Abstract
Aims:
The present study was aimed to investigate the effects of Emblica officinalis against fluoride-induced alterations in the behavioral and biochemical abnormalities in rats.
Design:
The healthy adult albino rats of Wistar strain (Rattus norvegicus) weighed 200–250 g were used for experiments. The animals were divided into three groups. Group I, control rats received only drinking water (F 0.9 ppm). Group II rats were exposed to fluoride (10 ppm) water for 60 days. Group III rats were treated with E. officinalis (100 mg/day/rat) along with fluoride water for 60 days. In order to investigate the effects of elevated levels of fluoride (10 ppm) in drinking water on behavioral pattern of rat, a maze test was carried out in all three groups from day 1 till completion of treatment.
Materials and Methods:
The animals were weighed before and after treatment. After respective treatment, the animals were autopsied. The blood was collected through cardiac puncture and brain was excised blotted free of blood weighed and used for biochemical parameters. The estimation of protein, enzyme activity of cholinesterase (ChE), and lipid peroxidation were carried out in brain using standard techniques.
Results:
The tissue (brain) and serum fluoride was estimated by a fluoride-specific electrode (Orion). Learning and memory abilities assessed during maze test showed reduced memory retention in rats exposed to fluoride water in comparison to control whereas amla powder (E. officinalis) fed rats showed increased memory retention than fluoride water exposed rats. The protein content and ChE enzyme activity in brain of fluoride exposed rats diminished as compared to control whereas the same was found to be elevated in E. officinalis fed rats. The level of malanoaldehyde showed a significant increase in fluoride-treated group and decrease in E. officinalis treated group.
Conclusions:
Our results suggest that exposure of rats to Na-F has detrimental effects on the brain as reflected in diminished learning and memory. Administration of E. officinalis during fluoride exposure significantly overcome neuronal fluoride toxicity and, therefore, may be used as a therapeutic agent for fluorotic victims.
Keywords: Antioxidant, Emblica officinalis, fluoride, maze test, neurotoxicity
INTRODUCTION
The widespread distribution of fluoride in nature is a direct source of adverse health effects in human populations. Fluoride crosses the blood-brain barrier raises the possibility that fluoride can affect the structure and functions of the central or peripheral nervous system. Earlier reports of scientist[1,2] on the effects of NaF on rat brain and other tissues and on neuronal cell bodies in the hippocampus suggest that excess fluoride intake has deleterious effects on central nervous system (CNS). Accumulation of fluoride has also been observed in the brains of experimental animals exposed to high doses of fluoride for a prolonged period.[1,2] Some scientists[3,4] observed that the severity of the adverse effects of F on the behavior of rats is directly correlated with the concentration of F ion in the plasma and in specific regions of the brain. The levels of mental work capacity of adults with chronic fluorosis and the intelligent quotient of children born and raised in the areas with endemic fluorosis were found to be lower than normal.[5,6] These findings are also consistent with animal studies in rats.[7] The dietary supplementations of vitamins and calcium have an important role in alleviating fluoride-induced toxic effects in brain.
The present investigation was undertaken to focus on brain functions affected by fluoride and learning and memory ability in fluorotic rats and its mitigation through Emblica officinalis powder.
MATERIALS AND METHODS
We used 30 healthy male and female Swiss albino rats of Wistar strain (Rattus norvegicus), with a mean body weight of 200–250. The animals were maintained on standard diets and water ad libitum. They were housed under standard conditions of temperature (22–25°C), 12/12-h light/dark cycle, ventilation and hygiene. After 1-week, the rats were randomly divided into three groups of 10 animals each. Group I is of control rats which received <0.5 mg NaF/L in their drinking water. Group II is fluoride exposed group with 10 ppm NaF in their drinking water for 60 days. Group III rats were treated with E. officinalis (100 mg/day/rat) along with fluoride water (10 ppm) for 60 days. The dried amla powder was given as a whole to the animals for 60 days. The rats were tested for learning and memory from day 1 till completion of treatment and sacrificed for biochemical study of their brain tissue.
