Abstract
A nosocomial outbreak of Serratia marcescens in respiratory samples predominantly from patients in a surgical intensive care unit is reported. Most of these patients were cardiac surgical patients. Initially, a vigorous but inconclusive investigation was implemented on the basis of standardized (according the US Centers for Disease Control and Prevention) steps of outbreak investigation. Then, a systemic quality management approach with “fishbone” analysis was added. As a consequence, plausible causes for the outbreak were identified: (i) S marcescens was found on the transesophageal echocardiography probe used during cardiac surgery; and (ii) the quality of the surface disinfection was insufficient due to multiple reasons and was completely reengineered. In conclusion, in addition to the standardized steps of outbreak investigation, the complementary use of quality management tools such as the Ishikawa “fishbone” analysis is helpful for outbreak control. The complete reengineering of the disinfectant procurement and logistics is assumed to have been the most effective measure to control the described outbreak.
Keywords: Ishikawa's “fishbone” analysis, outbreak control, Serratia marcescens
Serratia marcescens is a gram-negative bacterium belonging to the Enterobacteriaceae family. It is well recognized as a causative agent of severe nosocomial infections, including pneumonia, meningitis, bacteremia, urinary tract infections, endocarditis, conjunctivitis, and surgical site infections. In hospitals, Serratia species survive and grow in moist environments and sites and not uncommonly colonize human bowel, bladder, upper respiratory tract, and skin.
Different environmental sources for S marcescens infection outbreaks have been described, including contaminated medical devices,1 intravenous and topical solutions,2 liquid soap,3 and air-conditioning.4
In this report, the investigation and control process concerning an outbreak of S marcescens lasting 12 months in an acute care hospital with 600 beds are presented and discussed.
METHODS
Setting
The outbreak occurred in the Luzerner Kantonsspital, which is a 600-bed public acute care teaching hospital serving a population of about 0.5 million people in central Switzerland. The surgical department includes all surgical subspecialties, although with no transplant unit. Approximately 35 000 surgical procedures are performed annually. Surgical intensive care patients are cared for in a specialized 12-bed intensive care unit (ICU).
Outbreak investigation
The “ten steps of an outbreak investigation” were followed, as presented by the Centers for Disease Control and Prevention (CDC)5:
Prepare for field work
Establish the existence of an outbreak
Verify the diagnosis
Define an identify cases
Describe and orient the data in terms of time, place, and person
Develop hypotheses
Evaluate hypotheses
Refine hypothesis and carry out of additional studies
Implement control and prevention measures
Communicate findings
Ishikawa fishbone approach
The fishbone diagram6,7 was used as a visual tool to better portray contributing factors as follows: people, process, equipment, material, and management.
Microbiologic methods
During the outbreak, patient screening included throat swabbing or sampling tracheal secretions when patients were intubated. The samples were inoculated on MacConkey agar and incubated at 37°C for 24 to 48 hours. Identification of bacterial strains was made with MALDI/TOF (matrix-assisted laser desorption-ionization/time-of-flight) mass spectrometry.
All isolates with S marcescens were genotyped and compared using pulsed-field gel electrophoresis (PFGE) using XbaI restriction enzyme. The patterns were compared using the GelCompar software. Interpretation was done as described elsewhere.8
Case-controlled comparison
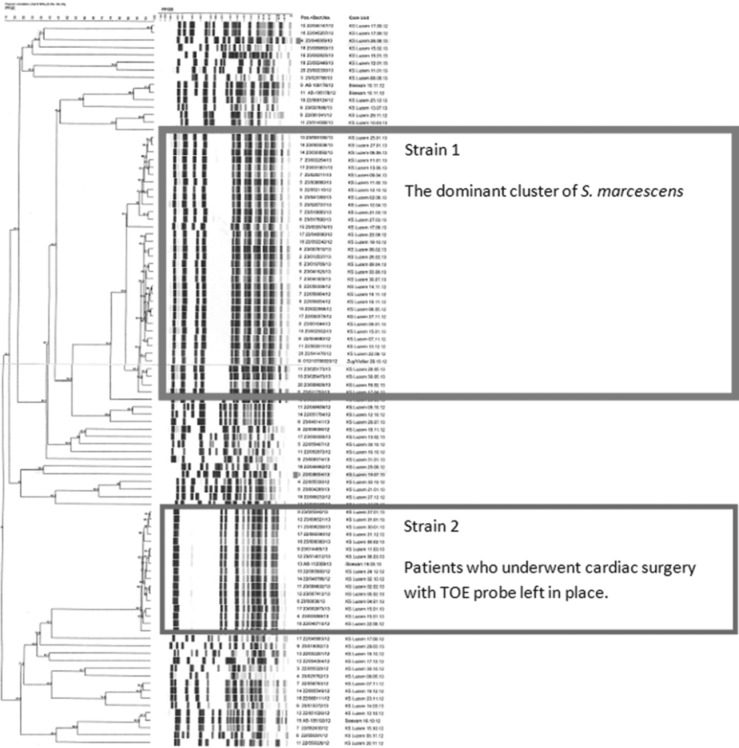
Patients (N = 27) with S marcescens strain 1 in PFGE were matched with 2 controls and them compared with their control cases. Controls were matched for age (±5 years), time of hospital stay (±2 weeks), and ward. Patients with a sample of S marcescens with the same PFGE pattern (see Figure 3) but heterogeneous epidemiologic background were included.
