Abstract
Division of labour is of fundamental importance for the success of societies, yet little is known about how individual specialization affects the fitness of the group as a whole. While specialized workers may be more efficient in the tasks they perform than generalists, they may also lack the flexibility to respond to rapid shifts in task needs. Such rigidity could impose fitness costs when societies face dynamic and unpredictable events, such as an attack by socially parasitic slavemakers. Here, we experimentally assess the colony-level fitness consequences of behavioural specialization in Temnothorax longispinosus ants that are attacked by the slavemaker ant T. americanus. We manipulated the social organization of 102 T. longispinosus colonies, based on the behavioural responses of all 3842 workers. We find that strict specialization is disadvantageous for a colony's annual reproduction and growth during slave raids. These fitness costs may favour generalist strategies in dynamic environments, as we also demonstrate that societies exposed to slavemakers in the field show a lower degree of specialization than those originating from slavemaker-free populations. Our findings provide an explanation for the ubiquity of generalists and highlight their importance for the flexibility and functional robustness of entire societies.
Keywords: behavioural specialization, division of labour, dynamic conditions, colony fitness, social insects, slavemaker ants
1. Introduction
Division of labour into specialized units is a highly successful strategy, found at all levels of biological organization. It is associated with the major evolutionary transitions, such as those from prokaryotes to eukaryotes, single-cell to multi-cellular organisms and solitary to social life [1]. The economist Adam Smith proposed that societies in which each worker specializes on a subset of tasks capitalize on increased individual efficiency and avoid the costs of task switching [2]. These benefits however fail to explain the enormous variation in division of labour observed in nature. Ant colonies typically face between 20 and 40 different tasks, yet the maximum number of morphologically differentiated worker castes is only three, and 80% of the ant genera lack such physical worker castes altogether [3]. Even among these monomorphic species, the number of tasks greatly exceeds the number of distinct behavioural castes, which is rarely more than six [3]. Despite this variation, little is known about the constraints on division of labour and how it affects the fitness of societies as a whole. Indeed, over 35 years ago, Oster & Wilson highlighted that the relationship between division of labour and fitness awaits empirical testing [3], and little progress has been made since [4,5].
Empirical evidence shows that the presumed advantage of behavioural specialization for individual efficiency is not universal, even in ants, the exemplars of division of labour [6]. Moreover, the adaptive significance of division of labour has been repeatedly questioned on theoretical grounds, as specialization is thought to constrain a worker's flexibility in task performance, and hence the ability of societies to cope with change [7,8]. Indeed, the major factors underlying worker specialization, such as morphology, genes or age [3,9,10], only enable societies to respond to environmental change over relatively long time spans, sometimes generations. This is hard to reconcile with the need to respond to short-term fluctuations in task needs. Theoretical models indeed predict that selection for flexibility and functional robustness under dynamic conditions counteracts the evolution of division of labour [11–13]. Yet an ecological perspective on the social organization of insect colonies is largely missing from the empirical literature [14].
Here, we experimentally assess the colony-level fitness consequences of behavioural specialization in Temnothorax longispinosus ants during an attack by the slavemaker ant T. americanus (formerly genus Protomognathus) [15]. To exploit the work force of its Temnothorax hosts, this social parasite conducts slave raids on neighbouring host colonies, capturing brood and frequently killing adult workers and the queen [16–18]. Slave raids thus impose severe costs in terms of the host's annual reproductive success and the potential for colony survival and growth—the three main fitness components of insect societies. Importantly, slave raids are representative of dynamic conditions frequently faced by societies, causing dramatic changes in task needs (electronic supplementary material, video S1). As a failure to respond probably results in severe fitness losses, flexibility in task performance is expected to confer the largest fitness benefits to T. longispinosus colonies under slavemaker attack. We therefore test experimentally whether colonies composed of specialized workers suffer higher fitness losses during slave raids than colonies composed of less specialized workers. In addition, we use a population-comparative approach to investigate whether natural levels of worker specialization are related to slavemaker presence in the local ant community.
2. Material and methods
(a). Experimental study design
We manipulated the social organization of 102 T. longispinosus colonies, based on the behavioural responses of all 3842 workers. Mechanistically, division of labour is often explained by response threshold models, which assume that workers differ in their intrinsic responsiveness towards stimuli, determining the likelihood that they perform the associated tasks [19,20]. To assess worker specialization, we therefore tested their responsiveness towards two types of stimulus: an opponent and a brood stimulus (figure 1a). These stimuli were chosen because they are associated with the two main, fitness-determining tasks during slave raids: nest defence and brood evacuation [17]. Workers were grouped into four categories, based on whether they were more responsive than the average response of their colony towards the brood stimulus (brood-responsive), the opponent stimulus (opponent-responsive), neither of the stimuli (unresponsive), or both stimuli (both-responsive; figure 1b). We established generalist colonies by removing workers responding to only one stimulus type and specialist colonies by removing workers responding to neither or both stimuli (figure 1c). One day after the manipulation of colony composition, each experimental colony was exposed to a slavemaker colony (figure 1d). A major experimental benefit of slave raids is that fitness losses become immediately apparent and can therefore be quantified before workers change their behavioural profile following experimental manipulation of the composition of their colony.
