Abstract
Analyses of senescence in social species are important to understanding how group living influences the evolution of ageing in society members. Social insects exhibit remarkable lifespan polyphenisms and division of labour, presenting excellent opportunities to test hypotheses concerning ageing and behaviour. Senescence patterns in other taxa suggest that behavioural performance in ageing workers would decrease in association with declining brain functions. Using the ant Pheidole dentata as a model, we found that 120-day-old minor workers, having completed 86% of their laboratory lifespan, showed no decrease in sensorimotor functions underscoring complex tasks such as alloparenting and foraging. Collaterally, we found no age-associated increases in apoptosis in functionally specialized brain compartments or decreases in synaptic densities in the mushroom bodies, regions associated with integrative processing. Furthermore, brain titres of serotonin and dopamine—neuromodulators that could negatively impact behaviour through age-related declines—increased in old workers. Unimpaired task performance appears to be based on the maintenance of brain functions supporting olfaction and motor coordination independent of age. Our study is the first to comprehensively assess lifespan task performance and its neurobiological correlates and identify constancy in behavioural performance and the absence of significant age-related neural declines.
Keywords: senescence, neurodegeneration, biogenic amines, task performance, ants
1. Introduction
Evolutionary theories of senescence predict behavioural declines in old age [1–3] in association with physiological and genetic processes that deteriorate over the lifespan [4,5]. Ageing nervous systems may functionally decline, leading to sensory and motor deficits [6,7] due to neurodegeneration, manifest as apoptosis (programmed cell death), which may increase with age [8], disease [9] or development [10]. Few studies have examined the influence of sociality on ageing and life history [11–13], and little is understood about cognitive decline and neurobiological changes accompanying senescence in social animals, apart from humans [12,14–16]. Social insects have striking lifespan polyphenisms [17–20]: queens often live more than a decade, whereas workers may live only several months [21], indicating that the differentiation of reproductive and sterile castes has had profound effects on senescence, which may be influenced by the social organization of colony labour. The relationship of task performance to age-related changes in the worker brain, however, is not well understood.
The ant Pheidole dentata has served as a model to explore the evolution and ecology of social structure, as well as the neurobiological underpinnings of age-related worker behaviour [22–27]. Minor workers expand their task repertoires from eclosion to approximately three weeks of age [23] while their brains undergo synaptic remodelling [28] and serotonergic systems that influence behavioural development and task performance mature [25,29–31]. How do workers age behaviourally, and do age-related and brain-based neuroanatomical and neurochemical changes occur that could negatively impact lifespan task performance? Using individuals of known age and robust and comprehensive assays to quantify effectiveness of task performance in minor workers, we tested the hypothesis that behavioural performance declines across the P. dentata minor worker lifespan in association with age-related patterns of apoptosis and cellular modifications in synaptic complexes considered significant to sensory processing, integration and motor control, and thus behavioural efficacy [32,33]. In addition, we quantified levels of neuromodulators known to influence neural structure and signalling [34] that could alter circuit function in an age-dependent manner to regulate task performance or alter other physiological processes associated with ageing. We predicted that if workers functionally senesce, nursing and foraging performance should decrease, and sensory and motor processes regulating worker responsiveness to social signals and cues that guide these tasks should covary with age-related neurobiological declines. We localized cell death in functionally specialized brain compartments—the optic lobes (OL; vision), antennal lobes (AL; olfaction), mushroom bodies (MB; integration, learning and memory), central complex (CC; motor response and integration), suboesophageal zone (SEZ; control of mouthparts) and the remainder of the central brain (RCB) [35]—to quantify neural changes that could underscore senescence and impact task performance. We hypothesized that apoptosis in the brain would increase with age, and the density of synaptic complexes of microglomeruli (MG) associated with cognitive function [33,36] would decrease prior to or concurrent with age-related declines in task performance. Finally, we predicted that brain biogenic amine titres, notably serotonin (5HT) and dopamine (DA), would decrease as workers aged and compromise the functionality of systems under neuromodulatory control, as well as neuroplasticity [37,38].
2. Material and methods
(a). Ant husbandry
Colonies of P. dentata were collected in and around Alachua County, Florida, and reared in Harris environmental chambers maintained at 25°C and 40–55% relative humidity and cultured as described in Seid et al. [28]. Subcolonies with minors of known age were established with 40–70 pupae within 72 h of eclosion, the queen, brood and approximately 150 marked workers. Workers were sampled from subcolonies derived from five to 15 parent colonies and assigned haphazardly to assays; numbers of subcolonies are provided for each assay below. Details of subcolony establishment and marking are provided in the electronic supplementary material. Minor workers of known ages (20–22, 45–47, 95–97 and 120–122 days) were selected for behavioural analyses that encompass the breadth of the minor worker repertoire [22,23]. Workers reared in our queenright subcolonies live up to 140 days; because few workers survived to 120 days, sample sizes were smaller at this age than for other worker ages due to the diminishing return on attempting to acquire samples of very old workers.
(b). Behavioural assays
(i). Nursing effort
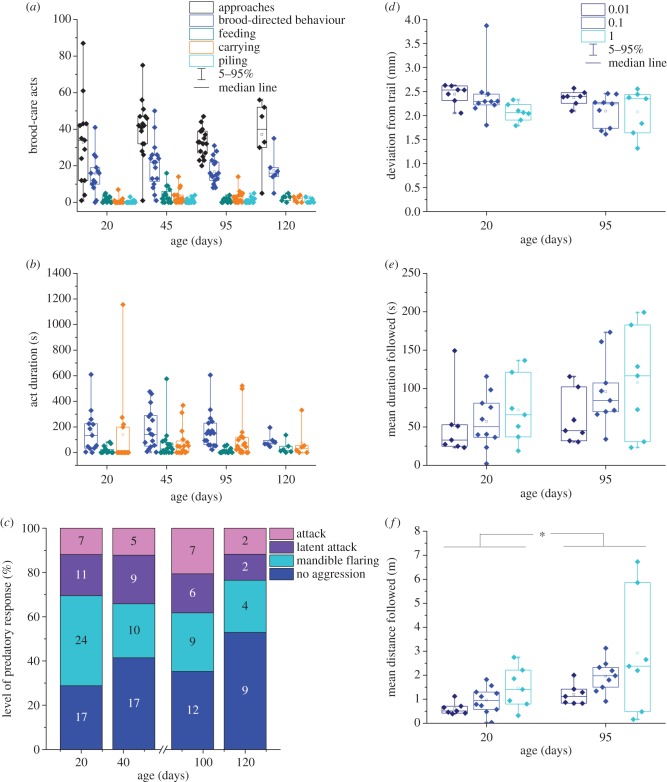
Nursing ability was assayed in a cylindrical chamber (34 mm inner diameter) with a humidified plaster-like bottom (dental stone) to prevent larvae from desiccating. Sides were coated with Fluon® to prevent escape. Eight 2nd to 4th instar larvae with dark guts were added to the chamber and evenly distributed along the circumference of a 20 mm circle. A cover bisected by a 10 mm diameter tube was placed over the chamber, and a single minor worker was introduced to the central tube using featherweight forceps. After the worker acclimated for 5 min, the lid was gently removed, allowing access to larvae. Responses were recorded for 20 min using a Canon FS400 digital camcorder placed above the assay chamber. The number and duration of approaches and acts to larvae were scored manually using JWatcher Video (www.jwatcher.ucla.edu) by an observer blind to the age of the ant (N = 13, 16, 17, 6 for 20-, 45-, 95- and 120-day-old ants, respectively, from 10 subcolonies). We quantified the number of times a worker approached, carried, fed, piled or otherwise provided care to larvae and recorded the duration of feeding, carrying and unspecified brood-directed behaviour, which could include licking or other manipulation of brood items. Details are provided in the electronic supplementary material. Two-way χ2 was used for brood-care act frequencies, and two-way ANOVA was used for durations to test for effects of age, differences in frequencies and durations of behaviours and interaction effects. Statistical analyses were performed in JMP®.
