Abstract
Objective:
The main objective of this preliminary study is to confirm the synergistic anticarcinogenic potential of Vitex trifolia and Triticum aestivum ethanolic extracts.
Materials and Methods:
Rat hepatic microsomal degranulation is a short - term technique that has been used for the detection of potential chemical carcinogens, in vitro. The present study has been carried out to study the inhibition of ribosome- membrane disruption against 3, 8-Diamino-5-ethyl-6-pheylphenanthridinium bromide (EB), as the degranulating agent, by measuring the RNA/protein ratios of microsomal membranes in the presence or absence of V.trifolia and T. aestivum extracts. These two extracts were further evaluated for cytotoxic effect in HCT 116 and A549 cell lines.
Results:
V. trifolia and T. aestivum protects hepatic microsomes against the degranulatory attack by the carcinogen EB showed a significant reduction in the proliferation of the HCT 116 and A549 cancer cell lines.
Conclusion:
The ethanolic extracts of the plants, V. trifolia and T. aestivum individually possessed anti-degranulatory potential. Importantly they act synergistically, possess appreciable anticarcinogenic properties, based on their ability to inhibit EB induced liver microsomal degranulation. Further these extracts inhibit cell proliferation of cancer cell lines.
Keywords: 3, 8-diamino-5-ethyl-6-phenylphen-anthridinium bromide, degranulation, Vitex trifolia, Triticum aestivum
INTRODUCTION
Herbal And food - based medicines obtained from plant extracts have been traditionally utilized to treat a wide variety of diseases and clinical disorders and yet relatively little knowledge is available about their mode of action, specific molecules involved, and exact effective doses. For instance, plants that are rich in polyphenolic compounds, especially flavonoids, are used to benefit clinically human health by reducing the incidence of cancer.[1] The plant Vitex trifolia L., (Verbenaceae) is a flavonoid rich medicinal plant which has been traditionally used to treat rheumatic pain and inflammation.[2] Also, pharmacological properties have been linked with Vitex viz., antibacterial.,[3] and hepatoprotective activity.[4] Recently the flavonoids have been shown as novel cell cycle inhibitors.[5] The shoot of Triticum aestivum L., (Wheat grass) contains flavonoids which possess anti-cancer activity, ulcer-alleviating ability, antioxidant activity, anti-arthritic activity, and blood refreshing activity in Thalassemia Major. The key clinical utilization of wheat grass might be due to the presence of high content of bioflavonoids such as apigenin, quercitin, luteoline. Furthermore, indole compounds namely choline and laetrile present in it might also be responsible for its therapeutic potential.[6] Since very few studies have been made on these very promising herbal drugs, it was thought worthwhile to study the anticarcinogenic effect of V.trifolia and T. aestivum against a provenchemical carcinogen by a short - term technique, under in vitro conditions, using rat liver microsomes. Also, synergistic actions of these extracts were investigated in this study.
MATERIALS AND METHODS
Preparation of the extract
Leaves of V. trifolia L., (Verbenaceae) and Shoot of T. aestivum L were collected from VIT university plant nursery, Vellore, Tamil Nadu. India during the month of September and were identified by the botanist, Professor Dr. Ayyanar, Loyola college, Chennai, India. The V. trifolia leaves and T. aestivum shoots were shade-dried for 1 week and ground into fine powder. The ground leaf powder (25g) was extracted with 250 ml of 90% ethanol in soxhlet apparatus. The ethanolic mixture was transferred into rotary evaporator for removal of solvent from sample. The extract (around 5 g) was stored at -20°C before assay.
Chemicals
The chemicals used in the present study were purchased from Sigma-Aldrich (India) and S. D. Fine Chem Ltd and were of analytical grade.
Animals
Male Wistar rats, weighing 120-150g were used. The animals were cared in accordance with the guidelines provided by the Committee for the Purpose of Control and Supervision on Experiments on Animals and the Institutional Animal Ethics Committee approved the entire study (Approval no.VIT/IAEC/VIIth/34/2013).
