Abstract
Objectives:
Intentional and accidental intoxication with aluminium phosphide (ALP) remains a clinical problem, especially in the Middle East region. Considering the high mortality rate besides lack of any recommended first option drug for its treatment, this study was aimed to compare the therapeutic effects of N-acetylcysteine (NAC), vitamin C (Vit C), and methylene blue; both in isolate and also in combination, for the treatment of ALP intoxication in a rat model.
Materials and Methods:
In this experimental animal study, 80 male Wistar rats in eight groups were intoxicated with ALP (12.5 mg/kg) and treated with a single dose of NAC (100 mg/kg) or Vit C (500–1,000 mg/kg) or methylene blue (1 mg/kg/5 min, 0.1%) or two of these agents or all three of them (controls were not treated). Rats were monitored regarding the parameters of drug efficacy as increased survival time and reduced morbidity and mortality rate for 3 consecutive days to ensure toxin neutralization. Macroscopic changes were recorded and biopsy sections were taken from brain, cerebellum, kidney, liver, and heart for microscopic evaluation regarding cellular hypoxia.
Results:
The mean survival times of rats exposed to ALP and treated with VitC + NAC was 210.55±236.22 minutes. In analysis of survival times, there was a significant difference between Group 5 which received VitC + NAC and the other groups (P < 0.01). Serum magnesium levels after death were higher than normal (P = 0.01).
Conclusions:
Despite the higher survival rate of antioxidant-treated rats compared with controls, this difference was not statistically significant.
Keywords: Aluminium phosphide, methylene blue, N-acetylcysteine, vitamin C
INTRODUCTION
Aluminium phosphide (ALP) tablets and pearls are still used worldwide against rodents, insects, and pests for herbs and stored food materials as corns and rice.[1,2] The widespread use of this agent is mostly due to its inexpensive price, high efficiency, and easy application.[3] The most common mode of ALP intoxication is suicidal attempts which has a higher prevalence in Northern Iran, India, and the Middle East;[4,5] with the mortality rates of 40–80%.[6]
Fatality of ALP tablets is thought to be related to the release of the highly toxic hydrogen phosphine gas (PH3), especially in humid conditions. In this clinical condition breakage of mitochondrial oxidative phosphate system leads to multiorgan failure (MOF), which manifests as pulmonary edema; cardiogenic shock; and congestion in liver, kidneys, adrenals, spleen, gastrointestinal system, and brain.[7] It also mimics ischemic heart disease, which leads to unsatisfied treatment with streptokinase. These catastrophic events follow cardiovascular collapse and sever hypotension.[8] One of the main underlying mechanisms of MOF following ALP intoxication is tissue hypoxia due to methemoglobinemia and intravascular hemolysis.[9,10,11]
Since there is no effective antidote drug for the treatment of PH3 or ALP intoxication, therapeutic interventions for these patients are based on the recovery of oxidative phosphorylation.[12,13,14] Therefore, the administration of methylene blue and vitamin C (Vit C) may have beneficial effects and maydecrease the fatality rate of ALP intoxication.[15,16] Previously, it has been reported that N-acetylcysteine (NAC) usage may lead to improvement of hepatic complications following ALP intoxication.[17,18] Positive effects of magnesium sulfate are evident by 24% reduction of mortality rate.[19]
ALP intoxication not always happens due to suicidal attempts, but it also occurs accidentally as an occupational intoxication following inhalation of toxic PH3 gas in ALP manufacturing factories.[20,21] Children may also have fatal accidental contacts with ALP.[22] Unfortunately, there is still no advised “ first option drug” in the treatment of ALP intoxication.[1,13,23,24]
In view of the facts that the intentional and accidental intoxication with ALP remains a clinical problem, especially in this part of the world, the dangerous complications of its toxicity and especially its high mortality rate besides lack of any advised first option drug for its treatment, this study was aimed to compare the therapeutic effects of NAC, ascorbic acid (Vit C), and methylene blue; both in isolate and also in combination, for the treatment of ALP intoxication in a rat model.