(ii). Pheromone trail-following ability
To determine if workers experienced age-related declines in sensorimotor functions associated with the perception of trail pheromone and trail-following ability, individual ants were presented with artificial pheromone trails at concentrations of 1, 0.1 or 0.01 poison glands per trail [25]. Minor workers did not follow trails at 0.001 glands per trail. Details are provided in the electronic supplementary material. We video recorded each assay of worker responsiveness to an artificial trail and subsequent orientation using a Canon FS400 digital camcorder for 5 min and used Ctrax [39] to quantify worker movement. Worker age was masked with a random number so that analyses were conducted blind. Trail following was assessed by tracking activity within and outside a 1 cm diameter annulus digitally drawn along the chemical trail to estimate the active space of the pheromone. Annulus size was determined conservatively after observing numerous occurrences of trail-following behaviour. Using larger annuli did not significantly affect results (data not shown). Of the twenty-three 95-day-old minors sampled, six were raised in a subcolony lacking a queen. These ants were not significantly different in any trail-following metrics than age-matched workers from queenright subcolonies tested at the same pheromone concentrations, and data were therefore pooled.
Trail-following metrics—mean deviation from the trail axis (accuracy), duration and distance of following activity within the annulus, and mean and maximum duration and distance of individual following bouts—were used to quantify the integration of sensory and motor activity. Details are provided in the electronic supplementary material. We tested 20- and 95-day-old minors using pheromone concentrations of 1.0, 0.1 and 0.01 gland per trail (20-day: N = 7, 10, 7; 95-day: N = 7, 9, 7 for 1.0, 0.1 and 0.01 gland/trail trail-pheromone concentration (TPC), respectively, sampled from seven subcolonies). Accuracy, duration, distance, mean and maximum distance and duration of individual following bouts were analysed using a two-way ANOVA for age and pheromone concentration in JMP; to correct for multiple tests, a false-discovery rate (FDR) correction was applied [40].
(iii). Worker predatory behaviour
Ants were isolated for 2 min in a small Fluon-lined Petri dish. A live fruit fly, tethered by its wings or abdomen end with fine watchmakers forceps affixed to a small platform, was then introduced into the arena. Only flies that responded to touch with movement were used. Each ant was assigned a random number, and worker response was video recorded for 2 min. Predatory response by the worker was scored on a four-point scale: 1: no aggression, 2: mandible flaring, 3: latent attack (delayed or not sustained for the duration of the assay), 4: attack. Because this assay was scored preliminarily in real time, it was not always possible to record responses blind to the age of the ant, for instance, if only one age was being tested on a day. However, videos that did not involve immediate and consistent attack, which was unambiguous, were reviewed at a later date by a blinded observer. Chi-square tests were performed in JMP to examine the effect of age (N = 59, 41, 34, 17 for each age group from 20 to 120 days from 15 subcolonies) on predatory response.
(c). Age-related changes in neuroanatomy
(i). Measurement of apoptosis in the brain
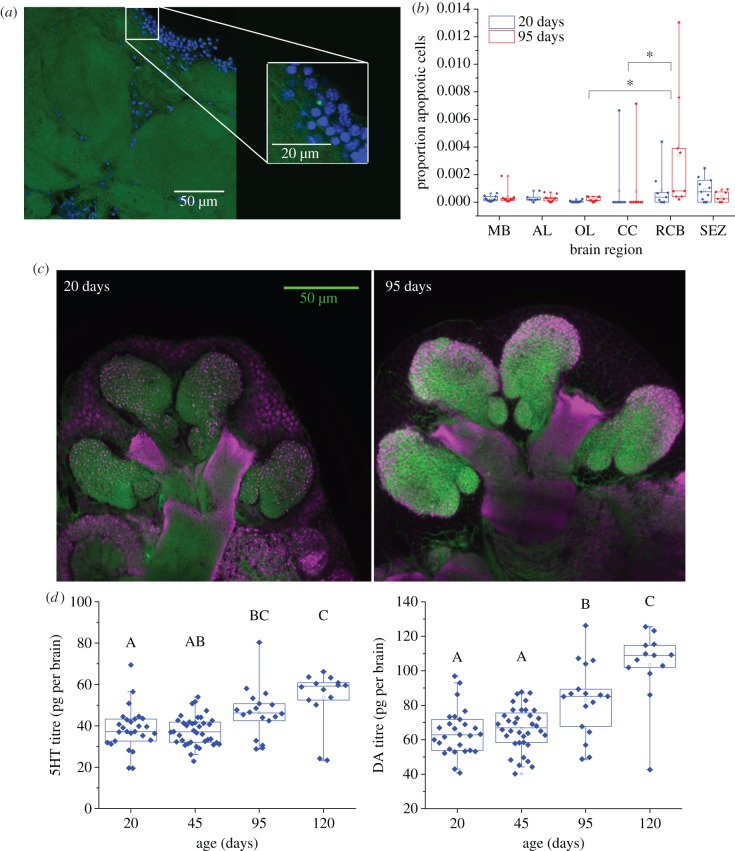
Brains of minor workers aged 20–22 or 95–97 days were selected for analysis by Tdt-mediated dUTP-biotin nick end labelling (TUNEL). TUNEL identifies fragmented DNA characteristic of apoptotic cell death [41] and serves as a primary method for identification of apoptosis in a variety of tissues [42,43]. Negative controls, brain tissue not treated with the active TUNEL enzyme, terminal transferase, were generated according to manufacturer instructions (Roche In Situ Cell Death Detection Kit, Fluorescein, Cat. no. 11 684 795 910). For positive controls, we adapted procedures developed for Drosophila to confirm assay function in Pheidole brains. Workers of unknown age were selected for positive controls and were briefly cold immobilized and then impaled in the head with a metal pin (0.1 mm diameter, Fine Science Tools), between the mandibles and into the brain. Injured ants were maintained in groups with food and water for 16–20 h prior to dissection. TUNEL reactions were performed according to manufacturer instructions, vibratome sectioned at 100 µm, incubated with DAPI and mounted in 80% glycerol. Details are provided in the electronic supplementary material. All control brain sections were imaged on an Olympus FluoView® 10i confocal microscope using a 10× objective. TUNEL-positive cells were confirmed using a 60× objective and examination of nuclear morphology. A set of controls and experimental brains was processed at one time; all brains were discarded from any set in which positive or negative controls failed for a total of 22 usable brains (N = 12, 10 for 20 and 95 days from six subcolonies). Known-age ant brain sections were scanned in entirety with a 60× objective using 2 µm optical sections.