Estimation of total phenolic content
Total phenolic content was determined by reaction with Folin-Ciocalteu reagent, as described by Singleton and Rossi[7] and Kahkonen et al.[8] Leaf extract (1mg/ml) was mixed with 2 ml of Folin-Ciocalteu reagent (which had previously been diluted 10-fold with distilled water) and allowed to stand at room temperature for 8 min; 1.6 ml of 7.5% Na2 CO3 solution was added to the mixture. Contents of the tubes were mixed and allowed to stand in the dark for 30 min, after which the absorbance was measured at 765 nm (Hitachi U-2001). The results were expressed as gallic acid equivalents per 100 mg of crude extract (mg gallic acid equivalents/100 mg extract).
Estimation of flavonoid content
Flavonoid content was determined by the procedure described by Zhishen et al.[9]
Leaf extract (1mg/ml) was mixed with 0.3 ml of 5% aqueous NaNO2 (w/v) and allowed to stand at room temperature for 5 min; 0.6 ml of 10% AlCl3 solution (w/v) was added to the mixture. 2 ml of 1M NaOH and 2.1 ml of water were added to the mixture after 6 min. The absorbance was measured at 510 nm (Hitachi U-2001). The results were expressed as quercetin (Q) equivalents per 100 mg of crude extract (mg Q/100 mg extract).
Microsomal degranulation assay
Five rats were sacrificed for each experiment and their livers were chopped. To 0.5gm of liver 1.25 volume of 0.225 M sucrose tris (ST) buffer (pH 7.4) were added and the mixture was homogenized in chilled condition and processed for microsomal degranulation.[10,11] Tissue homogenates were centrifuged for 20 min at 9000 rpm at 4°C, the post mitochondrial supernatant collected and mixed with 0.5 g calcium chloride. After that the tubes were kept in ice for 20 min, centrifuged at 4°C, 10,000 rpm for 20 min. The pelleted microsomes were resuspended in 0.225 M ST buffer (pH 7.4) and protein[12] and RNA[13] were estimated as per the standard methods.
3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide assay
Cell viability was determined by using [3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide] MTT assay according to a previously described protocol.[14] HCT116 and A549 cells, both cancer cells originated respectively from lung and colon cancer, were harvested by trypsinization and resuspended at a final concentration of 2 × 104 cells/ml in fresh DMEM with 2% Fetel bovine serum. Aliquots of 100 µl cell suspension were plated in 96-well tissue culture plates. In order to detect the cytotoxicity of the cells, cells were treated with V.trifolia at a concentration range 20, 40, 60 and 80µg/ml together with 100, 200, 400, 500µg/ml of T. aestivum for 24 hours. After 24h, 20 µl of a 5 mg/ml MTT solution was added to each well, and the plate was incubated for 4h, allowing viable cells to reduce the yellow MTT to dark-blue formazan crystals, which were dissolved in 100 µl of DMSO. The absorbance in the individual well was determined at 570 nm using microplate reader [ELx-800 biotek absorbance reader]. The cell viability was calculated as percentage of viable cells and then plotted on a graph.
Growth inhibition (%) = (A570 nm of treated cells/A570 nm of control cells) ×100
Statistical analysis
Data are expressed as the mean ± standard deviation of the mean (SD).
RESULTS
Phenolic and flavonoid content
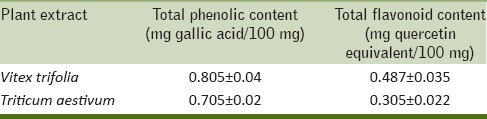
We initially estimated the phenolic and flavonoid content of V. trifolia and T. aestivum as these compounds may contribute directly to the net anticarcinogenic potential of leaf extract. Accordingly, the total phenolic content of V. trifolia was 0.805 ± 0.04 mg gallic acid per 100 mg extract and T. aestivum was 0.705 ± 0.022 mg gallic acid per 100 mg extract [Table 1]. Similarly the flavonoid content of the extracts were investigated to explore the quenching ability of V. trifolia and T. aestivum in reducing the oxidative damage to cells as demonstrated before.[9] The total flavonoid content of V. trifolia was 0.487 ± 0.035 mg quercetin equivalent per 100mg extract and T. aestivum was 0.305 ± 0.022 mg quercetin equivalent per 100mg extract [Table 1].
Table 1.
