Abstract
Purpose of Review
Hematopoietic stem (HSCs), and progenitor (HPCs) cells reside in a hypoxic (lowered oxygen (O2) tension) environment, in vivo. We review literature on growth of HSCs and HPCs under hypoxic and normoxic (ambient air) conditions with a focus on our recent work demonstrating the detrimental effects of collecting and processing cells in ambient air through a phenomenon termed Extra Physiologic Oxygen Shock/Stress (EPHOSS), and we describe means to counteract EPHOSS for enhanced collection of HSCs.
Recent Findings
Collection and processing of bone marrow (BM) and cord blood (CB) cells in ambient air causes rapid differentiation, and loss of HSCs, with increases in HPCs. This apparently irreversible EPHOSS phenomenon results from increased mitochondrial ROS, mediated by a p53-Cyclophilin D – mitochondrial permeability transition pore (MPTP) axis, and involves hif-1α and miR210. EPHOSS can be mitigated by collecting and processing cells in lowered (3%) O2, or in ambient air in the presence of, cyclosporine A (CSA) which effects the MPTP, resulting in increased HSC collections.
Summary
Our recent findings may be advantageous for HSC collection for hematopoietic cell transplantation, and likely for enhanced collection of other stem cells types. EPHOSS should be considered when ex-vivo cell analysis is utilized for personalized medicine, since metabolism of cells and their response to targeted drug treatment ex-vivo may not mimic what occurs in vivo.
Keywords: Hematopoietic stem and progenitor cells, microenvironment, O2 tension, EPHOSS and its mitigation
Introduction
Complex multicellular organisms began to evolve as the atmospheric oxygen (O2) started increasing to reach its present levels of approximately 21% [1,2]. However, and perhaps ironically, the production of blood cells in vivo in neonates and adults occurs in a microenvironment that is hypoxic [3*,4*,5, 6, 7**,8]. Blood cell production is dependent on critical cell-cell and cytokine-cell interactions between hematopoietic stem (HSCs) and progenitor (HPCs) cells, and their precursors and more mature cell offspring [9–11] and occurs mainly in the bone marrow (BM) microenvironment where HSCs and HPCs are near or in contact with stromal cells, osteoblasts and endothelial cells in a low O2 environment that ranges from 1–4% with perhaps some slightly lower or higher O2 levels [5,6,7**,8]. While HSCs and HPCs can be grown ex-vivo in atmospheric O2, these rare life-saving cells proliferate better in vitro in hypoxia (usually ≤ 5% O2), compared to normoxia (defined as atmospheric O2), [12–17]. Colony assays of BM HPCs from mice or humans, or cord blood (CB) cells from humans demonstrate increased numbers and cell cycling of granulocyte macrophage (CFU-GM), granulocyte (CFU-G), macrophage (CFU-M), erythroid (BFU-E), megakaryocytic (CFU/BFU-Meg), and multipotential (CFU-GEMM; CFU-Mix) HPCs when in vitro culture conditions are hypoxic. Expansion of HPCs and HSCs ex-vivo are superior under hypoxic culture conditions [15,17].
Studies have evaluated the distribution of HSCs and HPCs in relationship to BM microenvironmental cells in context of regional O2 levels. HSCs and cells within BM that support HSCs are mainly present in a niche predominately located at a lower region of the O2 gradient, suggesting that regional hypoxia plays an important role in regulating HSC function [5]. More recent studies have refined concepts of HSC localization. One study, defined HSC phenotype within endosteal BM regions as being superior for homing and proliferative capacity, compared to these same phenotyped cells isolated from the central BM [18]. Another group performed in vivo measurements of local O2 tension in live mice [7**] using two-photon phosphorescence lifetime microscopy to determine that absolute local O2 tension of the BM was low (<32mm Hg) even though there was a very high vascular density. Although the BM as a whole was hypoxic, they found heterogeneity in local O2 levels with the lowest (about 9.9mm Hg, or 1.3% O2) present in deeper peri-sinusoidal regions. Under conditions of post-chemotherapy stress, HSCs and HPCs did not seek out specific niches defined by low O2 for their preferential homing. Another group used 5-color imaging cytometric analysis to quantitate the distribution of HSCs and HPCs in femoral BM cavities [6]. HSCs and HPCs localized preferentially in endosteal zones, where they interacted closely with sinusoidal and non-sinusoidal BM microvessels. HSCs/HPCs exhibited a hypoxic metabolic profile defined by strong retention of pimonidazole and expression of hypoxia inducing factor (hif)-1α, regardless of: location in the BM, position next to vascular structures, or cell cycle state. Thus, the hypoxic phenotype of HSCs and HPCs in BM was cell, rather than location, specific. Endosteal BM areas did not contain the most hypoxic HSCs/HPCs, and hif-1α stabilization in these cells occurred independent of differences in O2 levels at different anatomical sites.