Figure 1.
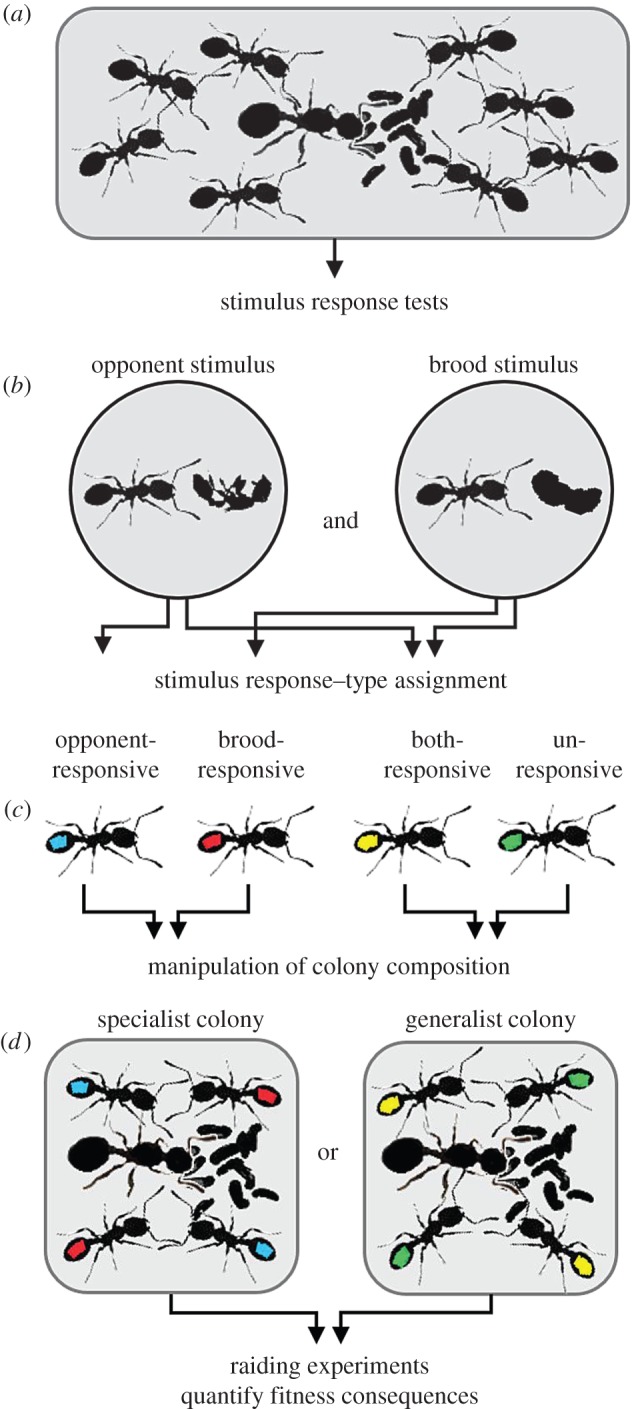
Experimental procedure. (a) Shortly before the emergence of the new, annual worker generation, each Temnothorax longispinosus worker was subjected to an opponent and a brood stimulus test, (b) based on which individuals were assigned to one of four response groups. Arrows indicate that workers were more responsive than their colony's average response. (c) Specialist colonies were established by the removal of both-responsive and unresponsive group members, generalist colonies by the removal of opponent- and brood-responsive group members (see methods section for further details). (d) The fitness consequences of colony composition were assessed during raiding experiments.
(b). Colony collection and maintenance
In June 2013, we collected 159 T. longispinosus ant colonies containing 30–60 workers and 100 T. americanus colonies with 3–10 slavemaker workers at the E. N. Huyck preserve, Rensselearville, New York, USA (42°31′26″, −074°9′20″). Colonies were transported to the laboratory, where they were counted, transferred to artificial nest sites and housed in plastered boxes to prevent desiccation. Prior to the experiments, T. longispinosus colonies were kept at a constant 14°C and a 12 L : 12 D cycle to delay brood development. Five days before the stimulus response tests, they were gradually acclimatized to the experimental temperature regime of 27°C. Temnothorax americanus slavemaker colonies were kept at a constant 25°C and a 12 L : 12 D cycle until the start of the raiding experiments, when temperatures were increased to 27°C to promote raid willingness. Colonies kept at temperatures below 20°C were fed weekly, and those kept at higher temperatures twice a week, with honey and crickets. For our experiments, we selected 102 T. longispinosus colonies with 37.6 ± 7.6 (mean ± s.d.) workers.
(c). Stimulus response tests
One day prior to the raiding trials, each T. longispinosus worker was isolated and subjected to a standardized opponent and brood stimulus test (figure 1a) [21]. For the opponent stimulus test, we introduced a freshly frozen, non-nest-mate worker into an 8 mm diameter circular arena with the focal worker. For each focal worker, we recorded the responsiveness to the opponent stimulus by scoring the occurrence of aggression (i.e. biting, holding, dragging and stinging) during 20 scan observations (i.e. every 10 s for 3 min 20 s). We chose a dead opponent to exclude effects of variation in opponent aggression on the responsiveness of the focal ant. An earlier study confirmed that responses towards live opponents were correlated with those towards freshly frozen, dead opponents [22]. Opponents originated from the same population but a different collection site as the focal ant to ensure that focal ants were not tested against a colony member from a polydomous nest part. Each opponent was used only once.