Concentration of total phenolic compounds in gallic acid equivalents and flavonoids in quercetin equivalents in GLE
Microsomal degranulation
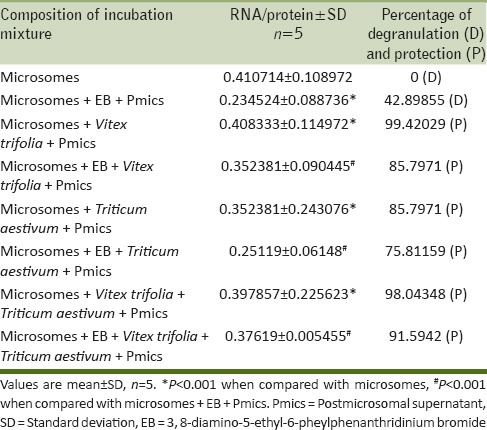
The observations recorded indicate that 3,8-diamino-5-ethyl-6-phenylphenanthridinium bromide (EB) is capable of inducing microsomal degranulation extensively (P < 0.001) (62% on the basis of RNA/protein ratio) [Table 1]. Application of whole extract from V. trifolia shows 85% protection against EB mediated degranulation whereas T. aestivum shows 75% protection (P < 0.001). Cumulatively both V. trifolia and T. aestivum shows 91% protection (P < 0.001) [Table 2].
Table 2.
Hepatic microsomal degranulation by EB and its inhibition by Vitex trifolia and Triticum aestivum
Antiproliferative activity
Basically, MTT proliferation assay is based on the ability of viable cells with active mitochondrial dehydrogenase enzyme which cleave the tetrazolium rings of MTT where the optical density obtained was proportional to the number of healthy viable cells. In this study, HCT116 and A549 cancer cells were treated with V. trifolia at a concentration range 20, 40, 60 and 80µg/ml together with100, 200, 400, 500µg/mlof T. aestivum for 24 hours. Based on the graph plotted, IC50 value was determined. Figure 1 shows, the IC50values after 24 hour treatment with a combination of40µg/ml V.trifolia and 200 µg/ml T. aestivum. The synergic combination of these two extracts showed a significant reduction in the proliferation of A549 and HCT116 cell lines.
Figure 1.
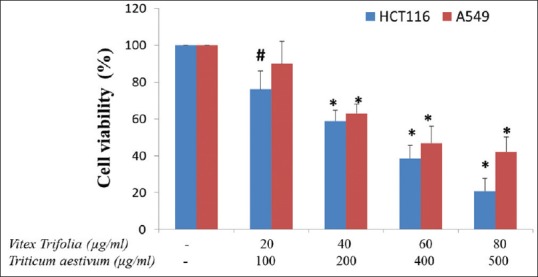
Inhibition of A549 and HCT-116 cell growth by V.trifolia and T. aestivum as determined by the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide assay. Cells were exposed for 24 h to the indicated concentrations of V. trifolia and T. aestivum. Values are the mean from three experiments; bars, standard deviation *P < 0.001 when compared with control; #P < 0.01 when compared with control
Statistical analysis
Data are expressed as the mean ± (SD) of the mean. Vertical error bars denote the SD. The significance of differences between groups was evaluated by analysis of variance with post hoc Tukey's test when comparing three or more groups. A P < 0.05 was considered statistically significant.