Figure 3.
Pulsed-field gel electrophoresis pattern of patients with Serratia marcescens infection: strain 1 is the dominant cluster, but patients have heterogeneous epidemiologic background. Strain 2 samples are all from patients who underwent cardiac surgery with the transesophageal echocardiography probe left in place.
All statistical analyses were performed using Intercooled Stata Software version 11 for Windows (Stata Corp, College Station, Texas). We used univariate conditional logistic regression analysis for the calculation of risk factors. The low number of cases precluded any multivariable analyses. A 2-tailed test of significance with a P value of less than .05 was considered statistically significant.
RESULTS
Outbreak investigation
In July 2012, 3 cardiac surgical ICU patients had respiratory colonization or infection with S marcescens. Because of the accumulation of diagnosed cases, this cluster was defined as an outbreak by the hospital's infectious disease department, and the standardized CDC steps of outbreak investigation were initiated.5
Prepare for field work
The ICU attending physician informed the infection control team about 3 patients with S marcescens in respiratory samples in the cardiac surgical unit in 1 week.
Establish the existence of an outbreak
Three patients colonized or infected with S marcescens at the same time in the same ward is absolutely unexpected, so an outbreak situation was assumed.
Verify the diagnosis
Diagnosis was verified by culture-proven colonization or infection with S marcescens.
Define and identify cases
The case definition was as follows: S marcescens in a respiratory sample during hospitalization on the ICU. Throughout the next 4 weeks, all cardiac surgical patients were screened for S marcescens by a pharyngeal swab postoperatively. In addition, the infection control team collected clinical and epidemiologic information, studied risk factors, and documented all findings in a database.
Describe and orient the data in terms of time, place, and person
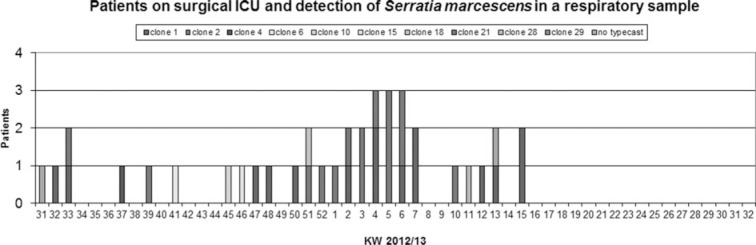
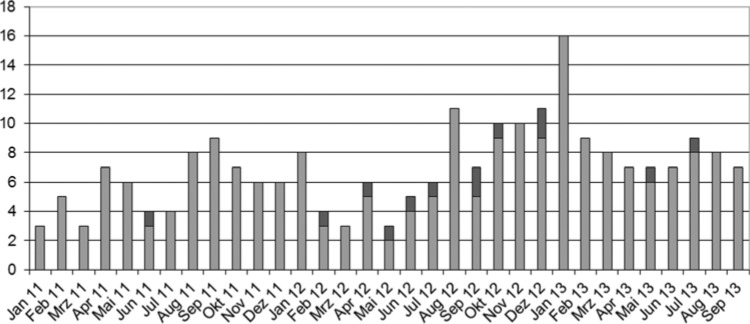
The outbreak was visualized by 2 epidemic curves over time: the first with the case definition “Sample with S marcescens in a respiratory probe” (see Figure 5), and the second with all samples of S marcescens (see Figure 6).
Figure 5.
Epicurve with the case definition “sample with Serratia marcescens” in a respiratory probe.
Figure 6.
Epicurve with all samples of Serratia marcescens.
