Abstract
The present study highlights fluoride -induced toxicity and the protective role of ascorbic acid in the liver and ovary of freshwater fish, Heteropneustis fossilis. The fish specimens were exposed to different concentrations (35 mg F/L and 70 mg F/L) of fluoride. Parameters related to oxidative stress were studied at the end of the experiment. The biomarkers selected for the study were thiobarbituric acid reactive substances for assessing the extent of lipid peroxidation (LPO) and antioxidant defense system such as reduced glutathione (GSH), superoxide dismutase (SOD) catalase (CAT) glutathione peroxidase (GPx), and glutathione S-transferase (GST) activities. The fluoride exposure significantly elevated the level of LPO, CAT, SOD, and GST in the tissues of treated group as well as modulated the activities of GSH and level of GPx after exposure as compared to the control. A significant decrease in GPx activity was found in these tissues suggesting that fluoride exposure increases the level of free radical, as well as CAT activity. Pre- and post treatment with ascorbic acid decreased the LPO, SOD, CAT, GST level, and increased GSH, GPx levels in the liver and ovary.
Keywords: Ascorbic acid, biomarkers, Heteropneustis fossilis, oxidative stress, sodium fluoride
INTRODUCTION
In biological systems, oxidative stress has become an area of significant interest for aquatic toxicological studies. The problems associated with fluoride exposure indicate the biochemical stress in the body by generating imbalance between reactive oxygen species (ROS) and antioxidants thereby inducing oxidative stress. Fluorosis is a disease arising due to excess intake of fluoride. There is an evidence that fluoride consumption causes crippling disease in man.[1,2] There are several routes of fluoride intake such as water, food, air, medicaments, and cosmetics. Out of these, water is the main source of fluoride exposure.[3]
There are strong evidences suggesting the involvement of oxidative stress in the mechanism of fluoride toxicity. Lipid peroxidation (LPO) is one of the main manifestations of oxidative damage induced by various compounds, including metals[4] hence, it has been used as a biomarker of pollution.[5,6,7] Superoxide dismutase (SOD) is the enzyme that catalyzes the dismutation of the superoxide anion to O2 and H2O2 and catalase (CAT) reacts with H2O2 to form water and oxygen molecule.[8] SOD is a group of metalloenzymes that plays a crucial antioxidant role and constitutes the primary defense against the toxic effects of superoxide radical in aerobic organisms.[9] CAT is one of the sensitive enzymes and its activity is modulated by various factors including overproduction of superoxide radicals.[10,11] Glutathione (GSH) plays an important part in modulating metal-induced LPO through its role as a reducing substrate in oxidative reactions and its sequestering capacity of metals.[12] The antioxidant defense system includes enzymes such as SOD, CAT, glutathione peroxidase (GPx), glutathione S-transferase (GST), and other low molecular weight scavengers such as GSH.[13] The GPx detoxifies H2O2 or organic hydroperoxides that are produced in LPO.[14] Reduced GSH is an important antioxidant, which is readily oxidized by ROS to oxidized GSH.[15] GST form a family of multifunctional Phase II biotransformation enzymes present in the cytosol of most cells, which catalyze the conjugation of GSH to a variety of compounds and also involved in the transport and elimination of reactive compounds that carry other indirect antioxidant functions.[15,16]
Ascorbic acid is a water-soluble ROS scavenger with high potency. The role of antioxidants is to balance the cellular production of ROS, maintaining the intracellular redox balance by preventing the cellular damage caused by ROS. Adequate amount of Vitamin C is another important content which can ameliorate fluoride toxicity.
MATERIALS AND METHODS
Experimental animals and chemicals
The freshwater catfish Heteropneustis fossilis (mean weight 28.04 ± 0.20 g and length 17.09 ± 0.20 cm) were purchased from the local market in Lucknowand used for the experiment. They were acclimated to laboratory conditions for 1-month prior to the experiments. The specimens were given prophylactic treatment by bathing them twice in 0.05% KMnO4 solution for 2 min to avoid any dermal infections. The NaF (AR grade) obtained from Qualigens Fine Chemicals Limited, Mumbai, India. Ascorbic acid was purchased from HiMedia.