DISCUSSION
In the present study the model utilizing the carcinogenic potential of (EB) was assessed by measuring the detachment of ribosomes from rough endoplasmic reticulum (RER). Earlier studies have reported that carcinogens degranulated RER under in vivo and in vitro conditions[15,16] resulting in a decreased RNA/Protein ratio and provides the basis of ascreening test for environmental or chemical carcinogens.[17] Liver provides a good model for the study of carcinogen-induced degranulation, as it is a rich source of RER and it has the metabolic transformation capacity required to generate active forms of electrophilic carcinogens from precursors. In the present study V. trifolia and T. aestivum exposure, both individually and when given together, resulted in an increased RNA/Protein ratio which has been taken as an index of protection of microsomal degranulation. Our results are in accordance to the earlier findings[18] an increase in RNA/Protein ratio of treated rats due to direct protection of membrane degranulation has been reported. Researchers have demonstrated that electrophiles of a carcinogen cause degranulation (removal of ribosomes) from RER, involved in protein synthesis for export from cytosol. Ribosome loss is monitored by determining RNA/protein ratio for membranes due to loss of RNA with the ribosomes.[19] Detachment of ribosomes from the extravesicular surfaces of RER may lead to alterations in the pattern of cellular protein synthesis and alteration in gene expression leading to carcinogenesis. In this study we found that V.trifolia and T. aestivum significantly inhibited carcinogen induced microsomal degranulation which points to the negative regulatory role of these extracts during the carcinogenesis process. It has been reported that the plant flavonoids showed marked reduction in the degree of ribosomal detachment from the microsomes.[20]
Moreover, treatment with combination of Triticum and Vitex extract was found to inhibit cell proliferation in HCT116 and A549 cancer cells. From our findings, it is clear that the flavonoids present in the Triticum and Vitex spp possess considerable anticarcinogenic role. V. trifolia (80mg/ml)[21] and T. aestivum (600mg/ml)[22] has been reported for its anticarcinogenic properties. Synergistic effect showed that the addition of T. aestivum extract appreciably enhanced the anticarcinogenic potential of V. trifolia. This is the first report to show the synergistic effect of these herbs and the finding could have very important applications in the design of functional foods and in the exploration of novel lead moieties against cancer and other disorders. Thus, in phytotherapy, there are potentially significant advantages associated with the synergistic interactions of different plant extracts such as increased efficiency, reduction of undesirable effect, increase in stability and obtaining an adequate therapeutic effect with relatively small doses, when compared with a synthetic medication.[23]
CONCLUSION
Also to the encouraging anti-carcinogenic activity of individual extracts using microsomal degranulation studies, when combined at the whole extract level, there exists enhanced synergy between V.trifolia and T. aestivum. Our study highlights the mechanistic role viz., hepatoprotective, anti-carcinogenic prospective of these plants extracts and their application in traditional medicines. Interestingly the enhanced level of anti-carcinogenic activity confirms the unexplored anti-cancerous potential behind these customary formulations. Also there is an urgent need for more studies concerning the molecular basis of synergistic interactions, and to understand the individual component in the development of pharmacological agents to treat cancer and other chronic inflammatory disorders. Fulfilling these goals could bring more druggable lead molecules from the traditional medicinal formulations followed with ancient Chinese and Indian treatment modalities. Incidentally these treatment measures are effective only when given as the combinatorial formulation, not as the individual entity.
Financial support and sponsorship
The authors are grateful to VIT University, Vellore, Tamilnadu, India for providing financial support for this investigation
Conflicts of interest
The authors have declared that there is no conflict of interest
REFERENCES
- 1.Scalbert A, Manach C, Morand C, Remesy C. Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr. 2005;45:287–306. doi: 10.1080/1040869059096. [DOI] [PubMed] [Google Scholar]
