Abstract
Background:
Pergularia daemia (Asclepiadacea) is a fetid- smelling perennial herb growing well along the river bang and road sides of India. Naturally the plant has powerful antioxidants including polyphenols, flavanoids, steroids and terpenoids.
Objective:
The aim of this study is to evaluate the in vitro antioxidant potential and to determine the median lethal dose (LD50) of crude ethyl acetate and methanol extracts of Pergularia daemia. The plant Pergularia daemia possess effective scavenging activity against 2, 2' azino bis (3 ethylbenzothiazoline 6 sulfonic acid (ABTS), nitric oxide and reducing power radicals at different concentrations (100, 200, 300, 400 & 500 µg/mL) of both extracts.
Results:
From the above in vitro assay we have exposed that the methanolic extract exert higher antioxidant activity at 400 µg/mL than ethyl acetate extract. Acute toxicity study revealed that the extracts showed no signs of toxicity upto a dose level of 2500 mg/kg b.wt.
Conclusion:
Thus our findings provide that both extracts of Pergularia daemia possess a strong antioxidant capacity and are relatively has high margin of safety.
Keywords: Acute toxicity (LD50), in vitro antioxidants, Pergularia daemia, phytochemical screening
INTRODUCTION
Reactive oxygen species (ROS) are a family of molecules that are continuously generated, transformed, and consumed in all living organisms as a consequence of aerobic life.[1] Recent studies have shown strong evidence that biological reactive oxygen species (ROS) such as 2,2'-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid (ABTS), nitric oxide, and reducing power radicals are involved in the development of cancer.[2] Antioxidants are nutritional substances which could prevent or slow down the oxidative damage and act as free radical scavengers, preventing and repairing cellular and molecular damage.[3] In recent years, much attention has been devoted to natural antioxidant and their association with health benefits. Numerous ethnopharmacological surveys were conducted and revealed that large numbers of lactiferous plant species are used as a source of herbal therapies. India is a country which is rich in indigenous herbal resources and it grows on their varied topography and under changing agro climate condition, permitting the growth of almost 20,000 plants which are of medicinal value. Higher plants produce a variety of antioxidant compounds such as polyphenols and have been found to possess numerous biological activities.[4] The plant Pergularia daemia (Asclepiadacea), known as veliparuthi in Tamil, which is traditionally used as anthelmintic, laxative, antipyretic, and expectorant; is also used to treat infantile diarrhea and intermittent fevers.[5] The plant is widely consumed and used in folk medicine for the treatment of inflammation, diabetes, malaria, asthma and liver disorders.[6] Phytochemically the plant has been investigated for cardenolides, alkaloid, flavonoids, saponins, triterpenes and steroidal compounds.[7]
Although herbal products act as healer for various diseases, some kind of plant products could cause toxic effects, when given as overdose. The term acute toxicity is defined as adverse effect of the substances which could cause an opposing effect acutely and mortality at particular concentrations.[8] The present study has therefore been designed to concrete the in vitro antioxidant potential and determine the toxicity limit (LD50) of ethyl acetate and methanolic extracts of P. daemia (PDEAE and PDME).
MATERIALS AND METHODS
Chemicals
ABTS, 2, 2-diphenyl-1-picrylhydrazyl, nitroblue tetrazolium, ascorbic acid, potassium per sulfate, and NADH was purchased from Sigma Aldrich Co., St Louis, USA. All other chemicals used in this study were of analytical grade, and the assay kits for toxicological studies were purchased from Human Diagnostics, Faridabad, India.
Plant materials
Matured P. daemia (Forsk.) plant was collected from river banks of Pudukkottai District, Tamil Nadu, India. The plant was identified by Dr. V Venkatesalu, Professor, Department of Botany, Annamalai University. A voucher specimen (ACC: 196) was deposited in the herbarium of Department of Botany, Annamalai University.
