Abstract
Objectives:
Asafetida is traditionally used in folklore medicine for the treatment of various ailments. To validate its use in traditional medicine, it is important to evaluate its toxicity in the animal system. Therefore, this study aimed to evaluate the toxicological effects of asafetida in Wistar albino rats.
Materials and Methods:
Acute toxicity tests were conducted by the oral administration of 250, 500, and 1,000 mg/kg body weight of the animal. In chronic study, animals were administered with various doses of asafetida (25, 50, 100, and 200 mg/kg body weight) for a period of 6 weeks. At end of experiment, the effects of asafetida on hematological, renal, and hepatic markers and histological parameters were analyzed.
Results:
In acute toxicity study, no mortality was seen up to 72 h of the administration of asafetida. No signs of neurological and behavioral changes were noticed within 24 h. In the chronic study, the asafetida intake has changed the hematological parameters such as red blood cell (RBC), white blood cell (WBC), hematocrit (HCT), and platelets. Aspartate aminotransferase (AST) and lactate dehydrogenase (LDH) were significantly increased in treated animals. The plasma level of urea and creatinine were not altered by the administration of asafetida throughout the study. Histopathology study indicates hepatotoxicity, but no signs of prominent pathological changes in kidney.
Conclusions:
Asafetida did not show any acute toxicity, but chronic administration could have undesirable effects on hepatocytes and hematological factors.
Keywords: Asafetida, hematology, kidney, liver, toxicity
INTRODUCTION
Asafetida, an oleo-gum-resin, is extracted by incisions in the stem and roots of Ferula assafoetida L. and some others Ferula species.[1] The resin is an important pharmacological and industrial agent and is being used in traditional medicines for treatment of various disorders like stomach ache, indigestion, bronchitis, asthma, and whooping cough;[2] and for treatment of intestinal parasites.[3] Nepalian people use asafetida 50–200 mg twice a week mainly as sedative, carminative, antispasmodic, diuretic, antihelmintic, emmenagogue, and aphrodisiac agent.[4] In Ayurveda, asafetida is introduced as a valuable remedy for flatulence, hysteria, gastrointestinal disorders, inflammation, stomach ache, spasmodic, and helminthic.[1] New studies showed that this oleo-gum-resin has antiviral, antifungal, cancer chemopreventive, antidiabetic, and cytotoxicity effect.[1] It also has antispasmodic, anticonvulsant, and antinociceptive effects.[5,6,7] Although there are different studies about pharmacological properties of asafetida, to our knowledge, there is no comprehensive toxicological study of asafetida on animal models. There is only one case report that methemoglobinemia has been observed after administration of asafetida in a 5-week-old black male infant.[8] It is also recommended that asafetida should not be used during pregnancy as it may increase the risk of abortion.[9]
The toxicity effects of asafetida have been studied mainly on parasites and some protozoan animals suggesting it antiparasitic,[10,11,12] antifungal,[13] and antibacterial.[14] Kumar and Singh reported that different root extracts of Ferula assafoetida have anti molluscicidal activity against the snail Lymnaea acuminate.[10] Bagheri et al., showed that asafetida has cytotoxicity effect on the brine shrimp.[15] Some of old studies showed that asafetida has a weak exchange-inducing on sister chromatid in spermatogonia[16] and clastogenicity in mouse spermatocytes.[17] The study is aimed to study the toxic effects of asafetida on blood parameters, kidney and liver factors, and histopathology of kidney and liver in male Wistar rats.
MATERIALS AND METHODS
Animals
Male Wistar rats weighing between 150 and 180g and 6–8 weeks old were bred and maintained in the animal house unit of the faculty of medicine under controlled temperature at 21 ± 1°C in 12 h light: 12 h darkness schedule. Animals were housed in plastic cages and food and water was made available ad libitum. The study was approved by Institutional Animal Ethical Committee of Shahid Sadoughi University of Medical Science.
Acute toxicity studies
For acute toxicity studies, rats were divided into four groups (n = 5; each) and were administered orally with three doses of asafetida corresponding to 250, 500, and 1,000 mg/kg body weight. The control group received 1 ml of physiological saline. They were all placed under observation immediately for 24 h for any behavioral, neurological changes and then mortality for the next 72 h.[18]
Chronic toxicity studies
The rats were divided into five groups (n = 4; each) and were administrated four different doses of asafetida corresponding to 25, 50, 100, and 200 mg/kg orally every day for 6 weeks' duration. Another group of five rats that received 1 ml of physiological saline served as control.