- 2.Matsui M, Adib-Conquy M, Coste A, Kumar-Roiné S, Pipy B, Laurent D, et al. Aqueous extract of Vitex trifolia L.(Labiatae) inhibits LPS-dependent regulation of inflammatory mediators in RAW 264.7 macrophages through inhibition of Nuclear Factor kappa B translocation and expression. J Ethnopharmacol. 2012;143:24–32. doi: 10.1016/j.jep.2012.05.043. [DOI] [PubMed] [Google Scholar]
- 3.Hossain MM, Paul N, Sohrab MH, Rahman E, Rashid MA. Antibacterial activity of Vitex trifolia. Fitoterapia. 2001;72:695–7. doi: 10.1016/s0367-326x(01)00304-5. [DOI] [PubMed] [Google Scholar]
- 4.Manjunatha BK, Vidya SM. Hepatoprotective Activity of Vitex trifolia against Carbon Tetrachloride-induced Hepatic Damage. Indian J Pharm Sci. 2008;70:241–5. doi: 10.4103/0250-474X.41466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li WX, Cui CB, Cai B, Wang HY, Yao XS. Flavonoids from Vitex trifolia L. inhibit cell cycle progression at G2/M phase and induce apoptosis in mammalian cancer cells. J Asian Nat Prod Res. 2005;7:615–26. doi: 10.1080/10286020310001625085. [DOI] [PubMed] [Google Scholar]
- 6.Sachin S, Vivek Kumar S, Archana S, Shrivastav BR. Therapeutic potential of wheatgrass (Triticum aestivum L.) for the treatment of chronic diseases. South Asian J Exp Biol. 2013;3:308–13. [Google Scholar]
- 7.Singleton VL, Rossi JA., Jr Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–58. [Google Scholar]
- 8.Kähkönen MP, Hopia AI, Vuorela HJ, Rauha JP, Pihlaja K, Kujala TS, et al. Antioxidant activity of plant extracts containing phenolic compounds. J Agric Food Chem. 1999;47:3954–62. doi: 10.1021/jf990146l. [DOI] [PubMed] [Google Scholar]
- 9.Zhishen H, Mengcheng T, Jianmin W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–9. [Google Scholar]
- 10.Puri S, Dani HM, Singh J. Assay of carcinogenicity of tobacco metabolites employing microsomal degranulation technique. Indian J Exp Biol. 1998;36:483–7. [PubMed] [Google Scholar]
- 11.Mehta M, Hundal SS. Assessment of genotoxic potential of arsenic in female albino rats at permissible dose levels. Toxicol Int. 2014;21:24–8. doi: 10.4103/0971-6580.128787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 13.Fleck A, Munro HN. The precision of ultraviolet absorption measurements in the schmidt-thannhauser procedure for nucleic acid estimation. Biochim Biophys Acta. 1962;55:571–83. doi: 10.1016/0006-3002(62)90836-3. [DOI] [PubMed] [Google Scholar]
- 14.Roudkenar MH, Halabian R, Roushandeh AM, Nourani MR, Masroori N, Ebrahimi M, et al. Lipocalin 2 regulation by thermal stresses: Protective role of Lcn2/NGAL against cold and heat stresses. Exp Cell Res. 2009;315:3140–51. doi: 10.1016/j.yexcr.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 15.Sood M, Singh AG, Dani HM, Sharma R. Prediction of anticarcinogenic potential of garlic by in vitro rat liver microsomal degranulation. Asian J Microbiol Biotechnol Environ Sci. 2007;9:211–4. [Google Scholar]
- 16.Sahambi SK, Dhanju C. Evidences of microsomal degranulation in the liver of rats treated with organophosphates. Nat Acad Sci Lett. 2005;28:45–7. [Google Scholar]
- 17.Williams DJ, Rabin BR. Distruption by carcinogens of the hormone dependent association of membranes with polysomes. Nature. 1971;232:102–5. doi: 10.1038/232102a0. [DOI] [PubMed] [Google Scholar]
- 18.Jagota SK, Dani HM. Microsomal degranulation by Isatin and its inhibitiors by ascorbic acid. Curr Sci. 1981;50:721–5. [Google Scholar]
- 19.Orrenius S, Ericsson JL. Enzyme-membrane relationship in phenobarbital induction of synthesis of drug-metabolizing enzyme system and proliferation of endoplasmic membranes. J Cell Biol. 1966;28:181–98. doi: 10.1083/jcb.28.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dani HM, Sharma S, Neelam H. Anticarcinogenicity of Flavonoids as Studied by Inhibition of Lipid Peroxidation, Microsomal Degranulation and Their Interactions with Benzo (A) Pyrene Metabolites. Lipid-Soluble Antioxidants: Biochemistry and Clinical Applications Molecular and Cell Biology Updates. 1992:320–9. [Google Scholar]
- 21.Vasanthi VJ, Radhjeyalakshmi R, Nasrin F. Evaluation of anticancer activity using hexanic extract of Vitex trifolia on two different cancer cell lines. Int J Pharm Phytochem Res. 2014;6:449–53. [Google Scholar]
- 22.Garima S, Sankar P, Muddasarul H, Varalakshmi D, Rukkumani R. GC-MS Analysis, in vitro antioxidant and cytotoxic studies of wheatgrass extract. Am J Phytomed Clin Ther. 2014;2:877–93. [Google Scholar]
- 23.Maique WB. Synergy: An old wisdom, a new paradigm for pharmacotherapy. Braz J Pharm Sci. 2009;45:371–8. [Google Scholar]


