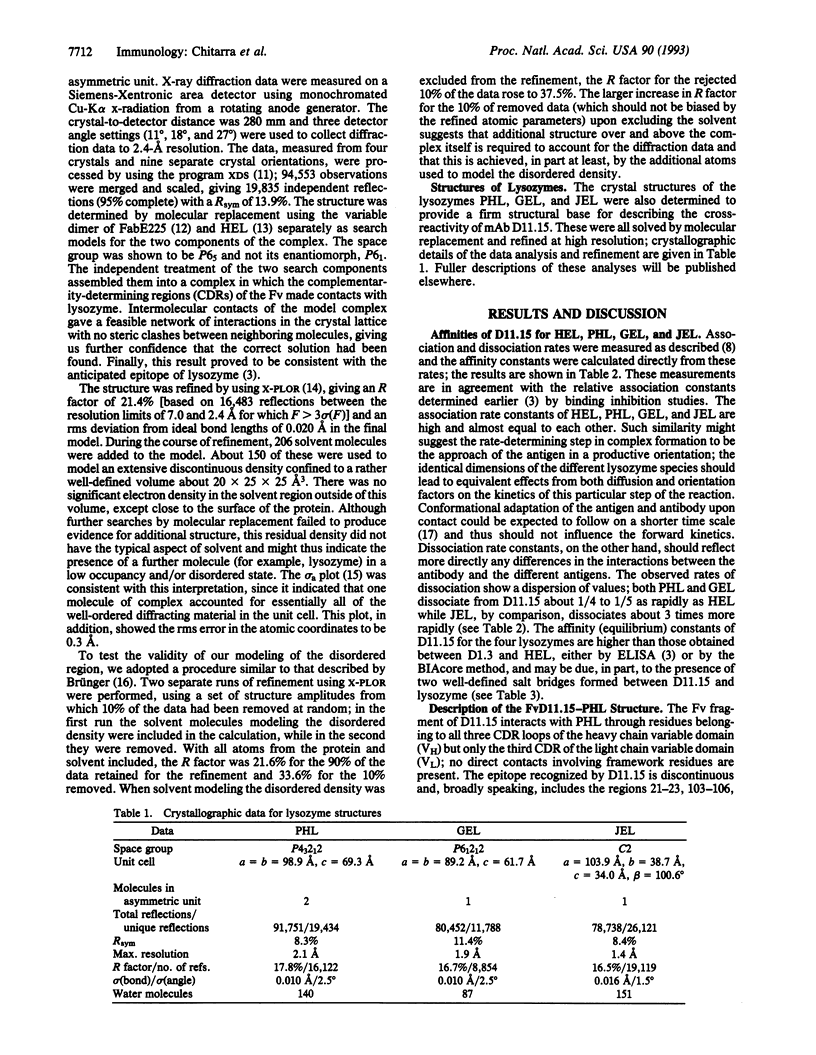
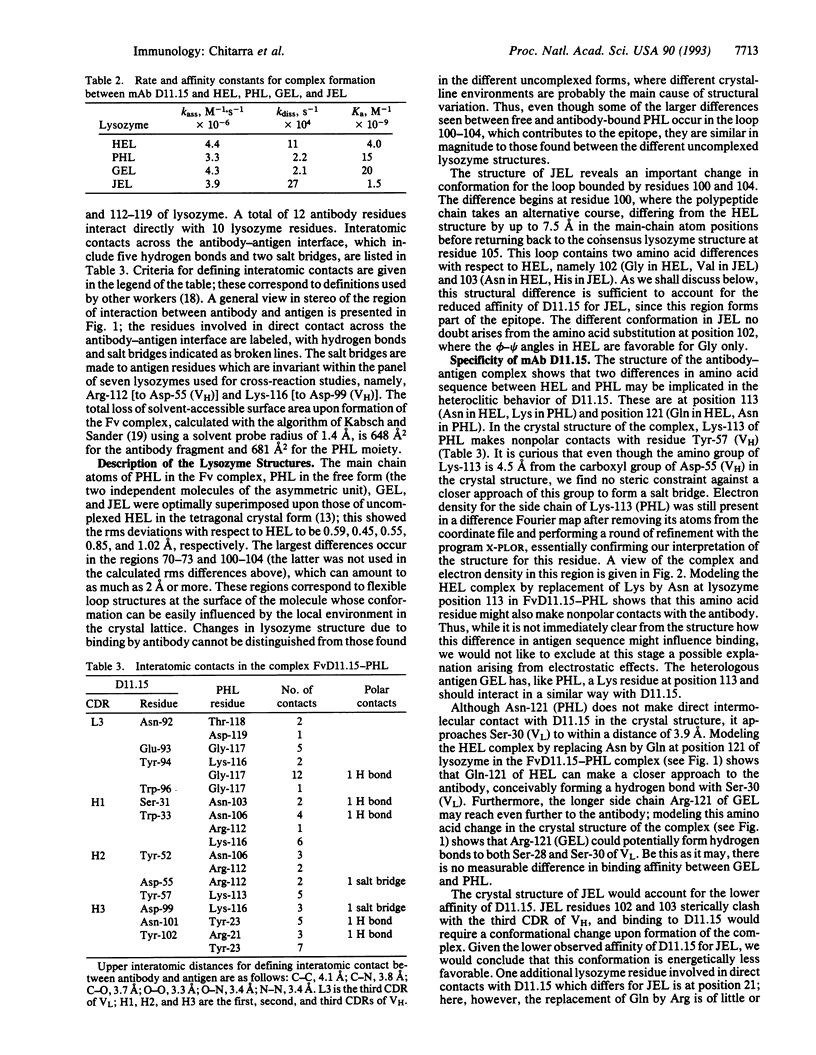
Abstract
Although antibodies are highly specific, cross-reactions are frequently observed. To understand the molecular basis of this phenomenon, we studied the anti-hen egg lysozyme (HEL) monoclonal antibody (mAb) D11.15, which cross-reacts with several avian lysozymes, in some cases with a higher affinity (heteroclitic binding) than for HEL. We have determined the crystal structure of the Fv fragment of D11.15 complexed with pheasant egg lysozyme (PHL). In addition, we have determined the structure of PHL, Guinea fowl egg lysozyme, and Japanese quail egg lysozyme. Differences in the affinity of D11.15 for the lysozymes appear to result from sequence substitutions in these antigens at the interface with the antibody. More generally, cross-reactivity is seen to require a stereochemically permissive environment for the variant antigen residues at the antibody-antigen interface.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amit A. G., Mariuzza R. A., Phillips S. E., Poljak R. J. Three-dimensional structure of an antigen-antibody complex at 2.8 A resolution. Science. 1986 Aug 15;233(4765):747–753. doi: 10.1126/science.2426778. [DOI] [PubMed] [Google Scholar]
- Bentley G. A., Boulot G., Riottot M. M., Poljak R. J. Three-dimensional structure of an idiotope-anti-idiotope complex. Nature. 1990 Nov 15;348(6298):254–257. doi: 10.1038/348254a0. [DOI] [PubMed] [Google Scholar]
- Bhat T. N., Bentley G. A., Fischmann T. O., Boulot G., Poljak R. J. Small rearrangements in structures of Fv and Fab fragments of antibody D1.3 on antigen binding. Nature. 1990 Oct 4;347(6292):483–485. doi: 10.1038/347483a0. [DOI] [PubMed] [Google Scholar]
- Brünger A. T., Kuriyan J., Karplus M. Crystallographic R factor refinement by molecular dynamics. Science. 1987 Jan 23;235(4787):458–460. doi: 10.1126/science.235.4787.458. [DOI] [PubMed] [Google Scholar]
- Fischmann T. O., Bentley G. A., Bhat T. N., Boulot G., Mariuzza R. A., Phillips S. E., Tello D., Poljak R. J. Crystallographic refinement of the three-dimensional structure of the FabD1.3-lysozyme complex at 2.5-A resolution. J Biol Chem. 1991 Jul 15;266(20):12915–12920. [PubMed] [Google Scholar]