Experimental plan
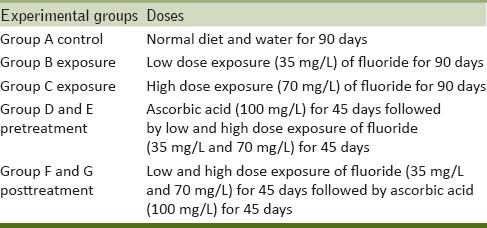
Fishes were divided into five groups with 12 fish in each group. Group A served as the control (without F added to the water) and Group B was exposed to a concentration of fluoride 35 mg F ion/L (one-tenth of 96-h LC50 value). The Group C was exposed to a concentration of fluoride 70 mg F ion/L (one-fifth of 96-h LC50 value). Group D and E pretreatment (100 mg/L ascorbic acid + low and high dose fluoride) and Group F and G posttreatment (low and high dose fluoride + 100 mg/L ascorbic acid). At the end of the experiment, the fish were sacrificed. The liver and ovary of both control and treated fish were dissected. The organs were washed in ice-cold physiological saline solution and stored at − 80°C until analysis [Table 1].
Table 1.
Experimental plan
Preparation of homogenate
The liver and ovary were separately homogenized in 10% (w/v) ice-cold chilled sodium phosphate buffer (0.1 M, pH 7.4) using a Potter-Elvehjem homogenizer. A part of this homogenate was used for biochemical estimations and the other part was centrifuged at 9000 rpm for 30 min at 4°C to using a Sigma Refrigerated Centrifuge (model 3K30) to obtain the supernatant which was further used for SOD, CAT, GPx, GST, and protein estimations.
Biochemical and enzyme estimation
LPO was estimated by the method of Ohkawa et al.[17] GSH was assayed by the method of Ellman.[18] SOD activity was analyzed using the method of Kakkar et al.[19] The activity of CAT was measured according to the method of Sinha.[20] GPx activity was measured using the method of Rotruck et al.[21] GST was estimated by the method of Habig et al.[22] Protein estimation was determined using a colorimetric method and BSA as standard.[23]
Statistical analysis
The results were expressed as mean and standard error (mean ± standard error mean) and determined for all the parameters. The data were analyzed employing the analysis of variance (ANOVA) using Graph Pad in Stat Software Inc., version 3.06, San Diego, USA. The Dunnett's test for multiple comparisons of groups against the control was performed, to determine the significant differences among the groups.
RESULTS
Lipid peroxidation and nonenzymatic antioxidant
The LPO level was significantly increased (P < 0.01) in exposure Group B and C of the liver and ovary as compared to the control Group A. A nonsignificant (P > 0.05) decrease in LPO was observed in pre- and post-treatment in Groups D, E, F, and G [Figure 1].
Figure 1.
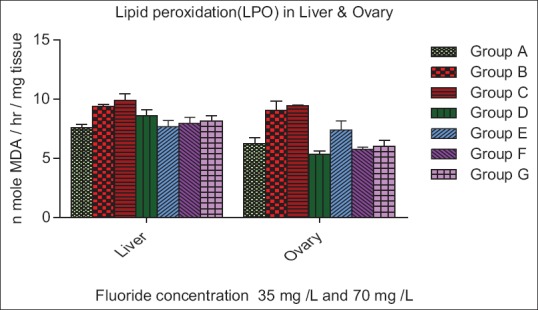
Effect of ascorbic acid and fluoride on the lipid peroxidation level. The value represents the mean ± standard error mean (n = 6). Group A: Control, Group B: Fluoride (35 mg F ion/L), Group C: Fluoride (70 mg F ion/L), Group D and E: Pretreatment, Group F and G: Post treatment
In liver, the GSH level was decreased nonsignificantly (P > 0.05) in Group B and C, while significantly (P > 0.05) increase in Group D, E, and F, whereas increase significantly (P < 0.05) in Group G as compare to control. In ovary, the GSH level was nonsignificantly (P > 0.05) decrease in Group B while increase significantly (P < 0.01) in Group C. In pretreatment group the GSH level was increased nonsignificantly (P > 0.05) in contrast significant (P < 0.01) increased level was recorded in posttreatment group as compared to control group respectively [Figure 2].
Figure 2.