The sequential screening identified the operating room (OR) as the location of S marcescens acquisition because the first probes while entering the ward and the second by entering the OR were always negative (no proof of S marcescens). Two patients had positive samples when entering the ICU after surgery. Therefore, the identification of a point source in the OR was forced by observing the process of anesthesia induction and monitoring and swabbing the transesophageal echocardiography (TOE) probe before and after use. At that point, 44 patients had S marcescens in a respiratory sample. All S marcescens strains from patients in our hospital were sent to an external reference laboratory for molecular typing by PFGE.
The molecular typing by PFGE showed 2 dominant clusters of S marcescens with a same PFGE pattern. The first cluster with S marcescens was difficult to interpret. Epidemiologically, these cases were too heterogeneous (Figure 3). Notably, the same type of S marcescens strain was also found at least in another tertiary care hospital in Switzerland.
The second cluster of 17 S marcescens isolates with the same pattern included patients who underwent cardiac surgery with the TOE probe left in place during surgery. The same strain was also found on the TOE probe when screened by several moistened swabs. The probe was replaced. The original TOE probe surface was then assessed using a microscope, and the flexible part of this probe showed a brittle and cracked surface on light microscopy (Figures 1 and 2).
Figure 1.
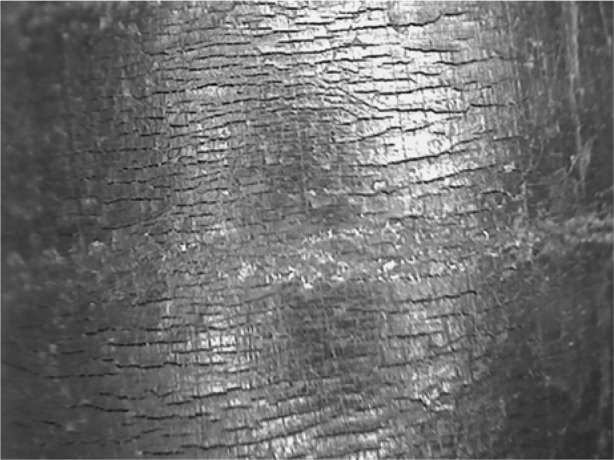
The surface of a used transesophageal echocardiography probe under the microscope during an outbreak: The surface is scratched and broken.
Figure 2.

The surface of a new transesophageal echocardiography probe under the microscope.
Using some of the patients from the first cluster of S marcescens infection, we started a case-control study. No source for the acquisition with S marcescens strain 1 in the PFGE (Figure 3) could be detected with the case-control study. The study showed 2 risk factors for the acquisition of S marcescens: reoperation 4/17 cases (19%) versus 0/34 (0%) (P < .001) and hospitalization in the ICU before the detection of S marcescens (17% controls versus 56% cases (P = .002). Hospitalization in the recovery room before detection of S marcescens was shown to be protective: 65% (controls) versus 26% (cases) (P = .002).
Develop hypotheses
The interpretation of the collected data was suggestive of a point source of outbreak. Therefore, further steps were initiated and samples taken for analysis: tap water in the ICU was sampled for S marcescens and Pseudomonas aeruginosa, the environment (soaps, disinfection wipes, creams, ultrasonic gel, and TOE probe) was also sampled using swabs moistened with sterile saline water, and cleaning and disinfection in the ICU were intensified in frequency.
The infection control team audited the care process in the ICU. Patient care was observed closely in the search for hygiene lapses. Moreover, the importance of the hand hygiene was emphasized and hand hygiene compliance was monitored at the bedside.
Evaluate hypotheses
None of the analyses of samples from the sources mentioned earlier revealed S marcescens. In tap water, different bacteria, including Stenotrophomonas maltophilia, Pseudomonas aeruginosa, and Bacillus sp, were found, but these were determined to be incidental and not the source of the outbreak. Despite all measures, the number of patients with S marcescens in a respiratory probe was still increasing, and a point source was still assumed, although not clearly identified. To control the ongoing outbreak, CDC's step 5 was reconsidered, but data collection was modified:
People who underwent cardiac surgery were sequentially screened by a pharyngeal swab at 4 times: entering the hospital, entering the OR, while leaving the OR and entering the ICU, and leaving the ICU.
The method for sampling disinfection wipes was changed according to a recent publication.9 This study showed a lower yield of sampling by swabs than by culturing the disinfection solution.