- Guillon V., Alzari P. M., Poljak R. J. Preliminary crystallographic study of a complex between an heteroclitic anti-hen egg-white lysozyme antibody and the heterologous antigen pheasant egg-white lysozyme. J Mol Biol. 1987 Sep 20;197(2):375–376. doi: 10.1016/0022-2836(87)90132-x. [DOI] [PubMed] [Google Scholar]
- Gurd F. R., Rothgeb T. M. Motions in proteins. Adv Protein Chem. 1979;33:73–165. doi: 10.1016/s0065-3233(08)60459-3. [DOI] [PubMed] [Google Scholar]
- Harper M., Lema F., Boulot G., Poljak R. J. Antigen specificity and cross-reactivity of monoclonal anti-lysozyme antibodies. Mol Immunol. 1987 Feb;24(2):97–108. doi: 10.1016/0161-5890(87)90081-2. [DOI] [PubMed] [Google Scholar]
- Kabsch W., Sander C. Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers. 1983 Dec;22(12):2577–2637. doi: 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]
- Karlsson R., Michaelsson A., Mattsson L. Kinetic analysis of monoclonal antibody-antigen interactions with a new biosensor based analytical system. J Immunol Methods. 1991 Dec 15;145(1-2):229–240. doi: 10.1016/0022-1759(91)90331-9. [DOI] [PubMed] [Google Scholar]
- Kundrot C. E., Richards F. M. Crystal structure of hen egg-white lysozyme at a hydrostatic pressure of 1000 atmospheres. J Mol Biol. 1987 Jan 5;193(1):157–170. doi: 10.1016/0022-2836(87)90634-6. [DOI] [PubMed] [Google Scholar]
- Mäkelä O. Single lymph node cells producing heteroclitic bacteriophage antibody. J Immunol. 1965 Aug;95(2):378–386. [PubMed] [Google Scholar]
- Richards F. F., Konigsberg W. H., Rosenstein R. W., Varga J. M. On the specificity of antibodies. Science. 1975 Jan 17;187(4172):130–137. doi: 10.1126/science.46122. [DOI] [PubMed] [Google Scholar]
- Tulip W. R., Varghese J. N., Webster R. G., Air G. M., Laver W. G., Colman P. M. Crystal structures of neuraminidase-antibody complexes. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):257–263. doi: 10.1101/sqb.1989.054.01.032. [DOI] [PubMed] [Google Scholar]
- Tulip W. R., Varghese J. N., Webster R. G., Laver W. G., Colman P. M. Crystal structures of two mutant neuraminidase-antibody complexes with amino acid substitutions in the interface. J Mol Biol. 1992 Sep 5;227(1):149–159. doi: 10.1016/0022-2836(92)90688-g. [DOI] [PubMed] [Google Scholar]