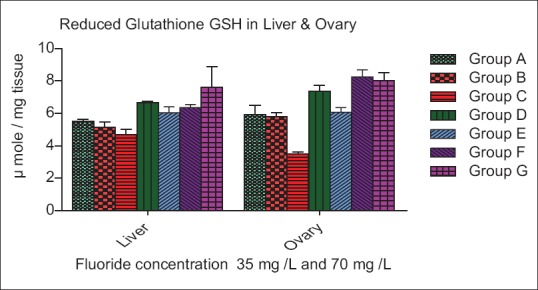
Effect of ascorbic acid and fluoride on the reduced glutathione level. The value represents the mean ± standard error mean (n = 6). Group A: Control, Group B: Fluoride (35 mg F ion/L), Group C: Fluoride (70 mg F ion/L), Group D and E: Pretreatment, Group F and G: Post treatment
Enzymatic antioxidants
The SOD activity increase insignificantly (P > 0.05) in Group B, whereas a significant increase (P < 0.01) in Group C of liver, while a nonsignificant (P > 0.05) increase was seen in Group B and C of ovary as compared to control. In both organ liver and ovary of Group D, E, F, and G decrease nonsignificantly (P > 0.05) as compare to control group [Figure 3]. The CAT activities were increase very significantly (P < 0.01) in Group B and C of liver whereas in Group D, E increase insignificantly (P < 0.01) and Group F, G decreased nonsignificantly (P < 0.05) as compare to control. The CAT activity was increased nonsignificantly (P > 0.05) and (P < 0.01) in Group B and C of ovary, while Group D and E decreased significantly (P < 0.01) and (P < 0.05) whereas in Group F and G decreased nonsignificant (P > 0.05) and (P < 0.05) as compare to control [Figure 4]. The GPx activity in Groups B and C of the liver was decreased significantly (P < 0.01), while a nonsignificant (P > 0.05) increase in Group D of the liver. In Group E, F, and G of liver increase significantly (P < 0.05) as compared to the control. The GPx level in ovary increase significantly (P < 0.01) in Group B and C, whereas decreased nonsignificantly (P > 0.005) in Group D, E, F, and G as compare to control group [Figure 5].
Figure 3.
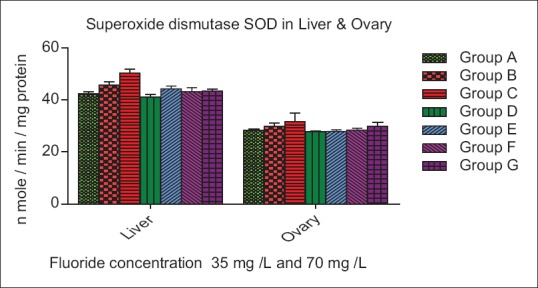
Effect of ascorbic acid and fluoride on the superoxide dismutase level. The value represents the mean ± standard error mean (n = 6). Group A: Control, Group B: Fluoride (35 mg F ion/L), Group C: Fluoride (70 mg F ion/L), Group D and E: Pretreatment, Group F and G: Post treatment
Figure 4.
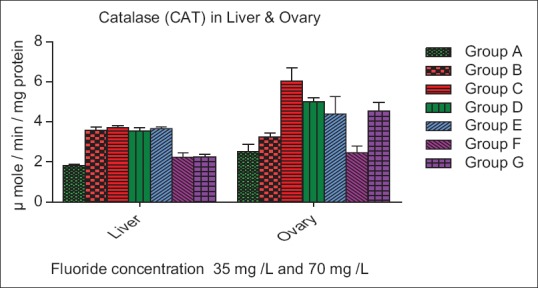
Effect of ascorbic acid and fluoride on the Catalase level. The value represents the mean ± standard error mean (n = 6). Group A: Control, Group B: Fluoride (35 mg F ion/L), Group C: Fluoride (70 mg F ion/L), Group D and E: Pretreatment, Group F and G: Post treatment
Figure 5.
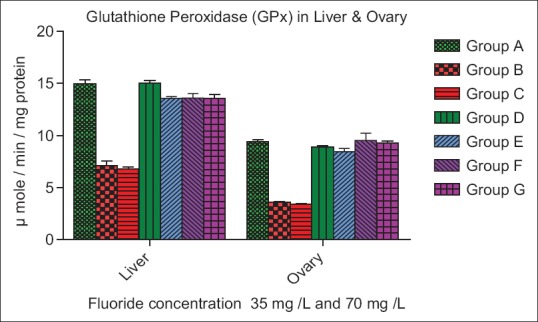
Effect of ascorbic acid and fluoride on the glutathione peroxidase level. The value represents the mean ± standard error mean (n = 6). Group A: Control, Group B: Fluoride (35 mg F ion/L), Group C: Fluoride (70 mg F ion/L), Group D and E: Pretreatment, Group F and G: Post treatment
The GST activity increased significantly (P > 0.05) and (P < 0.01) in Groups B and C of liver while decreased nonsignificantly (P < 0.05) in Group D, E, F, and G as compared to control. The GST level in ovary increased significantly (P < 0.01) in Group B and C, while GST level was decreased significantly (P < 0.05) in Group D, E, F, and G as compared to control [Figure 6]. The protein level decreased nonsignificantly (P > 0.05) in Group B and C of liver and ovary as compared to the control. A nonsignificant (P > 0.05) increase in protein activity was observed in Group D, E, F, and G.