Plant oleo-gum resin
Ferula assafoetida oleo-gum-resin was collected from Tabas region (Yazd province, Iran) during the summer and the plant species was botanically identified by Dr. Abbas Zarezadeh in Yazd Agricultural Research Center. The dried powder of asafetida was soaked in distilled water overnight at room temperature and the yielded suspension was used orally. Concentrations and dosages of the extract were expressed as crude amount of the dried oleo-gum-resin used in preparing the stock solution.
Biochemical analysis
Blood was collected from orbital sinus of rats. Serum was prepared by centrifugation (3,000 rpm, 20 min) and stored frozen until biochemical assay. The urea, creatinine, lactate dehydrogenase (LDH), and aspartate aminotransferase (AST) were determined using the suitable kits and according to the manufacturer's instructions.
Hematological tests
The blood with ethylenediaminetetraacetic acid (EDTA) was used for the counting of RBC and total count of WBC and platelet by standard procedures. Hematocrit percentage (HCT %) was also determined.[19]
Histopathology evaluation
For histopathological studies, liver and kidney tissue were collected immediately in 10% neutral buffered formalin. The tissues were processed by routine histological methods and embedded in paraffin blocks. The sections were cut by a rotary microtome and subsequently stained with Ehlrich's hematoxylin and eosin (H and E). The histological sections were studied under Olympus light microscopy (Olympus, Japan, magnification ×40) to evaluate histopathological changes.
Statistical analysis
Statistical data were assessed with one-way analysis of variance (ANOVA), followed by post hoc Tukey's test using Graph pad prism version 5. Results were expressed as mean ± standard error of the mean (SEM). A value of P < 0.05 was considered significant.
RESULTS
Acute toxicity studies
After the administration of different doses of asafetida, the rats were monitored for the signs of toxicity. The animals showed normal activity and no mortality were observed till the end of the study.
Biochemical factors
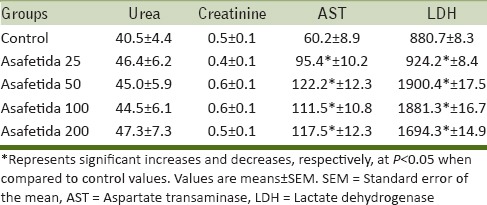
The result of serum kidney and liver parameters analysis in chronic study was shown in Table 1. There is no significant difference between the urea and creatinine concentration in control and treated animals with asafetida. Our results also showed that AST and LDH significantly increased in all treated animals with asafetida compared to control group (P < 0.05).
Table 1.
Effect of asafetida on kidney and liver biochemical parameters in male rats
Hematological changes
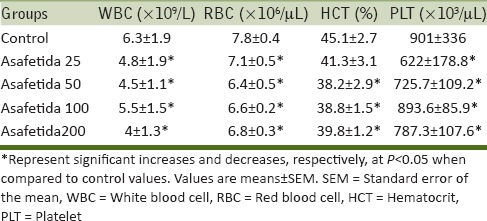
Table 2 reveals that the asafetida significantly decreased the concentrations of WBC, RBC, platelets, and HCT % after 6 weeks of treatment with asafetida. The HCT % in asafetida 25 group did not show any significant difference.
Table 2.
Effect of oral consumption of asafetida on hematological parameters in male rats
Histopathological findings of liver
The liver sections of rats in control group showed normal hepatic architecture and normal hepatocytes with prominent nucleus, central vein, and portal areas with no sign of inflammation or necrosis [Figure 1a]. Histopathological examination of extract treated rats showed little degenerative changes in some hepatic cells [Figure 1b]. Findings demonstrated that with increase in the extract doses (50,100, and 200 mg/kg respectively), hepatocytes became larger in size with prominent nucleus compared to control group. Hypotrophied Kupffercells were also prominent with increase in extract doses and blood vessels expansion and dilated hepatic sinusoids were noticed [Figure 1c–e]. However, the general structures of lobules of liver are normal and clear.
Figure 1.