Brains were assigned a random number and images were analysed manually using FluoView® software (FV10-ASW v. 3.0 Viewer) by an observer blind to worker age. TUNEL-positive cells were identified visually by their brightly fluorescent cell bodies. In some instances, only a portion of the nucleus appeared TUNEL-positive. Apoptosis was confirmed by observing nuclear morphology: apoptotic nuclei often show enhanced DNA condensation [44]. Cells identified as apoptotic were TUNEL-positive, co-localized with a nucleus and exhibited atypical nuclear morphology. Cells were counted and categorized according to the nearest neuropil. Although we could not always be certain that cell bodies projected to an adjacent neuropil region, our method reasonably estimated brain compartment variation in cell death.
To compare the frequency of apoptosis between functional brain regions that differ in volume and cell number, we employed an unbiased stereological approach to estimate total cell number. Volumes were measured directly on a subset of brains by an experimenter blind to brain age, and means for each age group calculated. Apoptosis was then scaled to total cell number in each brain region. Cell number per region was estimated using an optical disector [45] on one brain of each age group and extrapolated for other brains. We were thus able to calculate the proportion of apoptotic cells across age groups and brain regions. Proportions of apoptotic cells (number of apoptotic cells/estimated cell number) were calculated for each brain region. Because the total number of cells per region is large relative to the total number of apoptotic cells, small errors in total cell estimation have negligible effects on results. A mixed-effects ANOVA was run in SPSS to test for effects of age, brain region and age × brain region interaction effects on the log-transformed proportion of apoptotic cells with age as a between-subjects factor and brain region as a within-subject factor. Differences between brain regions were identified using pairwise comparisons and Bonferroni corrections. Details are provided in the electronic supplementary material.
(ii). Quantification of microglomeruli in the mushroom body lip
MG, synaptic complexes characterized by a synapsin-immunoreactive presynaptic bouton surrounded by a halo of phalloidin-labelled postsynaptic terminals, were immunohistochemically labelled in 20- and 95-day-old minors (N = 28, 18 from 14 subcolonies) following the protocols of Groh et al. [46]. Details are provided in the electronic supplementary material. To quantify age-related changes in MG densities, the imaging plane where the peduncle completely bisects the calyx and the collar is maximally visible was selected because it could be easily identified across samples. We thus minimized potential spatial effects by selecting approximately the same location. All images were taken using an Olympus FluoView 10i confocal microscope with a 60× objective and analysed blind to brain age in ImageJ [47]. Global adjustments were made to brightness and contrast in single-channel images, which were then merged and pseudocoloured to count MG in two circles (400 µm diameter each) drawn over the lip region. Details of placement and counting criteria are provided in the electronic supplementary material. Mean counts per calyx (lateral or medial) and means of both calyces were analysed with a one-way ANOVA. Counts were converted to MG per µm3 using calyx neuropil measurements from Muscedere & Traniello [31].
(d). Quantification of biogenic amines
Biogenic amine titres of individual brains were measured using high-performance liquid chromatography with electrochemical detection as described in Muscedere et al. [25] by an experimenter blind to worker age. Details are provided in the electronic supplementary material. Volume measurements of the rind, the outer region of the brain where cell bodies are located, in 20- and 95-day-old minors show no significant differences with age; neuropil volumes would be expected to show similar patterns. Therefore, it was not necessary to correct for brain size. Eleven of 98 minor workers sampled for our assessment of amine titres across age and social role were from a colony lacking a queen. Neither 5HT nor DA titres in these workers differed significantly from titres of age-matched minors from queenright colonies; data were therefore pooled. Amine titres are reported in pg/brain for workers from 13 subcolonies (5HT: N = 23, 31, 18, 14, DA: N = 23, 29, 17, 13 for each age group from 20 to 120 days). Octopamine titres were not measured because levels of this monoamine are very low and do not change with age from eclosion to 20 days in P. dentata, a period during which 5HT and DA titres both significantly increase [29]. Effects of age on amine titres were tested with an ANOVA in JMP and significant differences between groups determined with Tukey's post hoc test.
(e). Power analysis
To be confident that negative results biologically reflected lack-of-age effects, we performed retrospective power analyses using suggested effect sizes from Cohen ([48]; see also [49–51]). Power, expressed as a proportion ranging from 0 to 1, reflects the probability of correctly rejecting the null hypothesis [49] and therefore the ability to detect a statistically significant result. Power greater than 0.8 is generally considered sufficient to detect a specified effect size [48]. We used standardized effect sizes to estimate the power of our analyses and hence our ability to detect significance. For TUNEL analysis, we calculated power using a simulation method for an effect size of 0.5 as well as 2.5, which we found between brain regions. A 2.5-fold difference between ages was used as benchmark for detecting apoptotic cells in association with negligible senescence [52] and an effect size of approximately 2.5 was found for age-related changes in MG density [53]. Our effect sizes of 0.3 and 0.5 are thus conservative. All other tests had power of minimally 0.77 for an effect size of 0.5. We are thus confident that we could detect effects of relevant size in all assays. All power analyses were conducted in PASS14 (NCSS Statistical Software, Kaysville, UT, USA).
3. Results
(a). Task performance
We measured the efficacy of tasks performed within and outside the nest that comprise the breadth of the P. dentata minor worker repertoire at four ages: 20–22, 45–47, 95–97 and 120–122 days. Laboratory mortality of minors is 50% at 77 days and 25% at 117 days (Kaplan–Meier survival estimate), with a maximum lifespan of 140 days.
(i). Nursing effort
Behavioural components of nursing involving sensory and motor functions did not significantly vary with worker age and the frequencies of behaviours were not significantly different from each other (two-way χ2 test; age: 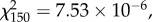 p = 1.00; behaviour:
p = 1.00; behaviour: 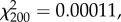 p = 1.00; age × behaviour:
p = 1.00; age × behaviour: 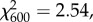 p = 1.00; 20, 45, 95 and 120 days: N = 13, 16, 17, 6, figure 1a). Durations of unspecified brood-directed behaviour, feeding and carrying did not change with age and workers spent significantly more time providing unspecified brood-directed behaviour than feeding (two-way ANOVA; age: F3,144 = 0.398, p = 0.755; behaviour: F2,144 = 6.542, p = 0.0019; age × behaviour: F6,144 = 0.557, p = 0.764, N = 13, 16, 17, 6; Tukey's post hoc, figure 1b; power = 0.942 and 0.366, respectively, for 0.5 and 0.3 effect sizes).
p = 1.00; 20, 45, 95 and 120 days: N = 13, 16, 17, 6, figure 1a). Durations of unspecified brood-directed behaviour, feeding and carrying did not change with age and workers spent significantly more time providing unspecified brood-directed behaviour than feeding (two-way ANOVA; age: F3,144 = 0.398, p = 0.755; behaviour: F2,144 = 6.542, p = 0.0019; age × behaviour: F6,144 = 0.557, p = 0.764, N = 13, 16, 17, 6; Tukey's post hoc, figure 1b; power = 0.942 and 0.366, respectively, for 0.5 and 0.3 effect sizes).