Figure 6.
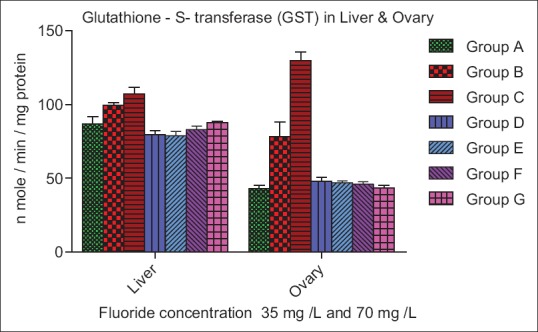
Effect of ascorbic acid and fluoride on the glutathione-S-transferase level. The value represents the mean ± standard error mean (n = 6). Group A: Control, Group B: Fluoride (35 mg F ion/L), Group C: Fluoride (70 mg F ion/L), Group D and E: Pretreatment, Group F and G: Posttreatment
DISCUSSION
A significant increase in the LPO level was observed after fluoride exposure in the liver and ovary of fishes. Increase in MDA was an indicator of enhanced oxidative stress.[24] Fluoride induced increase in LPO and disturbance in the integrity of the cell membranes leading to inhibition of the membrane-bound enzymes has already been reported.[25] Similar result shows dichlorvos causes a significant increase in MDA content in H. fossilis.[26] Increased MDA level observed with similar results of the study carried out by[27] in Cyprinus carpio and Ictalurus nebulosus following the exposures to dichlorvos. Similar result has been reported with Diazinon exposure in the liver of C. carpio.[28]
Increase in ROS and LPO resulted in the depletion of GSH in the liver and ovary of exposed fish, in this study GSH is an endogenous, peptidal, antioxidant, which prevents damage to the cellular components by ROS and peroxides.[29] In addition to working as a direct free-radical scavenger, GSH also functions as a substrate for GPx and GST. The reduction in GSH level may be due to the direct conjugation of GSH with electrophiles generated after due to fluoride exposure. Decrease in the GSH level has already been reported after exposure to other organophosphate methyl parathion in freshwater fish.[30]
SOD is a class of enzymes that catalyze the binding of ROS with water to generate H2O2 and it is the first enzymatic defense against the superoxide anion. CAT is responsible for the breakdown of H2O2 to water and oxygen, protecting the cell from the damaging action of H2O2 and the hydroxyl radical. In the present study, SOD, CAT, and GST level increased significantly in liver and ovary with increased concentration of fluoride and exposure duration. Similar result was found with most investigations.[31,32] Dabas et al.[33] reported a similar result was an assessment of tissue specific effect of cadmium in freshwater fish Channa punctuatus. GPx catalyzes the reaction of hydroperoxides with reduced GSH to form glutathione disulfide and the reduction product of the hydroperoxide. In the present study, different doses of fluoride concentration decreased the GPx activity in liver and ovaries of fish as compared to control group. It may be due to reduced activity of GSH which is used as a substrate for GPx. Similar results were reported by Sharma et al.[34]
Ascorbic acid has been reported to exhibit good antioxidant and free-radical scavenging activities. These antioxidants exhibit LPO lowering effect by radical scavenging activity.[35] The MDA level was brought to a normal level by pre- and post-treatment of ascorbic acid, in the present study pre- and post-treatment with ascorbic acid replenished the decreased GSH level, possibly due to its capability of scavenging the free-radical generated after fluoride exposure. Scavenging of ROS by ascorbic acid may be responsible for the decrease in SOD and CAT activity and replenishment of GSH may have helped in regaining the GPx activity. Pre- and post-treatment with ascorbic acid helps in decreasing the GST level. Tripathi and Shasmal,[36] also reported that Vitamin C can repair the impairment of antioxidant enzymes when induced with chlorpyrifos in H. fossilis. This study shows that ascorbic acid possesses moderate antioxidant activity which is clearly evident by the investigations in the liver and ovary of fluoride exposed fish. Exposure of normal fish with ascorbic acid does not exhibit any type of oxidative stress-related toxicity.