MATERIALS AND METHODS
This experimental animal study was performed in the Department of Physiology, School of Medicine at the Isfahan University of Medical Sciences, Isfahan, Iran (2012–2013). All the experiments performed in this study have been carried out according to the World Medical Association statement on animal use in biomedical research and the protocol of our study was approved by the institutional board of ethics for animal research.
Eighty male Wistar rats, with the average weight between 200 and 250 g and approximate age of 1 year, were divided randomly[25] into eight groups with 10 rats in each cage. Each rat was intoxicated with ALP (Phostoxin, 3 mg tablet, ALP 56%, Alcan, Romania). ALP dissolved in normal saline was administered intragastrically by using gavages' syringe and oral devices. In Groups 1–3, single dose of NAC, methylene blue, and Vit C were administered, respectively. Rats in groups 4–6, received single dose of NAC + Methylene blue, NAC and Vit C and methylene blue and Vit C, respectively. The dose of applied agents in this study was as follows: 12.5 mg/kg of ALP, 100 mg/kg of NAC, 1 mg/kg/5 min (0.1%) of methylene blue, and 500–1,000 mg/kg of Vit C. Rats in Group 7, received single dose of NAC + methylene blue + Vit C. In Group 8, no drug was administered (control group). Due to the absence of effective agent in treatment of ALP intoxication, positive control group was not needed. The route of administration of applied agents was by feeding tube for ALP and intraperitoneal for NAC, Vit C, and methylene blue. All agents were dissolved in saline. During and after intoxication, vital signs and behavioral changes of rats were fully controlled. In intoxicated rats, survival count was evaluated. Survival count in various groups was compared with the average death time of the control group. Rats were monitored regarding parameters of drug efficacy as increased survival time and reduced morbidity and mortality rate for 3 consecutive days to ensure toxin neutralization. All rats underwent autopsy after sacrifice under anesthesia. Indeed, magnesium level of samples taken from peritoneum was also measured after death. Macroscopic changes were recorded and biopsy sections were taken from brain, cerebellum, kidney, liver, and heart for microscopic evaluation regarding cellular hypoxia.
Statistical analysis was done using comparison of mentioned parameters in treated groups vs control group through Statistical Package for Social Sciences (SPSS) v 17.0 by Cox regression, hazard ratio, median ratio, and median difference. Survival time in various groups was demonstrated as mean (minutes) ± standard deviation (SD). P - values less than 0.05 were considered as statistically significant.
RESULTS
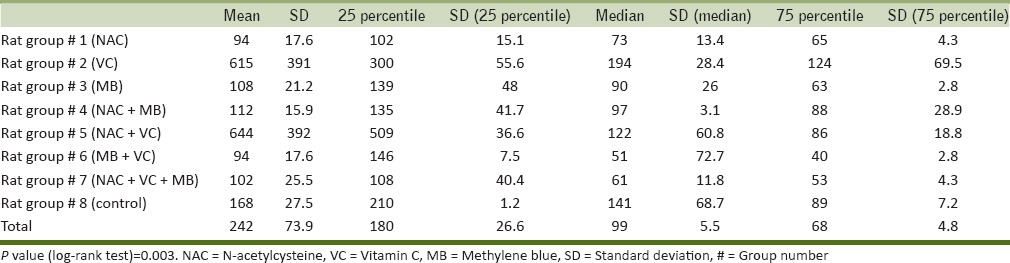
Survival time was counted sine the gavage of ALP to the occurrence of death time using chronometer. In rats treated with NAC, mean survival time (MST) was 94 min. Minimum and maximum survival time was 22 and 223 min, respectively. In this group, all rats expired. In methylene blue-treated rats, one rat was survived after 3 days (survival count = 1). After 1 h passed from intoxication, reduced body movements and fatigue was seen in this survived rat, but gradually consciousness and body movements were increased. And finally, after 5 hrat became completely normal and survival time was considered 4,320 min. Minimum and maximum survival time was 265 and 4,320 min, respectively. In this group, one rat showed typical tonic-colonic seizure after 2 h which lasted for 20 s. After 1 min, loss of consciousness and fatigue happened. Second episode of similar seizure attack occurred after 40 min of passing in normal states. In postictal phase, apnea occurred for 30 s. This rat, survived for 265 