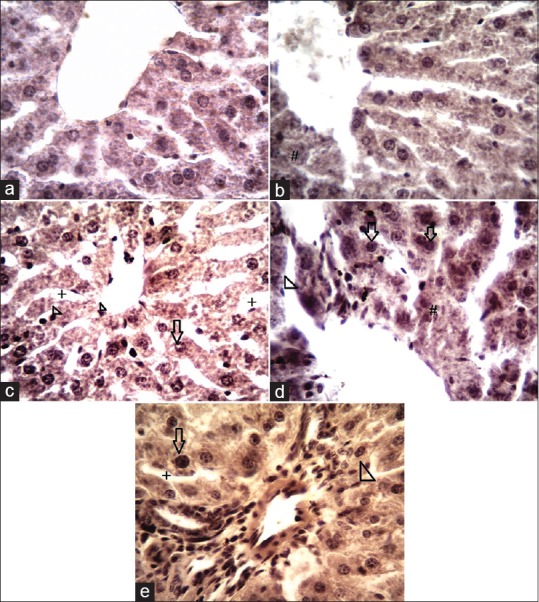
Histological architecture of the liver in different experimental groups. (a) No histopathological changes were seen in control group and the structures of lobules and hepatocyte of liver are normal. (b) Little degenerative changes of some hepatic cells (#) were seen. (c-e) In treatment groups (50, 100, and 200 mg/kg respectively) an increase in size of hepatocytes (arrows) were noticed. Hypotrophied Kupffer's cells (head arrow), dilated hepatic sinusoids (+) were also seen with increase in extract doses (H and E, ×400). H and E = hematoxylin and eosin
Histopathological findings of kidney
Histological examination of the control group showed normal appearance and there were no changes in the renal tubular and glomerulus of kidneys [Figure 2a]. Sections of kidneys from extract groups (25, 50, 100, and 200 mg/kg) showed no signs of prominent pathological changes except occasional renal tubular necrosis [Figure 2b–d]. In some sections of extract groups, mild infiltration of inflammatory cells around the blood vessels and in the interstitial spaces was found. In extract group (200 mg/kg), some of the glomeruli appeared to be slightly expanded and there were little signs of tubular degenerative changes [Figure 2e].
Figure 2.
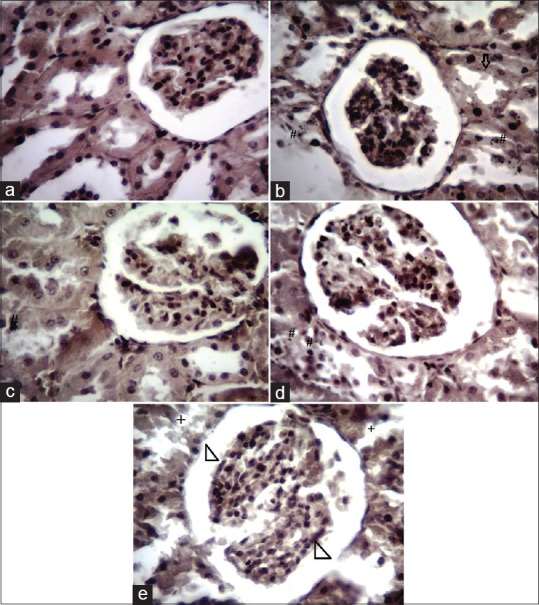
(a) Paraffin section of kidney from control group showing normal histology. (b-d) Treatment groups (25, 50, and 100 mg/kg, respectively) showing no prominent histopathological changes except little renal tubular necrosis (#) and mild infiltrations of inflammatory cells in the interstitial spaces (arrow). (e) Showing mild degeneration of the cells lining the kidney tubules (+) and some of the slightly expanded glomeruli (head arrow) in treatment group at 200 mg/kg (H and E, ×400)
DISCUSSION
The herbal drugs are playing an important role in healthcare programs throughout the world with a general belief that herbal drugs are always safe as these are natural. However, adverse effects do occur which are usually mild and affect a small number of people. Asafetida is used in different countries not only as a culinary spice but also traditionally used to treat various diseases. Although there are no experimental data about toxicity effects of asafetida, in Iranian traditional, it was emphasized that intake of larger dosages of asafetida can lead to swelling of the lips, digestive complaints such as flatulence and diarrhea, discomfort, and headache.[3] The nontoxic nature of the asafetida and its oral safety at selected dose level was confirmed as the administration of the drug does not cause mortality in rats. According to Clarke and Clarke,[20] any compound or drug with the oral LD50 estimate greater than 1,000 mg/kg could be considered of low toxicity. This supports that asafetida was found to be safe up to 1,000 mg/kg body weight in terms of mortality. In the present study, acute toxicity of asafetida caused a significant increase in AST and LDH level in treated animals as compared to control group [Table 1]. There are many enzymes found in the serum that did not originally originate from the serum. During tissue damage, some of these enzymes find their way into the serum, probably by leakage.[21] Serum enzyme measurements are therefore a valuable tool in clinical diagnosis, providing information on the effect and nature of pathological damage to any tissue. The increase in serum LDH and AST activity may indicate hepatic damage probably by the altered cell membrane permeability leading to the leakage of the enzymes from the tissues to the serum.