Evaluation of learning and memory in young rats: Rats of all groups were deprived from feed for a 23 h period before start of testing. The maze experiment was performed every alternate day till the completion of treatment. Rats were given their daily feed amount as a reward at the end of the maze. The rat starts in one location, runs through the maze and finishes at a reward in another location. How many trials does it take for a hungry rat to run the maze to the food reward at the end, with no mistakes? How quickly does the rat complete the maze each time? Over time rats tends to run the maze with fewer and fewer errors, more and more quickly. By graphing the number of errors over time, we can generate a learning curve for the rats.
Biochemistry
After the respective treatment, the animals were autopsied the blood was collected through cardiac puncture brain was excised blotted free of blood and weighed.
The biochemical parameters studied were protein,[8] acetylcholinesterase (Ellaman' method, 1961),[9] Lipid peroxidation (LPO)-Thiobarbituric acid reactive species assay,[10] tissue fluoride,[11] serum fluoride (Ion–Selective Electrode Method using Orion Model 94-09 Ion meter).
For each biochemical parameter, a minimum of 6 replicates were assayed. The statistical significance of the results was analyzed by Student's t- test.
RESULTS
The results revealed that there was a significant decrease (P < 0.01) in the final body weight of rat treated with 10 ppm fluoride water for 60 days, whereas a non-significant change in the brain weight was observed as compared to control group. Further, the treatment with E. officinalis along with fluoride water resulted in normal body and organ weight of the animal [Table 1].
Table 1.
Body (g) and brain weights (mg/100 g bwt) of control, fluoride (10 ppm) water, and fluoride (10 ppm) water + Emblica officinalis (100 mg/day/rat) treated rats
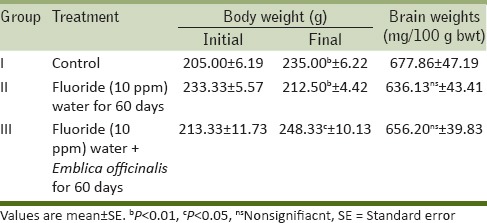
The total protein content and enzyme activity of acetylcholinesterase (AChE) was found to be diminished. The significant (P < 0.01) decline was observed in protein content in the brain of fluorotic rats. The feeding with E. officinalis resulted in increased total protein concentration and enzyme activity as compared to control group. Whereas the concentration of TBARS was found to be elevated significantly (P < 0.05) in fluoride-treated group in comparison to control group indicating intensified LPO. However on supplementation of E. officinalis, the elevated concentration of TBARS was declined significantly (P ≤ 0.05) [Table 2].
Table 2.
Content of protein (mg/g), level of lipid peroxidation and activities of AChE (U/L) in brain tissue of control, fluoride (10 ppm) water and F.W. (10 ppm) + Emblica officinalis (100 mg/day/rat) treated group
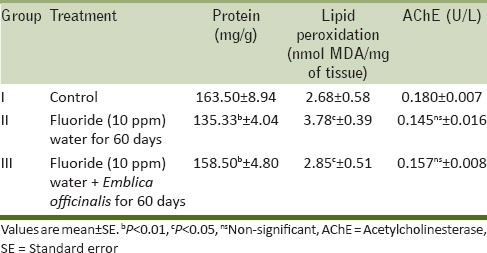
The fluoride concentration of brain and serum was enhanced significantly (P ≤ 0.001, P ≤ 0.05) influorotic rats as compared to the control group. On supplementation of E. officinalis along with fluoride water reduced the deposition of fluoride in brain and serum significantly (P ≤ 0.001) as compared to the fluorotic rats. The decreased serum and brain fluoride in E. officinalis treated rats revealed the importance of E. officinalis in mitigation of fluoride toxicity.