min (survival rate = 1). This data is in favor of beneficial effects of methylene blue in the treatment of ALP intoxication. In Vit C-treated rats; the MST and minimum and maximum survival time were 108.7, 45, and 272 min, respectively. Signs of death in one of the rats in this group were severe respiratory distress with gasping, bradycardia and tachypnea in about 0.5 h before death. Another rat manifested severe respiratory distress 1 h after toxin gavage (respiratory rate >135 per minute). This rat survived for 272 min. In this group, rats without tachypnea survived for 152 min, in which 3 min before death deep breathes with bradypnea occurred. Two rats experienced seizure in last minutes of life as stretched movements followed by tonic movements (survival time = 63 min). In rats treated with NAC + methylene blue, MST, and minimum and maximum survival time were 112.7, 60, and 235 min, respectively (survival count = 0). No behavioral changes were seen in these rats. In rats treated with NAC + Vit C; MST and minimum and maximum survival time were 764.6, 229, and 4,320 min, respectively (survival count = 1). Increased survival time in these rats implies to the beneficial effects of this drug combination in the treatment of ALP intoxication. In rats treated with methylene blue + Vit C; MST and minimum and maximum survival time were 84.1, 38, and 140 min, respectively (survival count = 0). No behavioral changes were seen in these rats. In rats treated with methylene blue + Vit C + NAC; MST and minimum and maximum survival time were 168.5, 79, and 334 min, respectively (survival count = 0). No behavioral changes were seen in these rats. In the control group; MST and minimum and maximum survival time were 168.5, 79, and 334 min, respectively (survival count = 0); and also no behavioral changes were seen in these rats. The average values of MST and minimum and maximum survival time in eight groups were 276.31, 22, and 4,320 min, respectively (survival count = 0). Analysis of mean, median, and quartile of survival time for each group is included in Table 1, in which the only statistically significant measure was log-rank test (P = 0.003).
Table 1.
Statistical data for survival in studied rat groups
Blood magnesium level in three subcategories of hypo- (< 3.66), normal, and hyper-magnesemia (> 5.73) were investigated using Cox regression analysis. Hazard ratio for hypo-vshypermagnesemia was 3.12. Despite the protective role of hypermagnesemia in ALP intoxication, it was statistically insignificant (P = 0.06).
Pathological study of the lung tissue of the samples first showed follicular bronchitis followed by hemorrhage in all samples. Mild brain edema was seen, which was more pronounced in rats treated with NAC. Liver changes was seen in two-third of rats, mostly as liver congestion. Almost all the rats showed myocardial congestion. Myocardial hemorrhage was seen in some of them. In kidneys, congestion and hemorrhage were seen. Myocytolysis, myocyte maculation, and tissue degeneration was seen in myocardial tissue of intoxicated rats. Brain tissue showed disorganization in various layers, disappearance of glial cells, neuronal degeneration, and appearance of nectoric pieces. Tubular degeneration was seen in kidneys of intoxicated rats.
Results of survival rate analysis in rats (Cox regression, hazard ratio, mean, and median survival ratio) have been presented in Tables 1 and 2 and Figure 1. Survival rate in Groups 2 and 5 were higher than other, but this difference was statistically insignificant which might be due to the low sample volume. Rats in other groups had lower survival rate compared with control group, in which rats in Groups 6 and 7 had lower survival rate. This difference was just significant in Group 6 (P = 0.03).
Table 2.
