specialist (Neotoma stephensi) and generalist (Neotoma albigula) in response to juniper (Juniperus monosperma) ingestion. Comp. Biochem. Physiol. D, Genomics Proteomics 2, 34–43. ( 10.1016/j.cbd.2006.11.001) [DOI] [PubMed] [Google Scholar]
- 15.Keplinger ML, Lanier GE, Deichmann WB. 1959. Effects of environmental temperature on the acute toxicity of a number of compounds in rats. Toxicology 1, 156–161. ( 10.1016/0041-008x(59)90136-x) [DOI] [PubMed] [Google Scholar]
- 16.Kaplanski J, Ben-Zvi Z. 1980. Effect of chronic heat exposure on in vitro drug metabolism in the rat. Life Sci. 26, 639–642. ( 10.1016/0024-3205(80)90240-4) [DOI] [PubMed] [Google Scholar]
- 17.Aldrich CG, Paterson JA, Tate JL, Kerley MS. 1992. The effects of endophyte-infected tall fescue consumption on diet utilization and thermal regulation in cattle. J. Anim. Sci. 71, 164–170. [DOI] [PubMed] [Google Scholar]
- 18.Settivari R, Evans T, Eichen P, Rottinghaus G, Spiers D. 2008. Short-and long-term responses to fescue toxicosis at different ambient temperatures. J. Therm. Biol. 33, 213–222. ( 10.1016/j.jtherbio.2007.12.001) [DOI] [Google Scholar]
- 19.Settivari R, Evans T, Yarru L, Eichen P, Sutovsky P, Rottinghaus G, Antoniou E, Spiers D. 2009. Effects of short-term heat stress on endophytic ergot alkaloid-induced alterations in rat hepatic gene expression. J. Anim. Sci. 87, 3142–3155. ( 10.2527/jas.2008-1684) [DOI] [PubMed] [Google Scholar]
- 20.Gordon CJ, Johnstone A, Aydin C. 2014. Thermal stress and toxicity. Compr. Physiol. 4, 995–1016. ( 10.1002/cphy.c130046) [DOI] [PubMed] [Google Scholar]
- 21.Dearing MD. 2013. Temperature-dependent toxicity in mammals with implications for herbivores: a review. J. Comp. Physiol. B 183, 43–50. ( 10.1007/s00360-012-0670-y) [DOI] [PubMed] [Google Scholar]
- 22.Dearing MD, Forbey JS, McLister JD, Santos L. 2008. Ambient temperature influences diet selection and physiology of an herbivorous mammal, Neotoma albigula. Physiol. Biochem. Zool. 81, 891–897. ( 10.1086/588490) [DOI] [PubMed] [Google Scholar]
- 23.McLister J, Sorensen J, Dearing M. 2004. Effects of consumption of juniper (Juniperus monosperma) on cost of thermoregulation in the woodrats Neotoma albigula and Neotoma stephensi at different acclimation temperatures. Physiol. Biochem. Zool. 77, 305–312. ( 10.1086/380211) [DOI] [PubMed] [Google Scholar]
- 24.Kurnath P, Dearing MD. 2013. Warmer ambient temperatures depress liver function in a mammalian herbivore. Biol. Lett. 9, 20130562 ( 10.1098/rsbl.2013.0562) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cameron GN, Rainey DG. 1972. Habitat utilization by Neotoma lepida in the Mohave desert. J. Mammal 53, 251–266. ( 10.2307/1379160) [DOI] [Google Scholar]
- 26.Karasov WH. 1989. Nutritional bottleneck in a herbivore, the desert wood rat (Neotoma lepida). Physiol. Zool. 62, 1351–1382. [Google Scholar]
- 27.Mabry TJ, Hunziker JH, Difeo D Jr. 1977. The natural products chemistry of Larrea In Creosote bush: biology and chemistry of Larrea in New World deserts (eds TJ Mabry, JH Hunziker, DR DiFeo), pp. 115–134. New York, NY: Stroudsberg, Dowden, Hutchinson & Ross. [Google Scholar]
- 28.Goodman T, Grice H, Becking G, Salem F. 1970. A cystic nephropathy induced by nordihydroguaiaretic acid in the rat. Light and electron microscopic investigations. Lab. Invest. 23, 93–107. [PubMed] [Google Scholar]
- 29.Rios JM, Mangione AM, Gianello JC. 2008. Effects of natural phenolic compounds from a desert dominant shrub Larrea divaricata Cav. on toxicity and survival in mice. Revista chilena de historia natural 81, 293–302. ( 10.4067/S0716-078X2008000200011) [DOI] [Google Scholar]
- 30.Brown JH. 1968. Neotorna cinerea and N. albigula. Ann Arbor, MI: Museum of Zoology, University of Michigan. [Google Scholar]
- 31.Schmidt-Nielsen K. 1997. Animal physiology: adaptation and environment. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 32.Torregrossa A-M, Dearing MD. 2009. Nutritional toxicology of mammals: regulated intake of plant secondary compounds. Funct. Ecol. 23, 48–56. ( 10.1111/j.1365-2435.2008.01523.x) [DOI] [Google Scholar]
- 33.Hafner MS. 1977. Density and diversity in Mojave desert rodent and shrub communities. J. Anim. Ecol. 46, 925–938. ( 10.2307/3650) [DOI] [Google Scholar]
- 34.Rundel PW, Gibson AC. 2005. Ecological communities and processes in a Mojave Desert ecosystem. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 35.Smith FA, Betancourt JL. 2006. Predicting woodrat (Neotoma) responses to anthropogenic warming from studies of the palaeomidden record. J. Biogeogr. 33, 2061–2076. ( 10.1111/j.1365-2699.2006.01631.x) [DOI] [Google Scholar]
- 36.Moritz C, Patton J, Conroy C, Parra J, White G, Beissinger S. 2008. Impact of a century of climate change on small-mammal communities in Yosemite National Park, USA. Science 322, 261–264. ( 10.1126/science.1163428) [DOI] [PubMed] [Google Scholar]
- 37.Cahill A, et al. 2013. How does climate change cause extinction? Proc. R. Soc. B 280, 20121890 ( 10.1098/rspb.2012.1890) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malenke JR, Skopec MM, Dearing MD. 2014. Evidence for functional convergence in genes upregulated by herbivores ingesting plant secondary compounds. BMC Ecol. 14, 23 ( 10.1186/1472-6785-14-23) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flanagan S, Ryan A, Gisolfi C, Moseley P. 1995. Tissue-specific HSP70 response in animals undergoing heat stress. Am. J. Physiol. Regul. Integr. Comp. Physiol. 268, R28–R32. [DOI] [PubMed] [Google Scholar]
- 40.Chatelain M, Halpin CG, Rowe C. 2013. Ambient temperature influences birds' decisions to eat toxic prey. Anim. Behav. 86, 733–740. ( 10.1016/j.anbehav.2013.07.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parry ML, Canziani OF, Palutikof JP, Van der Linden PJ, Hanson CE. 2007. Climate Change 2007: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 42.Field CB, et al. 2014. Part A: global and sectoral aspects. In Climate change 2014: impacts, adapation, and vulnerability. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, pp. 1–32. New York, NY: Cambridge University Press. [Google Scholar]
- 43.Zvereva EL, Kozlov MV. 2006. Consequences of simultaneous elevation of carbon dioxide and temperature for plant–herbivore interactions: a metaanalysis. Glob. Change Biol. 12, 27–41. ( 10.1111/j.1365-2486.2005.01086.x) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and R code: Dryad: http://dx.doi.org/10.5061/dryad.6q16d.