Preparation of plant extracts by successive solvent extraction
The shade-dried plant materials (root, stem, leaves, flower, and bark) of P. daemia of about 1,000 g were subjected for size reduction to coarse powder. The powdered plant material was defatted by using petroleum ether (60–80°C) and then extracted with methanol and ethyl acetate using Soxhlet apparatus for about 72 h at 40°C. After that the sediment was filtered with Whatman no. 1 filter paper (Whatman Ltd, England). Both PDEAE and PDME were further concentrated under vacuum using rotary vacuum evaporator (Buchi R-V120, Switzerland) at 40°C and then reconstituted in minimum amount of dimethyl sulfoxide (DMSO) and stored at 4°C for further use.[9] The percentage yield of ethyl acetate and methanol extracts were found to be 4.5% (w/w) and 8.1% (w/w), respectively.
Preliminary phytochemical analysis
Test for flavonoids
A few drops of 1% NH3 solution were added to the ethyl acetate and methanolic extract of plant leaves in a test tube. A yellow coloration was observed if flavonoid compounds are present.
Test for tannins
0.5 g of powdered sample of plant leaves was boiled in 20 ml of distilled water in a test tube and then filtered. The filtration method used here was the normal method, which included a conical flask and filter paper. 0.1% of FeCl3 was added to the filtered samples and observed for brownish green or a blue black coloration, which showed the presence of tannins.
Test for carbohydrates
To 0.5 ml of powdered sample of extract, 5 ml of Benedict's reagent was added and boiled for 5 min. Formation of bluish green color showed the presence of carbohydrate solution and was boiled for few more minutes. In the presence of flavonoids, reddish pink or dirty brown color was produced.
Test for alkaloids
A 5 ml of the extract was added to 2 ml of HCl. To this acidic medium, 1 ml of Dragendroff's reagent was added. An orange or red precipitate produced immediately indicates the presence of alkaloids.
Test for steroids
A 1 ml of extract was dissolved in 10 ml of chloroform and equal volume of concentrated H2 SO4 was added by sides of the test tube. The upper layer turns red and sulfuric acid layer showed yellow with green fluorescence. This indicated the presence of steroids.
Test for proteins
To a small amount of methanolic and ethyl acetate extract, five to six drops of Millon's reagent was added. A white precipitate which turns red on heating was formed and it is indicated the presence of proteins.
Test for terpenoids
A 5 ml of methanolic and ethyl acetate extract was mixed with 2 ml of CHCl3 in a test tube and 3 ml of concentrated H2 SO4 was carefully added to the mixture to form a layer. An interface with reddish brown coloration is formed if terpenoids constituent is present.[10]
In vitro antioxidant assays
ABTS radical scavenging assay
A 7 mM of ABTS was prepared and the stock was mixed with 2.5 mM of potassium persulfate. The mixture was then allowed to stand for 12–16 h in dark room at 37°C. In this condition the radicals were stable for more than 2 days. The incubation mixture contained 0.54 mL of ABTS, 0.5 mL of phosphate buffer, and varying concentrations of PDEAE and PDME (100, 200, 300, 400, and 500 µg/mL). The blank contained water instead of PDEAE and PDME. The absorbance was observed spectrophotometrically at 734 nm and compared with standard ascorbic acid.[11] The percentage of inhibition was calculated using the following formula:
% of scavenging activity= (Acontrol − Atest/Acontrol) ×100,
Where Acontrol is the absorbance of ABTS and ATest is the absorbance of ABTS radical + sample extract/standard.
Nitric oxide radical scavenging assay
The procedure was based on the principle that sodium nitroprusside in aqueous solution at physiological pH spontaneously generates nitric oxide which interacts with oxygen to produce nitrite ions that can be estimated using Griess reagent. Scavengers of nitric oxide compete with oxygen, leading to reduced production of nitrite ions. For the experiment, sodium nitroprusside (10 mM) in phosphate-buffered saline were mixed with different concentrations of each extracts dissolved in water and incubated at room temperature for 150 min. Following the incubation period, 0.5 mL of Griess reagent was added. The absorbance of the chromophore formed was read at 546 nm and ascorbic acid was used as a standard.[12]
% of scavenging = (Acontrol − ATest/Acontrol) ×100,
Where Acontrol is the absorbance of control and ATest is the absorbance of sample extract/standard.