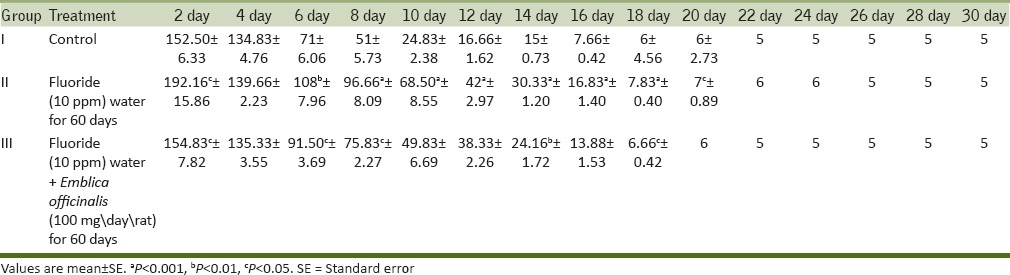
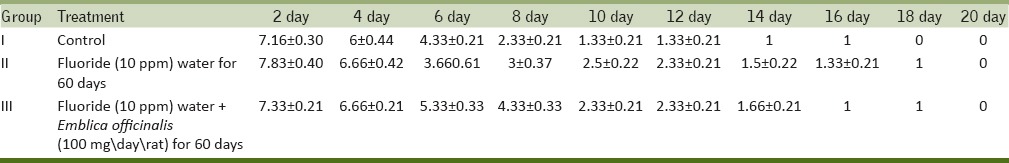
The maze test conducted on all three groups of rat showed that the time taken by the control rats to approach food material gradually decreases from day 2 to day 30. The fluorosed rats took more time to reach the food material as compared to control rats and took more days in learning to approach the food material and committed a greater number of errors as compared to control group. The ingestion of antidote E. officinalis helps in recovery to some extent and the time taken by the rats to reach the food material was less than fluorosed rats and they took fewer days in learning with lesser no of errors [Table 4].
Table 4a.
Maze test of rats for time taken to reach the food material
DISCUSSION
This study was conducted to study the effects of E. officinalis on fluoride-induced neurotoxicity in rats. Reactive oxygen species (ROS) are implicated as important pathologic mediators in many neuronal disorders. Generation of free radicals, LPO, and altered antioxidant systems are considered to play a vital role in posing toxic effects of fluoride.
High fluoride intake resulted in significant reduction in body weight, but the weight of brain showed nonsignificant reduction which may be due to decrease of food intake by the animals. The fluoride ions act as enzymatic poisons, interrupting protein synthesis, increasing glycolysis, activating antioxidative pathways,[12,13] these changes may influence organ weight and body weight. E. officinalis fruit as a food supplement is beneficial in reducing fluoride-induced alterations in body and brain weight and recovery of health of the animal.
Brain protein and AChE activity are important to maintain normal brain physiological function and learning-memory ability.[14] In the present study, the brain protein contents decreased significantly in rats treated with 10 ppm NaF as compared to controls. This may be due to the inhibition of some enzymes of protein or alteration in protein synthesis.[15]
Significant inhibition of the activities of AChE was detected in brain tissue of the rats with NaF in their drinking water as compared to controls. AChE seems to be the most sensitive parameter for monitoring intoxication due to toxic compounds. Fluoride has an inhibitory effect on AChE enzyme. The inhibition of AChE might alter the synaptic transmission of impulses[16] and weaken the CNS. Activity of acetylcholine esterase and concentration of protein was restored to normal level on parallel supplementation of E. officinalis suggesting its role in the amelioration of F-induced stress.
Concentration of TBARS was used as an indicator of LPO. In the present study, TBARS concentration increased significantly in investigated tissue of animals exposed to fluoride, thereby indicating an enhancement of LPO. Administration of E. officinalis powder to the fluoride exposed animals resulted in significant decrease in tissue LPO. Ascorbic acid content of the fruit powder promotes elimination of ROS, thereby helping to reduce oxidative stress.
From the results of our study, we observed that there was highly significant increase in fluoride concentration in the brain tissue and serum [Table 3],[17] which is in agreement with the previous findings. It has been shown that F can cross the blood-brain barrier and accumulate in the brain of rats exposed to high fluoride.[18] Fluoride in high doses can induce the deterioration of learning and memory capability in animals and in humans with chronic fluorosis.[19]
Table 3.
Fluoride content in rat brain and serum of control, fluoride (10 ppm) water, and F.W. (10 ppm) + Emblica officinalis (100 mg/day/rat) treated group
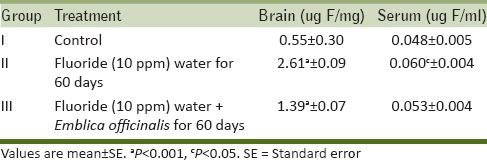
Table 4b.