For the brood stimulus test, we introduced a focal ant into the arena with a worker pupa from its own colony. For each focal worker, we recorded the responsiveness to the brood stimulus by scoring the occurrence of pupa grooming or carrying during 20 scan observations (i.e. every 10 s for 3 min 20 s). Brood in the pupal stage undergoes restructuring during which it cannot actively signal its needs or be fed. This precludes that variation in begging intensity of the brood affected the focal ant's responsiveness to the brood stimulus. If there were fewer worker pupae than workers in a colony, we re-used pupa in the brood stimulus tests. The average responsiveness to the brood stimulus did not differ between colonies where pupae were re-used or not (F1,100 = 1.96, p = 0.165). Stimuli were provided in the same order as they are perceived by workers during a raid (i.e. the opponent stimulus test preceded the brood stimulus tests with a 30 min interval).
In total, we recorded 20 observations per worker per stimulus, based on which we grouped each worker into one of four response groups (figure 1b). For each experimental colony, the opponent-responsive group consisted of workers that were more responsive to the opponent stimulus, but less responsive to the brood stimulus, than the average colony responsiveness (workers assigned to the opponent-responsive group: mean ± s.d. = 14.1 ± 5.3%). Vice versa, the brood-responsive group consisted of workers that were above averagely responsive to the brood stimulus but below averagely responsive to the opponent stimulus (33.7 ± 8.7%). Workers with a lower or higher than average responsiveness to both stimuli were assigned to the unresponsive (29.5 ± 8.5%) or both-responsive group (22.7 ± 6.1%), respectively. See the electronic supplementary material, figure S1 for the stimulus response distribution of the four stimuli response groups. Workers were marked on the gaster with Edding 750 paint marker in accordance with their response group. Colours were randomized between colonies to allow for our blind experimental design.
(d). Manipulation of colony composition
Prior to the stimulus response tests, colonies were randomly assigned to either specialist (n = 50) or generalist (n = 52) colony-type treatment. Colony composition was manipulated by excluding opponent- and brood-responsive group members from generalist colonies, or unresponsive or both-responsive group members from specialist colonies (figure 1c). The reason we also removed the unresponsive group from specialist colonies was not because these workers were considered to be generalists, but because a pilot study showed that this approach equalized the average responsiveness towards the two stimuli in the two colony treatment groups (see the electronic supplementary material, figure S2 and the methods and results for pre- and post-manipulation colony characteristics and stimulus-response distributions). The number of excluded workers equalled twice the number of workers of the smallest response group. If this resulted in only the partial removal of a response group, we excluded those workers that showed the most extreme responsiveness in the stimuli tests. For instance, if the smallest response group of the original colony contained five workers, then we established a generalist colony by excluding from the opponent-responsive group the five workers that were most responsive to the opponent stimulus and from the brood-responsive group the five workers that were most responsive to the brood stimulus. Alternatively, to establish a specialist colony, we excluded from the both-responsive group the five workers with the strongest bivariate response and from the unresponsive group, the five workers with the weakest bivariate response. On average, we excluded 25.6 ± 5.1% (s.d.) of the workers from generalist colonies and 26.1 ± 5.3% from specialist colonies. This way, we avoided that natural variation in the relative abundance of the different response groups caused consistent biases in experimental colony sizes between generalist and specialist colonies.
For each colony, we calculated a Dy|x index [23] before and after manipulation. Although the Dy|x index was developed to quantify division of labour among members of a social group, we apply it to reflect worker specialization in their responses towards the two stimuli. Hence, an index value of 1 represents maximal specialization, where each worker in the colony is responsive to only a single stimulus type, whereas a value of 0 means that each worker is equally responsive to both stimuli. This index is invariant to the number of workers in a colony and thus allowed for direct comparisons despite variation in colony size [23].
Manipulation of colony composition resulted in a 39.8 ± 2.8% lower Dy|x index for generalist colonies compared with specialist colonies (t-test: t100 = 14.36, p < 0.0001; figure 2). Manipulation did not affect the level of behavioural variation within colonies, nor was intra-colonial behavioural variation related to the fitness outcomes of the raiding experiments described below (see electronic supplementary material, methods and results).
Figure 2.
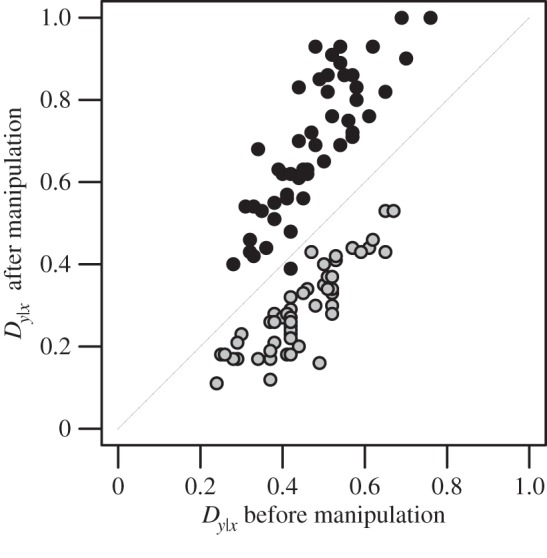
The effect of manipulation on the level of specialization in generalist and specialist colonies. Each point depicts a Temnothorax longispinosus colony. The level of specialization is represented by the Dy|x index. Black symbols denote specialist colonies; grey symbols, generalist colonies. Dy|x of specialist and generalist colonies differed after manipulation (t-test: t100 = 14.36, p < 0.0001), but not before manipulation (t100 = 1.27, p = 0.207).