Reducing power
Various concentrations of PDEAE and PDME (100, 200, 300, 400, and 500 µg/mL) in double distilled water was mixed with 2.5 mL of phosphate buffer and 2.5 mL of potassium ferricyanide. The mixture was incubated at 50°C for 20 min after which, 1.5 mL of trichloroacetic acid (TCA) was added and centrifuged at 3,000 rpm for 10 min. From all the tubes, 0.5 mL of supernatant was taken and mixed with 1 mL of distilled water and 0.5 mL of ferric chloride. The absorbance was measured spectrophotometrically at 700 nm. Ascorbic acid was used as standard for comparison. Increased absorbance of the reaction mixture indicated increasing reducing power. Incubation with water in place of additive was taken as blank.[13]
% of scavenging = (Acontrol − ATest/Acontrol) ×100,
Where, Acontrol is the absorbance of control and ATest is the absorbance of sample extract/standard.
Determination of lethal dose (LD50)
Experimental animals
Acute toxicity study was conducted on 84 male golden Syrian hamsters weighing 120–150 g, obtained from the Central Animal House, Department of Experimental Medicine, Rajah Muthiah Institute of Health Sciences, Annamalai University, Tamil Nadu, India. The whole experiment was carried out according to the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals, New Delhi, India and approved by the Animal Ethical Committee of Annamalai University (Proposal no. 647: Dated 25.09.2009). Animals were kept in polypropylene cages (47 × 34 × 20 cm; six hamsters/cage) layered with husk, renewed every 24 h under a 12:12 h light dark cycle at around 22°C. The animals were fed on a standard pelleted diet (Sai Enterprises, Chennai, India).
Preparation of doses and administration
In this study, the hamsters were fasted prior to sample administration for 18 h. The plant extracts (PDME and PDEAE) were mixed with 0.5% DMSO, and then administered intragastrically by infant feeding tube (Number 7). The volume of the dose depends on the size of the animals. Approximately 1 ml/100 g. bwt of sample was administered to each animal.[14]
LD50 evaluation
A total number of 84 animals were split into 14 groups (six animals in each group). Group I–VII (Set 1) and VII–XIV (Set 2) animals were treated with PDME (Set 1) and PDEAE (Set 2) at the dose of 100, 200, 400, 800, 1,600, 2,200, and 4,400 mg/kg. bwt, respectively. The plant extract-administered animals were closely observed for 2 h then at 12 h and finally 24 h for toxic signs and symptoms. The percentage of mortality was calculated by counting the death of animals in each group. Determination of LD50 was done by Miller and Tainter graphical plot method.[15]
Subacute toxicity studies
The hepatic and renal marker levels were observed in the serum of the experimental animals that were treated with 100, 200, and 300 mg/kg. bwt of PDME and PDEAE. Blood samples were collected by single orbital sinus and 0.8 ml (10% from total volume of 7.2 ml) of blood was taken from each animal. The blood sample was then centrifuged at 2,000 rpm for 15 min. The supernatant was taken for analysis. Activity of serum alkaline phosphatase (ALP) was estimated by the method of Kind and King.[16] The serum gamma-glutamyl transferase (GGT) was determined by the method of Rosalki and Rau 1972.[17] Serum glutamic oxaloacetic transaminase (SGOT) was determined by the method of Schumann and Klauke.[18] Total bilirubin level was evaluated by the method of Weigl et al.,[19] serum albumin level was estimated by the method of Doumas et al.,[20] and estimation of renal functional markers such as urea and creatinine were determined by Fawcett and Scott[21] and Jeffe's method.[22]
RESULTS
Preliminary phytochemical analysis
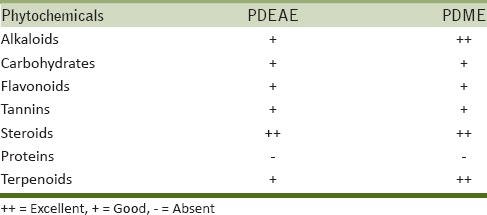
Results revealed the accumulation of alkaloids, carbohydrates, flavonoids, tannins, steroids, and terpenoids. The amount of alkaloids and terpenoids in methanol extract varied from that of ethyl acetate extract. Methanol extract provided rich amount of alkaloids and terpenoids as compared to ethyl acetate extract [Table 1].