The screening of probes from the TOE probe was also changed: instead of only 1, several moistened swabs at different locations of the TOE probe were used.
Inhalation therapy was assumed to be a risk factor for the acquisition of S marcescens, but no source was identified and that specific hypothesis was abandoned.
Infection control measures
The processes of cleaning and disinfection of TOE probes were reassessed and optimized. It is very difficult to clean and disinfect such an instrument because there is no automated purpose-built processor as there is, for example, for endoscopes. As a first step in a setup procedure after use, mechanical cleaning is mandatory to remove any biofilm that could interfere with probe disinfection. After this step, the whole probe in a length of (about) 1.5 m must be rinsed with sterile water to remove the disinfectant. Also, the storage of the probes was improved: the probe now hangs in a box avoiding humidity and avoiding any unnecessary contacts. Finally, the use of a single-use sheet (condom) was strongly recommended to clinicians.
During our investigation, an unexpected reason for the first cluster of S marcescens identified in this outbreak was found. The results from the samples of the disinfection wipes were evaluated, and the solution in the disinfection boxes was tested. Several wipe boxes refilled with aldehyde disinfectant were contaminated by Pseudomonas aeruginosa, Pseudomonas putida, and Bacillus cereus. In the alcohol-based disinfection wipe boxes, there was no growth of germs. As a consequence, the use of wipe dispensers with aldehyde disinfectant was no longer allowed and alcohol-based wipe dispensers were used instead.
Refine hypotheses and carry out additional studies
The contaminated disinfection solution forced a check of the quality of surface disinfection in the entire hospital. In the OR and ICUs, proportioning dispensers were used for preparing the disinfectant. But the solution in the bottles had different color gradations, suggesting different disinfectant concentrations. The concentration of aldehyde in several samples was measured. The range of measured concentrations did not reach the target value of 0.5% and had a wide variation. Some of the dispensers for preparing this disinfectant were not authorized for hospital use.10 Some of the proportioning dispensers had trouble with the disinfectant containers that the Luzerner Kantonsspital used: the seals became brittle very often and had to be replaced, although a compatible disinfection concentrate was used.
Usage of all of these dispensers was suspended, and the teams were instructed how to prepare the disinfectant solutions manually and to install single-use bottles for use on-site.
In addition, the infection control team scrutinized the disinfection process outside the OR and ICUs in our hospital. A canister of 8-L content was used to prepare the use solution of disinfectant from the concentrate. From this bottle, the solution was poured into smaller bottles that were used in patient rooms or for direct use from this canister. This canister was never reprocessed because there was no convenient possibility of reprocessing. The smaller bottles used in the patient rooms were also not reprocessed routinely.9
In addition, canisters for preparing the disinfectant solution were emptied and dried after filling the smaller bottles for the patient rooms. These bottles were replaced by disposable single-use bottles.
A spot-check of concentration from this disinfection solution showed varying and insufficient concentrations. Diluting the disinfectant on the ward was no longer allowed and replaced by central production with a proportioning automat. A trained person produces the disinfectant in single-use bottles for the whole hospital except the facility management. Finally, the ready-to-use disinfectant solution was replaced by commercially diluted disinfectant packed in ready-to-use bottles.
The new information about this low-quality disinfectant helped view the outbreak of S marcescens in a new light. The infection control team is convinced that contaminated and low concentrated disinfectant solutions contributed to the observed outbreak. A higher colonization pressure together with other not identified problems may then have promoted the outbreak. Accordingly, the so-called “fishbone diagram” by Ishikawa was used to evaluate and improve our process.6 Usually, not one but several factors (eg, human, environmental, management, equipment) contribute to a problem (Figure 4). A recommended approach is to check and improve all the “fishbone” items at the same time without knowing the exact contribution of each factor to the problem under investigation.
Figure 4.