Hazard ratio, median ratio, and median difference of survival of studied rat groups in comparison to the control group
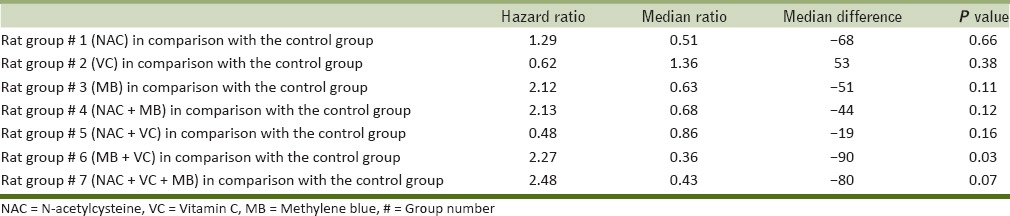
Figure 1.

Survival pattern of the studied groups of rats (Group 8 = control)
DISCUSSION
Acute intoxication with ALP in some part of Northern Iran occurs commonly, both intentional and accidental, due to its inexpensive price, high toxicity, and easy access. Unfortunately, most of the intoxicated cases die rapidly. Despite of its high mortality rate, there is still no known antidote for phosphine or ALP, but in literature there are some successful reported cases and suggestions.[2,13,26,27,28]
For the treatment of ALP-intoxicated rat, we applied Vit C and NAC to scavenge free radicals and methylene blue as an antidote for methemoglobinemia. Our data demonstrates positive effects of combined NAC and Vit C for increasing the survival time and in one case we observed a complete survival. This data is consistent with Findings of Kariman et al., which emphasizes on the role of oxidative stress in pathophysiology of ALP intoxication.[12] Indeed, considering the absence of enhanced survival in rats treated with Vit C, which is in parallel with the findings of Shadnia et al., it could be concluded that Vit C by itself is not effective.[27] However, Moghaddamnia et al., have shown the partial efficacy of Vit C in delaying death time compared with nontreated cases.[29]
There is a huge controversy regarding the efficacy of Vit C and methylene blue in the treatment of ALP intoxication. One of the underlying mechanisms of ALP intoxication is shown to be creation of reactive oxygen species and lipid peroxidation in biologic systems by the action of phosphine and organic phosphides. Hassanian-Moghaddam et al., has demonstrated the release of phosphine gas from hydrolysis of metallic phosphides, lipid proxidesand8-hydroxyguaninein DNA materials of 1c1c7 cells.[30] Kariman et al., have demonstrated the significant increase in lipid peroxidation and decreased total antioxidant levels after ALP intoxication.[12] Thus, antioxidants remained as the main therapy for the treatment of ALP intoxication at present time. In contrast to surveys which imply on the role of methemoglobinemia in ALP intoxication, we did not reach beneficial effects from administration of methylene blue. But we had observed one case of complete survival in rats treated with methyleneblue, which is consistent with Shadnia et al.,'s observation.[27] In contrast to Azad et al., based on the efficacy of NAC on increased survival time and decreased hepatic complications, we showed inefficacy of NAC on survival time of treated rats.[17]
Blood level of magnesium was increased in rats intoxicated with ALP. Interestingly, this parameter was higher in rats treated with Vit C and NAC. Moghaddamnia et al., showed that hepatic congestion may present by high doses of ALP (40 mg/kg), while in lower dose (20 mg/kg) hepatic necrosis was considered mainly.[29] Similarly, since we used high dose of ALP (50 mg/kg), hepatic congestion was seen mainly; and in minority centrilobular necrosis was seen. Pathologic changes in lung were follicular bronchiolitis, diffuse vascular damage with atelectasia, edema, and hemorrhage; while Moghaddamnia et al., showed congestion and neutrophilic infiltration.[29] We observed hemorrhage and congestion in heart and kidney, in contrast with others which showed no change in these organs. Histological changes in vital organs which lead to death suggest tissue hypoxia.
Despite the higher survival rate of antioxidant-treated rats compared with control ones; this difference was not statistically significant. Interestingly, hypermagnesemia which occurs after ALP intoxication seems to be a protective mechanism for ALP intoxication.
Financial support and sponsorship
Nil
Conflicts of interest
There are no conflicts of interest
Acknowledgments
We wish to thank the personnel of animal laboratory of the department of Physiology (School of Medicine) at the Isfahan University of Medical Sciences for their sincere collaboration. This research was financially supported by Legal Medicine Research Centre, Tehran, Iran (Project No. 101943) and the vice-chancellery for research and technology at the Isfahan University of Medical Sciences. Authors would like to thank Mr. Rory O'Conner for his kind help in English editing of the manuscript.