Extra Physiologic Oxygen Shock/Stress (EPHOSS)
While the biology of HSCs/HPCs and other stem cells (embryonic, mesenchymal, and neural) are now considered in context of anatomical site positioning in vivo, and in context of O2 tension for growth differences ex-vivo [8,19], no attempts have been made to assess initial effects of even brief exposure of HSCs and HPCs to ambient atmospheric O2 regardless of whether or not the cells collected in ambient air are subsequently processed, cultured or injected into animals under normoxia or hypoxia. Our most recent studies [20**] now demonstrate that even very brief exposure to ambient air has a rapid and apparently irreversible effect that changes the metabolism of HSCs and HPCs. Through a phenomenon that we termed EPHOSS, this results in rapid loss of HSC numbers with concomitant increases in HPCs, due to rapid differentiation of HSCs. Mechanisms of EPHOSS encompass ambient air-induced production of mitochondrial reactive oxygen species (ROS), and induction of the mitochondrial permeability transition pore (MPTP) opening. This occurs with BM and also human CB cells, which is consistent with reports that human CB cells are also in a hypoxic environment [21]. EPHOSS is mediated by interactions with the MPTP and cyclophilin D (CypD) and p53, with links to expression of hif-1α, and the hypoxamir, miR210. This information is important for hematopoietic cell transplantation (HCT), especially for CB HCT where numbers of cells from single collections are low. While efforts have focused on enhancing the current clinical efficacy of these cells for HCT via ex-vivo expansion of these cells, or by increasing their homing capabilities [22,23], to compensate low collection numbers, being able to collect more HSCs in a CB collection could greatly enhance the efficacy of CB for HCT. In fact, EPHOSS, and means to prevent its action, will likely extend to many other stem cell types, including embryonic stem cells (ESCs), mesenchymal stem cells (MSCs), adipose stem cells (HSCs), and other tissue specific stem cells that normally reside in a hypoxic environment in vivo.
ROS can be toxic, but also has differentiation inducing activity [24*]. We reasoned that collecting mouse BM cells and doing all processing and eventual culture of the cells or their injection into mice under low O2 tension might mitigate production of mitochondrial ROS, and subsequent ROS-induced differentiation of the mouse BM HSCs [20**]. For this to be successful, it was necessary for all procedures to be done in a hypoxic chamber in which everything used (media, plastic ware, glassware, syringes, etc.) was pre-equilibrated to 3% O2 in the hypoxic chamber for 18 hrs prior to collection, processing, and eventual culture or injection into mice under 3% O2. This resulted in ~3–5 fold enhanced collection of mouse BM long-term repopulating (LT)-HSCs, rigorously determined by phenotype and functional engraftment of competitive repopulating units (CRU) as defined by donor cell chimerism and limiting dilution analysis, with concomitant decreases in HPCs, defined by phenotype for short-term repopulating (ST)-HSCs and multi-potential progenitor (MPPs) cells, and by function using colony assays for CFU-GM, BFU-E, and CFU-GEMM.
Collection of cells in 3% O2 and then placing them in ambient air for as short as 20–30 minutes (the shortest time in which we were able to process the cells) resulted in greatly reduced numbers of HSCs and increased numbers of HPCs. Additionally, collection and processing of cells in air, or collecting of cells at 3% O2 and placement in ambient air greatly increased production of mitochondrial ROS, mitochondrial mass/activity, and high mitochondrial membrane potential. EPHOSS did not link to apoptosis, nor did it influence the homing efficiency of the collected cells. Collection and processing of human CB CD34+ cells in 3% O2 also resulted in about a 3 fold increase in rigorously defined human HSCs [25], demonstrating that effects of EPHOSS were not limited to BM [20**].
Mechanisms of EPHOSS
We evaluated mechanisms of EPHOSS for obtaining insight into its biology, and also for potential alternative means of collecting HSCs in order to mimic the enhancing effects of HSC collection at low O2 tension [20**]. Collection of BM or CB cells at low O2 would present a logistical problem that even if solved would make collection of cells cumbersome and expensive. We focused on the MPTP as a potential key to EPHOSS [20**]. Although oxidative stress favors induction of the MPTP opening which can result in the swelling of mitochondria, and uncoupling of OXPHOS, that leads to apoptosis and necrosis [26,27], this MPTP opening can be transient and function in a regulatory capacity conducive to modulating differentiation of stem cells. A key regulatory component of the MPTP is CypD, which regulates induction of the MPTP [28,29]. Interestingly, cyclosporine A (CSA), a small molecule inhibitor of CypD which binds CypD and antagonizes induction of the MPTP [30,31], is FDA approved, and is used as an immunosuppressant to treat graft vs. host disease for HCT, as well as a treatment possibility for heart attack and stroke [32,33]. We reasoned that CSA might be useful to protect against effects of MPTP induction, and if this was successful it might be rapidly considered for collection of HSCs in ambient air by mimicking effects of low O2 tension. We found that collection and processing of mouse BM or human CB in ambient air but in the immediate and continued presence of CSA resulted respectively in greatly enhanced numbers of phenotypically identified HSCs and functional CRUs for mouse BM, and SCID Repopulating Cells (SRCs) for human CB [20**]. To maintain HSC numbers in CB through the cryopreservation and thaw procedures necessary for CB banking, it is likely that CSA may have to be present throughout the freeze/thaw procedures.