Figure 1.
(a) Number and (b) duration of nursing behaviours (boxes show quartiles and whiskers 95% CIs) of minor workers of different ages are maintained from 20 to 120 days (N = 13, 16, 17, 6 for 20-, 45-, 95- and 120-day-old minors, respectively). (c) Percentage of each age group (20, 45, 95 and 120 days; N = 59, 41, 34, 17) exhibiting each type of predatory response. Number of minors for each response type and age are indicated. (d–f) Worker trail-following improves with age. (d) Trail-following accuracy by 20- and 95-day-old minor workers. (e) Duration within the active space of an artificial trail. (f) Trail-following distance (20 days: N = 7, 10, 7; 95 days: N = 7, 9, 7 for 0.01, 0.1 and 1 TPC, respectively). Significant effect of age on trail-following distance is indicated by an asterisk. All data points shown. Boxes reflect first and third quartiles and whiskers show 95% CIs. (Online version in colour.)
(ii). Responsiveness to social signals regulating foraging and other collective actions
We examined the impact of worker age on fundamental sensorimotor functions (olfactory responsiveness, motor activity and motion coordination) and thus the ability of workers to participate in cooperative foraging by quantifying osmotropotactic pheromone-trail following. Neither worker age nor TPC significantly affected trail-following accuracy, without an interaction effect (two-way ANOVA, F1,41 = 1.444; age: p = 0.2104; TPC: F2,41 = 2.995, p = 0.1895; age × TPC: F2,41 = 0.903, p = 0.4809, all FDR corrected; 20-day N = 7, 10, 7; 95-day N = 7, 9, 7 for 1.0, 0.1 and 0.01 gland/trail TPC, respectively; figure 1d). Duration within the active space of pheromone was not significantly affected by age and there was no significant effect of TPC or interaction effect (two-way ANOVA, age: F1,41 = 4.2404, p = 0.1607; TPC: F2,41 = 1.899, p = 0.20; age × TPC: F2,41 = 0.4644, p = 0.6318; all p-values are FDR corrected, figure 1e). Mean and maximum durations of following did not differ significantly with age or TPC and did not show significant interaction effects (two-way ANOVA; age: F1,41 = 0.0132, TPC: F2,41 = 1.388, age × TPC: F2,41 = 1.574, all p > 0.2). Older workers followed trails for longer distances and minors of both ages tended to follow trails for greater distances at higher concentrations (two-way ANOVA, age: F1,41 = 9.684, p = 0.0238; TPC: F2,41 = 4.863, p = 0.0889; age × TPC: F2,41 = 0.545, p = 0.6318; all p-values FDR corrected, figure 1f; power = 0.768 and 0.366, respectively, for effect sizes of 0.5 and 0.3 for all trail-following metrics).
(iii). Age-related changes in predatory behaviour
Ageing minors showed no decline in responsiveness to prey or predatory attack (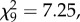 p = 0.61; 20, 45, 95 and 120 days: N = 59, 41, 34, 17, figure 1c; power = 0.977 and 0.728, respectively, for effects sizes of 0.5 and 0.3).
p = 0.61; 20, 45, 95 and 120 days: N = 59, 41, 34, 17, figure 1c; power = 0.977 and 0.728, respectively, for effects sizes of 0.5 and 0.3).
(b). Age-related neurobiological changes
(i). Minor worker age and distribution of apoptosis in brain compartments
Across all ages, minor workers had very few apoptotic cells in all brain regions (figure 2a). The proportion of apoptotic cells was highly variable and did not differ between 20- and 95-day-old workers. Apoptotic cell counts differed by brain region (mixed-effects ANOVA, age: F1,78 = 2.570, p = 0.120; brain region: F5,27.8 = 5.403, p < 0.001; age x brain region: F5 = 1.974, p = 0.1863; 20 days: N = 11, 7, 9, 8, 9, 11; 95 days: 10, 10, 10, 9, 9, 7 for the MB, AL, OL, CC, RCB and SEZ, respectively, figure 2b). The CC and OLs had a significantly lower proportion of apoptotic cells than the RCB. For an effect size of 0.5, power was 0.16 for age, 0.33 for brain region and 0.20 for interaction effect. Assuming an effect size of 2.5, as we found between the CC and RCB and was the smaller of our two significant differences, power was 0.79, 1.0 and 1.0 for age, brain region and age × brain region.
Figure 2.
Neuroanatomical and neurochemical metrics. (a) Representative apoptotic cell in the MB rind (cell body region) in a 95-day-old minor. Cell bodies labelled with DAPI (blue); TUNEL-positive cell (green). Inset: TUNEL-positive cell co-localized showing condensed nucleus. (b) Proportion of apoptotic cells in relation to total cells/brain region (95% CIs [whiskers]) in 20- and 95-day-old ants (20 days: N = 11, 7, 9, 8, 9, 11; 95 days: 10, 10, 10, 9, 9, 7 for the MB, AL, OL, CC, RCB and SEZ, respectively). Age did not significantly affect proportion of cells but brain regions differed (20 days left, 95 days right for each brain region). MB, mushroom bodies; AL, antennal lobes; OL, optic lobes; CB, central body; RCB, remainder central brain; SEZ, suboesophageal zone. (c) Representative micrographs illustrating densities of MG in a 20- (left) and 95-day-old minor (right). Brains are pseudocoloured; phallodin (green), synapsin (magenta). (d,e) Brain titres of 5HT (d) and DA (e) significantly increase with age (p < 0.001; 5HT: N = 23, 31, 18, 14; DA: N = 23, 29, 17, 13, for 20-, 45-, 95- and 120-day-old minors, respectively). Boxes show first and third quartiles; whiskers = 95% CIs. (Online version in colour.)
(ii). Density of synaptic complexes
MG density did not significantly change with worker age (mean ± s.e., 20 days: 0.0891 ± 0.00179, 95 days: 0.0859 ± 0.00224 MG µm−3 of calyx, N = 28, 18; ANOVA, F1,44 = 1.211, p = 0.28, figure 2c; power = 0.9125 and 0.512 for effect sizes of 0.5 and 0.3, respectively).
(iii). Brain titres of dopamine and serotonin
Brain 5HT and DA levels significantly increased with age (ANOVA, 5HT: F3,82 = 10.443, p < 0.0001, N = 23, 31, 18, 14; power = 0.973 and 0.597 for 0.5 and 0.3 effect sizes; DA: F3,78 = 20.921, p < 0.0001, N = 23, 29, 17, 13; figure 2d; power = 0.970 and 0.585 for effect sizes of 0.5 and 0.3). 5HT was significantly higher in brains of 95- and 120-day-old minors than 20- and 45-day-old minors. DA titre increased beyond 45 days of age.
