Abstract
Background:
Morbidity and mortality related to acute poisoning is a serious health concern worldwide. Paraquat is known to be responsible for a number of acute poisonings in south India.
Aim:
The study aims at presenting the various aspects of paraquat poisoning that include patient profile, clinical presentation, end-organ complications, and observations at autopsy.
Materials and Methods:
The present registry-based retrospective research was conducted in a tertiary care teaching hospital in south India. All the confirmed cases of paraquat poisoning were included in the present study. The postmortem and hospital records of these patients were retrieved and relevant information was collected and analyzed.
Results:
Paraquat poisonings constituted 14.4% of the total poisoning fatalities during the study period. Equal number of males and females were observed in the present study. The victims were aged between 17 and 65 years (mean ± SD = 30.2 ± 13.1 years). Manner of death was suicidal in 92.9% cases. Common presenting symptoms after ingestion of paraquat included vomiting, followed by difficulty in breathing. In the present series, overall survival post paraquat consumption ranged between 10 h and 25 days. Half of the victims died within 2 days of consumption of poison. The underlying cause of death included acute renal failure (ARF), adult respiratory distress syndrome (ARDS), multiorgan failure (MOF), acute liver failure, etc., In all the cases, brain was congested and edematous, and visceral organs showed marked congestion at autopsy. Lungs were congested with marked edema in 10 cases.
Conclusion:
It is recommended that the availability of this highly toxic substance be restricted so as to prevent its misuse as a method of suicide.
Keywords: Autopsy, Manipal, paraquat, poisoning, South India
INTRODUCTION
Morbidity and mortality related to acute poisoning is a serious health concern worldwide. Acute poisoning results in a large number of fatalities, especially in the developing countries where poisoning is reported as a preferred means of suicide. Poisoning is one of the preferred methods for committing suicide in India.[1] In a study from the region, poisoning was reported as the second most common cause of non-natural deaths,[2] with fatal self-poisoning reported in more than 90% of the cases.[3] The nature and cause of acute poisoning may vary regionally and globally. Agrochemicals and pesticides are most commonly implicated in acute poisonings in India.[4,5] Though most of the fatal poisonings in the region are reportedly due to organophosphate compounds,[3] other agents such as paraquat are known to be responsible for a number of acute poisonings in southern Karnataka.[6]
The National Institute for Occupational Safety and Health (NIOSH) describes paraquat dichloride (CH3(C5H4N)2 CH3• 2Cl) as a yellow colored salt with an ammonia-like odor having serious effects on the various organ systems; progressive pulmonary fibrosis as being the cause of death in most cases of paraquat toxicity.[7,8] Whereas the use of paraquat is banned in the European Union since 2007, in the United States it is classified as 'restricted use', so that it is used by licensed applicators only. In third-world countries; however, its unrestricted availability, low cost, low toxic dose, and efficacy makes it a popular poison for committing suicide.
Though studies from the region mention incidents of paraquat poisonings,[3,6] its details have rarely been elaborated. The present study aims at presenting various aspects of paraquat poisoning that include patient profile, clinical presentation, end-organ complications, and observations at autopsy.
MATERIALS AND METHODS
In India, autopsy is mandatorily performed in all poisoning deaths, following which the relevant viscera, and blood are sent to Regional Forensic Science Laboratory (RFSL) for chemical analysis. Toxicological analysis is performed using color tests and thin layer chromatographic (TLC) methods at the RFSL for identification of the poison responsible for the fatal outcome. The present registry-based retrospective research was conducted in a tertiary care teaching hospital in south India and confirmed cases of paraquat poisoning were included in the study. The postmortem records of all the confirmed paraquat poisoning cases that were autopsied at our center during the period of 2 years from January, 2009 to December, 2010 were analyzed. Hospital records of these patients were retrieved and relevant information including age, gender, possible intent behind consumption, approximate volume consumed (in mL), time interval between consumption and hospitalization, time interval to death, and presenting symptoms were recorded. Details of investigations including clinician/intensivist diagnosis of adult respiratory distress syndrome (ARDS), chest radiograph reports, arterial blood gas (ABG) reports, number of patients put on invasive mechanical ventilation or on supplemental oxygen therapy, number of patients with acute renal failure (ARF), number of patients needing dialysis, number of patients who had hepatic dysfunction/failure, peak bilirubin levels, and peak creatinine levels during hospital stay were used for analysis. In addition, the cause of death as documented in the autopsy reports was recorded for mortality assessment.