CONCLUSION
The present study revealed that fluoride is responsible for oxidative stress in various tissues of the fish. It has been shown by the increase in LPO and in response the antioxidant defense mechanisms were induced. The effect of chronic exposure of fluoride on LPO, nonenzymatic antioxidant, and enzymatic antioxidant in liver and ovary. SOD, CAT, and GST level increase significantly while GSH and GPx decrease nonsignificantly and significantly, respectively. Pre- and post-treatment was observed after 90 days duration decrease LPO, SOD, CAT, and GST level in both organ while increase GPx and GSH level.
Financial support and sponsorship
Nil
Conflicts of interest
There are no conflicts of interest
Acknowledgment
The authors are thankful to the Head, Department of Zoology University of Lucknow, for providing the necessary facilities and suggestions. We are also thankful to the Director, CSIR-IITR, Lucknow for rendering essential research facilities and support.
REFERENCES
- 1.Susheela AK, Ghosh G. Fluoride: Too much can cripple you. Health for the million. IAHS publications no. 260. 1990 Sep-Oct; ISSN No. 0144-7815. [Google Scholar]
- 2.Tripathi M. Fluorosis a crippling disease. Everyman's sci. 2007;XLI:340–5. [Google Scholar]
- 3.Susheela AK. A Treatise on Fluorosis. 2nd ed. Delhi, India: Fluorosis Research and Rural Development Foundation; 2003. pp. 69–71. [Google Scholar]
- 4.Ercal N, Gurer-Orhan H, Aykin-Burns N. Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem. 2001;1:529–39. doi: 10.2174/1568026013394831. [DOI] [PubMed] [Google Scholar]
- 5.Blaha L, Kopp R, Simkova K, Mares J. Oxidative stress biomarkers are modulated in silver carp (Hypophthalmichthys molitrix Val) exposed to microcystin-producing cyanobacterial water bloom. Acta Vet Brno. 2004;73:477–82. [Google Scholar]
- 6.Sayeed I, Parvez S, Pandey S, Bin-Hafeez B, Haque R, Raisuddin S. Oxidative stress biomarkers of exposure to deltamethrin in freshwater fish, Channa punctatus Bloch. Ecotoxicol Environ Saf. 2003;56:295–301. doi: 10.1016/s0147-6513(03)00009-5. [DOI] [PubMed] [Google Scholar]
- 7.Almroth BC, Sturve J, Berglund A, Förlin L. Oxidative damage in eelpout (Zoarces viviparus), measured as protein carbonyls and TBARS, as biomarkers. Aquat Toxicol. 2005;73:171–80. doi: 10.1016/j.aquatox.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Ozmen I, Bayir A, Cengiz M, Sirkecioglu AN, Atamanalp M. Effects of water reuse system on antioxidant enzymes of rainbow trout (Oncorhynchus mykiss W, 1792) Vet Med Czech. 2004;49:373–8. [Google Scholar]
- 9.Kohen R, Nyska A. Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol. 2002;30:620–50. doi: 10.1080/01926230290166724. [DOI] [PubMed] [Google Scholar]
- 10.Kono Y, Fridovich I. Superoxide radical inhibits catalase. J Biol Chem. 1982;257:5751–4. [PubMed] [Google Scholar]
- 11.Pandey S, Ahmad I, Parvez S, Bin-Hafeez B, Haque R, Raisuddin S. Effect of endosulfan on antioxidants of freshwater fish Channa punctatus Bloch: 1. Protection against lipid peroxidation in liver by copper preexposure. Arch Environ Contam Toxicol. 2001;41:345–52. doi: 10.1007/s002440010258. [DOI] [PubMed] [Google Scholar]
- 12.Schlenk D, Rice CD. Effects of zinc and cadmium treatment on hydrogen peroxide-induced mortality and expression of glutathione and metallothionein in a teleost hepatoma cell line. Aquat Toxicol. 1998;43:121–9. [Google Scholar]
- 13.Storey KB. Oxidative stress: animal adaptations in nature. Braz J Med Biol Res. 1996;29:1715–33. [PubMed] [Google Scholar]
- 14.Halliwell B, Gutteridge HM. Free Radicals in Biology and Medicine. Oxford: Oxford University Press; 1999. pp. 1–936. [Google Scholar]
- 15.Livingstone DR. Oxidative stress in aquatic organisms in relation to pollution and aquaculture. Rev Med Vet. 2003;154:427–30. [Google Scholar]
- 16.Sies H. Strategies of antioxidant defense. Eur J Biochem. 1993;215:213–9. doi: 10.1111/j.1432-1033.1993.tb18025.x. [DOI] [PubMed] [Google Scholar]