4. Discussion
With advancing age, P. dentata minor workers showed no apparent deficits in the suite of behaviours we assayed, no increase in apoptosis in brain compartments that regulate task performance, and no decline in densities of synaptic structures considered significant to cognitive ability. In aggregate, our behavioural and neurobiological results capture the pattern of minor worker ageing through comprehensive assays of task performance and suggest negligible senescence throughout at least 85% of the minor worker lifespan. Results of behavioural assays were consistent with records of low levels of apoptosis throughout the brain and particularly in the MBs, and ALs, and OLs of ageing workers. The age-invariance of MG density suggests olfactory processing abilities remain intact in old age. Extranidal work involves individual and collective actions requiring orientation and navigation, and the sampling of diverse sensory environments. Responsiveness to light, which cues the performance of outside-nest work, did not decline with age, and old minors showed positive phototaxis, providing evidence that light level discrimination does not senesce (electronic supplementary material, figure S1). Our results indicate that old workers retain the range of sensory abilities necessary to be behaviourally pluripotent and switch tasks having different spatial distributions. Neither the efficacy nor plasticity of task performance appeared to be compromised in ageing workers. Our results suggest age-related losses in cell numbers in the brain were extremely low and thus insignificant, and appear to have no impact on behavioural functions. Therefore, processing power underscoring social signal and cue perception and response appears to be maintained in old workers. The CC and OLs had lower levels of apoptosis than the RCB, suggesting that brain regions critically important to information processing and responsiveness to task stimuli may be particularly buffered from decline. The CC is being increasingly implicated in integrative functions as well as motor output [54], and maintenance of the OL neuropil with increasing age could be particularly important to extranidal task performance. Pheidole dentata have relatively small eyes and OLs [31] which could contribute to lower levels of redundancy in these circuits and hence greater importance for their protection. High variability in the proportion of apoptotic cells in the CC likely stems from the small number of cells that comprise this region, causing a small absolute change in the number of dying cells to more strongly influence this proportion. Nevertheless, the CC showed significantly lower levels of apoptosis than the RCB. Titres of 5HT—a neuromodulator significant for olfactory social functions in P. dentata [25]—and DA increased with age, rather than showing senescence-associated declines [37,55]. DA can have neurotrophic functions, and 5HT and DA may interact to promote circuit development and neuroplasticity [34] as well as modulate sensory [56,57] and motor function [58,59], perhaps contributing to the preservation of task performance in ageing workers. In sum, we did not identify age-related deficits in behavioural abilities or aminergic or cell deterioration, suggesting that P. dentata minor workers do not exhibit senescence throughout much of their lifespan and are able to continue to effectively contribute to colony labour well into their final weeks of life.
Older P. dentata minors are more efficient at nursing [24] and show a significantly increased response compared with young minors (less than approx. 10–15 days of age) to colony conditions that reflect greater brood-care needs [23]. Our results demonstrate that minor workers retain the olfactory sensitivity required to assess brood welfare and nurture growth and development, as well as provide motor control to mouthparts to manipulate delicate eggs and immatures, thus allowing them to remain effective alloparents late in life.
As in many ant species, olfaction is the predominant sensory modality in P. dentata. Responsiveness to chemical cues and pheromones can develop with maturity [60] and decline with senescence [61]. However, results suggest that old P. dentata minors do not decline in performing foraging or nursing tasks that differ qualitatively in olfactory social cue and signal arrays. Odorant inputs are transduced to trigger motor output through the SEZ to control the mouthparts [62] and thoracic ganglia to direct leg movements [63]. Our assessment of predation showed no effect of worker age on sensory responsiveness to prey or the motor execution of attack. Minors of all ages did not appear impaired in attack mechanics, including biting behaviour, suggesting that mandibular muscle functions and SEZ neuropil appear well maintained with age. Again, contrary to senescence theory, we found improvements in trail following and activity level (electronic supplementary material, figure S1) in ageing minors, suggesting continued development and/or experience-dependent enhancements of behaviour into old age. Workers did not decrease in trail-following accuracy; surprisingly, 95-day minors followed trails for longer distances than younger individuals, perhaps due to their higher activity level (electronic supplementary material, figure S1), enhanced olfactory responsiveness to trail pheromone, or both. Therefore, older minors not only did not exhibit senescence, but in fact improved in certain functions, suggesting selection for effective task performance throughout the lifespan. This does not imply behavioural specialization by older workers on outside-nest tasks; indeed, P. dentata do not appear to exhibit strong age-related variation in worksites [64]. Specialized workers may in fact not be more efficient in task performance in at least some taxa (Temnothorax albipennis; [65]). Pharmacological depletion of 5HT in P. dentata decreases responsiveness to trail-pheromone, likely through modulation of olfactory sensitivity [25]. Although declining monoamine titres could cause olfactory and hence behavioural deficits, 5HT and DA titres increased with age, potentially enabling olfaction to remain intact.
Social insect workers have been hypothesized to experience programmed death at an age marginally greater than their life expectancy in nature [66], implying that task performance abilities either gradually diminish or abruptly decline with age. Although worker longevity in the field is not known in P. dentata, it is reasonably anticipated to be shorter than the 140-day lifespan we recorded in the laboratory, given increased worker mortality associated with the transition to extranidal tasks performed in more unpredictable environments [20,67,68]. Although we cannot exclude the possibility that P. dentata minor workers decline rapidly just before death, this appears to be unlikely because precipitous senescence [69] is rare and associated with unusually high investment in reproduction [70], which is absent in sterile workers. The lack of functional senescence in P. dentata minors contrasts with the rapid ageing exhibited by most genetic gerontological models [71]. Studies on ageing honeybees are equivocal regarding functional senescence in workers [12,14–16], which do not decline in responsiveness to light and sucrose, olfactory associative learning or locomotion as they age [12]. However, chronological age is only one factor influencing senescence in honeybees: overwintering bees, which can be more than six months old, maintain tactile and olfactory learning but show deficits in olfactory long-term memory in comparison to chronologically younger foragers [72]. Our behavioural metrics encompassed measures of sensorimotor function similar to those used in studies of honeybees, as well as efficacy assessments of task performance inside and outside the nest required to fully evaluate worker capability in a species that retains a broad task repertoire throughout the worker lifespan. Such differences in hymenopteran ageing phenotypes may be related to the lack of temporal caste discretization in P. dentata [23,73] and contrasting nesting and foraging ecology.
Eusociality has had profound consequences for the evolution of behavioural development, immune function and genetic regulation of ageing [17,18,19]. Social interactions appear to mediate metabolic homeostasis and affect mortality rates [74]. Division of labour could in part lead to selection for the maintenance of individual functionality throughout the sterile worker lifespan [20]. In ants, specialized morphologies and task performance are key to social complexity; worker polymorphism, task repertoire development and behavioural specialization are underscored by brain neuropil growth and investment patterns [31]. Complex task repertoires are generated by a miniature brain that even at an extremely small size does not appear to compromise cognitive ability [75], suggesting that ant brains may operate with a level of metabolic efficiency that could enable conserved energy to be distributed to provide molecular protection against neural and behavioural senescence. The lack of functional senescence in P. dentata minor workers, underscored by the maintenance of neuroanatomical and neurochemical substrates that activate and support task performance, suggests that the ant nervous system has evolved robust functionality throughout the relatively short sterile worker lifespan. This resilience may be associated with energy savings resulting from the absence of reproductive costs of workers, reduced neuron and neural circuit size, and lower requirements for redundancy and information storage that could minimize neurometabolic costs in individual worker brains [76–79]. Additionally, the benefits of living in a highly integrated homeostatic social system capable of collective information processing and emergent cognition may allocate cost reductions in brain metabolism to neural systems operational maintenance and thus effective lifetime behavioural performance.