To implicate the MPTP in EPHOSS further, we assessed whether or not CypD deletion (−/−), which is known to prevent induction of the MPTP [34–36], might protect against effects of EPHOSS for enhanced collection of HSCs from mouse BM. CypD −/− mouse BM cells collected and processed in air were greatly increased in phenotypically-defined and functional HSCs, with decreased numbers of HPC compared to CypD +/+ mouse BM. CypD −/− BM LT-HSC were also significantly reduced in production of mitochondrial ROS. Evaluating mouse CypD −/− spleen cells by the Seahorse XF96 flux analyzer, demonstrated that basal respiration and maximal respiratory capacity was higher in CypD −/− cells than in wild type control cells [20**].
We were also able to link p53 −/− BM cells to a p53-CypD-MPTP axis in mechanisms of EPHOSS [20**]. Using hif-1α and miR210 −/− mouse BM cells we also linked hif-1α and miR210 to EPHOSS [20**], although exact mechanisms have not yet been worked out. CypD −/− and p53 −/− had EPHOSS-protective effects, while hif-1α −/− and miR210 −/− abrogated the protective effect seen under hypoxic harvesting and processing of the cells. Our studies highlight how interpretation of experimental results of mouse gene deletion models can be influenced once EPHOSS is considered.
Broad Implications for Role of EPHOSS in Interpretation of Studies of HSCs/HPCs and Other Stem/Progenitor Cell Types
Our studies on EPHOSS [20**] clearly link this phenomenon to HSCs, HPCs, and regulation of hematopoiesis. However, we believe that this phenomenon has much broader implications, not only for understanding the potential true in vivo numbers, characterization, and function of HSCs and HPCs, but also in the context of development and pathologic cell types. Many types of adult stem cells exist naturally in niches in vivo that are hypoxic [37], and ESCs, which are found in the inner mass of blastocytes, and cancer stem cells (CSCs), reside in hypoxic environments [38–40]. ROS is important in the growth, differentiation and the regulation of these cells [24*,41–43]. Ex-vivo growth in lowered O2 tension favors the growth of ESCs, induced pluripotent stem cells (iPSCs), CSCs, as well as MSCs, ASCs, and other cells [24*,44–47]. While much has been written about the metabolism of HSCs, HPCs, ESCs, CSCs, and MSCs amongst a plethora of other stem/progenitor cell types [48*,49–53], critical consideration should now be given as to how accurate these measurements and analyses are with regards to the metabolism of these cells and their function in vivo in hypoxic environments. Thus, many studies on metabolism of stem/progenitor cells may have to be re-evaluated in context of EPHOSS. This is especially of relevance for future efforts of personalized medicine, since such treatments would be based on gene expression patterns and response of a person's tissue to ex-vivo treatment. However, metabolic profiling for the development of specific therapeutic strategies meant to target, for example, CSCs [54–56] may not accurately represent the metabolism of these cells as they exist in their microenvironment in vivo, since these cells are harvested and studied in atmospheric oxygen, and have already been subjected to consequences of EPHOSS.
Another area to consider for effects of EPHOSS would be aging and senescence and its effects on the metabolism, and response of stem cells from aged animals or humans to cytokines/growth modulating factors. Aging has detrimental effects on HSCs and many other tissue-specific stem cells [57–65]. Since ROS has been linked as a driver in the aging process, it is possible that stem cells from aged animals and humans may be especially susceptible to EPHOSS-linked production of mitochondrial ROS after collection and processing of these cells in ambient air. The therapeutic potential of even aged HSCs may be enhanced if EPHOSS is mitigated during their collection/harvest.
Many factors influence the regulation of stem/progenitor cells in vivo. For example, the enzyme, Dipeptidylpeptidase (DPP)4 which can truncate and change the functional activity of a large number of cytokines/growth factors, and other growth modulating proteins [66–69]. DPP4 is found within cells and in the serum, and is also present on cell surfaces of HSCs, HPCs, mature hematopoietic, and other cells as CD26. How DPP4 works in vivo and in context of EPHOSS remains to be determined.