Table 1.
Qualitative identification of phytochemicals in PDEAE and PDME
In vitro antioxidant activity
ABTS radical scavenging assay
PDEAE and PDME exhibit effective scavenging activity on ABTS radicals and the activity was comparable to that of ascorbic acid. The 50% of inhibition concentration (IC50) value indicate that the ABTS free radical scavenging activity with a IC50 of 349.65 µg/ml of PDME and 387.59 µg/ml of PDEAE was almost equivalent to that standard ascorbic acid (IC50-318.47 µg/ml) [Figure 1].
Figure 1.
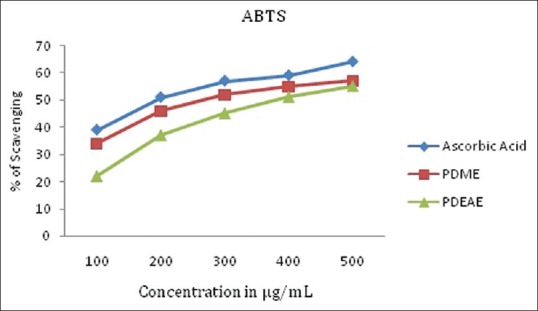
ABTS radical scavenging activity of PDME and PDEAE and the standard ascorbic acid. Results represent means of triplicates of different concentrations analyzed. PDEAE = Ethyl acetate extracts of Pergularia daemia, PDME = methanolic extracts of P. daemia, ABTS = 2,2'-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid
Nitric oxide radical scavenging assay
In the present study, PDEAE and PDME were subjected to determine the inhibitory effect on nitric oxide formulation. The results from the present study reveal that the extracts have a moderate activity in scavenging nitric oxide radical. Figure 2 illustrates a significant decrease in the nitric oxide radical due to scavenging ability of extracts and ascorbic acid.
Figure 2.
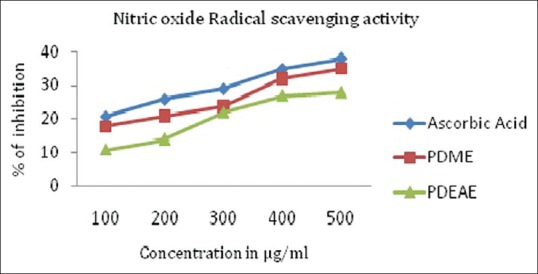
Nitric oxide radical scavenging activity of PDME and PDEAE and the standard ascorbic acid. Results represent means of triplicates of different concentrations analyzed
Reducing power
Fe3+ ions were potentially reduced by the plant extracts in different concentrations (100, 200, 300, 400, and 500 µg/mL). Further, methanolic extract has higher ability to reduce Fe3+ ions than ethyl acetate extract. The ascorbic acid was used as a comparable control [Figure 3].
Figure 3.
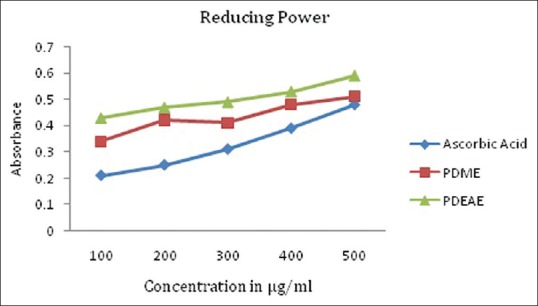
Reducing power activity scavenging activity of PDME and PDEAE and the standard ascorbic acid. Results represent means of triplicates of different concentrations analyzed
Acute toxicity
Toxic signs
There were no toxic signs or central nervous system (CNS) abnormalities observed in both PDME and PDEAE treated hamsters at the dose of 100, 200, 400, and 800 mg/kg. bwt. However, 1,600, 2,200, and 4,400 mg/kg. bwt treated hamsters showed behavioral abnormalities after 8 h of extract administration; the animals exhibited salivation, arching and rolling, lack of breathing and food consumption, gasping, and finally died.