RESULTS
The present research constituted of a series of 14 cases of fatal paraquat poisoning during 2009–2010 as shown in Table 1. Paraquat poisonings constituted 14.4% of the total poisoning fatalities (n = 97) during the study period. Equal number of males and females (n = 7 each) were the victims of fatal paraquat poisoning in the present study. The victims were aged between 17 and 65 years (mean ± SD = 30.2 ± 13.1 years). Mean age of males (33.3 ± 17.4 years) was higher than the females (27.1 ± 6.9 years). While the majority of the victims were Hindus (n = 13, 92.9%) and one victim was a follower of Islam (7.1%). Most of the victims consumed the poison during the morning hours between 6 am and 12 pm (n = 8, 57.14%). A large number of cases (n = 6, 42.9%) were reported during the last quarter of the year (October–December). Manner of death was reported as suicidal in 92.9% cases (n = 13) and accidental in one case (7.1%). Profile of victims of fatal paraquat poisoning is detailed in Table 1. Paraquat poisoning was confirmed based on the history, clinical features, and investigations that included chemical analysis of the first gastric lavage and postmortem analysis of blood and viscera at the RFSL. Paraquat was identified in the postmortem analysis of blood and viscera in cases with a survival period of less than 4 days. It could not be detected on postmortem in cases with survival of more than 4 days.
Table 1.
Profile of the victims of fatal paraquat poisoning
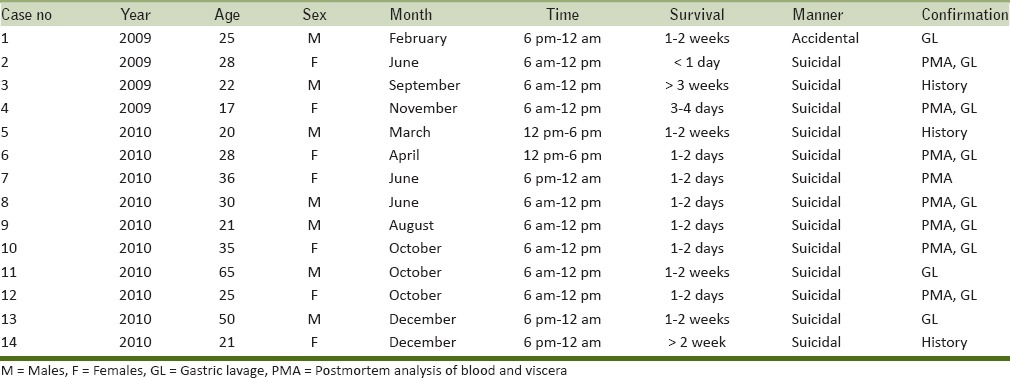
The exact dose of paraquat ingested in the present series was not detailed in 10 cases. In the other four cases, based on the history given by the patients/attendants, the amount consumed was approximately 50, 100, 150, and 250 ml, respectively. The concentration of paraquat present in the consumed liquids ranged from 5 to 30%. The patients presented to the hospital after a median of 7 h (range: 0–48 h). Common presenting symptoms after ingestion included vomiting as observed in seven patients (50.0%), followed by difficulty in breathing in five cases (35.7%). One patient (7.1%) was febrile at admission. Clinical examination showed oral cavity erosions in three patients (21.4%) and icterus in two patients (14.2%). The results of clinical presentation and key laboratory investigations are shown in Table 2 and the pattern of organ involvement and mortality assessment are outlined in Table 3. Hypoxia (defined in present series as PaO2 < 80 mmHg) was seen in seven (50%) patients. Chest radiographs and/or a clinician diagnosis suggestive of ARDS was seen in six (42.8%) patients, while one patient was hypoxic due to aspiration pneumonitis. Of these, three patients needed invasive mechanical ventilation. Renal failure was observed in 11 (78.5%) patients. The mean peak creatinine level in the present series was 380.12 µmol/L (4.3 mg/dL). Two patients needed hemodialysis and another two patients underwent hemoperfusion. Four (28.5%) patients had acute liver failure subsequent to ingestion of paraquat. The mean peak bilirubin observed in these patients was 61.57 µmol/L (3.6 mg/dL). Elevations in creatinine kinase (CK) levels were observed in seven patients.
Table 2.
Clinical presentation and key laboratory investigations in paraquat toxicity
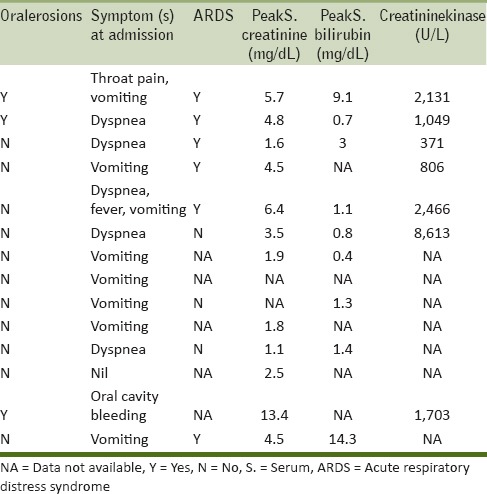
Table 3.