Conclusion
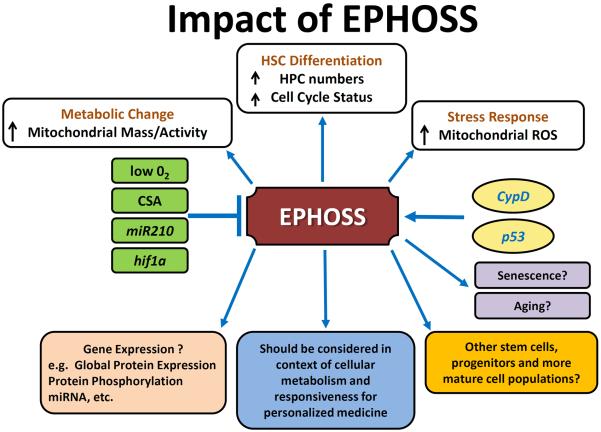
EPHOSS is a new, interesting, and likely important phenomenon which will have relevance to understanding the true physiology and pathology of stem and progenitor cells and how they will best be assessed for future therapeutic modalities [20**]. Information on HSCs, HPCs, and other stem and progenitor cells and their interactions with microenvironmental niche cells, which have reached extremely high levels of sophistication [70–73], may now also have to be considered for re-evaluation in the context of present and future knowledge of the effects of EPHOSS on cellular processes. EPHOSS may also influence metabolism and differentiation of lymphocytes, monocytes and granulocytes, and other mature tissue cells. Figure 1 diagrams the potential impact of EPHOSS, and implications of EPHOSS for different stem, progenitor, and more mature cell types, whether normal or from patients or animals with malignant and non-malignant disorders, and should be considered as an important and worthy pursuit. More mechanistic insight into EPHOSS is warranted.
Figure 1.
A Diagrammatic representation of the impact of EPHOSS on cells and cellular processes. Abbreviations: EPHOSS, extra physiologic oxygen shock/stress; ROS, reactive oxygen species; miR, micro RNA; hif1α, hypoxia inducing factor 1α; O2, oxygen; CSA, cyclosporine A; cypD; cyclophilin D; HSC, hematopoietic stem cell; HPC, hematopoietic progenitor cell.
Key points.
-
-
HSCs and HPCs reside in vivo in a microenvironment that is quite hypoxic compared to that of ambient atmospheric O2.
-
-
Collection and processing of mouse BM and human CB in ambient air underestimates numbers of HSCs in these tissues due to EPHOSS that is triggered by higher O2 than that found in vivo. This results in increased production of mitochondrial ROS, and differentiation of HSCs.
-
-
EPHOSS appears to be irreversible and mediated by a p53-cyclophilin D- MPTP axis, and is associated with expression of hif-1α, and miR210.
-
-
Mitigation of EPHOSS by collection and processing of BM and CB in hypoxia, or alternatively in air in the continuous presence of CSA, results in higher numbers of HSCs.
Acknowledgements
We thank Scott Cooper for helping with the Figure.
Financial support and sponsorship The published studies by the authors were supported by the following US Public Health Service Grants from the NIH to HEB: R01 HL056416, R01 HL67384, R01 HL112669, and P01 DK090948. HAO was supported by NIH T32 training grant DK07519 to HEB.
Footnotes
Conflicts of Interest HEB is a member of the Medical Scientific Advisory Board of Corduse, a public cord blood banking company, and is a Founder of the Corduse family cord blood bank.
References and recommended reading
Papers of particular interest, published within the annual period of review have been highlighted as:
* Of special interest
** Of outstanding interest
- 1.Gramling C. Geochemistry. Low oxygen stifled animals' emergence, study says. Science. 2014;346:537. doi: 10.1126/science.346.6209.537. [DOI] [PubMed] [Google Scholar]
- 2.Planavsky NJ, Reinhard CT, Wang X, Thomson D, McGoldrick P, Rainbird RH, Johnson T, Fischer WW, Lyons TW. Earth history. Low mid-Proterozoic atmospheric oxygen levels and the delayed rise of animals. Science. 2014;346:635–638. doi: 10.1126/science.1258410. [DOI] [PubMed] [Google Scholar]
- *3.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327–334. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]; An excellent review on the intricacies of the microenvironmental niche in bone marrow that sustains HSCs.
- *4.Mendelson A, Frenette PS. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat Med. 2014;20:833–846. doi: 10.1038/nm.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]; An excellent review on the intricacies of the microenvironmental niche in bone marrow that sustains HSCs.
- 5.Parmar K, Mauch P, Vergilio JA, Sackstein R, Down JD. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc.Natl.Acad.Sci.U.S.A. 2007;104:5431–5436. doi: 10.1073/pnas.0701152104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nombela-Arrieta C, Pivarnik G, Winkel B, Canty KJ, Harley B, Mahoney JE, Park SY, Lu J, Protopopov A, Silberstein LE. Quantitative imaging of haematopoietic stem and progenitor cell localization and hypoxic status in the bone marrow microenvironment. Nat.Cell Biol. 2013;15:533–543. doi: 10.1038/ncb2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **7.Spencer JA, Ferraro F, Roussakis E, Klein A, Wu J, Runnels JM, Zaher W, Mortensen LJ, Alt C, Turcotte R, Yusuf R, Cote D, Vinogradov SA, Scadden DT, Lin CP. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature. 2014;508:269–273. doi: 10.1038/nature13034. [DOI] [PMC free article] [PubMed] [Google Scholar]; An outstanding in depth look at the oxygen status of HSC niches.