LD50 measurements
Table 2 shows the percentage mortality of PDEAE and PDME treated hamsters at the concentration of 100, 200, 400, 800, 1,600, 2,200, and 4,400 mg/kg. bwt. The probit values were plotted against extract concentration, then the dose corresponding to probit 5 in linear plotting method, that is, 50% was found [Figure 4]. LD50 values of both PDME and PDEAE were observed as 2,500 mg/kg. bwt on graphical plotting. Standard error for the LD50 was calculated using the formula,
Table 2.
Results of lethal dose (LD) of PDEAE and PDME for the determination of LD50 after intragastric administration in Hamster (n=6)
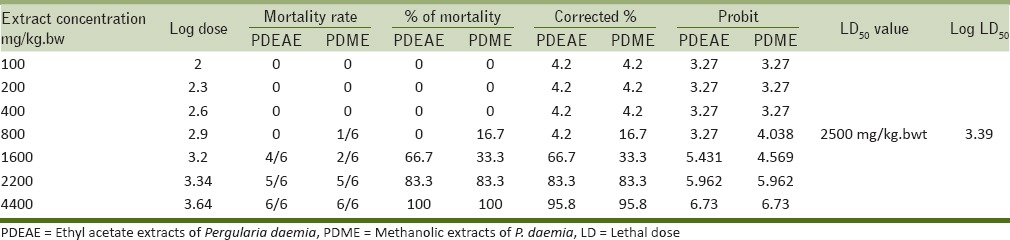
Figure 4.
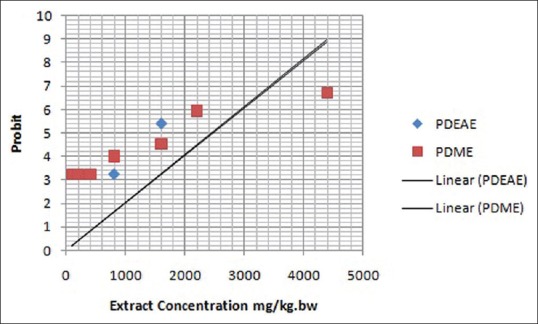
Plot of extract concentration versus probits for calculation of LD50 of PDEAE and PDME administered intragastrically. LD50 = Median lethal dose
Approximate standard error of LD50
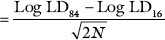
Therefore, LD50 of PDME and PDEAE was found to be 2,500 ± 296.15, with 95% confidence interval of 2,203.85–2,796.15.
Biochemical evaluation
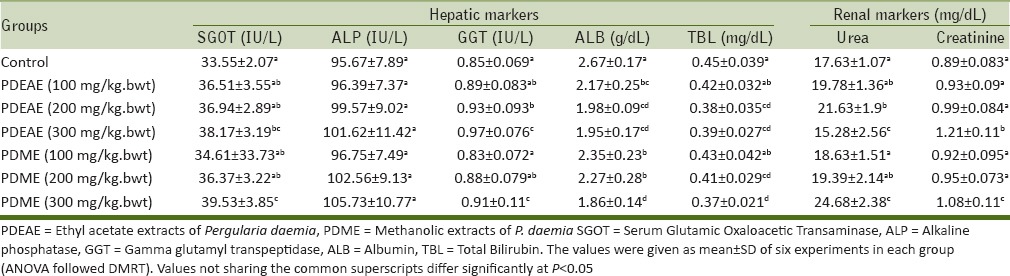
The functional alterations in liver and kidney were observed by analyzing the hepatic and renal markers in blood serum of the animals which were treated with 100, 200, and 300 mg/kg. bwt of PDEAE and PDME. However, there were no toxic changes in liver and kidney observed in both extracts treated hamsters [Table 3].