(e). Raiding trials
One to three days after manipulation, a T. longispinosus and a slavemaker colony were positioned in opposite corners of a 30 cm equilateral triangular arena. Each T. longispinosus colony was only allowed to be raided once but colonies that were not attacked on the first/second day were subjected to another raiding trial on the second/third day. In total, we staged 165 trials resulting in 77 raids: 55 raids on the first day, 13 on the second and nine on the third. Including trial day as random factor did not explain significant variation in colony fitness (all p > 0.9). At the onset of a raid, we provided an escape nest at a distance of 15 cm from the T. longispinosus nest. Since the confines of the arena prevented colonies from relocating outside the raiding radius of the slavemaker, raiders were physically restrained from attacking the escape nest by the observer. Successful trials were terminated 1 h after the last brood item was removed from the T. longispinosus nest site, at which point the slavemaker colony and any slavemakers inside the arena were removed. Trials that did not result in a raid were terminated at 18.00 h by returning the T. longispinosus and slavemaker colony, as well as any of their workers in the arena, to their own, separate nest-boxes.
(f). Verification that stimulus responsiveness reflects task performance
To confirm that stimulus responsiveness reflected task performance in the colony context during the experiments, we scored the number of workers per stimulus response group that showed aggression towards slaves and slavemakers in the arena. This scan sampling was repeated every 30 min up to the start of the raiding attack, yielding on average (±s.d.) 9.20 ± 9.00 scans for each of the 71 observed T. longispinosus colonies. We confirmed that workers that were above averagely responsive towards the opponent stimulus were also more likely to attack slaves and slavemakers when their colony was exposed to a slavemaker colony (electronic supplementary material, figure S3). Hence, stimulus responsiveness of individual workers outside the colony context reflected task performance in the colony context.
(g). Fitness consequences of colony composition
One day after a raiding trial, we counted the number of sexual and worker brood items that were saved by the T. longispinosus colony or lost to the slavemakers; the number of T. longispinosus worker and queen fatalities/survivors; and the number of fatalities among slavemaker colony members. This 1-day interval was chosen because many workers were still engaged in fights with slaves when a raiding trial was terminated, which often resulted in the death of combatants. We further recorded the time it took workers from T. longispinosus colonies to carry their first five brood items into the escape nest, once discovered, based on which we calculated the brood saving speed. Because not all raids were continuously observed when multiple raids occurred simultaneously, brood saving speed was documented for a random subset of the raids (n = 28).
We used generalized linear models (GLMs) with colony type as fixed predictor for the analyses of the relative number of saved brood items and worker fatalities (quasi-binomial GLMs with a logit-link function), as well as the likelihood that a queen died (binary GLM—logit-link). The square-root-transformed number of fatalities among slavemaker colony members and the log-transformed brood saving speed were analysed with t-tests. Residual distributions did not deviate significantly from normality (Kolmogorov–Smirnov tests). Where appropriate, we used jack-knife methods to assess the effect of influential data points, which did not yield qualitatively different results. For statistical analyses, we used R v. 3.1.1 [24].
(h). Worker specialization and slavemaker sympatry
We further assessed whether the predicted colony-level fitness costs of worker specialization during slave raids favour generalist strategies in the field. From each of six T. longispinosus populations (figure 4a), we selected 10 (MA and NH) to 15 (ME, NY, QC and VT) colonies. This was a subset of the minimally 100 colonies collected per community in June 2012 [25], based on which we determined slavemaker prevalence. As there was little variation in prevalence in parasitized populations (figure 4a), we used slavemaker presence/absence in the analyses. Colonies were collected shortly before the annual raiding season in summer and maintained under common garden conditions for three months. Because T. longispinosus colonies only encounter slavemakers in their natural habitat during the two month raiding season, the older worker generation could not have encountered slavemakers for over a year, whereas the young worker generation (which emerged after collection) did not have prior contact with slavemakers at all.
Figure 4.
(a) Natural variation in the level of worker specialization in relation to slavemaker presence, based on six Temnothorax longispinosus populations from Massachusetts (MA), Maine (ME), New Hampshire (NH), New York (NY), Quebec (QC) and Vermont (VT). Pie charts represent slavemaker prevalence (i.e. T. longispinosus colony to slavemaker colony ratio). (b) Temnothorax longispinosus colonies originating from communities where the slavemaker was present showed less division of labour. Symbols represent the estimates ± s.e.; numbers represent the ncolonies; *p < 0.05.