- 8.Mohyeldin A, Garzon-Muvdi T, Quinones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7:150–161. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Broxmeyer HE. Biomolecule-cell interactions. A review. Int. J. Cell. Cloning. 1986;4:378–405. doi: 10.1002/stem.5530040601. [DOI] [PubMed] [Google Scholar]
- 10.Broxmeyer HE, Williams DE. The production of myeloid blood cells and their regulation during health and disease. CRC Crit. Rev. Oncol./Hematol. 1988;8:173–226. doi: 10.1016/s1040-8428(88)80016-7. [DOI] [PubMed] [Google Scholar]
- 11.Shaheen M, Broxmeyer HE. Hematopoietic Cytokines and Growth Factors. In: Broxmeyer HE, editor. Cord Blood Biology, Transplantation, Banking, and Regulation. AABB Press; Bethesda, MD: 2011. pp. 35–74. [Google Scholar]
- 12.Bradley TR, Hodgson GS, Rosendaal M. The effect of oxygen tension on haemopoietic and fibroblast cell proliferation in vitro. J.Cell Physiol. 1978;97:517–522. doi: 10.1002/jcp.1040970327. [DOI] [PubMed] [Google Scholar]
- 13.Broxmeyer HE, Cooper S, Rubin BY, Taylor MW. The synergistic influence of human interferon-gamma and interferon-alpha on suppression of hematopoietic progenitor cells is additive with the enhanced sensitivity of these cells to inhibition by interferons at low oxygen tension in vitro. J.Immunol. 1985;135:2502–2506. [PubMed] [Google Scholar]
- 14.Lu L, Broxmeyer HE. Comparative influences of phytohemagglutinin-stimulated leukocyte conditioned medium, hemin, prostaglandin E, and low oxygen tension on colony formation by erythroid progenitor cells in normal human bone marrow. Exp.Hematol. 1985;13:989–993. [PubMed] [Google Scholar]
- 15.Smith S, Broxmeyer HE. The influence of oxygen tension on the long-term growth in vitro of haematopoietic progenitor cells from human cord blood. Br.J.Haematol. 1986;63:29–34. doi: 10.1111/j.1365-2141.1986.tb07491.x. [DOI] [PubMed] [Google Scholar]
- 16.Broxmeyer HE, Cooper S, Gabig T. The effects of oxidizing species derived from molecular oxygen on the proliferation in vitro of human granulocyte-macrophage progenitor cells. New York Acad. Sci. 1989;554:177–184. doi: 10.1111/j.1749-6632.1989.tb22419.x. [DOI] [PubMed] [Google Scholar]
- 17.Danet GH, Pan Y, Luongo JL, Bonnet DA, Simon MC. Expansion of human SCID-repopulating cells under hypoxic conditions. J.Clin.Invest. 2003;112:126–135. doi: 10.1172/JCI17669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grassinger J, Haylock DN, Williams B, Olsen GH, Nilsson SK. Phenotypically identical hemopoietic stem cells isolated from different regions of bone marrow have different biologic potential. Blood. 2010;116:3185–3196. doi: 10.1182/blood-2009-12-260703. [DOI] [PubMed] [Google Scholar]
- 19.Ivanovic Z. Hypoxia or in situ normoxia: The stem cell paradigm. J.Cell Physiol. 2009;219:271–275. doi: 10.1002/jcp.21690. [DOI] [PubMed] [Google Scholar]
- **20.Mantel CR, O'Leary HA, Chitteti BR, Huang X, Cooper S, Hangoc G, Brustovetsky N, Srour EF, Lee MR, Messina-Graham S, Haas DM, Falah N, Kapur R, Pelus LM, Bardeesy N, Fitamant J, Ivan M, Kim K-S, Broxmeyer HE. Extra Physiologic Oxygen Shock/Stress (EPHOSS) Limits Hematopoietic Stem Cell Collection and Function. Cell (in Press) 2015 doi: 10.1016/j.cell.2015.04.054. (CRM and HAO are equal co-first authors.) [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper has identified a new, and previously unrecognized detrimental effect of short exposure of tissue sources of hematopoietic stem cells (HSCs) to brief exposure to ambient air, as well as mechanisms involved and means to mitigate the effect for enhanced collections of HSCs, information of potential clinical relevance.