Error number of rats to reach the food material
Antioxidants play an important protective role against the fluoride poisoning[20] reports indicate that there is an inverse relationship between the dietary intake of antioxidant rich foods and the incidence of human diseases.[21] Ascorbic acid is an important antioxidant of plasma and in aqueous phase of cells. The ingestion of the antidote (E. officinalis) helps in cleaning the fluoride from the animal tissue and serum.[22]
In the present study, the classic maze test was used to measure the activity and response behavior of adult rats in response to exposure to fluoride. Learning-memory reflects the CNS function in animals. Concerning the associative learning memories are based on the acquisition of a predictive link between a specific event and a stimulus. Corresponding to the previous results,[22,23] where fluoride intoxicated rats performed poorly in maze test, rats with long exposure to NaF in the present study take significantly longer time in learning as compared to control. The number of errors was also higher as compared to control. Excessive production of ROS free radicals appears to play a role in diminishing cognitive ability processes such as learning and memory.
Similar to our work Niu et al.,[23] used an activity chamber and a Y-maze to measure the spontaneous activity and conditioned response behavior in rats. He showed that fluoride significantly increased the error number of rats during the days of training.
In recent years, it has been recognized that higher concentrations of fluoride produce neuronal dysfunction by mechanisms involving elevated levels of such free radicals. Fluoride poisoning can cause damage to the brain tissue resulting in low activation of Choline acetylase and causing poor memory retention. Choline in the nerves plays a key role in recognition and memory. The lack of cholinergic nerve transmitters resulting from the brain tissue damage caused by high fluoride may be one of the reasons the animals in the fluoride group showed poor learning ability.
The present study further suggests that the dietary supplementation of E. officinalis is beneficial in prevention of neuronal oxidative stress and degeneration. The E. officinalis contain tannoid principles emblicanin-A (37%)–B (33%) that exhibit antioxidant activity. Naturally there is a dynamic balance between the amount of free radicals generated in the body and anti-oxidant to quench them and protect the body against their deleterious effects. Fluoride produces an additional burden of free radicals in the body. E. officinalis administered orally significantly prevented the behavioral and biochemical abnormalities in albino rats.
In our previous work[24] we found that fluoride significantly decreased animal growth induced hematopoietic dysfunction and had adverse effects on serum indexes. However, the supplementation of E. officinalis significantly lowered the levels of oxidative stress by acting as a potential antioxidant in biological system.
CONCLUSION
Long-term exposure to high fluoride has been found to induce a decreased learning and memory capacity in rats, enhance LPO, and inhibit the activities of AChE in the brains of rats with fluorosis. The decline in learning and memory appears to be connected with the high level of oxidative stress and inhibited activity of AChE induced by F.[25] Exogenous supplementation of E. officinalis has been found to counter nutritional deficiency and to facilitate reduction of the toxic effects induced by F, thereby bolstering the cellular antioxidant defense.[26,27]
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Chirumari K, Reddy PK. Dose-dependent effects of F on the hippocampus and neocortex of rat brain. Fluroide. 2007;40:101–10. [Google Scholar]
- 2.Chulbek D. Fluoride and oxidative stress. Fluoride. 2003;36:217–28. [Google Scholar]
- 3.Dhanalakshmi S, Devi RS, Srikumar R, Manikandan S, Thangaraj R. Protective effect of Triphala on cold stress-induced behavioral and biochemical abnormalities in rats. Yakugaku Zasshi. 2007;127:1863–7. doi: 10.1248/yakushi.127.1863. [DOI] [PubMed] [Google Scholar]