REFERENCES
- 1.Li Q, Yu G, Jian X, Wang J, Sun J, Song C. The clinical characteristics of oral aluminium phosphide poisoning. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2014;32:545–6. [PubMed] [Google Scholar]
- 2.Moller Eggertsen P, Kristensen AK, Bredahl C. Survival after oral poisoning with insecticide against moles containing aluminium phosphide. Ugeskr Laeger. 2013;175:1704–5. [PubMed] [Google Scholar]
- 3.Mehrpour O, Keyler D, Shadnia S. Comment on Aluminum and zinc phosphide poisoning. Clin Toxicol (Phila) 2009;47:838–9. doi: 10.1080/15563650903203684. [DOI] [PubMed] [Google Scholar]
- 4.Anand R, Binukumar BK, Gill KD. Aluminum phosphide poisoning: An unsolved riddle. J Appl Toxicol. 2011;31:499–505. doi: 10.1002/jat.1692. [DOI] [PubMed] [Google Scholar]
- 5.Bajaj R, Wasir HS. Epidemiology of aluminium phosphide poisoning. Need for a survey. J Assoc Physicians India. 1990;38:197–8. [PubMed] [Google Scholar]
- 6.Bumbrah GS, Krishan K, Kanchan T, Sharma M, Sodhi GS. Phosphide poisoning: A review of literature. Forensic Sci Int. 2012;214:1–6. doi: 10.1016/j.forsciint.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 7.Proudfoot AT. Aluminium and zinc phosphide poisoning. Clin Toxicol (Phila) 2009;47:89–100. doi: 10.1080/15563650802520675. [DOI] [PubMed] [Google Scholar]
- 8.Soltaninejad K, Shadnia S, Ziyapour B, Brent J. Aluminum phosphide intoxication mimicking ischemic heart disease led to unjustified treatment with streptokinase. Clin Toxicol (Phila) 2009;47:908–9. doi: 10.3109/15563650903285657. [DOI] [PubMed] [Google Scholar]
- 9.Sanaei-Zadeh H. Aluminum phosphide poisoning and development of hemolysis and methemoglobinemia. Indian J Crit Care Med. 2012;16:248–9. doi: 10.4103/0972-5229.106519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shadnia S, Soltaninejad K, Hassanian-Moghadam H, Sadeghi A, Rahimzadeh H, Zamani N, et al. Methemoglobinemia in aluminum phosphide poisoning. Hum Exp Toxicol. 2011;30:250–3. doi: 10.1177/0960327110384287. [DOI] [PubMed] [Google Scholar]
- 11.Soltaninejad K, Nelson LS, Khodakarim N, Dadvar Z, Shadnia S. Unusual complication of aluminum phosphide poisoning: Development of hemolysis and methemoglobinemia and its successful treatment. Indian J Crit Care Med. 2011;15:117–9. doi: 10.4103/0972-5229.83021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kariman H, Heydari K, Fakhri M, Shahrami A, Dolatabadi AA, Mohammadi HA, et al. Aluminium phosphide poisoning and oxidative stress: Serum biomarker assessment. J Med Toxicol. 2012;8:281–4. doi: 10.1007/s13181-012-0219-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehrpour O, Jafarzadeh M, Abdollahi M. A systematic review of aluminium phosphide poisoning. Arh Hig Rada Toksikol. 2012;63:61–73. doi: 10.2478/10004-1254-63-2012-2182. [DOI] [PubMed] [Google Scholar]
- 14.Agarwal A, Robo R, Jain N, Gutch M, Consil S, Kumar S. Oxidative stress determined through the levels of antioxidant enzymes and the effect of N-acetylcysteine in aluminum phosphide poisoning. Indian J Crit Care Med. 2014;18:666–71. doi: 10.4103/0972-5229.142176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lall SB, Peshin SS, Mitra S. Methemoglobinemia in aluminium phosphide poisoning in rats. Indian J Exp Biol. 2000;38:95–7. [PubMed] [Google Scholar]