- 21.Sjostedt S, Rooth G, Caligara F. The oxygen tension of the blood in the umbilical cord and the intervillous space. Archives of Disease in Childhood. 1960;35:529–533. doi: 10.1136/adc.35.184.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ballen KK, Gluckman E, Broxmeyer HE. Umbilical cord blood transplantation: the first 25 years and beyond. Blood. 2013;122:491–498. doi: 10.1182/blood-2013-02-453175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munoz J, Shah N, Rezvani K, Hosing C, Bollard CM, Oran B, Olson A, Popat U, Molldrem J, McNiece IK, Shpall EJ. Concise review: umbilical cord blood transplantation: past, present, and future. Stem Cells Transl Med. 2014;3:1435–1443. doi: 10.5966/sctm.2014-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *24.Bigarella CL, Liang R, Ghaffari S. Stem cells and the impact of ROS signaling. Development. 2014;141:4206–4218. doi: 10.1242/dev.107086. [DOI] [PMC free article] [PubMed] [Google Scholar]; An excellent review of the role of ROS in stem cell function.
- 25.Doulatov S, Notta F, Laurenti E, Dick JE. Hematopoiesis: a human perspective. Cell Stem Cell. 2012;10:120–136. doi: 10.1016/j.stem.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Halestrap AP, Davidson AM. Inhibition of Ca2(+)-induced large-amplitude swelling of liver and heart mitochondria by cyclosporin is probably caused by the inhibitor binding to mitochondrial-matrix peptidyl-prolyl cis-trans isomerase and preventing it interacting with the adenine nucleotide translocase. Biochem.J. 1990;268:153–160. doi: 10.1042/bj2680153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaseva AV, Marchenko ND, Ji K, Tsirka SE, Holzmann S, Moll UM. p53 opens the mitochondrial permeability transition pore to trigger necrosis. Cell. 2012;149:1536–1548. doi: 10.1016/j.cell.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanveer A, Virji S, Andreeva L, Totty NF, Hsuan JJ, Ward JM, Crompton M. Involvement of cyclophilin D in the activation of a mitochondrial pore by Ca2+ and oxidant stress. Eur.J.Biochem. 1996;238:166–172. doi: 10.1111/j.1432-1033.1996.0166q.x. [DOI] [PubMed] [Google Scholar]
- 29.Connern CP, Halestrap AP. Recruitment of mitochondrial cyclophilin to the mitochondrial inner membrane under conditions of oxidative stress that enhance the opening of a calcium-sensitive non-specific channel. Biochem.J. 1994;302(Pt 2):321–324. doi: 10.1042/bj3020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGuinness O, Yafei N, Costi A, Crompton M. The presence of two classes of high-affinity cyclosporin A binding sites in mitochondria. Evidence that the minor component is involved in the opening of an inner-membrane Ca(2+)-dependent pore. Eur.J.Biochem. 1990;194:671–679. doi: 10.1111/j.1432-1033.1990.tb15667.x. [DOI] [PubMed] [Google Scholar]
- 31.Nicolli A, Basso E, Petronilli V, Wenger RM, Bernardi P. Interactions of cyclophilin with the mitochondrial inner membrane and regulation of the permeability transition pore, and cyclosporin A-sensitive channel. J.Biol.Chem. 1996;271:2185–2192. doi: 10.1074/jbc.271.4.2185. [DOI] [PubMed] [Google Scholar]
- 32.Junghanss C, Rathsack S, Wacke R, Weirich V, Vogel H, Drewelow B, Mueller S, Altmann S, Freund M, Lange S. Everolimus in combination with cyclosporin a as pre- and posttransplantation immunosuppressive therapy in nonmyeloablative allogeneic hematopoietic stem cell transplantation. Biol.Blood Marrow Transplant. 2012;18:1061–1068. doi: 10.1016/j.bbmt.2011.12.522. [DOI] [PubMed] [Google Scholar]
- 33.Kikuchi T, Mori T, Yamane A, Kato J, Kohashi S, Okamoto S. Variable magnitude of drug interaction between oral voriconazole and cyclosporine A in recipients of allogeneic hematopoietic stem cell transplantation. Clin.Transplant. 2012;26:E544–E548. doi: 10.1111/ctr.12016. [DOI] [PubMed] [Google Scholar]
- 34.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 35.Baines CP. The cardiac mitochondrion: nexus of stress. Annu.Rev.Physiol. 2010;72:61–80. doi: 10.1146/annurev-physiol-021909-135929. [DOI] [PubMed] [Google Scholar]
- 36.Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Cell biology of ischemia/reperfusion injury. Int.Rev.Cell Mol.Biol. 2012;298:229–317. doi: 10.1016/B978-0-12-394309-5.00006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo W. Concise review: breast cancer stem cells: regulatory networks, stem cell niches, and disease relevance. Stem Cells Transl Med. 2014;3:942–948. doi: 10.5966/sctm.2014-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown JM, Giaccia AJ. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Res. 1998;58:1408–1416. [PubMed] [Google Scholar]