- 4.Xiang Q, Liang Y, Chen L, Wang C, Chen B, Chen X. Effects of fluoride in drinking water on children's intelligence. Fluoride. 2003;36:84–94. [Google Scholar]
- 5.Wu CX, Gu XL, Ge YM, Zang JH, Wang JD. Effects of high fluoride and arsenic on brain biochemical indexes and learning-memory in rats. Fluoride. 2006;39:274–9. [Google Scholar]
- 6.Trivedi MH, Verma RJ, Chinoy NJ, Patel RS, Sathawara NG. Effects of high fluoride water on children's intelligence in India. Fluoride. 2007;40:178–83. [Google Scholar]
- 7.Shivarajashankara YM, Shivashankara AR, Rao HS, Bhat GP. Brain lipid peroxidation and antioxidant systems of young rats in chronic fluoride intoxication. Fluoride. 2002;35:197–203. [Google Scholar]
- 8.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin – Phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 9.Ellman GL, Courtney UD, Anders V, Jr, Featherstone RM. A new and colorimetric determination of acetylcholine esterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 10.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 11.Birkeland JM. Direct potentiometric determination of fluoride in soft tooth deposits. Caries Res. 1970;4:243–55. doi: 10.1159/000259645. [DOI] [PubMed] [Google Scholar]
- 12.Chlubek D, Nowacki P, Mikolajek W, Lagoska R, Jakubowaska K, Rzeuski R. Neurotoxicity of fluoride in rats-neuropathological studies. Fluoride. 1998;31:S24. [Google Scholar]
- 13.Gao Q, Liu Y, Guan Z. Decreased learning and memory ability in rats with fluorosis: Increased oxidative stress and reduced cholinesterase activity in the brain. Fluoride. 2009;42:277–85. [Google Scholar]
- 14.Bhatnagar M, Rao P, Saxena A, Bhatnagar R, Meena P, Barber S, et al. Biochemical changes in brain and other tissues of young adult female mice from fluoride in their drinking water. Fluoride. 2006;39:280–4. [Google Scholar]
- 15.Cheng XT, Wang SX, Gao JG. Analysis of MBP, NSE, F content and ChE activity in brain tissue of rats with chronic fluorosis. Chin J Endemiol. 2002;19:262–7. [Google Scholar]
- 16.Heba S, Lethey EL, Kamel MM, Shaheed IB. Neurobehavioral toxicity produced by sodium fluoride in drinking water of laboratory rats. J Am Sci. 2010;6:54–63. [Google Scholar]
- 17.Geeraerts F, Gijs G, Finne E, Crokaert R. Kinetics of fluoride penetration in liver and brain. Fluoride. 1986;19:108–12. [Google Scholar]
- 18.Chinoy NJ, Sharma M, Michael M. Beneficial effects of ascorbic acid and calcium on reversal of fluoride toxicity in male rats. Fluoride. 1993;26:45–56. [Google Scholar]
- 19.Chinoy NJ, Shah SD. Adverse effects of fluoride and/arsenic on the cerebral hemisphere of mice and recovery by some antidotes. Fluoride. 2004;37:162–71. [Google Scholar]
- 20.Madhusudhan N, Basha PM, Begum S, Ahmed F. Fluoride-induced neuronal oxidative stress and its amelioration by antioxidants in developing rats. Fluoride. 2009;42:179–87. [Google Scholar]
- 21.Chunxiang W, Xinil G, Yaming G, Jianhai Z, Jundong W. Effects of high fluoride and arsenic on brain biochemical indexes and learning-memory in rats. J Fluoride. 2006;39:274–9. [Google Scholar]
- 22.Mullenix PJ, Denbesten PK, Schunior A, Kernan WJ. Neurotoxicity of sodium fluoride in rats. Neurotoxicol Teratol. 1995;17:169–77. doi: 10.1016/0892-0362(94)00070-t. [DOI] [PubMed] [Google Scholar]
- 23.Niu RY, Sun ZL, Wang JM, Cheng Z, Wang JD. Effects of fluoride and lead on locomotor behaviour and expression of Nissl body in brain of adult rats. Fluoride. 2008;41:276–82. [Google Scholar]
- 24.Bhinda S, Sharma JD. Mitigation of fluoride toxicity through Emblica officinalis in rats. Int J Innov Res Tecnol Sci. 2014;2:5–10. [Google Scholar]
- 25.Vani ML, Reddy KP. Effects of fluoride accumulation on some enzymes of brain and gastrocnemius muscle of mice. Fluoride. 2000;33:17–26. [Google Scholar]
- 26.Vasant RA, Narasimhacharya AV. Alleviatory effects of Emblica officinalis as a food supplement in fluoride induced hyperlipemia and oxidative stress. Int J Pharm Pharm Sci. 2012;4:404–8. [Google Scholar]
- 27.Sharma JD, Bhinda S, Sharma PK, Kumari M, Boriwal R. Therapeutic efficacy of medicinal plants to mitigate fluorosis. J Glob Biosci. 2014;3:802–7. [Google Scholar]