Table 3.
Levels of hepatic and renal markers in serum of PDEAE and PDME treated hamsters
DISCUSSION
More than 80% of people utilize the medicinal plants for maintenance of good health in most developing countries. However, herbs have powerful natural antioxidant properties. Phenolic compounds like flavonoids, steroids, and tannins are the major source of antioxidants, which are highly present in medicinal plants.[23] Plant phenols are widely distributed in the tissue of plants as well as exposed a vital role in the free radical scavenging activity. Antioxidant activity of the phenols is mainly due to their redox, hydrogen donor and singlet oxygen quenching ability.[24] Two-third of worlds' plant species, out of which at least 35,000 are estimated to have medicinal value. The present study revealed that the presence of alkaloids, flavonoids, carbohydrates, steroids, proteins, tannins, and terpenoids in PDME and PDEAE. The amount present in the plant varied in methanolic extract from that of ethyl acetate extract, this is because of the polar nature of the solvent. The phenolic compounds were highly correlated with antioxidant ability. In the present study, the antioxidant properties of PDEAE and PDME by assessing the scavenging capacity of the extracts on ABTS, nitric oxide radicals, and reducing power activity have been evaluated.
ABTS is a chemical compound which is frequently used in food industry to determine the antioxidant capacity. The principle reaction is considered as the formation of colorless ABTS. Basically, ABTS reacts with potassium per sulfate and formed blue green chromophore. This then reacts with electron donors and may give colorless neutral solution.[25] In our study, the presence of hydrogen donating phenolics like flavonoids, steroids, tannins, and terpenoids were donating their electrons and neutralize the formation of ABTS. The colorless solution was observed at 734 nm.
Nitric oxide play an important role in various degenerative processes like carcinogenesis, juvenile diabetes, multiple sclerosis, and ulcerative colitis.[26] The radical has reported that it is a potent pleotropic inhibitor of physiological processes like muscle relaxation, neural signaling, and inhibition of platelet aggregation. Nitric oxide can contribute to reperfusion injury when an excessive amount produced during reperfusion reacts with superoxide to produce the damaging oxidant peroxyinitrite. The formation of nitric oxide radicals was effectively decreased by the presence of hydrogen donating particles in P. daemia. The reducing power activity was measured by potassium ferric cyanide reduction method. The yellow color of the solution changes to various shades of green and blue, depending upon the reducing power of each extract. The presence of antioxidants in the P. daemia causes the reduction of Fe3+/ferric cyanide complex to ferrous form.[27]
Acute toxicity of the PDEAE and PDME was evaluated using Miller and Tainter method. Seven doses were selected (100, 200, 400, 800, 1,600, 2,200, and 4,400 mg/kg. bwt) for the determination of LD50. At the end of the period (after 24 h), the number of dead animals were counted. The graphical representation of probit versus extract concentration showed a typical straight line, which was in agreement with the principle of probit analysis [Figure 4]. According to Miller and Tainter probit analysis method at 24 h, the acute oral LD50 value of PDME and PDEAE was calculated as 2,500 mg/kg. bwt. Overexposure of any substances can cause the functional disability of liver and kidney.[28] Hepatic (serum ALP, GGT, SGOT, total bilirubin, and albumin) and renal (urea and serum creatinine) markers are the major functional indicators of the liver and kidney, respectively. The functional biomarkers were analyzed for the determination of toxic changes during the extract administration. There were no toxic changes appeared at the dose level of 100, 200, and 300 mg/kg. bwt. The levels were showed in normal range in both PDEAE and PDME treated hamsters. Thus, it could be concluded that the PDEAE and PDME was nontoxic up to the dose range of 2,500 mg/kg. bwt. The plant P. daemia has a wide range of pharmacological activities. However, very less work has been done on this plant and there is a wide scope for investigation.
Financial support and sponsorship
DST- SERB.