- 39.Hill RP, Marie-Egyptienne DT, Hedley DW. Cancer stem cells, hypoxia and metastasis. Semin.Radiat.Oncol. 2009;19:06–111. doi: 10.1016/j.semradonc.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 40.Millman JR, Tan JH, Colton CK. The effects of low oxygen on self-renewal and differentiation of embryonic stem cells. Curr.Opin.Organ Transplant. 2009;14:694–700. doi: 10.1097/MOT.0b013e3283329d53. [DOI] [PubMed] [Google Scholar]
- 41.Mohyeldin A, Garzón-Muvdi T, Quiñones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7:150–161. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 42.Tothova Z, Gilliland DG. A radical bailout strategy for cancer stem cells. Cell Stem Cell. 2009;4:196–197. doi: 10.1016/j.stem.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 43.Jang YY, Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood. 2007;110:3056–3063. doi: 10.1182/blood-2007-05-087759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choi JR, Pingguan-Murphy B, Wan Abas WA, Noor Azmi MA, Omar SZ, Chua KH, Wan Safwani WK. Impact of low oxygen tension on stemness, proliferation and differentiation potential of human adipose-derived stem cells. Biochem.Biophys.Res.Commun. 2014;448:218–224. doi: 10.1016/j.bbrc.2014.04.096. [DOI] [PubMed] [Google Scholar]
- 45.Ye ZW, Zhang J, Townsend DM, Tew KD. Oxidative stress, redox regulation and diseases of cellular differentiation. Biochim.Biophys.Acta. 2014 doi: 10.1016/j.bbagen.2014.11.010. pii: S0304-4165(14)00387-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ng KP, Manjeri A, Lee KL, Huang W, Tan SY, Chuah CT, Poellinger L, Ong ST. Physiologic hypoxia promotes maintenance of CML stem cells despite effective BCR-ABL1 inhibition. Blood. 2014;123:3316–3326. doi: 10.1182/blood-2013-07-511907. [DOI] [PubMed] [Google Scholar]
- 47.Qi S, Fang Z, Wang D, Menendez P, Yao K, Ji J. Induced pluripotency by defined factors: Prey of oxidative stress. Stem Cells. 2015 Jan 16; doi: 10.1002/stem.1946. doi:10.1002/stem.1946. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- *48.Ito K, Suda T. Metabolic requirements for the maintenance of self-renewing stem cells. Nat.Rev.Mol.Cell Biol. 2014;15:243–256. doi: 10.1038/nrm3772. [DOI] [PMC free article] [PubMed] [Google Scholar]; An excellent review on the metabolism of HSCs and HSC function.
- 49.Kohli L, Passegue E. Surviving change: the metabolic journey of hematopoietic stem cells. Trends Cell Biol. 2014;24:479–487. doi: 10.1016/j.tcb.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galluzzi L, Pietrocola F, Levine B, Kroemer G. Metabolic Control of Autophagy. Cell. 2014;159:1263–1276. doi: 10.1016/j.cell.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oburoglu L, Tardito S, Fritz V, de Barros SC, Merida P, Craveiro M, Mamede J, Cretenet G, Mongellaz C, An X, Klysz D, Touhami J, Boyer-Clavel M, Battini JL, Dardalhon V, Zimmermann VS, Mohandas N, Gottlieb E, Sitbon M, Kinet S, Taylor N. Glucose and glutamine metabolism regulate human hematopoietic stem cell lineage specification. Cell Stem Cell. 2014;15:169–184. doi: 10.1016/j.stem.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 52.Harris JM, Esain V, Frechette GM, Harris LJ, Cox AG, Cortes M, Garnaas MK, Carroll KJ, Cutting CC, Khan T, Elks PM, Renshaw SA, Dickinson BC, Chang CJ, Murphy MP, Paw BH, Vander Heiden MG, Goessling W, North TE. Glucose metabolism impacts the spatiotemporal onset and magnitude of HSC induction in vivo. Blood. 2013;121:2483–2493. doi: 10.1182/blood-2012-12-471201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang YH, Israelsen WJ, Lee D, Yu VW, Jeanson NT, Clish CB, Cantley LC, Vander Heiden MG, Scadden DT. Cell-state-specific metabolic dependency in hematopoiesis and leukemogenesis. Cell. 2014;158:1309–1323. doi: 10.1016/j.cell.2014.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134:703–707. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 55.Kamleh MA, Spagou K, Want EJ. Metabolic profiling in disease diagnosis, toxicology and personalized healthcare. Curr.Pharm.Biotechnol. 2011;12:976–995. doi: 10.2174/138920111795909069. [DOI] [PubMed] [Google Scholar]