From each colony, we selected 10 focal workers, which were subjected to an opponent and a brood stimulus test as described above. To capture maximum behavioural variation, focal individuals were selected sequentially in the order in which they left the nest site upon opening it (e.g. for an average ± s.d. colony of 20.0 ± 3.8 workers, every second worker served as focal individual). Based on the two stimulus response tests, we calculated the Dy|x index per colony [23], which increases with the level of worker specialization in stimulus responsiveness.
Colony size did not differ between colonies originating from communities where slavemakers were present or absent (Poisson GLMM with population ID as random factor:  p = 0.474). Out of the 80 experimental colonies, 87.5% had a queen (or queens). Populations that occurred in the absence or the presence of slavemakers did not differ in queen presence (
p = 0.474). Out of the 80 experimental colonies, 87.5% had a queen (or queens). Populations that occurred in the absence or the presence of slavemakers did not differ in queen presence (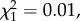 p > 0.999), although colonies were more often polygynous in slavemakers-free populations (binomial GLMM with population ID as random factor: estimate ± s.e.Δ = −2.10 ± 0.57, z = −3.70, p < 0.001; see also [26]).
p > 0.999), although colonies were more often polygynous in slavemakers-free populations (binomial GLMM with population ID as random factor: estimate ± s.e.Δ = −2.10 ± 0.57, z = −3.70, p < 0.001; see also [26]).
We used a linear mixed model [27] including the Dy|x index per colony as dependent variable, slavemaker presence as binary predictor, population ID as random factor and poly- versus monogyny as a binary covariate. We further fitted latitude (LR = 9.43, p = 0.002) and longitude (LR = 0.26, p = 0.605) of the collection sites as covariates in the initial model, but only the former was retained because the Dy|x index decreased with latitude (t3 = −3.74, p = 0.033; i.e. northern populations showed a lower level of worker specialization).
3. Results
(a). Fitness consequences of colony composition
We found no difference between generalist and specialist colonies in the likelihood that they were raided (71 and 80%, respectively; χ2-test: χ2 = 0.65, p = 0.419). However, generalist colonies that were attacked by slavemakers saved a larger fraction of their sexual (figure 3a; GLM: t51 = 2.75, p = 0.008) and worker brood (figure 3b; t75 = 3.44, p = 0.001) than specialist colonies. Moreover, generalist colonies were, on average, 2.6 times faster in carrying their brood to safety (figure 3c; t-test: t26 = 3.25, p = 0.003), which contributed to the higher fraction of brood saved (i.e. the faster colonies saved their brood, the more brood they managed to save in total; GLM: estimate ± s.e. = 0.028 ± 0.011, t26 = 2.50, p = 0.019). Generalist colonies did not trade off brood rescue for adult survival, as generalist and specialist colonies did not differ in the relative number of worker fatalities (GLM: t75 = 1.22, p = 0.228) or the likelihood that a queen died (GLM: z68 = −0.42, p = 0.673). However, slavemaker colonies that raided generalist T. longispinosus colonies suffered higher fatalities than those raiding specialists (figure 3d; t-test: t75 = −2.01, p = 0.048).
Figure 3.
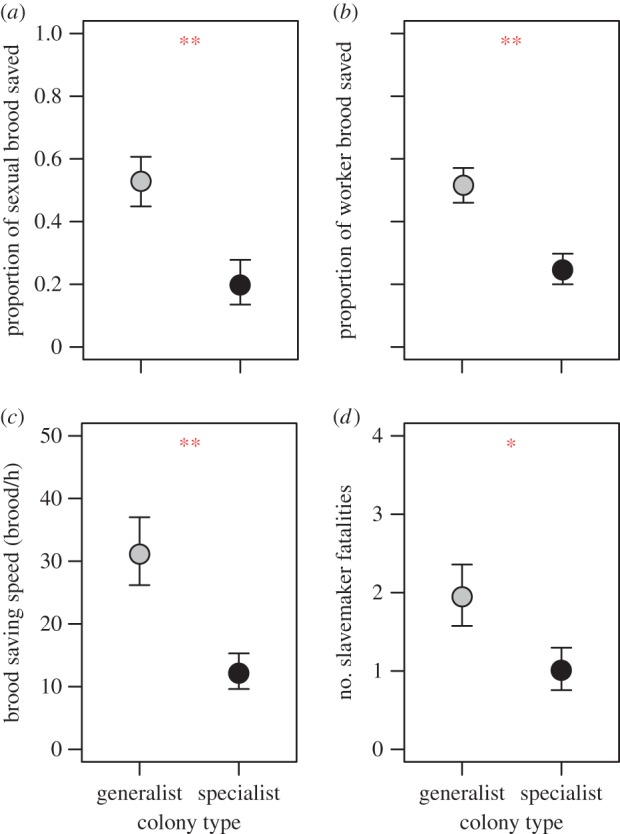
Fitness consequences of colony composition. Differences between specialist and generalist Temnothorax longispinosus colonies in the proportion of (a) sexual and (b) worker brood saved during slave raids. (c) Difference in the speed with which workers carried their brood to safety in an escape nest. (d) The number of fatalities among slavemaker colony members raiding specialist or generalist colonies. Symbols represent estimates ± s.e.; *p < 0.05, **p < 0.01.