- 16.Mostafazadeh B, Pajoumand A, Farzaneh E, Aghabiklooei A, Rasouli MR. Blood levels of methemoglobin in patients with aluminum phosphide poisoning and its correlation with patient's outcome. J Med Toxicol. 2011;7:40–3. doi: 10.1007/s13181-010-0121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Azad A, Lall SB, Mittra S. Effect of N-acetylcysteine and L-NAME on aluminium phosphide induced cardiovascular toxicity in rats. Acta Pharmacol Sin. 2001;22:298–304. [PubMed] [Google Scholar]
- 18.Chaudhry D, Rai AS. N-acetyl cysteine in aluminum phosphide poisoning: Myth or hope. Indian J Crit Care Med. 2014;18:646–7. doi: 10.4103/0972-5229.142172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Avasthi R, Sharma R. Aluminium phosphide poisoning and magnesium sulphate therapy. J Assoc Physicians India. 1994;42:670. [PubMed] [Google Scholar]
- 20.Sudakin DL. Occupational exposure to aluminium phosphide and phosphine gas. A suspected case report and review of the literature? Hum Exp Toxicol. 2005;24:27–33. doi: 10.1191/0960327105ht496oa. [DOI] [PubMed] [Google Scholar]
- 21.Shadnia S, Mehrpour O, Abdollahi M. Unintentional poisoning by phosphine released from aluminum phosphide. Hum Exp Toxicol. 2008;27:87–9. doi: 10.1177/0960327107086241. [DOI] [PubMed] [Google Scholar]
- 22.Sharma A, Gathwala G. Oral aluminium phosphide poisoning in Indian children. J Trop Med Hyg. 1992;95:221–2. [PubMed] [Google Scholar]
- 23.Marashi SM, Majidi M, Raji Asadabadi H, Nasri-Nasrabadi Z. A common misconception in the management of aluminium phosphide poisoning. Arh Hig Rada Toksikol. 2013;64:475–6. doi: 10.2478/10004-1254-64-2013-2404. [DOI] [PubMed] [Google Scholar]
- 24.Sanaei-Zadeh H. Acute aluminium phosphide poisoning: Can we predict survival? Indian J Anaesth. 2012;56:207–8. doi: 10.4103/0019-5049.96319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin RA, Daly A, DiFonzo CJ, de la Iglesia FA. Randomization of animals by computer program for toxicity studies. J Environ Pathol Toxicol Oncol. 1986;6:143–52. [PubMed] [Google Scholar]
- 26.Mehrpour O, Amouzeshi A, Dadpour B, Oghabian Z, Zamani N, Amini S, et al. Successful treatment of cardiogenic shock with an intraaortic balloon pump following aluminium phosphide poisoning. Arh Hig Rada Toksikol. 2014;65:121–6. doi: 10.2478/10004-1254-65-2014-2393. [DOI] [PubMed] [Google Scholar]
- 27.Shadnia S, Rahimi M, Pajoumand A, Rasouli MH, Abdollahi M. Successful treatment of acute aluminium phosphide poisoning: Possible benefit of coconut oil. Hum Exp Toxicol. 2005;24:215–8. doi: 10.1191/0960327105ht513oa. [DOI] [PubMed] [Google Scholar]
- 28.Moghadamnia AA. An update on toxicology of aluminum phosphide. Daru. 2012;20:25. doi: 10.1186/2008-2231-20-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moghaddamnia AA, Dibavand FA, Javadian SH. Aluminium phosphide poisoning in mice and the procedure for its managements. J Babol Univ Med Sci. 2000;2:25–33. [Google Scholar]
- 30.Hassanian-Moghaddam H, Pajoumand A, Dadgar SM, Shadnia Sh. Prognostic factors in methanol poisoning. Hum Exp Toxicol. 2007;26:583–6. doi: 10.1177/0960327106080077. [DOI] [PubMed] [Google Scholar]