Supplementary Material
Acknowledgements
Prof. Rhondda Jones and Dr Iulian Ilieş provided valuable statistical advice. We thank Drs Wulfila Gronenberg, Karen Warkentin and Kimberly McCall for their critical reading of earlier drafts of the manuscript and technical insights. Two anonymous reviewers provided constructive comments. Positive controls for TUNEL were adapted from procedures developed by Dr J. I. Etchegaray.
Data Accessibility
Data available from the Dryad Digital Repository: http://doi.org/10.5061/dryad.m280g.
Authors' Contributions
Y.M.G. conceived the study, designed and performed experiments, analysed data, performed statistics, and drafted and edited the manuscript. J.F.K. was involved in experimental design, data analysis and manuscript editing for the apoptosis study. V.F. and M.M. analysed trail-following assays. S.J.K.R. assisted with statistical analyses. A.R., L.W., A.D. and A.K. performed experiments, assisted in data analysis and contributed to drafting the manuscript. J.F.A.T. co-conceived the studies, designed experiments, and drafted and edited the manuscript. All authors gave final approval of the manuscript.
Competing Interests
We have no competing interests.
Funding
Y.M.G. was supported by the National Institute on Ageing of the National Institutes of Health (grant no. F31AG041589) and the National Science Foundation (grant nos. IOB 0725013 and IOS 1354291; J.F.T. sponsor). Support was also provided by Boston University Undergraduate Research Opportunity Program to A.R., A.K. and A.D. The work presented here is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Williams GC. 1957. Pleiotropy, natural selection, and the evolution of senescence. Evolution 11, 398–411. ( 10.2307/2406060) [DOI] [Google Scholar]
- 2.Medawar PB. 1952. An unsolved problem of biology. London, UK: H. K. Lewis. [Google Scholar]
- 3.Kirkwood TBL. 1977. Evolution of ageing. Nature 270, 301–304. ( 10.1038/270301a0) [DOI] [PubMed] [Google Scholar]
- 4.Guarente L, Kenyon C. 2000. Genetic pathways that regulate ageing in model organisms. Nature 408, 255–262. ( 10.1038/35041700) [DOI] [PubMed] [Google Scholar]
- 5.Martin GM. 2011. The biology of ageing: 1985–2010 and beyond. FASEB J. 25, 3756–3762. ( 10.1096/fj.11-1102.ufm) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeoman MS, Faragher RG. 2001. Ageing and the nervous system: insights from studies on invertebrates. Biogerontology 2, 85–97. ( 10.1023/A:1011597420036) [DOI] [PubMed] [Google Scholar]
- 7.Erickson CA, Barnes CA. 2003. The neurobiology of memory changes in normal ageing. Exp. Gerontol. 38, 61–69. ( 10.1016/S0531-5565(02)00160-2) [DOI] [PubMed] [Google Scholar]
- 8.Haddadi M, Jahromi SR, Sagar BKC, Patil RK, Shivanandappa T, Ramesh SR. 2014. Brain ageing, memory impairment and oxidative stress: a study in Drosophila melanogaster. Behav. Brain Res. 259, 60–69. ( 10.1016/j.bbr.2013.10.036) [DOI] [PubMed] [Google Scholar]
- 9.Friedlander RM. 2003. Apoptosis and caspases in neurodegenerative diseases. N. Engl. J. Med. 348, 1365–1375. ( 10.1056/NEJMra022366) [DOI] [PubMed] [Google Scholar]
- 10.Degterev A, Boyce M, Yuan J. 2003. A decade of caspases. Oncogene 22, 8543–8567. ( 10.1038/sj.onc.1207107) [DOI] [PubMed] [Google Scholar]
- 11.Rueppell O, Bachelier C, Fondrk MK, Page RE. 2007. Regulation of life history determines lifespan of worker honey bees (Apis mellifera L.). Exp. Gerontol. 42, 1020–1032. ( 10.1016/j.exger.2007.06.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rueppell O, Christine S, Mulcrone C, Groves L. 2007. Aging without functional senescence in honey bee workers. Curr. Biol. 17, R274–R275. ( 10.1016/j.cub.2007.02.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bourke AFG. 2007. Kin selection and the evolutionary theory of ageing. Annu. Rev. Ecol. Evol. Syst. 38, 103–128. ( 10.1146/annurev.ecolsys.38.091206.095528) [DOI] [Google Scholar]
- 14.Seehuus S-C, Krekling T, Amdam GV. 2006. Cellular senescence in honey bee brain is largely independent of chronological age. Exp. Gerontol. 41, 1117–1125. ( 10.1016/j.exger.2006.08.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Behrends A, Scheiner R, Baker N, Amdam GV. 2007. Cognitive ageing is linked to social role in honey bees (Apis mellifera). Exp. Gerontol. 42, 1146–1153. ( 10.1016/j.exger.2007.09.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Remolina SC, Hafez DM, Robinson GE, Hughes KA. 2007. Senescence in the worker honey bee Apis mellifera. J. Insect Physiol. 53, 1027–1033. ( 10.1016/j.jinsphys.2007.05.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keller L, Jemielity S. 2006. Social insects as a model to study the molecular basis of ageing. Exp. Gerontol. 41, 553–556. ( 10.1016/j.exger.2006.04.002) [DOI] [PubMed] [Google Scholar]
- 18.Rueppell O. 2009. Aging of social insects. In Organization of insect societies: from genome to sociocomplexity (eds Gadeau J, Fewell J, Wilson EO), pp. 51–73. Cambridge, MA: Harvard University Press. [Google Scholar]
- 19.Amdam GV. 2011. Social context, stress, and plasticity of ageing. Aging Cell 10, 18–27. ( 10.1111/j.1474-9726.2010.00647.x) [DOI] [PubMed] [Google Scholar]
- 20.Giraldo YM, Traniello JFA. 2014. Worker senescence and the sociobiology of ageing in ants. Behav. Ecol. Sociobiol. 68, 1901–1919. ( 10.1007/s00265-014-1826-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carey JR. 2001. Insect biodemography. Annu. Rev. Entomol. 46, 79–110. ( 10.1146/annurev.ento.46.1.79) [DOI] [PubMed] [Google Scholar]
- 22.Wilson EO. 1976. Behavioral discretization and the number of castes in an ant species. Behav. Ecol. Sociobiol. 1, 141–154. ( 10.1007/BF00299195) [DOI] [Google Scholar]
- 23.Seid MA, Traniello JFA. 2006. Age-related repertoire expansion and division of labor in Pheidole dentata (Hymenoptera: Formicidae): a new perspective on temporal polyethism and behavioral plasticity in ants. Behav. Ecol. Sociobiol. 60, 631–644. ( 10.1007/s00265-006-0207-z) [DOI] [Google Scholar]