(b). Worker specialization and slavemaker sympatry
As predicted, T. longispinosus colonies from communities where the slavemaker was present showed a lower Dy|xindex than those where the slavemaker was absent (figure 4; LMM: t3 = −3.90, p = 0.030). This difference was not driven by variation in the level of polygyny, and hence intra-colonial genetic variation, since the Dy|x index was unrelated to whether colonies were headed by a single or multiple queens (LR = 0.22, p = 0.636).
4. Discussion
Contrary to conventional wisdom that individual specialization provides societal benefits [2], our findings demonstrate that specialization can have detrimental consequences for the annual reproductive success of a colony and its potential for growth. Ant societies composed of specialists lost almost 80% of their worker and sexual brood during a raid, whereas generalist colonies managed to save more than half of their brood. Such heavy losses of sexual brood represent severe fitness costs to a species that only reproduces once per year. Moreover, the loss of a substantial part of the colony's new workforce probably compromises future colony survival and productivity. Further support for the ecological significance of these fitness costs of strict specialization is provided by our population comparison: workers from colonies that co-occur with slavemakers adopt a more generalist strategy compared with colonies from slavemaker-free sites. Hence, colonies exposed to the dynamic conditions posed by slavemaker attack appear to generate a robust, risk-tolerant organization of work by relying more on generalist workers.
Specialization is, in essence, a behavioural trait and variation in task performance among workers frequently occurs independently of their morphology, genes or age [28]. In the absence of unifying organizational principles, it is the fitness consequences of behavioural specialization per se that beg for experimental support [4]. Conveniently, T. longispinosus lacks physical worker castes, which rules out differences in task efficiency due to morphological adaptation. Moreover, we have shown that genetic variation is of minor importance for the division of labour in T. longispinosus, a species in which queens mate with a single male [29]. Thirdly, because our study population only produces a single worker generation per year, the assessment of worker specialization shortly before brood emergence ensures that all workers are at least 1 year old. This greatly limits the potential for age-dependent task performance, especially since there is little evidence for such age-polyethism in Temnothorax ants [30]. Hence, differences in worker behaviour alone are most likely to be responsible for the higher success of colonies composed of generalist workers compared with specialists during slave raids.
Although it is generally assumed that specialists are more efficient at their respective tasks than generalists, this ‘jack of all trades, master of none’ hypothesis has rarely been tested. A notable exception is a study by Dornhaus, who demonstrated that specialization in Temnothorax albipennis ants does not predict individual efficiency [6]. In our study, we investigated how worker specialization affects the efficiency of entire societies, which is the main unit of selection in social insects. We find that colonies composed of generalists inflicted more fatalities among slavemaker colony members than specialist colonies, without suffering higher losses among their own workers. This suggests that T. longispinosus colonies composed of specialists were actually less, not more efficient in their aggressive defences, which is at odds with the ‘jack of all trades, master of none’ hypothesis implicit in many discussion on division of labour.
Proximately, higher fitness losses suffered by societies composed of specialists probably arise when the coordination between workers fails. Successful division of labour requires close coordination when task performance by one group of specialists affects the efficiency of another. During raids, the re-appropriation of brood that was stolen requires first attacking the opponent and then bringing the brood to safety, which either necessitates the coordinated effort of two different specialists or a single generalist worker. Moreover, the costs of not performing a necessary task probably outweigh any advantages of specialization in terms of efficiency gain, especially since there is no evidence that specialization increases individual efficiency in Temnothorax ants [6]. Likewise, the potential benefits of specialization for the avoidance of costs associated with switching between different tasks [31] are likely to be negated during dynamic conditions such as slave raids, due to the disruption of the colony's spatial organization (electronic supplementary material, video S1). Indeed, spatial fidelity is a key regulator of social organization in ants because different sets of tasks are usually segregated within a colony [30,32]. In its absence, workers that perform any task they encounter probably contribute more to colony fitness than workers that reject tasks unless they fall within their narrow behavioural repertoire. This contribution of generalists to flexibility and functional robustness provides a key benefit of decentralized control of division of labour when groups face sudden shifts in group demand.
Fitness costs of division of labour probably apply especially to small societies, which are particularly prone to lose vital functions with the death of a few highly specialized workers. However, we do not believe this marginalizes the implications of our findings, because many taxa that exhibit division of labour are characterized by small group sizes. Indeed, the majority of social insect colonies do not resemble the large societies of honeybees or leafcutter ants [9,33,34], and social groups of cooperative birds and mammals rarely exceed several dozen of individuals. Moreover, most species that form large societies will cross a phase in their ontogeny where they are composed of only few individuals, during which the division of labour into highly specialized modules may jeopardize the survival of the group as a whole. Hence, small or young societies are expected to rely more on generalists in their work force to absorb fluctuations in the environment, and there is indeed ample empirical evidence supporting the positive association between group size and division of labour [34–36]. From an evolutionary perspective, the fitness pay-offs of division of labour in small societies are of particular interest, as social life probably evolved starting with small groups rather than large, complex societies. Hence, our findings provide insights into the evolutionary drivers of social life and the ultimate causes of variation in social organization.