- 56.Wood SL, Westbrook JA, Brown JE. Omic-profiling in breast cancer metastasis to bone: implications for mechanisms, biomarkers and treatment. Cancer Treat.Rev. 2014;40:139–152. doi: 10.1016/j.ctrv.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 57.Chambers SM, Shaw CA, Gatza C, Fisk CJ, Donehower LA, Goodell MA. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS.Biol. 2007;5:e201. doi: 10.1371/journal.pbio.0050201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ergen AV, Goodell MA. Mechanisms of hematopoietic stem cell aging. Exp.Gerontol. 2010;45:286–290. doi: 10.1016/j.exger.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mantel C, Broxmeyer HE. Sirtuin 1, stem cells, aging, and stem cell aging. Curr.Opin.Hematol. 2008;15:326–331. doi: 10.1097/MOH.0b013e3283043819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mantel C, Messina-Graham SV, Broxmeyer HE. Superoxide flashes, reactive oxygen species, and the mitochondrial permeability transition pore: potential implications for hematopoietic stem cell function. Curr.Opin.Hematol. 2011;18:208–213. doi: 10.1097/MOH.0b013e3283475ffe. [DOI] [PubMed] [Google Scholar]
- 61.Sudo K, Ema H, Morita Y, Nakauchi H. Age-associated characteristics of murine hematopoietic stem cells. J.Exp.Med. 2000;192:1273–1280. doi: 10.1084/jem.192.9.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nakamura-Ishizu A, Suda T. Aging of the hematopoietic stem cells niche. Int.J.Hematol. 2014;100:317–325. doi: 10.1007/s12185-014-1641-8. [DOI] [PubMed] [Google Scholar]
- 63.Flach J, Bakker ST, Mohrin M, Conroy PC, Pietras EM, Reynaud D, Alvarez S, Diolaiti ME, Ugarte F, Forsberg EC, Le Beau MM, Stohr BA, Méndez J, Morrison CG, Passegué E. Replication stress is a potent driver of functional decline in ageing haematopoietic stem cells. Nature. 2014;512:198–202. doi: 10.1038/nature13619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soulier J. When old hematopoietic stem cells get damaged. Cell Stem Cell. 2014;15:399–400. doi: 10.1016/j.stem.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 65.Oshima M, Iwama A. Epigenetics of hematopoietic stem cell aging and disease. Int.J.Hematol. 2014;100:326–334. doi: 10.1007/s12185-014-1647-2. [DOI] [PubMed] [Google Scholar]
- 66.Christopherson KW, II, Hangoc G, Mantel C, Broxmeyer HE. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 2004;305:1000–1003. doi: 10.1126/science.1097071. [DOI] [PubMed] [Google Scholar]
- 67.Broxmeyer HE, Hoggatt J, O'Leary HA, Mantel C, Chitteti BR, Cooper S, Messina-Graham S, Hangoc G, Farag S, Rohrabaugh SL, Ou X, Speth J, Pelus LM, Srour EF, Campbell TB. Dipeptidylpeptidase 4 Negatively Regulates Colony Stimulating Factor Activity and Stress Hematopoiesis. Nature Medicine. 2012;18:1786–1796. doi: 10.1038/nm.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ou X, O'Leary HA, Broxmeyer HE. Implications of DPP4 modification of proteins that regulate stem/progenitor and more mature cell types. Blood. 2013;122:161–169. doi: 10.1182/blood-2013-02-487470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.O'Leary HA, Ou X, Broxmeyer HE. The Role of DPP4 in hematopoiesis and transplantation. Current Opinions in Hematopoiesis (Hematology) 2013;20:314–319. doi: 10.1097/MOH.0b013e32836125ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cabezas-Wallscheid N, Klimmeck D, Hansson J, Lipka DB, Reyes A, Wang Q, Weichenhan D, Lier A, von PL, Renders S, Wunsche P, Zeisberger P, Brocks D, Gu L, Herrmann C, Haas S, Essers MA, Brors B, Eils R, Huber W, Milsom MD, Plass C, Krijgsveld J, Trumpp A. Identification of regulatory networks in HSCs and their immediate progeny via integrated proteome, transcriptome, and DNA Methylome analysis. Cell Stem Cell. 2014;15:507–522. doi: 10.1016/j.stem.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 71.Charbord P, Pouget C, Binder H, Dumont F, Stik G, Levy P, Allain F, Marchal C, Richter J, Uzan B, Pflumio F, Letourneur F, Wirth H, Dzierzak E, Traver D, Jaffredo T, Durand C. A systems biology approach for defining the molecular framework of the hematopoietic stem cell niche. Cell Stem Cell. 2014;15:376–391. doi: 10.1016/j.stem.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 72.Cahan P, Li H, Morris SA, Lummertz da Rocha E, Daley GQ, Collins JJ. CellNet: network biology applied to stem cell engineering. Cell. 2014;158:903–915. doi: 10.1016/j.cell.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morris SA, Cahan P, Li H, Zhao AM, San Roman AK, Shivdasani RA, Collins JJ, Daley GQ. Dissecting engineered cell types and enhancing cell fate conversion via CellNet. Cell. 2014;158:889–902. doi: 10.1016/j.cell.2014.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]