- 24.Muscedere ML, Willey TA, Traniello JFA. 2009. Age and task efficiency in the ant Pheidole dentata: young minor workers are not specialist nurses. Anim. Behav. 77, 911–918. ( 10.1016/j.anbehav.2008.12.018) [DOI] [Google Scholar]
- 25.Muscedere ML, Johnson N, Gillis BC, Kamhi JF, Traniello JFA. 2012. Serotonin modulates worker responsiveness to trail pheromone in the ant Pheidole dentata. J. Comp. Physiol. A 198, 219–227. ( 10.1007/s00359-011-0701-2) [DOI] [PubMed] [Google Scholar]
- 26.Muscedere ML, Djermoun A, Traniello JFA. 2013. Brood-care experience, nursing performance, and neural development in the ant Pheidole dentata. Behav. Ecol. Sociobiol. 67, 775–784. ( 10.1007/s00265-013-1501-1) [DOI] [Google Scholar]
- 27.Ilieş I, Muscedere ML, Traniello JFA. 2015. Neuroanatomical and morphological trait clusters in the ant genus Pheidole: evidence for modularity and integration in brain structure. Brain. Behav. Evol. 85, 63–76. ( 10.1159/000370100) [DOI] [PubMed] [Google Scholar]
- 28.Seid MA, Harris KM, Traniello JFA. 2005. Age-related changes in the number and structure of synapses in the lip region of the mushroom bodies in the ant Pheidole dentata. J. Comp. Neurol. 488, 269–277. ( 10.1002/cne.20545) [DOI] [PubMed] [Google Scholar]
- 29.Seid MA, Traniello JFA. 2005. Age-related changes in biogenic amines in individual brains of the ant Pheidole dentata. Naturwissenschaften 92, 198–201. ( 10.1007/s00114-005-0610-8) [DOI] [PubMed] [Google Scholar]
- 30.Seid MA, Goode K, Li C, Traniello JFA. 2008. Age- and subcaste-related patterns of serotonergic immunoreactivity in the optic lobes of the ant Pheidole dentata. Dev. Neurobiol. 68, 1325–1333. ( 10.1002/dneu.20663) [DOI] [PubMed] [Google Scholar]
- 31.Muscedere ML, Traniello JFA. 2012. Division of labor in the hyperdiverse ant genus Pheidole is associated with distinct subcaste- and age-related patterns of worker brain organization. PLoS ONE 7, e31618 ( 10.1371/journal.pone.0031618) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stieb SM, Hellwig A, Wehner R, Rössler W. 2012. Visual experience affects both behavioral and neuronal aspects in the individual life history of the desert ant Cataglyphis fortis. Dev. Neurobiol. 72, 729–742. ( 10.1002/dneu.20982) [DOI] [PubMed] [Google Scholar]
- 33.Groh C, Kelber C, Grübel K, Rössler W. 2014. Density of mushroom body synaptic complexes limits intraspecies brain miniaturization in highly polymorphic leaf-cutting ant workers. Proc. R. Soc. B 281, 20140432 ( 10.1098/rspb.2014.0432) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neckameyer WS, Bhatt P. 2012. Neurotrophic actions of dopamine on the development of a serotonergic feeding circuit in Drosophila melanogaster. BMC Neurosci. 13, 26 ( 10.1186/1471-2202-13-26) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strausfeld NJ. 2012. Arthropod brains: evolution, functional elegance, and historical significance. Cambridge, MA: Belknap Press of Harvard University Press. [Google Scholar]
- 36.Groh C, Rössler W. 2011. Comparison of microglomerular structures in the mushroom body calyx of neopteran insects. Arthropod. Struct. Dev. 40, 358–367. ( 10.1016/j.asd.2010.12.002) [DOI] [PubMed] [Google Scholar]
- 37.Rodríguez JJ, Noristani HN, Verkhratsky A. 2012. The serotonergic system in ageing and Alzheimer's disease. Prog. Neurobiol. 99, 15–41. ( 10.1016/j.pneurobio.2012.06.010) [DOI] [PubMed] [Google Scholar]
- 38.Kamhi JF, Traniello JFA. 2013. Biogenic amines and collective organization in a superorganism: neuromodulation of social behavior in ants. Brain. Behav. Evol. 82, 220–236. ( 10.1159/000356091) [DOI] [PubMed] [Google Scholar]
- 39.Branson K, Robie AA, Bender J, Perona P, Dickinson MH. 2009. High-throughput ethomics in large groups of Drosophila. Nat. Methods 6, 451–457. ( 10.1038/nmeth.1328) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pike N. 2011. Using false discovery rates for multiple comparisons in ecology and evolution. Methods Ecol. Evol. 2, 278–282. ( 10.1111/j.2041-210X.2010.00061.x) [DOI] [Google Scholar]
- 41.Gavrieli Y, Sherman Y, Ben-Sasson SA. 1992. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell Biol. 119, 493–501. ( 10.1083/jcb.119.3.493) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCall K, Peterson JS. 2004. Detection of apoptosis in Drosophila. Methods Mol. Biol. 282, 191–205. ( 10.1385/1-59259-812-9:191) [DOI] [PubMed] [Google Scholar]
- 43.Galluzzi L, et al. 2009. Guidelines for the use and interpretation of assays for monitoring cell death in higher eukaryotes. Cell Death Differ. 16, 1093–1107. ( 10.1038/cdd.2009.44) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robertson JD, Orrenius S, Zhivotovsky B. 2000. Review: nuclear events in apoptosis. J. Struct. Biol. 129, 346–358. ( 10.1006/jsbi.2000.4254) [DOI] [PubMed] [Google Scholar]
- 45.Gundersen HJ, et al. 1988. The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS 96, 857–881. ( 10.1111/j.1699-0463.1988.tb00954.x) [DOI] [PubMed] [Google Scholar]
- 46.Groh C, Tautz J, Rössler W. 2004. Synaptic organization in the adult honey bee brain is influenced by brood-temperature control during pupal development. Proc. Natl Acad. Sci. USA 101, 4268–4273. ( 10.1073/pnas.0400773101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675. ( 10.1038/nmeth.2089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohen J. 1988. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- 49.Thomas L. 1997. Retrospective power analysis. Conserv. Biol. 11, 276–280. ( 10.1046/j.1523-1739.1997.96102.x) [DOI] [Google Scholar]
- 50.Nakagawa S, Foster TM. 2004. The case against retrospective statistical power analyses with an introduction to power analysis. Acta Ethol. 7, 103–108. ( 10.1007/s10211-004-0095-z) [DOI] [Google Scholar]
- 51.Smith DR, Hardy ICW, Gammell MP. 2011. Power rangers: no improvement in the statistical power of analyses published in Animal Behaviour. Anim. Behav. 81, 347–352. ( 10.1016/j.anbehav.2010.09.026) [DOI] [Google Scholar]