[22,23] LDH and AST are considered to be sensitive indicators of hepatocellular damage and within limit can provide a quantitative evaluation of the degree of damage to the liver.[24] In the present study, there was not any significant difference in urea and creatinine concentrations following treatment with asafetida. Destruction of glomeruli causes significant decrease in the glomerular filtration rate (GFR) and increases the blood urea and creatinine resulting chronic renal failure.[25] Since the urea and creatinine are markers of kidney function,[26] the data suggest that asafetida is not nephrotoxic. Hematological data obtained in our work indicated a significant decline in RBC and WBC and platelet count [Table 2]. Altered RBC, WBC, and platelet counts reflect that asafetida would have some mild effect on the hematological parameter. Since blood cells are originated from bone marrow, the inertness of the extract on this organ is evident and its use in pharmacological evaluation is again certified. Histopathological observations revealed that treatment with asafetida resulted little degenerative changes of some hepatic cells. Sections of kidneys from extract groups (25, 50, 100, and 200 mg/kg) showed no signs of prominent pathological changes in the cortex and the medulla, but little renal tubular necrosis was seen. Our finding also demonstrated that these changes were dose-dependent, respectively, and these histopathological evidences were in agreement with biochemical results.
CONCLUSION
The data showed that asafetida did not have any acute toxicity, but the chronic consumption of this oleo-gum-resin caused reverse effects on liver and blood parameters. This study suggested that asafetida may be used in minimum possible doses.
Financial support and sponsorship
Nil
Conflicts of interest
There are no conflicts of interest
Acknowledgment
This research was supported by the Foundation of Shahid Sadoughi University of Medical Sciences and Health Services, Yazd, Iran.
REFERENCES
- 1.Iranshahy M, Iranshahi M. Traditional uses, phytochemistry and pharmacology of asafoetida (Ferula assa-foetida oleo-gum-resin) -a review. J Ethnopharmacol. 2011;134:1–10. doi: 10.1016/j.jep.2010.11.067. [DOI] [PubMed] [Google Scholar]
- 2.Zargari A. Medicinal Plants. Tehran: Tehran University Publications; 1996. pp. 529–602. [Google Scholar]
- 3.Emami A, Fasihi S, Mehregan I. Medicinal Plants. Vol. 1. Tehran: Andisheh Avar Publications; 2010. pp. 24–8. [Google Scholar]
- 4.Eigner D, Scholz D. The magic book of Gyani Dolma. Pharm Unserer Zeit. 1990;19:141–52. doi: 10.1002/pauz.19900190406. [DOI] [PubMed] [Google Scholar]
- 5.Bagheri S, Hejazian Sh, Dashti-R M. The Relaxant Effect of Seed's Essential Oil and Oleo-gum-resin of Ferula assa-foetida on Isolated Rat's Ileum. Ann Med Health Sci Res. 2014;4:238–41. doi: 10.4103/2141-9248.129050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bagheri SM, Rezvani ME, Vahidi AR, Esmaili M. Anticonvulsant effect of Ferula assa-foetida oleo gum resin on chemical and amygdala-kindled rats. N Am J Med Sci. 2014;6:408–12. doi: 10.4103/1947-2714.139296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bagheri SM, Dashti-R MH, Morshedi A. Antinociceptive effect of Ferula assa-foetida oleo-gum-resin in mice. Res Pharm Sci. 2014;9:207–12. [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly KJ, Nue J, Camitta BM, Honig GR. Methemoglobinemia in an infant treated with the folk remedy glycerited asafoetida. Pediatrics. 1984;73:717–9. [PubMed] [Google Scholar]