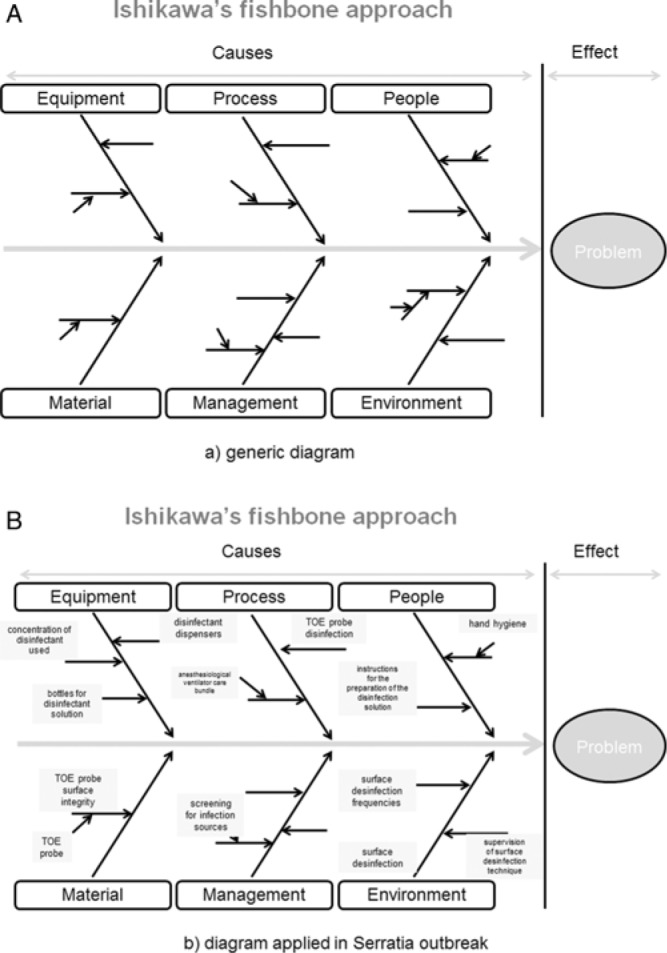
(A) Ishikawa fishbone approach model. (B) Ishikawa fishbone approach diagram applied in Serratia outbreak. TOE indicates transesophageal echocardiography.
After the implementation of the central disinfectant preparing station, the outbreak of S marcescens in a respiratory system of cardiac surgical ICU patients came under control. All the new cases found by the successive screening measures were colonized by strain 1, whereas strain 2 (associated with the TOE probe) was no longer detectable (Figures 5 and 6).
Implement control and prevention measures
There were several different prevention measures that we needed to implement because this outbreak had several causes as exemplified by the fishbone model. Ultimately, the disinfection process was optimized by using a commercialized diluted disinfectant. In addition, the preparation of TOE probes was optimized and periodic probe sampling was then conducted by the infection control team. Also, the whole-hospital hand hygiene compliance was measured and retrained on a periodic base. To confirm the stoppage of this outbreak, the monitoring process as described earlier to find S marcescens was sustained.
DISCUSSION
A hospital-wide outbreak with S marcescens involving 91 patients over 12 months is described. The molecular typing showed 2 clusters contributing to this outbreak. One included epidemiologically heterogeneous patients, and the second one was triggered by the point source of a contaminated TOE probe.
The initial approach of stepwise outbreak investigation according to the CDC guidelines (Table) was not successful in our hands for identifying the outbreak risks and eliminating them. Therefore, a systemic approach known in quality management using “fishbone” analysis as described by Ishikawa6 (Figure 4) was instituted. This universal tool for improving quality helps identify causes and effects of a problem in a structured manner. This framework enables amendments of several contributing factors simultaneously. The fishbone diagram is a visual tool that can facilitate grasp of multiple factors that contribute to an outbreak. The preparation of the fishbone diagram can be a format for team brainstorming and problem solving. It promotes systematic thinking through visual linkages, and it helps structure discussion and maintains focus on the current issues or problems.
Table. The 10 Steps for an Outbreak Investigation as Presented by the Centers for Disease Control and Preventiona.
| 1 | Prepare for field work |
| 2 | Establish the existence of an outbreak |
| 3 | Verify the diagnosis |
| 4 | Define an identify cases |
| 5 | Describe and orient the data in terms of time, place, and person |
| 6 | Develop hypotheses |
| 7 | Evaluate hypotheses |
| 8 | Refine hypothesis and carry out of additional studies |
| 9 | Implement control and prevention measures |
| 10 | Communicate findings |
aAdapted with permission from the Centers for Disease Control and Prevention.5
Using the fishbone diagram properly, the problem under investigation is defined and the search for causes is structured. The causes are grouped into major categories to identify the sources of variations. The categories are always the same and are as follows:
People: Anyone involved with the process
Process: Any critical point in the process
Equipment: Any equipment used in the process
Material: Any material used
Management: Data generated from the process used to evaluate its quality
All possible variables can be inserted into this diagram. The approach does not only help identify causes but also guide corrective measures as shown in our outbreak. By visualizing all the possible causal factors, measures to sample and measures to control were instituted in a coordinated fashion. In a first step, moistened swabs for searching S marcescens and Pseudomonas aeruginosa in disinfectant wipe boxes were taken. As an improvement, in a second step, the screening strategy was changed and the bacteria in the disinfectant solution were searched by membrane filtration of the fluid pressed from the wipes. Using this method, it was provable that the disinfection solution was insufficient. As a consequence, the “homemade disinfectant solutions” were replaced by a commercialized diluted ready-to-use product.