Our findings underline the importance of an ecological perspective on the organization of work and the behavioural rules of individuals that give rise to beneficial outcomes for societies as a whole. Although we focused on the enigmatic but idiosyncratic conditions caused by slave raids, social groups frequently face dynamic conditions, be it small day-to-day perturbations or sudden catastrophic shifts in task needs. Such conditions probably favour flexibility, not only in ant societies but across all levels of organization. For instance, metabolic networks that computationally evolve in fluctuating environments increase robustness through the acquisition of generalist enzymes [37], and the recent financial crisis has demonstrated how the reliance on a few specialized financial institutions can lead to the collapse of the global economy [38]. Hence, societal costs of strict division of labour probably apply whenever conditions demand flexible task performance by group members.
Acknowledgements
We thank Iris Kleudgen, Christoph Müller, Janine Ferreira and Isabelle Kleeberg for practical assistance. Thanks also to Joel Meunier, Barbara Feldmeyer, Jonathan Pruitt, Judith Korb, Dirk Metzler and Justyna Wolinska for comments on an earlier version of the manuscript.
Ethics
Ant collection permits were obtained from parks/preserves or we asked private land owners for permission to collect ant colonies. Import and export licences are not required for the transport of our study species. We followed the guidelines of the Study of Animal Behaviour and the legal and institutional rules.
Data accessibility
Data and R script are accessible via Dryad: http://dx.doi.org/10.5061/dryad.p064v.
Authors' contributions
Both authors designed the study and contributed to the manuscript. E.J. collected and analysed the data.
Competing interests
We declare we have no competing interests.
Funding
This study was funded by the Deutsche Forschungsgemeinschaft (Fo298/9-1 and Fo298/11-2; S.F.) and the E. N. Huyck preserve (E.J.).
References
- 1.Szathmáry E, Maynard Smith J. 1995. The major evolutionary transitions. Nature 374, 227–232. ( 10.1038/374227a0) [DOI] [PubMed] [Google Scholar]
- 2.Smith A. 1776. An inquiry into the nature and causes of the wealth of nations. London, UK: W. Strahan and T. Cadell. [Google Scholar]
- 3.Oster GF, Wilson EO. 1978. Caste and ecology in the social insects. Princeton, NJ: Princeton University Press. [PubMed] [Google Scholar]
- 4.Jeanson R, Weidenmüller A. 2014. Interindividual variability in social insects: proximate causes and ultimate consequences. Biol. Rev. 89, 671–687. ( 10.1111/brv.12074) [DOI] [PubMed] [Google Scholar]
- 5.Duarte A, Weissing FJ, Pen I, Keller L. 2011. An evolutionary perspective on self-organized division of labor in social insects. Annu. Rev. Ecol. Evol. Syst. 42, 91–110. () [DOI] [Google Scholar]
- 6.Dornhaus A. 2008. Specialization does not predict individual efficiency in an ant. PLoS Biol. 6, 2368–2375. ( 10.1371/journal.pbio.0060285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bourke FG, Franks NR. 1995. Social evolution in ants. Princeton, NJ: Princeton University Press. [Google Scholar]
- 8.Tofts C, Franks NR. 1992. Doing the right thing: ants, honeybees and naked mole-rats. Trends Ecol. Evol. 7, 346–349. () [DOI] [PubMed] [Google Scholar]
- 9.Hölldobler B, Wilson EO. 1990. The ants. Berlin, Germany: Springer. [Google Scholar]
- 10.Page R, Robinson GE. 1991. The genetics of division of labour in honey bee colonies. Adv. Insect Phys. 23, 117–169. ( 10.1016/S0065-2806(08)60093-4) [DOI] [Google Scholar]
- 11.Waibel M, Floreano D, Magnenat S, Keller L. 2006. Division of labour and colony efficiency in social insects: effects of interactions between genetic architecture, colony kin structure and rate of perturbations. Proc. R. Soc. B 273, 1815–1823. ( 10.1098/rspb.2006.3513) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wakano JY, Nakata K, Yamamura N. 1998. Dynamic model of optimal age polyethism in social insects under stable and fluctuating environments. J. Theor. Biol. 193, 153–165. ( 10.1006/jtbi.1998.0697) [DOI] [Google Scholar]
- 13.Rueffler C, Hermisson J, Wagner GP. 2012. Evolution of functional specialization and division of labor. Proc. Natl Acad. Sci. USA 109, 326–335. ( 10.1073/pnas.1110521109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordon DM. 2014. The ecology of collective behavior. PLoS Biol. 12, e1001805 ( 10.1371/journal.pbio.1001805) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ward PS, Brady SG, Fisher BL, Schultz TR. 2015. The evolution of myrmicine ants: phylogeny and biogeography of a hyperdiverse ant clade (Hymenoptera: Formicidae). Syst. Entomol. 40, 61–81. ( 10.1111/syen.12090) [DOI] [Google Scholar]