- 9.Eigner D, Scholz D. Ferula asa-foetida and Curcuma longa in traditional medical treatment and diet in Nepal. J Ethnopharmacol. 1999;67:1–6. doi: 10.1016/s0378-8741(98)00234-7. [DOI] [PubMed] [Google Scholar]
- 10.Kumar P, Singh DK. Molluscicidal activity of Ferula asafoetida, Syzygium aromaticum and Carum carvi and their active components against the snail Lymnaea acuminata. Chemosphere. 2006;63:1568–74. doi: 10.1016/j.chemosphere.2005.08.071. [DOI] [PubMed] [Google Scholar]
- 11.Ramadan NI, Abdel-Aaty HE, Abdel-Hameed DM, El Deeb HK, Samir NA, Mansy SS, et al. Effect of Ferula assafoetida on experimental murine Schistosoma mansoni infection. J Egypt Soc Parasitol. 2004;34:1077–94. [PubMed] [Google Scholar]
- 12.Ramadan NI, Al Khadrawy FM. The in vitro effect of Assafoetida on Trichomonas vaginalis. J Egyp Soc Parasitol. 2003;33:615–30. [PubMed] [Google Scholar]
- 13.Sitara U, Niaz I, Naseem J, Sultana N. Antifungal effect of essential oils on in vitro growth of pathogenic fungi. Pak J Bot. 2008;40:409–14. [Google Scholar]
- 14.Garg DK, Banerjea AC, Verma J. The role of intestinal Clostridia and the effect of asafoetida (Hing) and alcohol in flatulence. Indian J Microbiol. 1980;20:194–7. [Google Scholar]
- 15.Bagheri SM, Sahebkar A, Gohari AR, Saeidnia S, Malmir M, Iranshahi M. Evaluation of cytotoxicity and anticonvulsant activity of some Iranian medicinal Ferula species. Pharm Biol. 2010;48:242–6. doi: 10.3109/13880200903081796. [DOI] [PubMed] [Google Scholar]
- 16.Abraham SK, Kesavan PC. Genotoxicity of garlic, turmeric and asafetida in mice. Mutat Res. 1984;136:85–8. doi: 10.1016/0165-1218(84)90138-1. [DOI] [PubMed] [Google Scholar]
- 17.Walia K. Effect of asafoetida (7-hydroxycoumarin) on mouse spermatocytes. Cytologia (Tokyo) 1973;38:719–24. doi: 10.1508/cytologia.38.719. [DOI] [PubMed] [Google Scholar]
- 18.Devaki K, Beulah U, Akila G, Gopalakrishnan VK. Effect of Aqueous Extract of Passiflora edulis on Biochemical and Hematological Parameters of Wistar Albino Rats. Toxicol Int. 2012;19:63–7. doi: 10.4103/0971-6580.94508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanderson JH. Philips: An atlas of laboratory animal haematology. New York: Oxford University Press; 1981;1:150–55. [Google Scholar]
- 20.Clarke EG, Clarke ML. Landers veterinary toxicology in London. London: Bailliere Tindall; 1997;2:456–61. [Google Scholar]
- 21.Will's DE. Biochemical basis of medicine. Bristol: John Wright and Sons Ltd; 1985;1:45–66. [Google Scholar]
- 22.Appidi JR, Yakubu MT, Grierson DS, Afolayan AJ. Toxicological evaluation of aqueous extracts of Hermannia incana Cav. Leaves in male wistar rats. Afr J Biotechnol. 2009;8:2016–20. [Google Scholar]
- 23.Akanji MA, Yakubu MT. Alpha tocopherol protects against metabisulphite- induced tissue damage in rats. J Biochem Mol Biol. 2000;15:179–83. [Google Scholar]
- 24.Al-Habori M, Al-Aghbari A, Al-Mamary M, Baker M. Toxicological evaluation of Catha edulis leaves: A long term feeding experiment in animals. J Ethnopharmacol. 2002;83:209–17. doi: 10.1016/s0378-8741(02)00223-4. [DOI] [PubMed] [Google Scholar]
- 25.Ramakrishnan S, Swami R. Text Book of Clinical Biochemistry and Immunology. Vol. 3. Chennai: T.R. Publications; 1995. pp. 245–75. [Google Scholar]
- 26.Odoula T, Adeniyl FA, Bello IS, Subair HG. Toxicity studies on an unripe Carica papaya aqueous extract: Biochemical and haematological effects in Wister albino rats. J Med Plants Res. 2007;1:1–4. [Google Scholar]