Organ involvement and mortality assessment in paraquat poisoning

In the present series, overall survival post paraquat consumption ranged between 10 h and 25 days. Of the total fatalities, seven (50%) patients died within 2 days of paraquat consumption. The underlying cause of death was MOF in five patients, ARDS in six patients, ARF in 10 patients, acute liver failure in three patients, and nosocomial pneumonia in one patient. On autopsy, brain was congested and edematous, and visceral organs showed marked congestion in all the cases. Lungs were congested with marked pulmonary edema in 10 cases.
DISCUSSION
Paraquat, a dipyridyl compound, owes its name to the para positions of its quaternary nitrogens. It is widely used for weed control in about 100 countries, either to prepare the land before planting or for controlling weeds in more than 100 crop varieties. Its use is banned in 32 countries and sale restricted in a few others because of health concerns. Commercially, it is available in India in liquid form as GRAMOXONE (10–30% concentration) and in granule form as WEEDOL (5% concentration).[8] In developing countries like India, pesticides are a popular tool for farmer suicides due to their easy availability and unrestricted access. In India, the first case of paraquat poisoning was reported by Singh et al., in 1999.[9]
On ingestion, paraquat is rapidly but incompletely absorbed and is rapidly distributed to the lungs, liver, kidneys, and muscle. Ninety percent of it is eliminated unchanged by the kidneys in 12–24 h. Pulmonary concentrations of paraquat can be six to 10 times the plasma concentration. The primary injury, therefore, often is to the lung involving mainly the alveolar epithelium, where it may initially lead to an acute alveolitis and later, pulmonary fibrosis. Ingestion at high doses is known to injure other organ systems like kidney, liver, and heart with death in most cases resulting from respiratory or MOF. Paraquat toxicity at a cellular level is due to redox cycling and intracellular oxidative stress generation by accumulation of superoxide anion, hydrogen peroxide, and hydroxyl radicals. Additionally, oxidation of cellular Nicotinamide Adenine Dinucleotide Phosphate (NADPH) needed for intracellular reduction of paraquat leads to disruptions in vital NADPH mediated biochemical reactions.[10,11]
Paraquat is exceedingly toxic to humans, and as little as one teaspoonful of the active ingredient may be fatal with death known to occur as late as 30 days after the ingestion. The most likely route of paraquat poisoning is ingestion; however, poisoning may also occur after skin exposure or inhalation.[12] Although the WHO has estimated the lethal dose for humans to be 30–50 mg/kg, case studies have recorded the lowest fatal dose to be 17 mg/kg and even lower doses may be fatal among children.[13,14] In mild paraquat toxicity (< 20 mg paraquat ion/kg of body weight) patients are asymptomatic or have mild gastrointestinal symptoms and recover completely. Moderate to severe poisoning (20–40 mg paraquat ion/kg of body weight) is marked by nonspecific symptoms, local gastrointestinal effects, renal failure, and pulmonary fibrosis with delayed death in the majority of the patients. In acute fulminant poisonings (> 40 mg of paraquat ion/kg of body weight) multiple organ (cardiac, respiratory, renal, hepatic, adrenal, pancreatic, and neurological) failure occurs with early death in hours or days.[15] In the present series paraquat was ingested in all the cases with suicidal intent reported in all but one case.
Till date there is no single accepted guideline for treatment of paraquat poisoning. Gastrointestinal decontamination using Bentonite (7.5%), Fuller's Earth (15%), or activated charcoal is recommended for patients who present within 2–4 h of ingestion. ABCs of resuscitation should be followed as per routine guidelines. Oxygen therapy in mild to moderate hypoxia should be avoided as it may increase the ongoing oxidative stress. Hemodialysis and hemoperfusion may be considered in patients who present early, that is, within 2 h. Antioxidants like vitamin E, vitamin C, N-acetyl cysteine, salicylic acid, and desferrioxamine B; while showing promise in in vitro and animal studies have yet to be fully evaluated in humans. Similarly, immunosuppressive therapy with glucocorticoids and cyclophosphamide is widely used, but evidence for efficacy is weak.[16]
Acute poisonings are a cause of concern and hence, an analysis of potential poisons resulting in morbidity and mortality in different regions needs to be detailed for epidemiological purposes and effective management. Autopsy registers and treatment records play a vital role in providing detailed information in this regard. The present investigation is an attempt to gather the clinical and medicolegal details of fatal paraquat poisoning and present various features of fatal paraquat poisoning in the region. Such studies are essential to develop an understanding of poisoning caused by relatively less common agents in different regions and develop appropriate preventive strategies. Limitations exist in the present research and are primarily due to the retrospective nature of this hospital-based study where the information was retrieved from the treatment records and the medicolegal registers. Since these sources were not originally meant for research, there was nonuniformity in the details entered in the treatment files, and vital information such as amount of poison consumed was missing. Exact reason for suicide remained unanalyzed for lack of details in the files and because the relatives could not be contacted for further information. Nonetheless, the present study highlights various aspects of paraquat poisoning, which in spite of its associated morbidity and mortality is rarely described in literature. It is recommended that the availability of this highly toxic substance be restricted so as to prevent its misuse as a method of suicide.