- 52.Traniello IM, Sîrbulescu RF, Ilieş I, Zupanc GKH. 2014. Age-related changes in stem cell dynamics, neurogenesis, apoptosis, and gliosis in the adult brain: a novel teleost fish model of negligible senescence. Dev. Neurobiol. 74, 514–530. ( 10.1002/dneu.22145) [DOI] [PubMed] [Google Scholar]
- 53.Groh C, Ahrens D, Rossler W. 2006. Environment- and age-dependent plasticity of synaptic complexes in the mushroom bodies of honeybee queens. Brain. Behav. Evol. 68, 1–14. ( 10.1159/000092309) [DOI] [PubMed] [Google Scholar]
- 54.Pfeiffer K, Homberg U. 2014. Organization and functional roles of the central complex in the insect brain. Annu. Rev. Entomol. 59, 165–184. ( 10.1146/annurev-ento-011613-162031) [DOI] [PubMed] [Google Scholar]
- 55.Luijtelaar MGPA, van Steinbusch HWM, Tonnaer JADM. 1989. Similarities between aberrant serotonergic fibers in the aged and 5,7-DHT denervated young adult rat brain. Exp. Brain Res. 78, 81–89. ( 10.1007/BF00230689) [DOI] [PubMed] [Google Scholar]
- 56.Dacks AM, Christensen TA, Hildebrand JG. 2008. Modulation of olfactory information processing in the antennal lobe of Manduca sexta by serotonin. J. Neurophysiol. 99, 2077–2085. ( 10.1152/jn.01372.2007) [DOI] [PubMed] [Google Scholar]
- 57.Lazarini F, Gabellec M-M, Moigneu C, de Chaumont F, Olivo-Marin J-C, Lledo P-M. 2014. Adult neurogenesis restores dopaminergic neuronal loss in the olfactory bulb. J. Neurosci. 34, 14 430–14 442. ( 10.1523/JNEUROSCI.5366-13.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mustard JA, Pham PM, Smith BH. 2010. Modulation of motor behavior by dopamine and the D1-like dopamine receptor AmDOP2 in the honey bee. J. Insect Physiol. 56, 422–430. ( 10.1016/j.jinsphys.2009.11.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pearlstein E, Bras H, Deneris ES, Vinay L. 2011. Contribution of 5-HT to locomotion—the paradox of Pet-1−/− mice. Eur. J. Neurosci. 33, 1812–1822. ( 10.1111/j.1460-9568.2011.07679.x) [DOI] [PubMed] [Google Scholar]
- 60.Gadenne C, Renou M, Sreng L. 1993. Hormonal control of pheromone responsiveness in the male black cutworm Agrotis ipsilon. Experientia 15, 721–724. ( 10.1007/BF01923960) [DOI] [Google Scholar]
- 61.Cook-Wiens E, Grotewiel MS. 2002. Dissociation between functional senescence and oxidative stress resistance in Drosophila. Exp. Gerontol. 37, 1347–1357. ( 10.1016/S0531-5565(02)00096-7) [DOI] [PubMed] [Google Scholar]
- 62.Jørgensen K, Kvello P, Almaas TJ, Mustaparta H. 2006. Two closely located areas in the suboesophageal ganglion and the tritocerebrum receive projections of gustatory receptor neurons located on the antennae and the proboscis in the moth Heliothis virescens. J. Comp. Neurol. 496, 121–134. ( 10.1002/cne.20908) [DOI] [PubMed] [Google Scholar]
- 63.Niven JE, Graham CM, Burrows M. 2008. Diversity and evolution of the insect ventral nerve cord. Annu. Rev. Entomol. 53, 253–271. ( 10.1146/annurev.ento.52.110405.091322) [DOI] [PubMed] [Google Scholar]
- 64.Giraldo YM, Rusakov A, Diloreto A, Kordek A, Traniello JFA.Age, spatial location, neuromodulators, and task performance in the ant Pheidole dentata. In preparation. [DOI] [PMC free article] [PubMed]
- 65.Dornhaus A. 2008. Specialization does not predict individual efficiency in an ant. PLoS Biol. 6, 2368–2375. ( 10.1371/journal.pbio.0060285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oster GF, Wilson EO. 1978. Caste and ecology in the social insects. Princeton, NJ: Princeton University Press. [PubMed] [Google Scholar]
- 67.Schmid-Hempel P, Schmid-Hempel R. 1984. Life duration and turnover of foragers in the ant Cataglyphis bicolor (Hymenoptera, Formicidae). Insect. Soc. 31, 345–360. ( 10.1007/BF02223652) [DOI] [Google Scholar]
- 68.Kwapich CL, Tschinkel WR. 2013. Demography, demand, death, and the seasonal allocation of labor in the Florida harvester ant (Pogonomyrmex badius). Behav. Ecol. Sociobiol. 67, 2011–2027. ( 10.1007/s00265-013-1611-9) [DOI] [Google Scholar]
- 69.Morbey YE, Brassil CE, Hendry AP. 2005. Rapid senescence in pacific salmon. Am. Nat. 166, 556–568. ( 10.1086/491720) [DOI] [PubMed] [Google Scholar]
- 70.Finch CE. 1990. Longevity, senescence, and the genome. Chicago, IL: University of Chicago Press. [Google Scholar]
- 71.Austad SN. 2009. Comparative biology of ageing. J. Gerontol. Ser. A 64, 199–201. ( 10.1093/gerona/gln060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Behrends A, Scheiner R. 2010. Learning at old age: a study on winter bees. Front. Behav. Neurosci. 4, 15 ( 10.3389/fnbeh.2010.00015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Johnson BR. 2007. Within-nest temporal polyethism in the honey bee. Behav. Ecol. Sociobiol. 62, 777–784. ( 10.1007/s00265-007-0503-2) [DOI] [Google Scholar]
- 74.Koto A, Mersch D, Hollis B, Keller L. 2015. Social isolation causes mortality by disrupting energy homeostasis in ants. Behav. Ecol. Sociobiol. 69, 583–591. ( 10.1007/s00265-014-1869-6) [DOI] [Google Scholar]
- 75.Muscedere ML, Gronenberg W, Moreau CS, Traniello JFA. 2014. Investment in higher order central processing regions is not constrained by brain size in social insects. Proc. R. Soc. B 281, 20140217 ( 10.1098/rspb.2014.0217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chittka L, Niven J. 2009. Are bigger brains better? Curr. Biol. 19, R995–R1008. ( 10.1016/j.cub.2009.08.023) [DOI] [PubMed] [Google Scholar]
- 77.Eberhard WG, Wcislo WT. 2011. Grade changes in brain-body allometry. Morphological and behavioural correlates of brain size in miniature spiders, insects and other invertebrates. In Advances in insect physiology (ed. Casas J.), pp. 155–214. Burlington, NJ: Academic Press. [Google Scholar]
- 78.Seid MA, Castillo A, Wcislo WT. 2011. The allometry of brain miniaturization in ants. Brain. Behav. Evol. 77, 5–13. ( 10.1159/000322530) [DOI] [PubMed] [Google Scholar]
- 79.Wehner R, Fukushi T, Isler K. 2007. On being small: brain allometry in ants. Brain. Behav. Evol. 69, 220–228. ( 10.1159/000097057) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository: http://doi.org/10.5061/dryad.m280g.