- 17.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 18.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 19.Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–2. [PubMed] [Google Scholar]
- 20.Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–94. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 21.Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–90. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 22.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–9. [PubMed] [Google Scholar]
- 23.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 24.Buyukokuroglu ME, Taysi S, Polat F, Gocer F. Mechanism of the beneficial effects of dantrolene sodium on ethanol-induced acute gastric mucosal injury in rats. Pharmacol Res. 2002;45:421–5. doi: 10.1006/phrs.2002.0951. [DOI] [PubMed] [Google Scholar]
- 25.Basha PM, Sujitha NS. Combined influence of intermittent exercise and temperature stress on the modulation of fluoride toxicity. Biol Trace Elem Res. 2012;148:69–75. doi: 10.1007/s12011-012-9338-4. [DOI] [PubMed] [Google Scholar]
- 26.Vadhva P, Hasan M. Organophosphate dichlorvos induced dose-related differential alterations in lipid levels and lipid peroxidation in various regions of the fish brain and spinal cord. J Environ Sci Health B. 1986;21:413–24. doi: 10.1080/03601238609372534. [DOI] [PubMed] [Google Scholar]
- 27.Hai DQ, Varga SI, Matkovics B. Organophosphate effects of antioxidant system of carp (Cyprinus carpio) and catfish (Ictalurus nebulosus) Comp Biochem Physiol. 1997;117:83–8. doi: 10.1016/s0742-8413(96)00234-4. [DOI] [PubMed] [Google Scholar]
- 28.Oruc E. Effects of diazinon on antioxidant defense system and lipid peroxidation in the liver of Cyprinus carpio (L.) Environ Toxicol. 2011;26:571–8. doi: 10.1002/tox.20573. [DOI] [PubMed] [Google Scholar]
- 29.Pompella A, Visvikis A, Paolicchi A, De Tata V, Casini AF. The changing faces of glutathione, a cellular protagonist. Biochem Pharmacol. 2003;66:1499–503. doi: 10.1016/s0006-2952(03)00504-5. [DOI] [PubMed] [Google Scholar]
- 30.Monteiro DA, De Almeida JA, Rantin FT, Kalinin AL. Oxidative stress biomarkers in the freshwater characid fish, Brycon cephalus, exposed to organophosphorus insecticide Folisuper 600 (methyl parathion) Comp Biochem Physiol C Toxicol Pharmacol. 2006;143:141–9. doi: 10.1016/j.cbpc.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Atif F, Parvez S, Pandey S, Ali M, Kaur M, Rehman H, et al. Modulatory effect of cadmium exposure on deltamethrin-induced oxidative stress in Channa punctata Bloch. Arch Environ Contam Toxicol. 2005;49:371–7. doi: 10.1007/s00244-003-9231-4. [DOI] [PubMed] [Google Scholar]
- 32.Bouraoui Z, Banni M, Ghedira J, Clerandeau C, Guerbej H, Narbonne JF, et al. Acute effects of cadmium on liver Phase I and Phase II enzymes and metallothionein accumulation on sea bream Sparus aurata. Fish Physiol Biochem. 2008;34:201–7. doi: 10.1007/s10695-007-9177-y. [DOI] [PubMed] [Google Scholar]
- 33.Dabas A, Nagpure NS, Kumar R, Kushwaha B, Kumar P, Lakra WS. Assessment of tissue-specific effect of cadmium on antioxidant defense system and lipid peroxidation in freshwater murrel, Channa punctatus. Fish Physiol Biochem. 2012;38:469–82. doi: 10.1007/s10695-011-9527-7. [DOI] [PubMed] [Google Scholar]
- 34.Sharma P, Singh R, Jan M. Dose-dependent effect of deltamethrin in testes, liver, and kidney of wistar rats. Toxicol Int. 2014;21:131–9. doi: 10.4103/0971-6580.139789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma P, Shankar S, Agarwal A, Singh R. Variation in serum lipids and liver function markers in lindane exposed female wistar rats: Attenuating effect of curcumin, Vitamin C and Vitamin E. Asian J Exp Biol Sci. 2010;1:440–4. [Google Scholar]
- 36.Tripathi G, Shasmal J. Reparation of chlorpyrifos-induced impairment by thyroxine and Vitamin C in fish. Ecotoxicol Environ Saf. 2010;73:1397–401. doi: 10.1016/j.ecoenv.2010.07.022. [DOI] [PubMed] [Google Scholar]