Conflicts of interest
There are no conflicts of interest
Acknowledgment
We gratefully acknowledge the financial support of Department of Science and Technology-Science and Engineering Research Board (DST-SERB), New Delhi, India.
REFERENCES
- 1.Polefka TG, Meyer TA, Agin PP, Bianchini RJ. Cutaneous oxidative stress. J Cosmet Dermatol. 2012;11:55–64. doi: 10.1111/j.1473-2165.2011.00596.x. [DOI] [PubMed] [Google Scholar]
- 2.Zhao L, Chen J, Su J, Li L, Hu S, Li B, et al. In vitro antioxidant and antiproliferative activities of 5-hydroxymethylfurfural. J Agric Food Chem. 2013;61:10604–11. doi: 10.1021/jf403098y. [DOI] [PubMed] [Google Scholar]
- 3.Vaithiyanathan V, Mirunalini S. Chemo preventive potential of fruit juice of Phyllanthus emblica Linn.(amla) against mammary cancer by altering oxidant/antioxidantstatus, lipid profile levels and estrogen/progesterone receptor statusin female Sprague–Dawley rats. Biomed Prev Nutr. 2013;3:357–66. [Google Scholar]
- 4.Lo Scalzo R, Picchi V, Migliori CA, Campanelli G, Leteo F, Ferrari V, et al. Variations in the phytochemical contents and antioxidant capacity of organically and conventionally grown Italian cauliflower (Brassica oleracea L. subsp. botrytis): Results from a three-year field study. J Agric Food Chem. 2013;61:10335–44. doi: 10.1021/jf4026844. [DOI] [PubMed] [Google Scholar]
- 5.Bhaskar VH, Balakrishnan N. Pharmacognostic studies on Pergularia daemia roots. Pharm Biol. 2010;48:427–32. doi: 10.3109/13880200903160699. [DOI] [PubMed] [Google Scholar]
- 6.Sureshkumar SV, Mishra SH. Hepatoprotective effect of extracts from Pergularia daemia Forsk. J Ethnopharmacol. 2006;107:164–8. doi: 10.1016/j.jep.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 7.Gupta JC, Roy PK, Dutta A. Pharmacological action of an active-constituent isolated from Daemia extensa linn. (Syn. Pergularia extensa) Indian J Med Res. 1946;34:181–4. [PubMed] [Google Scholar]
- 8.Raj J, Chandra M, Dogra TD, Pahuja M, Raina A. Determination of median lethal dose of combination of endosulfan and cypermethrin in wistar rat. Toxicol Int. 2013;20:1–5. doi: 10.4103/0971-6580.111531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vyas B, Vyas R, Joshi S, Santani D. Antiurolithiatic activity of whole-plant hydroalcoholic extract of pergularia daemia in rats. J Young Pharm. 2011;3:36–40. doi: 10.4103/0975-1483.76417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karthishwaran K, Mirunalini S. GC-MS analysis of aerial parts of pergularia daemia (Forsk.) methanolic extract. E J Life Sci. 2012;2:50–5. [Google Scholar]
- 11.Wolfenden BS, Willson RL. Radical-cations as reference chromogens in kinetic studies of one- electron transfer reactions; pulse radiolysis studies of 2, 2’-azinobis-(3-ethylbenzothiazoline-6-sulfonate) J Chem Soc Perkin Trans. 1982;2:805–12. [Google Scholar]
- 12.Oyaizu M. Studies on products of browning reaction prepared from glucoseamine. Japan J Nut. 1986;44:307–15. [Google Scholar]
- 13.El SN, Karakaya S. Radical scavenging and iron-chelating activities of some greens used as traditional dishes in Mediterranean diet. Int J Food Sci Nutr. 2004;55:67–74. doi: 10.1080/09637480310001642501. [DOI] [PubMed] [Google Scholar]
- 14.Ghosh MN. Fundamentals of Experimental Pharmacology. 2nd Edn. Calcutta: Scientific Book Agency; 1984. p. 153. [Google Scholar]