Financial support and sponsorship
Nil
Conflicts of interest
There are no conflicts of interest
REFERENCES
- 1.Kanchan T, Menon A, Menezes RG. Methods of choice in completed suicides: Gender differences and review of literature. J Forensic Sci. 2009;54:938–42. doi: 10.1111/j.1556-4029.2009.01054.x. [DOI] [PubMed] [Google Scholar]
- 2.Kumar TS, Kanchan T, Yoganarasimha K, Kumar GP. Profile of unnatural deaths in Manipal, Southern India 1994-2004. J Clin Forensic Med. 2006;13:117–20. doi: 10.1016/j.jcfm.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Kanchan T, Menezes RG, Kumar TS, Bakkannavar SM, Bukelo MJ, Sharma PS, et al. Toxicoepidemiology of fatal poisonings in Southern India. J Forensic Leg Med. 2010;17:344–7. doi: 10.1016/j.jflm.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Kanchan T, Menezes RG. Suicidal poisoning in Southern India: Gender differences. J Forensic Leg Med. 2008;15:7–14. doi: 10.1016/j.jflm.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Bumbrah GS, Krishan K, Kanchan T, Sharma M, Sodhi GS. Phosphide poisoning: A review of literature. Forensic Sci Int. 2012;214:1–6. doi: 10.1016/j.forsciint.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 6.Ram P, Kanchan T, Unnikrishnan B. Pattern of acute poisonings in children below 15 years - A study from Mangalore, South India. J Forensic Leg Med. 2014;25:26–9. doi: 10.1016/j.jflm.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. NIOSH Pocket Guide to Chemical Hazards. [Lastaccessed on 2014 Jun 13]. Available from: http://www.cdc.gov/niosh/npg/npgd0478.html .
- 8.Reigart JR, Roberts JR, editors. Paraquat and diquat. Recognition and management of Pesticide Poisonings. (5th ed) 1999;Vol. 5:108–10. [Google Scholar]
- 9.Singh S, Bamberry P, Chaudhary D, Makharia G, Kakkar N, Singh D. Fatal paraquat poisoning: Report of two cases. J Assoc Physicians India. 1999;47:831–2. [PubMed] [Google Scholar]
- 10.Suntres ZE. Role of antioxidants in paraquat toxicity. Toxicology. 2002;180:65–77. doi: 10.1016/s0300-483x(02)00382-7. [DOI] [PubMed] [Google Scholar]
- 11.Dinis-Oliveira RJ, Duarta JA, Sanchez-Navarro A, Remiao F, Bastos ML, Carvalho F. Paraquat poisonings: Mechanisms of lung toxicity, clinical features, and treatment. Crit Rev Toxicol. 2008;389:13–71. doi: 10.1080/10408440701669959. [DOI] [PubMed] [Google Scholar]
- 12.CDC FACT SHEET. Facts about Paraquat. [Last accessed on 2014 Jun 13]. Available from: http://www.bt.cdc.gov/agent/paraquat/basics/facts.asp .
- 13.Wesseling C, van Wendel de Joode B, Ruepert C, Leon C, Monge P, Hermosillo H, et al. Paraquat in developing countries. Int J Occup Environ Health. 2001;7:275–86. doi: 10.1179/107735201800339209. [DOI] [PubMed] [Google Scholar]
- 14.International Programme on Chemical Safety. INCHEM website. [Last accessed on 2014 Jun 13]. Available from: www.inchem.org/documents/pds/pds/pest4_e.htm .
- 15.Vale JA, Meredith TJ, Buckley BM. Paraquat poisoning: Clinical features and immediate general management. Hum Toxicol. 1987;6:41–7. doi: 10.1177/096032718700600107. [DOI] [PubMed] [Google Scholar]
- 16.Gawarammana IB, Buckley NA. Medical management of paraquat ingestion. Br J Clin Pharmacol. 2011;72:745–57. doi: 10.1111/j.1365-2125.2011.04026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