- 16.Alloway TM. 1979. Raiding behaviour of two species of slave-making ants, Harpagoxenus americanus (Emery) and Leptothorax duloticus Wesson (Hymenoptera: Formicidae). Anim. Behav. 27, 202–210. ( 10.1016/0003-3472(79)90140-4) [DOI] [Google Scholar]
- 17.Foitzik S, DeHeer CJ, Hunjan DN, Herbers JM. 2001. Coevolution in host–parasite systems: behavioural strategies of slave-making ants and their hosts. Proc. R. Soc. Lond. B 268, 1139–1146. ( 10.1098/rspb.2001.1627) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wesson LG. 1939. Contributions to the natural history of Harpagoxenus americanus Emery (Hymenoptera: Formicidae). Trans. Am. Entomol. Soc. 65, 97–122. [Google Scholar]
- 19.Bonabeau E, Theraulaz G, Deneubourg JL. 1996. Quantitative study of the fixed threshold model for the regulation of division of labour in insect societies. Proc. R. Soc. Lond. B 263, 1565–1569. ( 10.1098/rspb.1996.0229) [DOI] [Google Scholar]
- 20.Beshers SN, Fewell JH. 2001. Models of division of labor in social insects. Annu. Rev. Entomol. 46, 413–440. ( 10.1146/annurev.ento.46.1.413) [DOI] [PubMed] [Google Scholar]
- 21.Modlmeier AP, Liebmann JE, Foitzik S. 2012. Diverse societies are more productive: a lesson from ants. Proc. R. Soc. B 279, 2142–2150. ( 10.1098/rspb.2011.2376) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Modlmeier AP, Foitzik S. 2011. Productivity increases with variation in aggression among group members in Temnothorax ants. Behav. Ecol. 22, 1026–1032. ( 10.1093/beheco/arr086) [DOI] [Google Scholar]
- 23.Gorelick R, Bertram SM, Killeen PR, Fewell JH. 2004. Normalized mutual entropy in biology: quantifying division of labor. Am. Nat. 164, 677–682. ( 10.1086/424968) [DOI] [PubMed] [Google Scholar]
- 24.R Core Team. 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 25.Jongepier E, Kleeberg I, Job S, Foitzik S. 2014. Collective defence portfolios of ant hosts shift with social parasite pressure. Proc. R. Soc. B 281, 20140225 ( 10.1098/rspb.2014.0225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foitzik S, Achenbach A, Brandt M. 2009. Locally adapted social parasite affects density, social structure, and life history of its ant hosts. Ecology 90, 1195–1206. ( 10.1890/08-0520.1) [DOI] [PubMed] [Google Scholar]
- 27.Pinheiro J, Bates D, DebRoy S, Sarkar D. 2013. nlme: linear and nonlinear mixed effects models. R package v. 3.1–118 Vienna, Austria: R Development Core Team. [Google Scholar]
- 28.Robinson GE. 1992. Regulation of division of labor in insect societies. Annu. Rev. Entomol. 37, 637–665. ( 10.1146/annurev.en.37.010192.003225) [DOI] [PubMed] [Google Scholar]
- 29.Foitzik S, Herbers JM. 2001. Colony structure of a slavemaking ant. II. Frequency of slave raids and impact on the host population. Evolution 55, 316–323. ( 10.1111/j.0014-3820.2001.tb01296.x) [DOI] [PubMed] [Google Scholar]
- 30.Sendova-Franks A, Franks NR. 1995. Spatial relationships within nests of the ant Leptothorax unifasciatus (Latr.) and their implications for the division of labour. Anim. Behav. 50, 121–136. ( 10.1006/anbe.1995.0226) [DOI] [Google Scholar]
- 31.Goldsby HJ, Dornhaus A, Kerr B, Ofria C. 2012. Task-switching costs promote the evolution of division of labor and shifts in individuality. Proc. Natl Acad. Sci. USA 109, 13 686–13 691. ( 10.1073/pnas.1202233109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mersch DP, Crespi A, Keller L. 2013. Tracking individuals shows spatial fidelity is a key regulator of ant social organization. Science 340, 1090–1093. ( 10.1126/science.1234316) [DOI] [PubMed] [Google Scholar]
- 33.Wilson EO. 1971. The insect societies. Cambridge, MA: Belknap Press of Harvard University Press. [Google Scholar]
- 34.Dornhaus A, Powell S, Bengston S. 2012. Group size and its effects on collective organization. Annu. Rev. Entomol. 57, 123–141. ( 10.1146/annurev-ento-120710-100604) [DOI] [PubMed] [Google Scholar]
- 35.Holbrook CT, Barden PM, Fewell JH. 2011. Division of labor increases with colony size in the harvester ant Pogonomyrmex californicus. Behav. Ecol. 22, 960–966. ( 10.1093/beheco/arr075) [DOI] [Google Scholar]
- 36.Ferguson-gow H, Sumner S, Bourke AFG, Jones KE. 2014. Colony size predicts division of labour in attine ants. Proc. R. Soc. B 281, 20141411 ( 10.1098/rspb.2014.1411) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nam H, Lewis NE, Lerman JA, Lee DH, Chang RL, Kim D, Palsson B. 2012. Network context and selection in the evolution to enzyme specificity. Science 337, 1101–1104. ( 10.1126/science.1216861) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arinaminpathy N, Kapadia S, May RM. 2012. Size and complexity in model financial systems. Proc. Natl Acad. Sci. USA 109, 18 338–18 343. ( 10.1073/pnas.1213767109) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and R script are accessible via Dryad: http://dx.doi.org/10.5061/dryad.p064v.