- 15.Miller LC, Tainter ML. Estimation of LD50 and its error by means of log-probit graph paper. Proc Soc Exp Biol Med. 1944;57:261. [Google Scholar]
- 16.Kind PR, King EJ. Estimation of plasma phosphatases by determination of hydrolysed phenol with amino-antipyrine. J Clin Pathol. 1954;7:322–6. doi: 10.1136/jcp.7.4.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosalki SB, Rau D. Serum-glutamyl transpeptidase activity in alcoholism. Clin Chim Acta. 1972;39:41–7. doi: 10.1016/0009-8981(72)90297-5. [DOI] [PubMed] [Google Scholar]
- 18.Schumann G, Klauke R. New IFCC reference procedures for the determination of catalytic activity concentrations of five enzymes in serum: Preliminary upper reference limits obtained in hospitalized subjects. Clin Chim Acta. 2003;327:69–79. doi: 10.1016/s0009-8981(02)00341-8. [DOI] [PubMed] [Google Scholar]
- 19.Weigl E, Bach H, Krieg D. Serum bilirubin in an obvious healthy population (author's transl) Med Klin. 1975;70:664–9. [PubMed] [Google Scholar]
- 20.Doumas BT, Watson WA, Biggs HG. Albumin standards and the measurement of serum albumin with bromcresol green. Clin Chim Acta. 1971;31:87–96. doi: 10.1016/0009-8981(71)90365-2. [DOI] [PubMed] [Google Scholar]
- 21.Fawcett JK, Scott JE. A rapid and precise method for the determination of urea. J Clin Pathol. 1960;13:156–9. doi: 10.1136/jcp.13.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeffe M. Concerning the precipitate produced in normal urine by picric acid and a new reaction of creatinine. Physiol Chem. 1886;10:91–400. [Google Scholar]
- 23.Di Pompo G, Poli F, Mandrone M, Lorenzi B, Roncuzzi L, Baldini N, et al. Comparative “in vitro” evaluation of the antiresorptive activity residing in four Ayurvedic medicinal plants. Hemidesmus indicus emerges for its potential in the treatment of bone loss diseases. J Ethnopharmacol. 2014;154:462–70. doi: 10.1016/j.jep.2014.04.033. [DOI] [PubMed] [Google Scholar]
- 24.Sivaci A, Duman S. Evaluation of seasonal antioxidant activity and total phenolic compounds in stems and leaves of some almond (Prunus amygdalus L.) varieties. Biol Res. 2014;47:9. doi: 10.1186/0717-6287-47-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haddouchi F, Chaouche TM, Ksouri R, Medini F, Sekkal FZ, Benmansour A. Antioxidant activity profiling by spectrophotometric methods of aqueous methanolic extracts of Helichrysum stoechas subsp. rupestre and Phagnalon saxatile subsp. saxatile. Chin J Nat Med. 2014;12:415–22. doi: 10.1016/S1875-5364(14)60065-0. [DOI] [PubMed] [Google Scholar]
- 26.Narasimhan MK, Pavithra SK, Krishnan V, Chandrasekaran M. In vitro Analysis of Antioxidant, Antimicrobial and Antiproliferative Activity of Enteromorpha antenna, Enteromorpha linza and Gracilaria corticata Extracts. Jundishapur J Nat Pharm Prod. 2013;8:151–9. doi: 10.17795/jjnpp-11277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chidambaram U, Pachamuthu V, Natarajan S, Elango B, Suriyanarayanan, Ramkumar KM. In vitro evaluation of free radical scavenging activity of Codariocalyx motorius root extract. Asian Pac J Trop Med. 2013;6:188–94. doi: 10.1016/S1995-7645(13)60021-8. [DOI] [PubMed] [Google Scholar]
- 28.Morales G, Paredes A, Olivares A, Bravo J. Acute oral toxicity and anti-inflammatory activity of hydroalcoholic extract from Lampaya medicinalis Phil in rats. Biol Res. 2014;47:6. doi: 10.1186/0717-6287-47-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