Sampling the TOE probes was also repeated. The technique remained unchanged, but more samples were taken and enabled the identification of S marcescens. This forced a complete reengineering of the cleaning process for the TOE probes. Finally, hand hygiene was also retrained and improved.
Under the pressure of all the instituted measures, the outbreak ceased. The complete reengineering of the disinfectant procurement and logistics, by implementing and delivering a ready-to-use commercially produced conveniently bottled disinfectant solution, was assumed to be the most effective measure to control the described outbreak. No further evidence of S marcescens was found by sustained monitoring process in cardiac surgical ICU patients.
As in most of the other reported outbreaks (www.outbreak-database.com), the reservoir and mode of transmission also remained unknown for the outbreak part with strain 1 in the PFGE pattern. Only for the cluster with strain 2, the TOE probe was identified as a point source.
Classical outbreak investigation recommendations focus on identifying the source of an outbreak. In contrast, the Ishikawa fishbone analysis considers different factors with influence on quality. This may help find the origin of a cluster of infections. But even in the absence of an identified source, quality can be improved. Changing simultaneously different factors precludes estimating the impact of a single measure.
CONCLUSIONS
In a complex hospital environment outbreak, investigation using a systemic quality management focus, based on the Ishikawa analysis in addition to the established stepwise CDC procedures, may be helpful and guide the search for causes. This approach also helps implement control measures in a structured way and supports a team approach.
Footnotes
The authors of this article have no conflict of interest to disclose.
REFERENCES
- 1.Sokalski SJ, Jewell MA, Asmus-Shillington AC, Mulcahy J, Segreti J. An outbreak of Serratia marcescens in 14 adult cardiac surgical patients associated with 12-lead electrocardiogram bulbs. Arch Intern Med. 1992;152(4):841–844. [PubMed] [Google Scholar]
- 2.Gupta N, Hocevar SN, Moulton-Meissner H, et al. Outbreak of Serratia marcescens bloodstream infections in patients receiving parenteral nutrition prepared by a compounding pharmacy. Clin Infect Dis. 2014;59(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rabier V, Bataillon S, Jolivet-Gougeon A, Chapplain JM, Beuchée A, Bétrémieux P. Hand washing soap as a source of neonatal Serratia marcescens outbreak. Acta Pedaitr. 2008;97(10):1381–1385. 10.1111/j.1651-2227.2008.00953.x. [DOI] [PubMed] [Google Scholar]
- 4.Uduman SA, Farrukh AS, Nath KN, et al. An outbreak of Serratia marcescens infection in a special-care baby unit of a community hospital in a United Arab Emirates: the importance of the air conditioner duct as a nosocomial reservoir. J Hosp Infect. 2002;52(3):175–180. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC). EXCITE: Epidemiology in the classroom outbreak steps. http://www.cdc.gov/excite/classroom/outbreak/steps.htm. Accessed September 14, 2014.
- 6.Fishbone diagram. http://www.siliconfareast.com/ishikawa.htm. Accessed September 14, 2014.
- 7.Drehobl P, Stover BH, Koo D. On the road to a stronger public health workforce: visual tools to address complex challenges [published online ahead of print October 16, 2014]. Am J Prev Med. 2014;47(5)(suppl 3):S280–S285. 10.1016/j.amepre.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 8.Tenover FC, Arbeit RD, Goering RV, et al. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33(9):2233–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.VAH Kontrollmassnahmen bei der Anwendung von Tuchspendersystemen für die Flächendesinfektion im Abhängigkeit vom Risikoprofil http://www.ihph.de/vah-online/.../2013_HM3_vah_Tuchspendesysteme_2.pdf. Accessed September 14, 2014.
- 10.RKI Bundesgesundheitsblatt-Gesundheitsforsch-Gesundheitss-chutz Anforderung an Gestaltung. Eigenschaften und Betrieb von dezentralen Desinfektionsmittel-Dosiergeräten. 2004;47:67–72. [DOI] [PubMed] [Google Scholar]